Exploring Prior Antibiotic Exposure Characteristics for COVID-19 Hospital Admission Patients: OpenSAFELY
Abstract
1. Introduction
2. Results
2.1. Study Participants
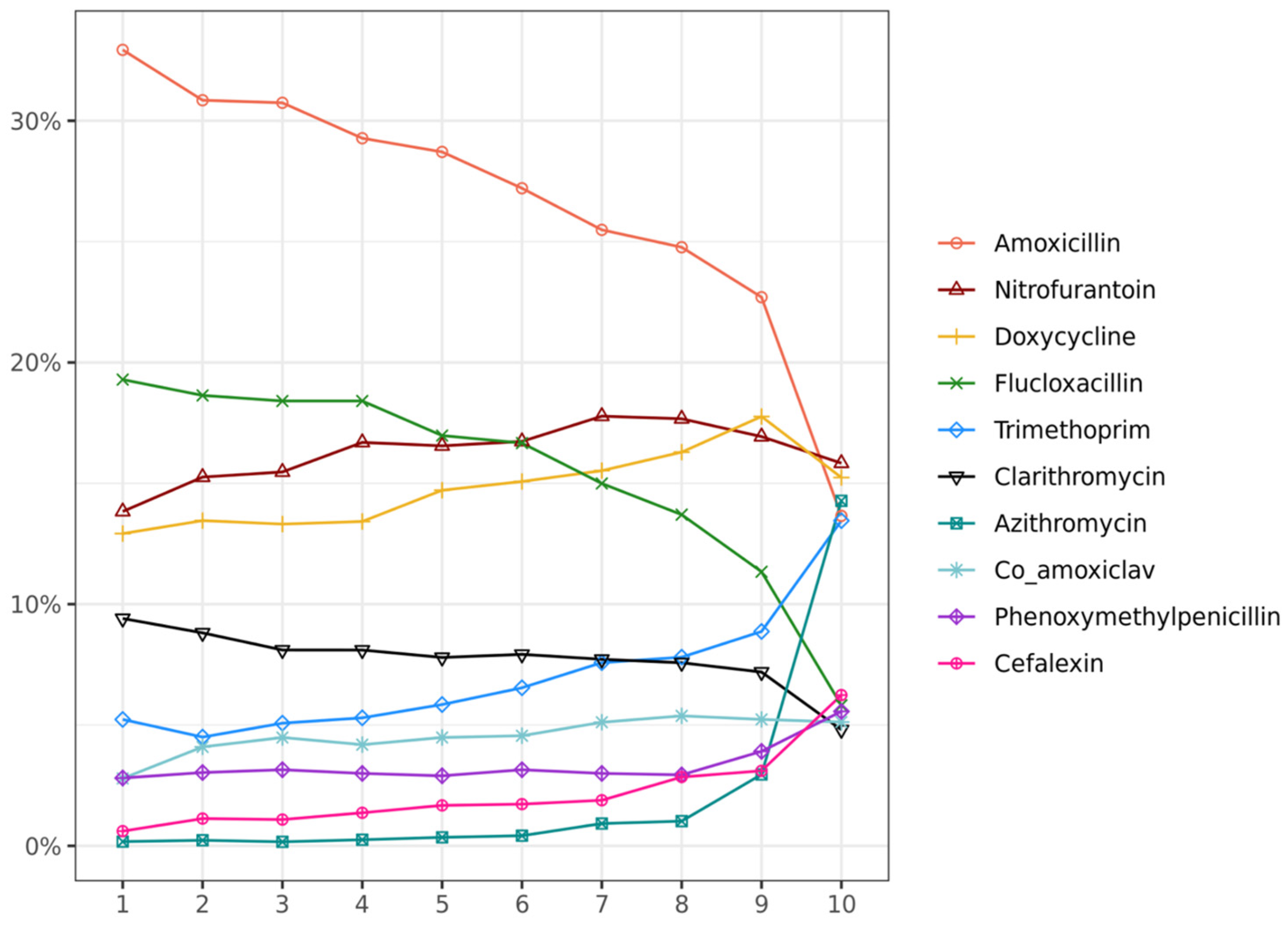
2.2. Antibiotic Exposure and Severe COVID-19 Outcome
2.3. Sensitivity Analysis for Antibiotic Exposure Interaction
3. Discussion
4. Materials and Methods
4.1. Data Sources
4.2. Study Design
4.3. Matching
4.4. Antibiotic Exposure
4.5. Confounding
4.6. Random Forest Model
4.7. Statistical Analysis
4.8. Sensitivity Analysis
4.9. Software and Reproducibility
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alimohamadi, Y.; Tola, H.H.; Abbasi-Ghahramanloo, A.; Janani, M.; Sepandi, M. Case fatality rate of COVID-19: A systematic review and meta-analysis. J. Prev. Med. Hyg. 2021, 62, E311–E320. [Google Scholar] [CrossRef]
- Wang, H.; Paulson, K.R.; Pease, S.A.; Watson, S.; Comfort, H.; Zheng, P.; Aravkin, A.Y.; Bisignano, C.; Barber, R.M.; Alam, T.; et al. Estimating excess mortality due to the COVID-19 pandemic: A systematic analysis of COVID-19-related mortality, 2020–2021. Lancet 2022, 399, 1513–1536. [Google Scholar] [CrossRef]
- Trougakos, I.P.; Stamatelopoulos, K.; Terpos, E.; Tsitsilonis, O.E.; Aivalioti, E.; Paraskevis, D.; Kastritis, E.; Pavlakis, G.N.; Dimopoulos, M.A. Insights to SARS-CoV-2 life cycle, pathophysiology, and rationalized treatments that target COVID-19 clinical complications. J. Biomed. Sci. 2021, 28, 9. [Google Scholar] [CrossRef]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.Y.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S.; et al. Gut microbiota Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021, 70, 698–706. [Google Scholar] [CrossRef]
- Sun, Z.; Song, Z.G.; Liu, C.; Tan, S.; Lin, S.; Zhu, J.; Dai, F.H.; Gao, J.; She, J.L.; Mei, Z.; et al. Gut microbiome alterations and gut barrier dysfunction are associated with host immune homeostasis in COVID-19 patients. BMC Med. 2022, 20, 24. [Google Scholar] [CrossRef]
- Albrich, W.C.; Ghosh, T.S.; Ahearn-Ford, S.; Mikaeloff, F.; Lunjani, N.; Forde, B.; Suh, N.; Kleger, G.R.; Pietsch, U.; Frischknecht, M.; et al. A high-risk gut microbiota configuration associates with fatal hyperinflammatory immune and metabolic responses to SARS-CoV-2. Gut Microbes 2022, 14, 2073131. [Google Scholar] [CrossRef]
- de Nies, L.; Galata, V.; Martin-Gallausiaux, C.; Despotovic, M.; Busi, S.B.; Snoeck, C.J.; Delacour, L.; Budagavi, D.P.; Laczny, C.C.; Habier, J.; et al. Altered infective competence of the human gut microbiome in COVID-19. Microbiome 2023, 11, 46. [Google Scholar] [CrossRef]
- Nel Van Zyl, K.; Matukane, S.R.; Hamman, B.L.; Whitelaw, A.C.; Newton-Foot, M. Effect of antibiotics on the human microbiome: A systematic review. Int. J. Antimicrob. Agents 2022, 59, 106502. [Google Scholar] [CrossRef]
- Schwartz, D.J.; Langdon, A.E.; Dantas, G. Understanding the impact of antibiotic perturbation on the human microbiome. Genome Med. 2020, 12, 82. [Google Scholar] [CrossRef]
- Llor, C.; Ouchi, D.; Giner-Soriano, M.; García-Sangenís, A.; Bjerrum, L.; Morros, R. Correlation between previous antibiotic exposure and covid-19 severity. A population-based cohort study. Antibiotics 2021, 10, 1364. [Google Scholar] [CrossRef]
- Yang, Y.T.; Wong, D.; Ashcroft, D.M.; Massey, J.; MacKenna, B.; Fisher, L.; Mehrkar, A.; Bacon, S.C.; Hand, K.; Zhong, X.; et al. Repeated antibiotic exposure and risk of hospitalisation and death following COVID-19 infection (OpenSAFELY): A matched case–control study. EClinicalMedicine 2023, 61, 102064. [Google Scholar] [CrossRef]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Malik, S.S.; Mundra, S. Increasing Consumption of Antibiotics during the COVID-19 Pandemic: Implications for Patient Health and Emerging Anti-Microbial Resistance. Antibiotics 2022, 12, 45. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Dong, X.; Liu, G.-H.; Gao, Y.-D. Risk and Protective Factors for COVID-19 Morbidity, Severity, and Mortality. Clin. Rev. Allergy Immunol. 2016, 64, 90–107. [Google Scholar] [CrossRef]
- Francino, M.P. Antibiotics and the human gut microbiome: Dysbioses and accumulation of resistances. Front. Microbiol. 2016, 6, 164577. [Google Scholar] [CrossRef]
- Elvers, K.T.; Wilson, V.J.; Hammond, A.; Duncan, L.; Huntley, A.L.; Hay, A.D.; Van Der Werf, E.T. Antibiotic-induced changes in the human gut microbiota for the most commonly prescribed antibiotics in primary care in the UK: A systematic review. BMJ Open 2020, 10, e035677. [Google Scholar] [CrossRef]
- Lekang, K.; Shekhar, S.; Berild, D.; Petersen, F.C.; Winther-Larsen, H.C. Effects of different amoxicillin treatment durations on microbiome diversity and composition in the gut. PLoS ONE 2022, 17, e0275737. [Google Scholar] [CrossRef]
- D’Agata, E.M.C.; Dupont-Rouzeyrol, M.; Magal, P.; Olivier, D.; Ruan, S. The impact of different antibiotic regimens on the emergence of antimicrobial-resistant bacteria. PLoS ONE 2008, 3, e4036. [Google Scholar] [CrossRef]
- Mo, Y.; Oonsivilai, M.; Lim, C.; Niehus, R.; Cooper, B.S. Implications of reducing antibiotic treatment duration for antimicrobial resistance in hospital settings: A modelling study and meta-analysis. PLoS Med. 2023, 20, e1004013. [Google Scholar] [CrossRef]
- Yoshida, H.; Motohashi, T.; De Bus, L.; De Waele, J.; Takaba, A.; Kuriyama, A.; Kobayashi, A.; Tanaka, C.; Hashi, H.; Hashimoto, H.; et al. Use of broad-spectrum antimicrobials for more than 72 h and the detection of multidrug-resistant bacteria in Japanese intensive care units: A multicenter retrospective cohort study. Antimicrob. Resist. Infect. Control 2022, 11, 119. [Google Scholar] [CrossRef]
- Spatz, M.; Da Costa, G.; Ventin-Holmberg, R.; Planchais, J.; Michaudel, C.; Wang, Y.; Danne, C.; Lapiere, A.; Michel, M.L.; Kolho, K.L.; et al. Antibiotic treatment using amoxicillin-clavulanic acid impairs gut mycobiota development through modification of the bacterial ecosystem. Microbiome 2023, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Van Staa, T.P.; Palin, V.; Li, Y.; Welfare, W.; Felton, T.W.; Dark, P.; Ashcroft, D.M. The effectiveness of frequent antibiotic use in reducing the risk of infection-related hospital admissions: Results from two large population-based cohorts. BMC Med. 2020, 18, 40. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Pate, A.; Yang, Y.T.; Fahmi, A.; Ashcroft, D.M.; Goldacre, B.; MacKenna, B.; Mehrkar, A.; Bacon, S.C.; Massey, J.; et al. The impact of COVID-19 on antibiotic prescribing in primary care in England: Evaluation and risk prediction of appropriateness of type and repeat prescribing. J. Infect. 2023, 87, 1. [Google Scholar] [CrossRef] [PubMed]
- Costelloe, C.; Metcalfe, C.; Lovering, A.; Mant, D.; Hay, A.D. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: Systematic review and meta-analysis. BMJ 2010, 340, c2096. [Google Scholar] [CrossRef] [PubMed]
- Vincent, E. Managing common infections: Guidance for primary care. Prim. Health Care 2016, 26, 14. [Google Scholar] [CrossRef]
- Antimicrobial stewardship: Systems and processes for effective antimicrobial medicine use. JAC Antimicrob. Resist. 2019, 1, dlz025. [CrossRef] [PubMed]
- Van Staa, T.; Li, Y.; Gold, N.; Chadborn, T.; Welfare, W.; Palin, V.; Ashcroft, D.M.; Bircher, J. Comparing antibiotic prescribing between clinicians in UK primary care: An analysis in a cohort study of eight different measures of antibiotic prescribing. BMJ Qual. Saf. 2022, 31, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.E.; Imai, K.; King, G.; Stuart, E.A. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. J. Stat. Softw. 2011, 42. [Google Scholar] [CrossRef]
- Deyo, R.A.; Cherkin, D.C.; Ciol, M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 1992, 45, 613–619. [Google Scholar] [CrossRef]
- Austin, P.C.; Tu, J.V.; Ho, J.E.; Levy, D.; Lee, D.S. Using methods from the data-mining and machine-learning literature for disease classification and prediction: A case study examining classification of heart failure subtypes. J. Clin. Epidemiol. 2013, 66, 398–407. [Google Scholar] [CrossRef]
- Hamilton, A.J.; Strauss, A.T.; Martinez, D.A.; Hinson, J.S.; Levin, S.; Lin, G.; Klein, E.Y. Machine learning and artificial intelligence: Applications in healthcare epidemiology. Antimicrob. Steward. Healthc. Epidemiol. 2021, 1, e28. [Google Scholar] [CrossRef] [PubMed]
- Probst, P.; Wright, M.N.; Boulesteix, A.L. Hyperparameters and tuning strategies for random forest. Wiley Interdiscip. Rev. Data Min. Knowl. Discov. 2019, 9, e1301. [Google Scholar] [CrossRef]
- Therneau, T.M. A Package for Survival Analysis in R. 2020. Available online: https://CRAN.R-project.org/package=survival (accessed on 4 July 2023).
- The NHS England OpenSAFELY COVID-19 Service—Privacy Notice. NHS Digital (Now NHS England). Available online: https://digital.nhs.uk/coronavirus/coronavirus-covid-19-response-information-governance-hub/the-nhs-england-opensafely-covid-19-service-privacy-notice (accessed on 4 July 2023).
- Data Security and Protection Toolkit—NHS Digital. NHS Digital (Now NHS England). Available online: https://digital.nhs.uk/data-and-information/looking-after-information/data-security-and-information-governance/data-security-and-protection-toolkit (accessed on 4 July 2023).
- ISB1523: Anonymisation Standard for Publishing Health and Social Care Data. NHS Digital (Now NHS England). Available online: https://digital.nhs.uk/data-and-information/information-standards/information-standards-and-data-collections-including-extractions/publications-and-notifications/standards-and-collections/isb1523-anonymisation-standard-for-publishing-health-and-social-care-data (accessed on 4 July 2023).
- Coronavirus (COVID-19): Notice under Regulation 3(4) of the Health Service (Control of Patient Information) Regulations 2002—General. 2022. Available online: https://www.gov.uk/government/publications/coronavirus-covid-19-notification-of-data-controllers-to-share-information/coronavirus-covid-19-notice-under-regulation-34-of-the-health-service-control-of-patient-information-regulations-2002-general--2 (accessed on 5 July 2023).
- Secretary of State for Health and Social Care—UK Government. COVID-19 Public Health Directions 2020: Notification to NHS Digital. Available online: https://digital.nhs.uk/about-nhs-digital/corporate-information-and-documents/directions-and-data-provision-notices/secretary-of-state-directions/covid-19-public-health-directions-2020 (accessed on 4 July 2023).
- Confidentiality Advisory Group. Health Research Authority. Available online: https://www.hra.nhs.uk/about-us/committees-and-services/confidentiality-advisory-group/ (accessed on 4 July 2023).

| Case (n 1 = 67,515) | Control (n 1 = 375,330) | |||
|---|---|---|---|---|
| n 1 | % | n 1 | % | |
| Age group | ||||
| 18–29 | 1665 | 2.5 | 9115 | 2.4 |
| 30–39 | 3205 | 4.7 | 18,350 | 4.9 |
| 40–49 | 4735 | 7.0 | 27,040 | 7.2 |
| 50–59 | 8310 | 12.3 | 48,065 | 12.8 |
| 60–69 | 11,070 | 16.4 | 63,885 | 17.0 |
| 70–79 | 16,535 | 24.5 | 94,630 | 25.2 |
| 80+ | 21,995 | 32.6 | 114,245 | 30.4 |
| Sex | ||||
| male | 36,555 | 54.1 | 207,450 | 55.3 |
| female | 30,960 | 45.9 | 167,880 | 44.7 |
| Ethnicity | ||||
| White | 57,400 | 85.0 | 305,410 | 81.4 |
| South Asian | 5495 | 8.1 | 28,565 | 7.6 |
| Black | 1310 | 1.9 | 4135 | 1.1 |
| Mixed | 560 | 0.8 | 2605 | 0.7 |
| Other | 1145 | 1.7 | 4760 | 1.3 |
| Unknown | 1600 | 2.4 | 29,860 | 8.0 |
| BMI category 2 | ||||
| Healthy weight (<18.5 kg/m2) | 13,490 | 20.0 | 82,845 | 22.1 |
| Underweight (18.5–24.9 kg/m 2) | 1580 | 2.3 | 7275 | 1.9 |
| Overweight (25–29.9 kg/m 2) | 16,935 | 25.1 | 109,010 | 29.0 |
| Obese (≥30 kg/m2) | 24,730 | 36.6 | 112,190 | 29.9 |
| Unknown | 10,780 | 16.0 | 64,010 | 17.1 |
| CCI group 3 | ||||
| No comorbidities (0) | 19,830 | 29.4 | 164,015 | 43.7 |
| Low (1–2) | 36,765 | 54.5 | 177,195 | 47.2 |
| Medium (3–4) | 9685 | 14.3 | 30,845 | 8.2 |
| High (5–6) | 1185 | 1.8 | 3085 | 0.8 |
| Very high (≥ 7) | 50 | 0.1 | 195 | 0.1 |
| Smoking status 4 | ||||
| Never | 22,425 | 33.2 | 142,935 | 38.1 |
| Current | 6145 | 9.1 | 34,385 | 9.2 |
| Former | 38,790 | 57.4 | 196,805 | 52.4 |
| Unknown | 160 | 0.2 | 1215 | 0.3 |
| IMD 5 | ||||
| 1 (least deprived) | 9370 | 13.9 | 62,170 | 16.6 |
| 2 | 11,470 | 17.0 | 70,280 | 18.7 |
| 3 | 13,420 | 19.9 | 77,720 | 20.7 |
| 4 | 14,380 | 21.3 | 77,020 | 20.5 |
| 5 (most deprived) | 17,605 | 26.1 | 79,830 | 21.3 |
| Unknown | 1265 | 1.9 | 8305 | 2.2 |
| Care home residents | 3010 | 4.5 | 31,875 | 8.5 |
| COVID-19 vaccine 6 | 29,100 | 43.1 | 181,090 | 48.2 |
| Flu vaccine 7 | 46,285 | 68.6 | 261,090 | 69.6 |
| Probability of COVID-19 Hospitalisation | Conditional Logistic Regression Model 1 | |||
|---|---|---|---|---|
| Risk Level | RF Estimated | Observed | OR | 95% CI |
| Decile 1 (lowest) | 0.09 | 0.08 | ref | |
| Decile 2 | 0.11 | 0.10 | 1.3 | 1.2–1.3 |
| Decile 3 | 0.12 | 0.11 | 1.5 | 1.4–1.5 |
| Decile 4 | 0.13 | 0.12 | 1.6 | 1.5–1.7 |
| Decile 5 | 0.14 | 0.13 | 1.7 | 1.6–1.8 |
| Decile 6 | 0.15 | 0.14 | 1.9 | 1.8–2.0 |
| Decile 7 | 0.16 | 0.16 | 2.1 | 2.1–2.2 |
| Decile 8 | 0.17 | 0.18 | 2.6 | 2.5–2.7 |
| Decile 9 | 0.19 | 0.22 | 3.1 | 3.0–3.2 |
| Decile 10 (highest) | 0.25 | 0.30 | 4.8 | 4.6–5.0 |
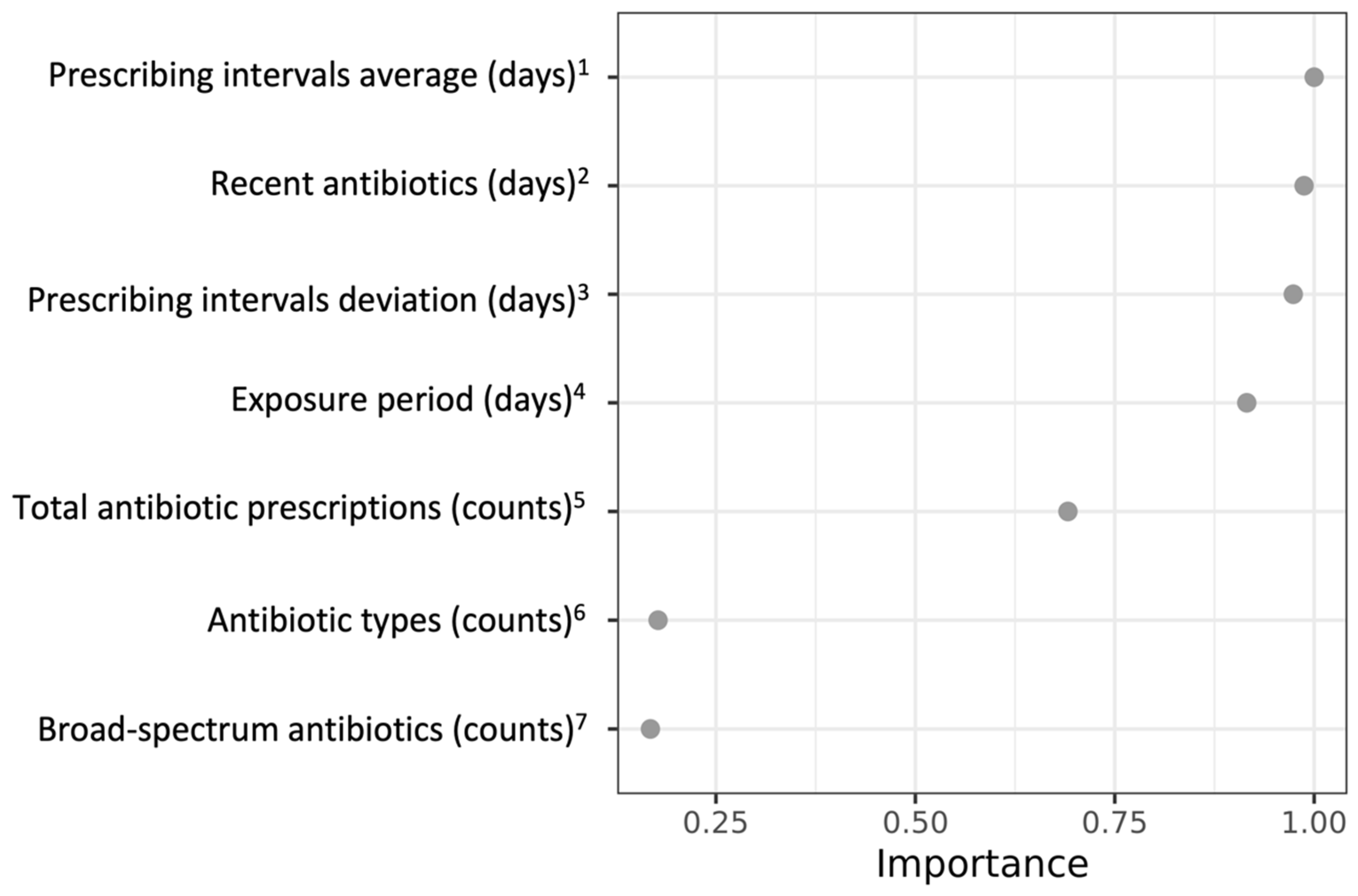
| Variables 1,2,3 | Decile 1 (Lowest Risk) | Decile 2 | Decile 3 | Decile 4 | Decile 5 | Decile 6 | Decile 7 | Decile 8 | Decile 9 | Decile 10 (Highest Risk) |
|---|---|---|---|---|---|---|---|---|---|---|
| Total antibiotics (count) 4 | 2 (2, 3) | 2 (2, 3) | 3 (2, 3) | 3 (2, 4) | 3 (2, 5) | 4 (3, 6) | 5 (4, 7) | 7 (5, 9) | 10 (7, 14) | 20 (13, 35) |
| Level 1 (2) (lowest) | 73.1% | 58.5% | 48.8% | 38.8% | 26.4% | 15.1% | 7.6% | 2.1% | 0.2% | 0% |
| Level 2 (3) | 25.6% | 37.7% | 43.3% | 45.9% | 45.3% | 41.4% | 30.3% | 16.6% | 5.6% | 0.6% |
| Level 3 (6) | 1.3% | 3.5% | 7.2% | 13% | 22.3% | 32% | 39.5% | 38.6% | 68.5% | 6.6% |
| Level 4 (13) (highest) | 0% | 0.3% | 0.6% | 2.3% | 6% | 11.5% | 22.6% | 42.7% | 23.7% | 92.8% |
| Antibiotic types (count) 5 | 2 (1, 2) | 2 (1, 2) | 2 (1, 2) | 2 (2, 3) | 2 (2, 3) | 2 (2, 3) | 2 (2, 3) | 3 (2, 4) | 3 (3, 4) | 4 (3, 6) |
| Level 1 (2) (lowest) | 91.9% | 83.9% | 78.7% | 73.6% | 64.1% | 53.8% | 44.9% | 33.7% | 23.7% | 14.8% |
| Level 2 (3) | 7.2% | 14.1% | 17.2% | 19.3% | 23.4% | 28.8% | 31.5% | 31.6% | 27.3% | 18.7% |
| Level 3 (4) (highest) | 0.8% | 2.0% | 4.2% | 7.1% | 12.5% | 17.4% | 23.6% | 34.8% | 49.0% | 66.5% |
| Broad-spectrum antibiotics (count) 6 | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 1) | 0 (0, 1) | 0 (0, 1) | 1 (0, 3) |
| Level 1 (0) (lowest) | 91.1% | 85.3% | 83.5% | 82.6% | 80.6% | 77.0% | 71.5% | 64.0% | 55.9% | 39.3% |
| Level 2 (1) | 7.2% | 12.1% | 13.3% | 13.6% | 14.3% | 15.8% | 17.3% | 19.9% | 21.4% | 18.8% |
| Level 3 (3) (highest) | 1.7% | 2.6% | 3.2% | 3.8% | 5.1% | 7.2% | 11.3% | 16.1% | 22.7% | 41.9% |
| Time between (day) 7 | 120 (11, 367) | 264 (58, 478) | 325 (116, 561) | 419 (180, 666) | 539 (268, 472) | 615 (402, 797) | 686 (477, 864) | 785 (583, 925) | 899 (743, 991) | 1010 (897, 1057) |
| Level 1 (75) (lowest) | 63.1% | 48.9% | 42.0% | 32.9% | 23.9% | 15.4% | 10.3% | 7.3% | 4.0% | 2.1% |
| Level 2 (423) | 24.9% | 33.2% | 35.1% | 34.9% | 31.8% | 30.7% | 26.3% | 17.9% | 9.3% | 6.2% |
| Level 3 (728) | 10.0% | 14.5% | 18.5% | 25.9% | 32.5% | 36.4% | 37.0% | 36.6% | 26.9% | 12.1% |
| Level 4 (977) (highest) | 2.0% | 3.4% | 4.4% | 6.3% | 11.8% | 17.4% | 26.3% | 38.2% | 59.8% | 79.5% |
| Recent antibiotics (day) 8 | 637 (388, 817) | 499 (292, 697) | 416 (252, 638) | 340 (199, 538) | 270 (150, 420) | 210 (188, 371) | 165 (95, 303) | 132 (80, 241) | 94 (62, 158) | 62 (51, 85) |
| Level 1 (65) (lowest) | 2.5% | 3.6% | 4.8% | 7.4% | 13.1% | 20.0% | 27.4% | 36.0% | 53.5% | 82.8% |
| Level 2 (155) | 6.8% | 12.9% | 17.5% | 24.0% | 30.3% | 33.9% | 37.1% | 38.0% | 33.6% | 14.8% |
| Level 3 (334) | 23.6% | 30.0% | 34.4% | 37.0% | 36.8% | 32.7% | 26.0% | 18.5% | 9.8% | 1.9% |
| Level 4 (678) (highest) | 67.1% | 53.5% | 43.3% | 31.6% | 19.8% | 13.4% | 9.6% | 7.5% | 3.0% | 0.5% |
| Prescribing intervals average (day) 9 | 98 (10, 238) | 151 (45, 314) | 175 (76, 324) | 190 (92, 316) | 186 (101, 306) | 170 (102, 284) | 145 (95, 236) | 122 (80, 174) | 94 (66, 138) | 50 (31, 76) |
| Level 1 (30) (lowest) | 43.7% | 29.8% | 22.2% | 17.2% | 13.3% | 11.6% | 11.8% | 14.6% | 22.0% | 63.8% |
| Level 2 (93) | 13.5% | 15.3% | 16.6% | 17.1% | 19.1% | 23.4% | 29.8% | 38.4% | 47.2% | 29.6% |
| Level 3 (171) | 17.6% | 20.6% | 24.2% | 26.3% | 29.2% | 31.3% | 33.5% | 34.1% | 27.1% | 6.0% |
| Level 4 (363) (highest) | 25.1% | 34.3% | 37.0% | 39.4% | 38.5% | 33.7% | 24.9% | 12.8% | 3.7% | 0.6% |
| Prescribing intervals deviation (day) 9 | 0 (0, 0) | 0 (0, 78) | 0 (0, 126) | 41 (0, 162) | 90 (0, 187) | 111 (40, 202) | 117 (63, 199) | 112 (71, 175) | 96 (62, 149) | 51 (25, 83) |
| Level 1 (0) (lowest) | 77.0% | 62.1% | 52.2% | 41.2% | 27.8% | 16.1% | 8.6% | 2.9% | 0.7% | 0.2% |
| Level 2 (37) | 7.6% | 11.7% | 12.8% | 14.8% | 16.3% | 18.0% | 18.2% | 20.0% | 27.9% | 64.1% |
| Level 3 (103) | 6.0% | 10.7% | 14.0% | 16.8% | 22.4% | 28.3% | 35.0% | 43.0% | 46.2% | 27.6% |
| Level 4 (226) (highest) | 9.4% | 15.5% | 21.0% | 27.2% | 33.5% | 37.6% | 38.2% | 34.2% | 25.3% | 8.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.-T.; Wong, D.; Zhong, X.; Fahmi, A.; Ashcroft, D.M.; Hand, K.; Massey, J.; Mackenna, B.; Mehrkar, A.; Bacon, S.; et al. Exploring Prior Antibiotic Exposure Characteristics for COVID-19 Hospital Admission Patients: OpenSAFELY. Antibiotics 2024, 13, 566. https://doi.org/10.3390/antibiotics13060566
Yang Y-T, Wong D, Zhong X, Fahmi A, Ashcroft DM, Hand K, Massey J, Mackenna B, Mehrkar A, Bacon S, et al. Exploring Prior Antibiotic Exposure Characteristics for COVID-19 Hospital Admission Patients: OpenSAFELY. Antibiotics. 2024; 13(6):566. https://doi.org/10.3390/antibiotics13060566
Chicago/Turabian StyleYang, Ya-Ting, David Wong, Xiaomin Zhong, Ali Fahmi, Darren M. Ashcroft, Kieran Hand, Jon Massey, Brian Mackenna, Amir Mehrkar, Sebastian Bacon, and et al. 2024. "Exploring Prior Antibiotic Exposure Characteristics for COVID-19 Hospital Admission Patients: OpenSAFELY" Antibiotics 13, no. 6: 566. https://doi.org/10.3390/antibiotics13060566
APA StyleYang, Y.-T., Wong, D., Zhong, X., Fahmi, A., Ashcroft, D. M., Hand, K., Massey, J., Mackenna, B., Mehrkar, A., Bacon, S., Goldacre, B., Palin, V., & van Staa, T. (2024). Exploring Prior Antibiotic Exposure Characteristics for COVID-19 Hospital Admission Patients: OpenSAFELY. Antibiotics, 13(6), 566. https://doi.org/10.3390/antibiotics13060566






