Obstructive Sleep Apnea and Acute Lower Respiratory Tract Infections: A Narrative Literature Review
Abstract
1. Introduction
2. Literature Search Strategy
3. Obstructive Sleep Apnea and Community-Acquired Pneumonia
4. Obstructive Sleep Apnea and Influenza Pneumonia
5. Obstructive Sleep Apnea and COVID-19 Pneumonia
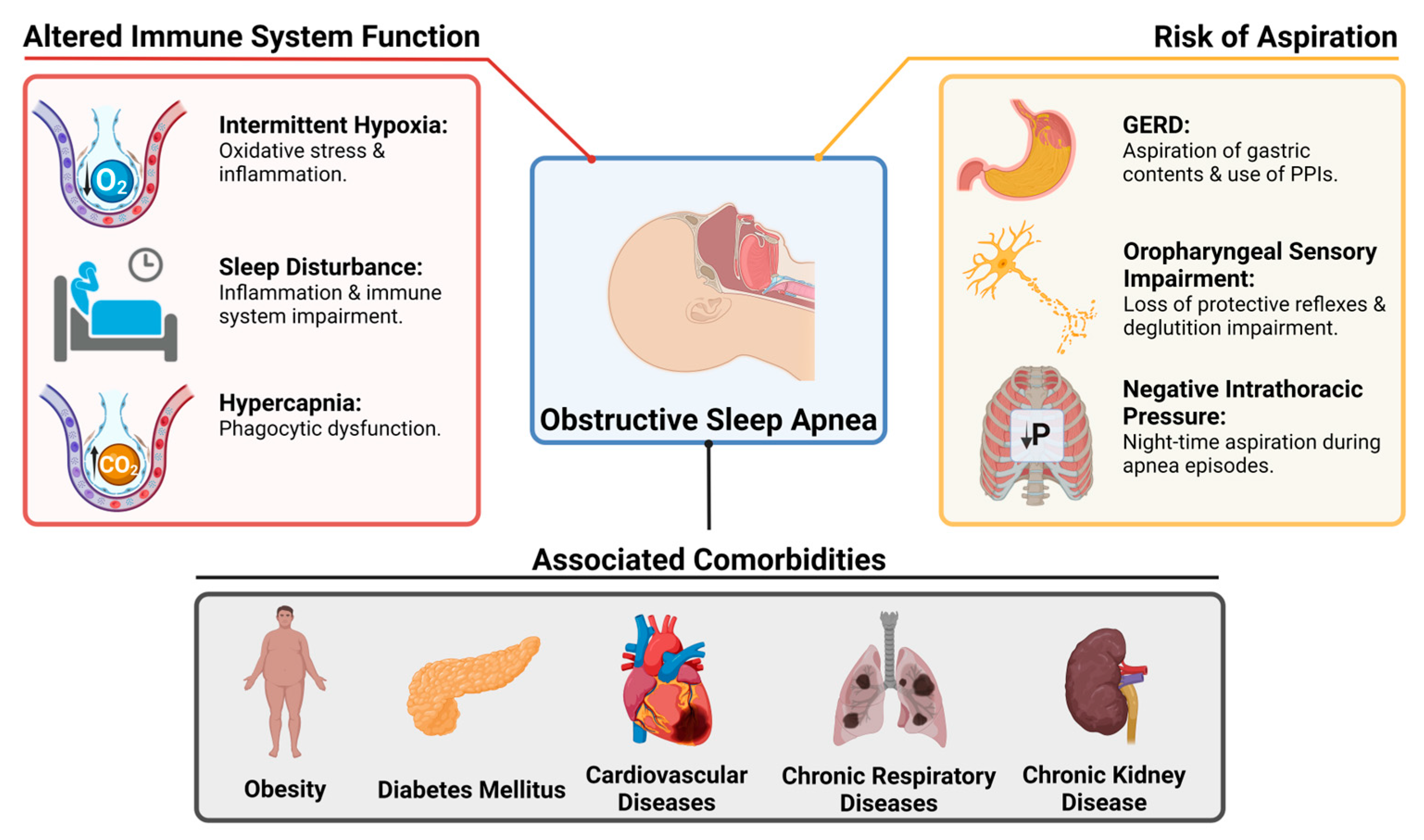
6. Obstructive Sleep Apnea and Lower Respiratory Tract Infections: Pathophysiology
6.1. Altered Immunity
6.2. Risk of Aspiration
6.3. The Role of Obesity and Other Comorbidities
7. Obstructive Sleep Apnea and Lower Respiratory Tract Infections: Treatment
7.1. Settings of Care and Empiric Antibiotics
7.2. Specific Risks Guiding Empiric Antibiotic Therapy
7.3. Antibiotic Pharmacokinetics, Side Effects, and Resistance
8. Discussion
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef]
- Heinzer, R.; Vat, S.; Marques-Vidal, P.; Marti-Soler, H.; Andries, D.; Tobback, N.; Mooser, V.; Preisig, M.; Malhotra, A.; Waeber, G.; et al. Prevalence of sleep-disordered breathing in the general population: The HypnoLaus study. Lancet Respir. Med. 2015, 3, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, J.A.; Veasey, S.C.; Morgan, B.J.; O’Donnell, C.P. Pathophysiology of sleep apnea. Physiol. Rev. 2010, 90, 47–112. [Google Scholar] [CrossRef]
- Eckert, D.J.; Malhotra, A. Pathophysiology of adult obstructive sleep apnea. Proc. Am. Thorac. Soc. 2008, 5, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Randerath, W.; Bassetti, C.L.; Bonsignore, M.R.; Farre, R.; Ferini-Strambi, L.; Grote, L.; Hedner, J.; Kohler, M.; Martinez-Garcia, M.A.; Mihaicuta, S.; et al. Challenges and perspectives in obstructive sleep apnoea: Report by an ad hoc working group of the Sleep Disordered Breathing Group of the European Respiratory Society and the European Sleep Research Society. Eur. Respir. J. 2018, 52, 1702616. [Google Scholar] [CrossRef] [PubMed]
- Bonsignore, M.R.; Baiamonte, P.; Mazzuca, E.; Castrogiovanni, A.; Marrone, O. Obstructive sleep apnea and comorbidities: A dangerous liaison. Multidiscip. Respir. Med. 2019, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Ko, I.; Kim, D.K. Association of Obstructive Sleep Apnea with the Risk of Affective Disorders. JAMA Otolaryngol. Head. Neck Surg. 2019, 145, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Feldman, C.; Shaddock, E. Epidemiology of lower respiratory tract infections in adults. Expert. Rev. Respir. Med. 2019, 13, 63–77. [Google Scholar] [CrossRef] [PubMed]
- International Respiratory Coalition (IRC). Lower Respiratory Tract Infections. Available online: https://international-respiratory-coalition.org/diseases/lower-respiratory-tract-infections/ (accessed on 10 April 2024).
- The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 10 April 2024).
- Docherty, A.B.; Harrison, E.M.; Green, C.A.; Hardwick, H.E.; Pius, R.; Norman, L.; Holden, K.A.; Read, J.M.; Dondelinger, F.; Carson, G.; et al. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ 2020, 369, m1985. [Google Scholar] [CrossRef]
- Mizgerd, J.P. Acute lower respiratory tract infection. N. Engl. J. Med. 2008, 358, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, K.; Huppertz, T.; Radsak, M.; Gouveris, H. Cellular Immune Dysfunction in Obstructive Sleep Apnea. Front. Surg. 2022, 9, 890377. [Google Scholar] [CrossRef] [PubMed]
- Faverio, P.; Zanini, U.; Monzani, A.; Parati, G.; Luppi, F.; Lombardi, C.; Perger, E. Sleep-Disordered Breathing and Chronic Respiratory Infections: A Narrative Review in Adult and Pediatric Population. Int. J. Mol. Sci. 2023, 24, 5504. [Google Scholar] [CrossRef] [PubMed]
- Keto, J.; Feuth, T.; Linna, M.; Saaresranta, T. Lower respiratory tract infections among newly diagnosed sleep apnea patients. BMC Pulm. Med. 2023, 23, 332. [Google Scholar] [CrossRef] [PubMed]
- Grant, L.R.; Meche, A.; McGrath, L.; Miles, A.; Alfred, T.; Yan, Q.; Chilson, E. Risk of Pneumococcal Disease in US Adults by Age and Risk Profile. Open Forum Infect. Dis. 2023, 10, ofad192. [Google Scholar] [CrossRef] [PubMed]
- Lutsey, P.L.; Zineldin, I.; Misialek, J.R.; Full, K.M.; Lakshminarayan, K.; Ishigami, J.; Cowan, L.T.; Matsushita, K.; Demmer, R.T. OSA and Subsequent Risk of Hospitalization with Pneumonia, Respiratory Infection, and Total Infection: The Atherosclerosis Risk in Communities Study. Chest 2023, 163, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Chiner, E.; Llombart, M.; Valls, J.; Pastor, E.; Sancho-Chust, J.N.; Andreu, A.L.; Sánchez-de-la-Torre, M.; Barbé, F. Association between Obstructive Sleep Apnea and Community-Acquired Pneumonia. PLoS ONE 2016, 11, e0152749. [Google Scholar] [CrossRef] [PubMed]
- Su, V.Y.; Liu, C.J.; Wang, H.K.; Wu, L.A.; Chang, S.C.; Perng, D.W.; Su, W.J.; Chen, Y.M.; Lin, E.Y.; Chen, T.J.; et al. Sleep apnea and risk of pneumonia: A nationwide population-based study. CMAJ 2014, 186, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Lindenauer, P.K.; Stefan, M.S.; Johnson, K.G.; Priya, A.; Pekow, P.S.; Rothberg, M.B. Prevalence, treatment, and outcomes associated with OSA among patients hospitalized with pneumonia. Chest 2014, 145, 1032–1038. [Google Scholar] [CrossRef]
- Beumer, M.C.; Koch, R.M.; van Beuningen, D.; OudeLashof, A.M.; van de Veerdonk, F.L.; Kolwijck, E.; van der Hoeven, J.G.; Bergmans, D.C.; Hoedemaekers, C.W.E. Influenza virus and factors that are associated with ICU admission, pulmonary co-infections and ICU mortality. J. Crit. Care 2019, 50, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Boattini, M.; Charrier, L.; Almeida, A.; Christaki, E.; Moreira Marques, T.; Tosatto, V.; Bianco, G.; Iannaccone, M.; Tsiolakkis, G.; Karagiannis, C.; et al. Burden of primary influenza and respiratory syncytial virus pneumonia in hospitalised adults: Insights from a 2-year multi-centre cohort study (2017–2018). Intern. Med. J. 2023, 53, 404–408. [Google Scholar] [CrossRef]
- Mok, E.M.; Greenough, G.; Pollack, C.C. Untreated obstructive sleep apnea is associated with increased hospitalization from influenza infection. J. Clin. Sleep Med. 2020, 16, 2003–2007. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.S.; Chen, H.C.; Li, H.Y.; Tsai, Y.T.; Yang, Y.H.; Liu, C.Y.; Lee, Y.C.; Hsu, C.M.; Lee, L.A. Sleep Apnea and Risk of Influenza-Associated Severe Acute Respiratory Infection: Real-World Evidence. Nat. Sci. Sleep 2022, 14, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.Y.; Chang, R.; Chiu, L.T.; Hung, Y.M.; Wei, J.C. Obstructive sleep apnea and influenza infection: A nationwide population-based cohort study. Sleep Med. 2021, 81, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Mashaqi, S.; Lee-Iannotti, J.; Rangan, P.; Celaya, M.P.; Gozal, D.; Quan, S.F.; Parthasarathy, S. Obstructive sleep apnea and COVID-19 clinical outcomes during hospitalization: A cohort study. J. Clin. Sleep Med. 2021, 17, 2197–2204. [Google Scholar] [CrossRef] [PubMed]
- Maas, M.B.; Kim, M.; Malkani, R.G.; Abbott, S.M.; Zee, P.C. Obstructive Sleep Apnea and Risk of COVID-19 Infection, Hospitalization and Respiratory Failure. Sleep Breath 2021, 25, 1155–1157. [Google Scholar] [CrossRef] [PubMed]
- Strausz, S.; Kiiskinen, T.; Broberg, M.; Ruotsalainen, S.; Koskela, J.; Bachour, A.; Palotie, A.; Palotie, T.; Ripatti, S.; Ollila, H.M. Sleep apnoea is a risk factor for severe COVID-19. BMJ Open Respir. Res. 2021, 8, e000845. [Google Scholar] [CrossRef] [PubMed]
- Rögnvaldsson, K.G.; Eyþórsson, E.S.; Emilsson, Ö.I.; Eysteinsdóttir, B.; Pálsson, R.; Gottfreðsson, M.; Guðmundsson, G.; Steingrímsson, V. Obstructive sleep apnea is an independent risk factor for severe COVID-19: A population-based study. Sleep 2022, 45, zsab272. [Google Scholar] [CrossRef] [PubMed]
- Cade, B.E.; Dashti, H.S.; Hassan, S.M.; Redline, S.; Karlson, E.W. Sleep Apnea and COVID-19 Mortality and Hospitalization. Am. J. Respir. Crit. Care Med. 2020, 202, 1462–1464. [Google Scholar] [CrossRef] [PubMed]
- Pena Orbea, C.; Wang, L.; Shah, V.; Jehi, L.; Milinovich, A.; Foldvary-Schaefer, N.; Chung, M.K.; Mashaqi, S.; Aboussouan, L.; Seidel, K.; et al. Association of Sleep-Related Hypoxia with Risk of COVID-19 Hospitalizations and Mortality in a Large Integrated Health System. JAMA Netw. Open 2021, 4, e2134241. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.K.; Song, I.A. Impact of coronavirus disease-2019 on chronic respiratory disease in South Korea: An NHIS COVID-19 database cohort study. BMC Pulm. Med. 2021, 21, 12. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, M.; Sansom, S.; Frankenberger, C.; Ward, E.; Hota, B. Clinical Course and Factors Associated with Hospitalization and Critical Illness Among COVID-19 Patients in Chicago, Illinois. Acad. Emerg. Med. 2020, 27, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Kendzerska, T.; Povitz, M.; Gershon, A.S.; Ryan, C.M.; Talarico, R.; Franco Avecilla, D.A.; Robillard, R.; Ayas, N.T.; Pendharkar, S.R. Association of clinically significant obstructive sleep apnoea with risks of contracting COVID-19 and serious COVID-19 complications: A retrospective population-based study of health administrative data. Thorax 2023, 78, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Peker, Y.; Celik, Y.; Arbatli, S.; Isik, S.R.; Balcan, B.; Karataş, F.; Uzel, F.I.; Tabak, L.; Çetin, B.; Baygül, A.; et al. Effect of High-Risk Obstructive Sleep Apnea on Clinical Outcomes in Adults with Coronavirus Disease 2019: A Multicenter, Prospective, Observational Clinical Trial. Ann. Am. Thorac. Soc. 2021, 18, 1548–1559. [Google Scholar] [CrossRef] [PubMed]
- Girardin, J.L.; Seixas, A.; Ramos Cejudo, J.; Osorio, R.S.; Avirappattu, G.; Reid, M.; Parthasarathy, S. Contribution of pulmonary diseases to COVID-19 mortality in a diverse urban community of New York. Chron. Respir. Dis. 2021, 18, 1479973120986806. [Google Scholar] [CrossRef] [PubMed]
- Gimeno-Miguel, A.; Bliek-Bueno, K.; Poblador-Plou, B.; Carmona-Pírez, J.; Poncel-Falcó, A.; González-Rubio, F.; Ioakeim-Skoufa, I.; Pico-Soler, V.; Aza-Pascual-Salcedo, M.; Prados-Torres, A.; et al. Chronic diseases associated with increased likelihood of hospitalization and mortality in 68,913 COVID-19 confirmed cases in Spain: A population-based cohort study. PLoS ONE 2021, 16, e0259822. [Google Scholar] [CrossRef] [PubMed]
- Cariou, B.; Hadjadj, S.; Wargny, M.; Pichelin, M.; Al-Salameh, A.; Allix, I.; Amadou, C.; Arnault, G.; Baudoux, F.; Bauduceau, B.; et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: The CORONADO study. Diabetologia 2020, 63, 1500–1515. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N.; Locke, E.; Green, P.; Berry, K.; O’Hare, A.M.; Shah, J.A.; Crothers, K.; Eastment, M.C.; Dominitz, J.A.; Fan, V.S. Risk Factors for Hospitalization, Mechanical Ventilation, or Death Among 10 131 US Veterans With SARS-CoV-2 Infection. JAMA Netw. Open 2020, 3, e2022310. [Google Scholar] [CrossRef]
- Izquierdo, J.L.; Ancochea, J.; Soriano, J.B. Clinical Characteristics and Prognostic Factors for Intensive Care Unit Admission of Patients with COVID-19: Retrospective Study Using Machine Learning and Natural Language Processing. J. Med. Internet Res. 2020, 22, e21801. [Google Scholar] [CrossRef] [PubMed]
- Lohia, P.; Sreeram, K.; Nguyen, P.; Choudhary, A.; Khicher, S.; Yarandi, H.; Kapur, S.; Badr, M.S. Preexisting respiratory diseases and clinical outcomes in COVID-19: A multihospital cohort study on predominantly African American population. Respir. Res. 2021, 22, 37. [Google Scholar] [CrossRef] [PubMed]
- Prasad, B.; Mechineni, A.; Talugula, S.; Gardner, J.; Rubinstein, I.; Gordon, H.S. Impact of Obstructive Sleep Apnea on Health Outcomes in Veterans Hospitalized with COVID-19 Infection. Ann. Am. Thorac. Soc. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Bailly, S.; Galerneau, L.M.; Ruckly, S.; Seiller, A.; Terzi, N.; Schwebel, C.; Dupuis, C.; Tamisier, R.; Mourvillier, B.; Pepin, J.L.; et al. Impact of obstructive sleep apnea on the obesity paradox in critically ill patients. J. Crit. Care 2020, 56, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Bolona, E.; Hahn, P.Y.; Afessa, B. Intensive care unit and hospital mortality in patients with obstructive sleep apnea. J. Crit. Care 2015, 30, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Webster, R.G.; Webby, R.J. Influenza Virus: Dealing with a Drifting and Shifting Pathogen. Viral Immunol. 2018, 31, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, C.S.; Allen, J.L.Y.; Gkrania-Klotsas, E. Influenza: Epidemiology and hospital management. Medicine 2021, 49, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Veerapandian, R.; Snyder, J.D.; Samarasinghe, A.E. Influenza in Asthmatics: For Better or for Worse? Front. Immunol. 2018, 9, 1843. [Google Scholar] [CrossRef] [PubMed]
- Rennard, S.; Decramer, M.; Calverley, P.M.; Pride, N.B.; Soriano, J.B.; Vermeire, P.A.; Vestbo, J. Impact of COPD in North America and Europe in 2000: Subjects’ perspective of Confronting COPD International Survey. Eur. Respir. J. 2002, 20, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Denson, J.L.; Gillet, A.S.; Zu, Y.; Brown, M.; Pham, T.; Yoshida, Y.; Mauvais-Jarvis, F.; Douglas, I.S.; Moore, M.; Tea, K.; et al. Metabolic Syndrome and Acute Respiratory Distress Syndrome in Hospitalized Patients With COVID-19. JAMA Netw. Open 2021, 4, e2140568. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Cappuccio, F.P. A systematic review of COVID-19 and obstructive sleep apnoea. Sleep Med. Rev. 2021, 55, 101382. [Google Scholar] [CrossRef] [PubMed]
- Bellou, V.; Tzoulaki, I.; van Smeden, M.; Moons, K.G.M.; Evangelou, E.; Belbasis, L. Prognostic factors for adverse outcomes in patients with COVID-19: A field-wide systematic review and meta-analysis. Eur. Respir. J. 2022, 59, 2002964. [Google Scholar] [CrossRef] [PubMed]
- Vardavas, C.I.; Mathioudakis, A.G.; Nikitara, K.; Stamatelopoulos, K.; Georgiopoulos, G.; Phalkey, R.; Leonardi-Bee, J.; Fernandez, E.; Carnicer-Pont, D.; Vestbo, J.; et al. Prognostic factors for mortality, intensive care unit and hospital admission due to SARS-CoV-2: A systematic review and meta-analysis of cohort studies in Europe. Eur. Respir. Rev. 2022, 31, 220098. [Google Scholar] [CrossRef] [PubMed]
- Hariyanto, T.I.; Kurniawan, A. Obstructive sleep apnea (OSA) and outcomes from coronavirus disease 2019 (COVID-19) pneumonia: A systematic review and meta-analysis. Sleep Med. 2021, 82, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Han, X.; Ren, J.; Wang, Y.; Yang, H. Significant association of obstructive sleep apnoea with increased risk for fatal COVID-19: A quantitative meta-analysis based on adjusted effect estimates. Sleep Med. Rev. 2022, 63, 101624. [Google Scholar] [CrossRef] [PubMed]
- Mandel, L.H.; Colleen, G.; Abedian, S.; Ammar, N.; Charles Bailey, L.; Bennett, T.D.; Daniel Brannock, M.; Brosnahan, S.B.; Chen, Y.; Chute, C.G.; et al. Risk of post-acute sequelae of SARS-CoV-2 infection associated with pre-coronavirus disease obstructive sleep apnea diagnoses: An electronic health record-based analysis from the RECOVER initiative. Sleep 2023, 46, zsad126. [Google Scholar] [CrossRef] [PubMed]
- Labarca, G.; Henríquez-Beltrán, M.; Lamperti, L.; Nova-Lamperti, E.; Sanhueza, S.; Cabrera, C.; Quiroga, R.; Antilef, B.; Ormazábal, V.; Zúñiga, F.; et al. Impact of Obstructive Sleep Apnea (OSA) in COVID-19 Survivors, Symptoms Changes Between 4-Months and 1 Year After the COVID-19 Infection. Front. Med. 2022, 9, 884218. [Google Scholar] [CrossRef] [PubMed]
- Chervin, R.D. Sleepiness, fatigue, tiredness, and lack of energy in obstructive sleep apnea. Chest 2000, 118, 372–379. [Google Scholar] [CrossRef] [PubMed]
- O’Mahoney, L.L.; Routen, A.; Gillies, C.; Ekezie, W.; Welford, A.; Zhang, A.; Karamchandani, U.; Simms-Williams, N.; Cassambai, S.; Ardavani, A.; et al. The prevalence and long-term health effects of Long Covid among hospitalised and non-hospitalised populations: A systematic review and meta-analysis. eClinicalMedicine 2023, 55, 101762. [Google Scholar] [CrossRef] [PubMed]
- Wulf Hanson, S.; Abbafati, C.; Aerts, J.G.; Al-Aly, Z.; Ashbaugh, C.; Ballouz, T.; Blyuss, O.; Bobkova, P.; Bonsel, G.; Borzakova, S.; et al. Estimated Global Proportions of Individuals with Persistent Fatigue, Cognitive, and Respiratory Symptom Clusters Following Symptomatic COVID-19 in 2020 and 2021. JAMA 2022, 328, 1604–1615. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, K.; Laffan, A.M.; Harrison, S.L.; Redline, S.; Spira, A.P.; Ensrud, K.E.; Ancoli-Israel, S.; Stone, K.L. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 2011, 306, 613–619. [Google Scholar] [CrossRef]
- Huang, L.; Yao, Q.; Gu, X.; Wang, Q.; Ren, L.; Wang, Y.; Hu, P.; Guo, L.; Liu, M.; Xu, J.; et al. 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet 2021, 398, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Wheaton, A.G.; Perry, G.S.; Chapman, D.P.; Croft, J.B. Sleep disordered breathing and depression among U.S. adults: National Health and Nutrition Examination Survey, 2005–2008. Sleep 2012, 35, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Rezaeitalab, F.; Moharrari, F.; Saberi, S.; Asadpour, H.; Rezaeetalab, F. The correlation of anxiety and depression with obstructive sleep apnea syndrome. J. Res. Med. Sci. 2014, 19, 205–210. [Google Scholar] [PubMed]
- Menzler, K.; Mayr, P.; Knake, S.; Cassel, W.; Viniol, C.; Reitz, L.; Tsalouchidou, P.E.; Janzen, A.; Anschuetz, K.; Mross, P.; et al. Undiagnosed obstructive sleep apnea syndrome as a treatable cause of new-onset sleepiness in some post-COVID patients. Eur. J. Neurol. 2024, 31, e16159. [Google Scholar] [CrossRef] [PubMed]
- Punjabi, N.M.; Caffo, B.S.; Goodwin, J.L.; Gottlieb, D.J.; Newman, A.B.; O’Connor, G.T.; Rapoport, D.M.; Redline, S.; Resnick, H.E.; Robbins, J.A.; et al. Sleep-disordered breathing and mortality: A prospective cohort study. PLoS Med. 2009, 6, e1000132. [Google Scholar] [CrossRef] [PubMed]
- Kheirandish-Gozal, L.; Gozal, D. Obstructive Sleep Apnea and Inflammation: Proof of Concept Based on Two Illustrative Cytokines. Int. J. Mol. Sci. 2019, 20, 459. [Google Scholar] [CrossRef] [PubMed]
- Dewan, N.A.; Nieto, F.J.; Somers, V.K. Intermittent hypoxemia and OSA: Implications for comorbidities. Chest 2015, 147, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Kimoff, R.J. Sleep fragmentation in obstructive sleep apnea. Sleep 1996, 19, S61–S66. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.; Savransky, V.; Nanayakkara, A.; Bevans, S.; Li, J.; Smith, P.L.; Polotsky, V.Y. Intermittent hypoxia has organ-specific effects on oxidative stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1274–R1281. [Google Scholar] [CrossRef] [PubMed]
- Imani, M.M.; Sadeghi, M.; Khazaie, H.; Emami, M.; Sadeghi Bahmani, D.; Brand, S. Evaluation of Serum and Plasma Interleukin-6 Levels in Obstructive Sleep Apnea Syndrome: A Meta-Analysis and Meta-Regression. Front. Immunol. 2020, 11, 1343. [Google Scholar] [CrossRef] [PubMed]
- Van der Touw, T.; Andronicos, N.M.; Smart, N. Is C-reactive protein elevated in obstructive sleep apnea? A systematic review and meta-analysis. Biomarkers 2019, 24, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Freund, A.; Orjalo, A.V.; Desprez, P.Y.; Campisi, J. Inflammatory networks during cellular senescence: Causes and consequences. Trends Mol. Med. 2010, 16, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Bientinesi, E.; Monti, D. Immunosenescence and inflammaging in the aging process: Age-related diseases or longevity? Ageing Res. Rev. 2021, 71, 101422. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.D.; Walters, E.H.; Simpson, J.L.; Keely, S.; Wark, P.A.B.; O’Toole, R.F.; Hansbro, P.M. Hypoxia-inducible factor and bacterial infections in chronic obstructive pulmonary disease. Respirology 2020, 25, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.R.; Peng, Y.J.; Nanduri, J. Hypoxia-inducible factors and obstructive sleep apnea. J. Clin. Investig. 2020, 130, 5042–5051. [Google Scholar] [CrossRef] [PubMed]
- Besedovsky, L.; Lange, T.; Born, J. Sleep and immune function. Pflugers Arch. 2012, 463, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Coronado, E.G.; Pantaleón-Martínez, A.M.; Velazquéz-Moctezuma, J.; Prospéro-García, O.; Méndez-Díaz, M.; Pérez-Tapia, M.; Pavón, L.; Morales-Montor, J. The Bidirectional Relationship between Sleep and Immunity against Infections. J. Immunol. Res. 2015, 2015, 678164. [Google Scholar] [CrossRef] [PubMed]
- Dopp, J.M.; Wiegert, N.A.; Moran, J.J.; Muller, D.; Weber, S.; Hayney, M.S. Humoral immune responses to influenza vaccination in patients with obstructive sleep apnea. Pharmacotherapy 2007, 27, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Tufik, S.; Andersen, M.L.; Rosa, D.S.; Tufik, S.B.; Pires, G.N. Effects of Obstructive Sleep Apnea on SARS-CoV-2 Antibody Response After Vaccination Against COVID-19 in Older Adults. Nat. Sci. Sleep 2022, 14, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Quach, H.Q.; Warner, N.D.; Ovsyannikova, I.G.; Covassin, N.; Poland, G.A.; Somers, V.K.; Kennedy, R.B. Excessive daytime sleepiness is associated with impaired antibody response to influenza vaccination in older male adults. Front. Cell Infect. Microbiol. 2023, 13, 1229035. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, S.; Lange, T.; Tieken, S.; Fehm, H.L.; Born, J. Sleep associated regulation of T helper 1/T helper 2 cytokine balance in humans. Brain Behav. Immun. 2004, 18, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Malhotra, A.; Gao, X.; Hu, F.B.; Neuman, M.I.; Fawzi, W.W. A prospective study of sleep duration and pneumonia risk in women. Sleep 2012, 35, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Resta, O.; Foschino Barbaro, M.P.; Bonfitto, P.; Talamo, S.; Mastrosimone, V.; Stefano, A.; Giliberti, T. Hypercapnia in obstructive sleep apnoea syndrome. Neth. J. Med. 2000, 56, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Budhiraja, R.; Siddiqi, T.A.; Quan, S.F. Sleep disorders in chronic obstructive pulmonary disease: Etiology, impact, and management. J. Clin. Sleep Med. 2015, 11, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Macavei, V.M.; Spurling, K.J.; Loft, J.; Makker, H.K. Diagnostic predictors of obesity-hypoventilation syndrome in patients suspected of having sleep disordered breathing. J. Clin. Sleep Med. 2013, 9, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Gates, K.L.; Howell, H.A.; Nair, A.; Vohwinkel, C.U.; Welch, L.C.; Beitel, G.J.; Hauser, A.R.; Sznajder, J.I.; Sporn, P.H. Hypercapnia impairs lung neutrophil function and increases mortality in murine pseudomonas pneumonia. Am. J. Respir. Cell Mol. Biol. 2013, 49, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Hua, L.; Chen, L.; Mu, T.; Dong, D.; Xu, J.; Shen, C. Causal association between obstructive sleep apnea and gastroesophageal reflux disease: A bidirectional two-sample Mendelian randomization study. Front. Genet. 2023, 14, 1111144. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.H.; Yang, X.P.; Niu, X.; Xiao, X.Y.; Chen, X. The relationship between obstructive sleep apnea hypopnea syndrome and gastroesophageal reflux disease: A meta-analysis. Sleep Breath 2019, 23, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Emilsson, Ö.I.; Bengtsson, A.; Franklin, K.A.; Torén, K.; Benediktsdóttir, B.; Farkhooy, A.; Weyler, J.; Dom, S.; De Backer, W.; Gislason, T.; et al. Nocturnal gastro-oesophageal reflux, asthma and symptoms of OSA: A longitudinal, general population study. Eur. Respir. J. 2013, 41, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- You, C.R.; Oh, J.H.; Seo, M.; Lee, H.Y.; Joo, H.; Jung, S.H.; Lee, S.H.; Choi, M.G. Association Between Non-erosive Reflux Disease and High Risk of Obstructive Sleep Apnea in Korean Population. J. Neurogastroenterol. Motil. 2014, 20, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.T.; Lai, C.C.; Wang, Y.H.; Tseng, P.H.; Wang, K.; Wang, C.Y.; Chen, L. Risk of pneumonia in patients with gastroesophageal reflux disease: A population-based cohort study. PLoS ONE 2017, 12, e0183808. [Google Scholar] [CrossRef] [PubMed]
- Fohl, A.L.; Regal, R.E. Proton pump inhibitor-associated pneumonia: Not a breath of fresh air after all? World J. Gastrointest. Pharmacol. Ther. 2011, 2, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Jobin, V.; Payne, R.; Beauregard, J.; Naor, N.; Kimoff, R.J. Laryngeal and velopharyngeal sensory impairment in obstructive sleep apnea. Sleep 2005, 28, 585–593. [Google Scholar] [CrossRef]
- Ghannouchi, I.; Speyer, R.; Doma, K.; Cordier, R.; Verin, E. Swallowing function and chronic respiratory diseases: Systematic review. Respir. Med. 2016, 117, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Pizzorni, N.; Radovanovic, D.; Pecis, M.; Lorusso, R.; Annoni, F.; Bartorelli, A.; Rizzi, M.; Schindler, A.; Santus, P. Dysphagia symptoms in obstructive sleep apnea: Prevalence and clinical correlates. Respir. Res. 2021, 22, 117. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, S.; Sudo, E.; Matsuse, T.; Ohga, E.; Ishii, T.; Ouchi, Y.; Fukuchi, Y. Impaired swallowing reflex in patients with obstructive sleep apnea syndrome. Chest 1999, 116, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Javaheri, S.; Barbe, F.; Campos-Rodriguez, F.; Dempsey, J.A.; Khayat, R.; Javaheri, S.; Malhotra, A.; Martinez-Garcia, M.A.; Mehra, R.; Pack, A.I.; et al. Sleep Apnea: Types, Mechanisms, and Clinical Cardiovascular Consequences. J. Am. Coll. Cardiol. 2017, 69, 841–858. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, K.; Eggli, D.F.; Maxwell, S.L. Quantitative aspiration during sleep in normal subjects. Chest 1997, 111, 1266–1272. [Google Scholar] [CrossRef]
- Beal, M.; Chesson, A.; Garcia, T.; Caldito, G.; Stucker, F.; Nathan, C.O. A pilot study of quantitative aspiration in patients with symptoms of obstructive sleep apnea: Comparison to a historic control group. Laryngoscope 2004, 114, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Chitose, S.I.; Sato, K.; Sato, F.; Ono, T.; Umeno, H. Recurrent aspiration pneumonia precipitated by obstructive sleep apnea. Auris Nasus Larynx 2021, 48, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Jehan, S.; Zizi, F.; Pandi-Perumal, S.R.; Wall, S.; Auguste, E.; Myers, A.K.; Jean-Louis, G.; McFarlane, S.I. Obstructive Sleep Apnea and Obesity: Implications for Public Health. Sleep Med. Disord. 2017, 1, 00019. [Google Scholar] [PubMed]
- Anderson, M.R.; Shashaty, M.G.S. Impact of Obesity in Critical Illness. Chest 2021, 160, 2135–2145. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, Z.; Yang, T.; Wang, M.; Xi, X. Is body mass index associated with outcomes of mechanically ventilated adult patients in intensive critical units? A systematic review and meta-analysis. PLoS ONE 2018, 13, e0198669. [Google Scholar] [CrossRef] [PubMed]
- Kahlon, S.; Eurich, D.T.; Padwal, R.S.; Malhotra, A.; Minhas-Sandhu, J.K.; Marrie, T.J.; Majumdar, S.R. Obesity and outcomes in patients hospitalized with pneumonia. Clin. Microbiol. Infect. 2013, 19, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Botros, N.; Concato, J.; Mohsenin, V.; Selim, B.; Doctor, K.; Yaggi, H.K. Obstructive sleep apnea as a risk factor for type 2 diabetes. Am. J. Med. 2009, 122, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Peppard, P.E.; Young, T.; Palta, M.; Skatrud, J. Prospective study of the association between sleep-disordered breathing and hypertension. N. Engl. J. Med. 2000, 342, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Marin, J.M.; Carrizo, S.J.; Vicente, E.; Agusti, A.G. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet 2005, 365, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, D.J.; Yenokyan, G.; Newman, A.B.; O’Connor, G.T.; Punjabi, N.M.; Quan, S.F.; Redline, S.; Resnick, H.E.; Tong, E.K.; Diener-West, M.; et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: The sleep heart health study. Circulation 2010, 122, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Teodorescu, M.; Broytman, O.; Curran-Everett, D.; Sorkness, R.L.; Crisafi, G.; Bleecker, E.R.; Erzurum, S.; Gaston, B.M.; Wenzel, S.E.; Jarjour, N.N. Obstructive Sleep Apnea Risk, Asthma Burden, and Lower Airway Inflammation in Adults in the Severe Asthma Research Program (SARP) II. J. Allergy Clin. Immunol. Pract. 2015, 3, 566–575.e561. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, P.L.; Miller, G.; Mendelson, W.B. Sleep apnea syndrome in chronic renal disease. Am. J. Med. 1989, 86, 308–314. [Google Scholar] [CrossRef]
- Torres, A.; Peetermans, W.E.; Viegi, G.; Blasi, F. Risk factors for community-acquired pneumonia in adults in Europe: A literature review. Thorax 2013, 68, 1057–1065. [Google Scholar] [CrossRef]
- Naqvi, S.B.; Collins, A.J. Infectious complications in chronic kidney disease. Adv. Chronic Kidney Dis. 2006, 13, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Viasus, D.; Garcia-Vidal, C.; Manresa, F.; Dorca, J.; Gudiol, F.; Carratalà, J. Risk stratification and prognosis of acute cardiac events in hospitalized adults with community-acquired pneumonia. J. Infect. 2013, 66, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, R.H.; Heerfordt, C.K.; Boel, J.B.; Dessau, R.B.; Ostergaard, C.; Sivapalan, P.; Eklöf, J.; Jensen, J.S. Inhaled corticosteroids and risk of lower respiratory tract infection with Moraxella catarrhalis in patients with chronic obstructive pulmonary disease. BMJ Open Respir. Res. 2023, 10, e001726. [Google Scholar] [CrossRef] [PubMed]
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019, 200, e45–e67. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.A.; Park, J.S.; Lee, C.W.; Choi, W.I. Pneumonia severity index in viral community acquired pneumonia in adults. PLoS ONE 2019, 14, e0210102. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, M.I.; Babu, B.L.; Reyes, L.F.; Chalmers, J.D.; Soni, N.J.; Sibila, O.; Faverio, P.; Cilloniz, C.; Rodriguez-Cintron, W.; Aliberti, S. Burden and risk factors for Pseudomonas aeruginosa community-acquired pneumonia: A multinational point prevalence study of hospitalised patients. Eur. Respir. J. 2018, 52, 1701190. [Google Scholar] [CrossRef] [PubMed]
- Srivali, N.; Chongnarungsin, D.; Ungprasert, P.; Edmonds, L.C. Two cases of Legionnaires’ disease associated with continuous positive airway pressure therapy. Sleep Med. 2013, 14, 1038. [Google Scholar] [CrossRef] [PubMed]
- Schnirman, R.; Nur, N.; Bonitati, A.; Carino, G. A case of legionella pneumonia caused by home use of continuous positive airway pressure. SAGE Open Med. Case Rep. 2017, 5, 2050313x17744981. [Google Scholar] [CrossRef] [PubMed]
- Brady, M.F.; Awosika, A.O.; Sundareshan, V. Legionnaires’ Disease. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Kato, H.; Hagihara, M.; Asai, N.; Shibata, Y.; Koizumi, Y.; Yamagishi, Y.; Mikamo, H. Meta-analysis of fluoroquinolones versus macrolides for treatment of legionella pneumonia. J. Infect. Chemother. 2021, 27, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Cesar, L.; Gonzalez, C.; Calia, F.M. Bacteriologic flora of aspiration-induced pulmonary infections. Arch. Intern. Med. 1975, 135, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Influenza Antiviral Medications: Summary for Clinicians|CDC. Available online: https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm#highrisk (accessed on 4 May 2024).
- People with Certain Medical Conditions|CDC. Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html (accessed on 4 May 2024).
- Zhang, X.B.; Chen, X.Y.; Chiu, K.Y.; He, X.Z.; Wang, J.M.; Zeng, H.Q.; Zeng, Y. Intermittent Hypoxia Inhibits Hepatic CYP1a2 Expression and Delays Aminophylline Metabolism. Evid. Based Complement. Alternat Med. 2022, 2022, 2782702. [Google Scholar] [CrossRef] [PubMed]
- Fradette, C.; Du Souich, P. Effect of hypoxia on cytochrome P450 activity and expression. Curr. Drug Metab. 2004, 5, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Fohner, A.E.; Sparreboom, A.; Altman, R.B.; Klein, T.E. PharmGKB summary: Macrolide antibiotic pathway, pharmacokinetics/pharmacodynamics. Pharmacogenet. Genom. 2017, 27, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Mesarwi, O.A.; Loomba, R.; Malhotra, A. Obstructive Sleep Apnea, Hypoxia, and Nonalcoholic Fatty Liver Disease. Am. J. Respir. Crit. Care Med. 2019, 199, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Elbarbry, F. Vancomycin Dosing and Monitoring: Critical Evaluation of the Current Practice. Eur. J. Drug Metab. Pharmacokinet. 2018, 43, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Munar, M.Y.; Singh, H. Drug dosing adjustments in patients with chronic kidney disease. Am. Fam. Physician 2007, 75, 1487–1496. [Google Scholar] [PubMed]
- Gorelik, E.; Masarwa, R.; Perlman, A.; Rotshild, V.; Abbasi, M.; Muszkat, M.; Matok, I. Fluoroquinolones and Cardiovascular Risk: A Systematic Review, Meta-analysis and Network Meta-analysis. Drug Saf. 2019, 42, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Cai, Y.; Chai, D.; Liang, B.; Bai, N.; Wang, R. The cardiotoxicity of macrolides: A systematic review. Pharmazie 2010, 65, 631–640. [Google Scholar] [PubMed]
- Filippone, E.J.; Kraft, W.K.; Farber, J.L. The Nephrotoxicity of Vancomycin. Clin. Pharmacol. Ther. 2017, 102, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef] [PubMed]
- McIsaac, D.I.; Gershon, A.; Wijeysundera, D.; Bryson, G.L.; Badner, N.; van Walraven, C. Identifying Obstructive Sleep Apnea in Administrative Data: A Study of Diagnostic Accuracy. Anesthesiology 2015, 123, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Steinhauer, K.; Goroncy-Bermes, P. Investigation of the hygienic safety of continuous positive airways pressure devices after reprocessing. J. Hosp. Infect. 2005, 61, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Ortolano, G.A.; Schaffer, J.; McAlister, M.B.; Stanchfield, I.; Hill, E.; Vandenburgh, L.; Lewis, M.; John, S.; Canonica, F.P.; Cervia, J.S. Filters reduce the risk of bacterial transmission from contaminated heated humidifiers used with CPAP for obstructive sleep apnea. J. Clin. Sleep Med. 2007, 3, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Sanner, B.M.; Fluerenbrock, N.; Kleiber-Imbeck, A.; Mueller, J.B.; Zidek, W. Effect of continuous positive airway pressure therapy on infectious complications in patients with obstructive sleep apnea syndrome. Respiration 2001, 68, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Jao, L.Y.; Su, W.L.; Chang, H.C.; Lan, C.C.; Wu, Y.K.; Yang, M.C. Pneumocystis jirovecii pneumonia presenting as a solitary pulmonary granuloma due to unclean continuous positive airway pressure equipment: A case report. J. Clin. Sleep Med. 2022, 18, 1717–1721. [Google Scholar] [CrossRef] [PubMed]
- Caiano Gil, J.; Calisto, R.; Amado, J.; Barreto, V. Eikenella corrodens and Porphyromonas asaccharolytica pleural empyema in a diabetic patient with obstructive sleep apnea syndrome on noninvasive ventilation. Rev. Port. Pneumol. 2013, 19, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R. Providing Cleaning Recommendations for Positive Airway Pressure Devices. Ann. Am. Thorac. Soc. 2024, 21, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Mercieca, L.; Pullicino, R.; Camilleri, K.; Abela, R.; Mangion, S.A.; Cassar, J.; Zammit, M.; Gatt, C.; Deguara, C.; Barbara, C.; et al. Continuous Positive Airway Pressure: Is it a route for infection in those with Obstructive Sleep Apnoea? Sleep Sci. 2017, 10, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Gavidia, R.; Shieu, M.M.; Dunietz, G.L.; Braley, T.J. Respiratory infection risk in positive airway pressure therapy users: A retrospective cohort study. J. Clin. Sleep Med. 2023, 19, 1769–1773. [Google Scholar] [CrossRef] [PubMed]
- Mutti, C.; Azzi, N.; Soglia, M.; Pollara, I.; Alessandrini, F.; Parrino, L. Obstructive sleep apnea, cpap and COVID-19: A brief review. Acta Biomed. 2020, 91, e2020196. [Google Scholar] [CrossRef]
- Feng, Z.; Glasser, J.W.; Hill, A.N. On the benefits of flattening the curve: A perspective. Math. Biosci. 2020, 326, 108389. [Google Scholar] [CrossRef] [PubMed]
- Klompas, M.; Baker, M.A.; Rhee, C. Airborne Transmission of SARS-CoV-2: Theoretical Considerations and Available Evidence. JAMA 2020, 324, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M. Sleep labs, lung function tests and COVID-19 pandemic—Only emergencies allowed! Pulmonology 2020, 26, 244–245. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.; Oyefeso, O.; Koeckerling, D.; Mudalige, N.L.; Pan, D. COVID-19: Community CPAP and NIV should be stopped unless medically necessary to support life. Thorax 2020, 75, 367. [Google Scholar] [CrossRef] [PubMed]
- Sampol, J.; Sáez, M.; Martí, S.; Pallero, M.; Barrecheguren, M.; Ferrer, J.; Sampol, G. Impact of home CPAP-treated obstructive sleep apnea on COVID-19 outcomes in hospitalized patients. J. Clin. Sleep Med. 2022, 18, 1857–1864. [Google Scholar] [CrossRef] [PubMed]
- Pépin, J.L.; Bailly, S.; Borel, J.C.; Logerot, S.; Sapène, M.; Martinot, J.B.; Lévy, P.; Tamisier, R. Detecting COVID-19 and other respiratory infections in obstructive sleep apnoea patients through CPAP device telemonitoring. Digit. Health 2021, 7, 20552076211002957. [Google Scholar] [CrossRef] [PubMed]
- Pneumococcal Vaccination: Who and When to Vaccinate|CDC. Available online: https://www.cdc.gov/vaccines/vpd/pneumo/hcp/who-when-to-vaccinate.html#adults-19-64 (accessed on 10 April 2024).
- Influenza Vaccination: A Summary for Clinicians|CDC. Available online: https://www.cdc.gov/flu/professionals/vaccination/vax-summary.htm#vaccinated (accessed on 10 April 2024).
- People at Higher Risk of Flu Complications|CDC. Available online: https://www.cdc.gov/flu/highrisk/index.htm (accessed on 10 April 2024).
- Kaku, Y.; Okumura, K.; Padilla-Blanco, M.; Kosugi, Y.; Uriu, K.; Hinay, A.A., Jr.; Chen, L.; Plianchaisuk, A.; Kobiyama, K.; Ishii, K.J.; et al. Virological characteristics of the SARS-CoV-2 JN.1 variant. Lancet Infect. Dis. 2024, 24, e82. [Google Scholar] [CrossRef] [PubMed]
- Song, X.D.; Yang, G.J.; Jiang, X.L.; Wang, X.J.; Zhang, Y.W.; Wu, J.; Wang, M.M.; Chen, R.R.; He, X.J.; Dong, G.; et al. Seroprevalence of SARS-CoV-2 neutralising antibodies and cross-reactivity to JN.1 one year after the BA.5/BF.7 wave in China. Lancet Reg. Health West. Pac. 2024, 44, 101040. [Google Scholar] [CrossRef] [PubMed]
- Jeworowski, L.M.; Mühlemann, B.; Walper, F.; Schmidt, M.L.; Jansen, J.; Krumbholz, A.; Simon-Lorière, E.; Jones, T.C.; Corman, V.M.; Drosten, C. Humoral immune escape by current SARS-CoV-2 variants BA.2.86 and JN.1, December 2023. Euro Surveill. 2024, 29, 2300740. [Google Scholar] [CrossRef] [PubMed]
- Troeger, C.; Blacker, B.; Khalil, I.A.; Rao, P.C.; Cao, J.; Zimsen, S.R.M.; Albertson, S.B.; Deshpande, A.; Farag, T.; Abebe, Z.; et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1191–1210. [Google Scholar] [CrossRef] [PubMed]
- Ford, N.D.; Patel, S.A.; Narayan, K.M. Obesity in Low- and Middle-Income Countries: Burden, Drivers, and Emerging Challenges. Annu. Rev. Public Health 2017, 38, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.; Rae, D.E.; Redman, K.N.; Knutson, K.L.; von Schantz, M.; Gómez-Olivé, F.X.; Scheuermaier, K. Sleep disorders in low- and middle-income countries: A call for action. J. Clin. Sleep Med. 2021, 17, 2341–2342. [Google Scholar] [CrossRef] [PubMed]
| (“Obstructive Sleep Apnea” OR “Sleep Apnea Syndromes” OR “Sleep-related breathing disorder” OR OSA) AND (pneumonia OR “acute pneumonia” OR “bacterial pneumonia” OR “community acquired pneumonia” OR CAP OR “lung infection” OR “respiratory infection” OR “bronchopneumonia”) |
| (“Obstructive Sleep Apnea” OR “Sleep Apnea Syndromes” OR “Sleep-related breathing disorder” OR OSA) AND (influenza OR “Influenza A” OR “Influenza B” OR “H1N1” OR “swine flu” OR “avian influenza” OR “H5N1” OR “seasonal influenza” OR “viral pneumonia” OR flu) |
| (“Obstructive Sleep Apnea” OR “Sleep Apnea Syndromes” OR “Sleep-related breathing disorder” OR OSA) AND (COVID-19 OR “SARS-CoV-2” OR “2019-nCoV” OR “coronavirus disease 2019” OR “novel coronavirus” OR “viral pneumonia”) |
| Author and Date | Design | Total N (OSA N) | Inclusion and Exclusion Criteria | Outcomes | Key Findings | Limitations |
|---|---|---|---|---|---|---|
| Keto et al., 2023 [15] | Case-control from Finland | 50,648 (25,324) | I: ICD code for OSA. E: OSA in the two years preceding the index date. | LRTI, recurring LRTI. | ↑ LRTI in the year preceding OSA RR 1.35, and during the year after OSA RR 1.39. | No PSG data, no data on OSA treatment, no BMI data. |
| Grant et al., 2023 [16] | Retrospective cohort from healthcare plans database | 38.62M PY (1.29M PY) | I: Minimum 1 year of enrollment in health plan. E: Death date before January 1st of the index year; Overlapping pneumonia inpatient admissions. | All-cause pneumonia, invasive pneumococcal disease, pneumococcal pneumonia. | OSA: ↑ pneumonia (18–49 y RR 3.6, 50–64 y RR 3.6, ≥65 y RR 3.4), ↑ invasive pneumococcal disease (18–49 y RR 5.7, 50–64 y RR 4.2, ≥65 y RR 4.2). | No PSG data, no data on OSA treatment, no BMI data. |
| Lutsey et al., 2023 [17] | Post-hoc analysis of the multicentric prospective cohort | 1586 (772) | I: Valid PSG data; Self-identify as White. E: CSA; Already had the outcome of interest at the time of visit. | Hospitalization: with pneumonia; with respiratory infection; with any infection. | OSA not linked to outcomes; T90 > 5% ↑ hospitalized pneumonia HR 1.59, ↑ hospitalized respiratory infection HR 1.53, ↑ hospitalized any infection HR 1.25. | No data on OSA treatment, mostly White population. |
| Chiner et al., 2016 [18] | Single center case-control | 123 (85) | I: Cases: Hospitalized for CAP; Controls: Hospitalized for non-respiratory/non-ENT infection. E: Previous OSA diagnosis and CPAP. | Pneumonia, PSI. | AHI ≥ 10: ↑ pneumonia OR 2.86; AHI ≥ 30: ↑ pneumonia OR 3.184; AHI positively correlated with PSI. | Small sample size, no data on OSA treatment. |
| Su et al., 2014 [19] | Retrospective cohort from Taiwan | 34,100 (6816) | I: ICD codes for OSA; E: ICD codes for pneumonia, lung abscess, empyema. | Pneumonia. | OSA: ↑ pneumonia HR 1.19; OSA requiring CPAP: ↑ pneumonia HR 1.32. | No PSG data, no BMI data. |
| Lindenauer et al., 2014 [20] | Multicenter, retrospective cohort | 250,907 (15,569) | I: ICD code for pneumonia; Chest radiography; Antibiotics within 48 h of admission. E: Transfers; Hospital LOS under 2 days; Cystic fibrosis; Pneumonia not present at admission. | ICU, MV, hospital mortality, hospital LOS, costs. | OSA: ↑ ICU OR 1.54, ↑ MV OR 1.68, ↑ hospital LOS RR 1.14, ↑ cost RR 1.22, ↓ mortality OR 0.90. | No PSG data, no data on OSA treatment, no BMI data. |
| Beumer et al., 2019 [21] | Two center, retrospective cohort | 199 (9) | I: Symptoms and positive influenza PCR; Transfers if not received antibiotics or antivirals. | ICU, ICU mortality. | OSA/CSA: ↑ ICU admission OR 9.73., not linked to mortality. | Small sample size, no PSG data, no data on OSA treatment. |
| Boattini et al., 2023 [22] | Post-hoc analysis of a multicentric, retrospective cohort | 356 (23) | I: Positive influenza or RSV PCR; Symptoms; Pulmonary infiltrate on imaging. E: Viral co-infections. | NIV failure, hospital mortality. | OSA/OHS: ↑ NIV failure OR 4.66, not linked to mortality. | No PSG data, no data on OSA treatment, no BMI data, no adjustments for obesity. |
| Mok et al., 2020 [23] | Single center, retrospective cohort | 53 (53) | I: ICD codes for OSA, influenza. E: No PSG data; No OSA treatment data; CSA on PSG. | Hospitalization, complications, hospital LOS. | OSA non-CPAP vs. CPAP: ↑ hospitalization OR 4.7. Severity of OSA not linked to hospitalization in CPAP-non adherent. | Small sample size, no adjustments for obesity and comorbidities. |
| Tsai et al., 2022 [24] | Retrospective cohort from Taiwan | 32,540 (6508) | I: Cases: ICD codes for OSA; Controls: No OSA; Randomly selected, matched by income, gender, urbanization, and age. E: influenza pneumonia before OSA. | Influenza-associated SARI. | OSA: ↑ influenza-SARI HR 1.98, ↑ cumulative incidence of influenza-SARI. | No PSG data, no data on OSA treatment, no BMI data. |
| Chen et al., 2021 [25] | Retrospective cohort from Taiwan | 27,501 (5483) | I: Cases: ICD codes for OSA; Controls: No OSA; Randomly selected, matched by age, sex, index years, and comorbidities. E: UPPP; influenza before OSA. | Influenza, composite (pneumonia, hospitalization). | OSA: ↑ influenza HR 1.18, ↑ pneumonia or hospitalization 1.79. | No PSG data, no data on OSA treatment, no BMI data. |
| Mashaqi et al., 2021 [26] | Multicentric, retrospective cohort | 1738 (139) | I: Hospitalized; ICD codes, PSG report, self-report, STOP-BANG for OSA; ICD codes COVID-19. E: ICD for CSA and unspecified sleep apnea. | MV, ICU, hospital mortality, hospital LOS. | OSA not linked to ICU admission, hospital LOS, MV, or mortality. | No PSG data, no data on OSA treatment. |
| Maas et al., 2021 [27] | Multicentric, retrospective cohort | 5544,884 (~44,877) | I: All patient encounters; January to June 2020. | COVID-19, hospitalization, respiratory failure. | OSA: ↑ COVID-19, OR 8.6, ↑ hospitalization, OR 1.65, ↑ respiratory failure, OR 1.98. | No PSG data, no data on OSA treatment. |
| Strausz et al., 2021 [28] | Retrospective cohort from FinnGen biobank | 445 (38) | I: All positive COVID-19 PCR from FinnGen biobank. | Hospitalization, COVID-19. | OSA not linked with COVID-19, ↑ hospitalization, OR 2.93. Link attenuated after adjustment for BMI in meta-analysis. | Small sample size, no PSG data, no data on OSA treatment. |
| Rögnvaldsson et al., 2022 [29] | Retrospective cohort from Iceland | 4756 (185) | I: Positive COVID-19 PCR. E: Nursing home; COVID-19 during hospitalization or rehabilitation. | Composite (hospitalization, mortality). | OSA: ↑ composite outcome (hospitalization and mortality) OR 2.0. OSA and CPAP: ↑ composite outcome (hospitalization and mortality) OR 2.4. | No PSG data for the control group, no BMI data for 30% of controls and 2% of the OSA group. |
| Cade et al., 2020 [30] | Multicentric, retrospective cohort | 4668 (443) | I: Positive COVID-19 PCR; A minimum of two clinical notes, two encounters, and three ICD diagnoses. | Mortality, composite (mortality, MV, ICU), hospitalization. | OSA or CPAP not linked with mortality, MV, ICU, and hospitalization. | No PSG data, no data on OSA treatment. |
| PenaOrbea et al., 2021 [31] | Multicentric, retrospective control and case-control | 5402 (2664) | I: Positive COVID-19 PCR; PSG record available. | COVID-19, WHO-designated COVID-19 clinical outcomes, composite (hospitalization, mortality). | AHI, T90, SaO2, ETCO2 and CPAP not linked with COVID-19. T90 and SaO2: ↑ WHO-designated COVID-19 outcomes ↑ hospitalization, ↑ mortality. | Included only patients who had indications for PSG. |
| Oh et al., 2021 [32] | Retrospective cohort from South Korea | 124,330 (550) | I: ICD codes for COVID-19, chronic respiratory diseases. E: COVID-19 still hospitalized as of June 26, 2020. | COVID-19; hospital mortality. | OSA: ↑ COVID-19, OR 1.65, not linked to mortality. | No PSG data, no data on OSA treatment, no BMI data. |
| Gottlieb et al., 2020 [33] | Retrospective cohort from Chicago, IL. | 8673 (288) | I: Positive COVID-19 PCR. E: Interhospital transfers. | Hospitalization, ICU. | OSA not linked to hospitalization, ↑ ICU, OR 1.58. | No PSG data, no data on OSA treatment. |
| Kendzerska et al., 2023 [34] | Retrospective cohort from Ontario, CA. | 4,912,229 (324,029) | I: Alive at the start of the pandemic; Followed until March 31, 2021, or death. | COVID-19, ED, hospitalization, ICU, 30-day mortality. | OSA: ↑ COVID-19, csHR 1.17, ↑ ED, csHR 1.62, ↑ hospitalizations csHR 1.50, ↑ ICU csHR 1.53, not linked to mortality. | No PSG data, no data on OSA treatment, no BMI data. |
| Peker et al., 2021 [35] | Multicenter, prospective, observational clinical trial | 320 (121) | I: Positive COVID-19 PCR and/or clinical/radiologic. | Clinical improvement, clinical worsening, hospitalization, oxygen, ICU. | OSA: ↑ delayed clinical improvement, OR 0.42, ↑ oxygen OR 1.95, ↑ clinical worsening. | No PSG data, no data on OSA treatment. |
| Girardin et al., 2021 [36] | Retrospective cohort from NYC and LI | 4446 (290) | I: Positive COVID-19 PCR. | Hospital mortality. | OSA not linked to mortality. | No PSG data, no data on OSA treatment, no BMI data. |
| Gimeno-Miguel et al., 2021 [37] | Retrospective cohort from Aragon, ES. | 68,913 (1231) | I: Positive COVID-19 PCR/antigen; E: Patients diagnosed from March to May 2020. | Composite (hospitalization, 30-day mortality) | OSA: ↑ composite outcome (hospitalization and 30-day mortality) in women OR 1.43, but not in men. | No PSG data, no data on OSA treatment, no BMI data. |
| Cariou et al., 2020 [38] | Multicentric, retrospective cohort | 1317 (114) | I: Positive COVID-19 PCR or clinical/radiological diagnosis, hospitalized, diabetics. | Composite (MV, 7-day mortality), mortality on day 7, MV on day 7, ICU, discharge on day 7. | OSA: ↑ mortality by day 7 OR 2.80, not linked to composite outcome (intubation and death within 7 days of admission). | No PSG data, no data on OSA treatment, diabetic population. |
| Ioannou et al., 2020 [39] | Longitudinal cohort from VA registry. | 10,131 (2720) | I: VA enrollees who had COVID-19 PCR test; E: VA employees. | Hospitalization, MV, mortality. | OSA: ↑ MV HR, 1.22, not linked to hospitalization, mortality. | No PSG data, no data on OSA treatment, male veterans. |
| Izquierdo et al., 2020 [40] | Multicentric, retrospective cohort | 10,504 (212) | I: Positive COVID-19 PCR or clinical/radiological diagnosis. | ICU. | OSA not linked to ICU admission. | No PSG data, no data on OSA treatment, no BMI data, no adjustments for obesity and comorbidities. |
| Lohia et al., 2021 [41] | Multicentric, retrospective cohort | 1871 (63) | I: Adults; Positive COVID-19 PCR; E: Readmission; Ambulatory surgery, pregnant, transferred-for-ECMO patients. | Mortality, MV, ICU. | OSA ↑ mortality OR 2.59, ↑ ICU OR 1.95, ↑ MV OR 2.20. | Small OSA sample size, no data on OSA treatment, mostly African Americans. |
| Prasad et al., 2024 [42] | Retrospective cohort from VA registry | 20,357 (6112) | I: Tested for COVID-19 by PCR; Until 16 December 2023. | COVID-19, LFNC, HFNC, NIV, MV, 30-day readmission; hospital LOS, ICU LOS, adapted WHO severity scale. | OSA ↑ COVID-19 OR 1.37, ↑ NIV OR 1.83, not linked to LFNC, HFNC, MV, 30-day readmission. CPAP adherence not linked to outcomes. | No PSG data. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemet, M.; Vukoja, M. Obstructive Sleep Apnea and Acute Lower Respiratory Tract Infections: A Narrative Literature Review. Antibiotics 2024, 13, 532. https://doi.org/10.3390/antibiotics13060532
Nemet M, Vukoja M. Obstructive Sleep Apnea and Acute Lower Respiratory Tract Infections: A Narrative Literature Review. Antibiotics. 2024; 13(6):532. https://doi.org/10.3390/antibiotics13060532
Chicago/Turabian StyleNemet, Marko, and Marija Vukoja. 2024. "Obstructive Sleep Apnea and Acute Lower Respiratory Tract Infections: A Narrative Literature Review" Antibiotics 13, no. 6: 532. https://doi.org/10.3390/antibiotics13060532
APA StyleNemet, M., & Vukoja, M. (2024). Obstructive Sleep Apnea and Acute Lower Respiratory Tract Infections: A Narrative Literature Review. Antibiotics, 13(6), 532. https://doi.org/10.3390/antibiotics13060532






