Clinical Evidence on Expansion of Essential Oil-Based Formulation’s Pharmacological Activity in Bovine Mastitis Treatment: Antifungal Potential as Added Value
Abstract
1. Introduction
2. Results
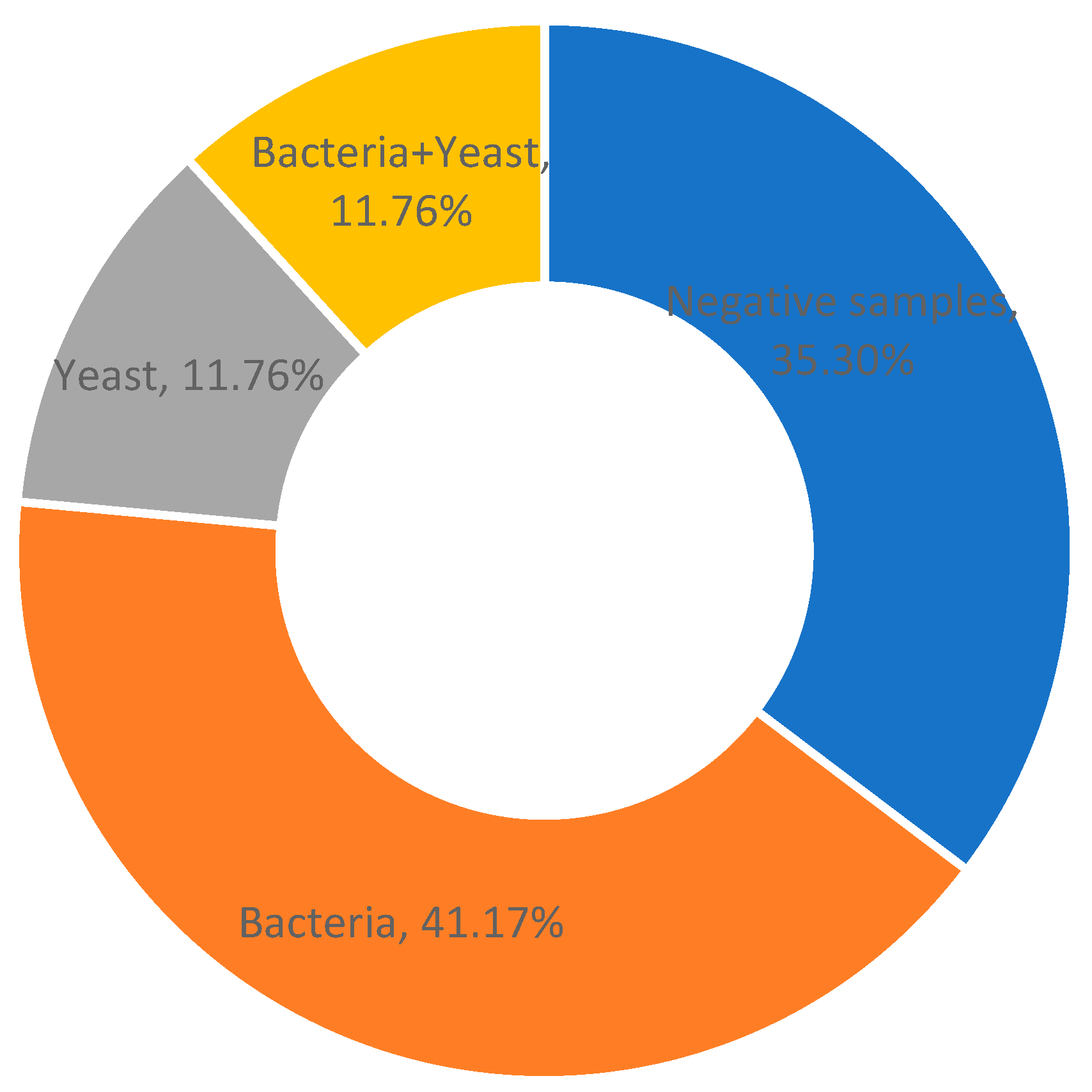
2.1. Prevalence of Mastitis-Associated Pathogens and In Vitro Antimicrobial Activity of Phyto-Bomat EOs
2.2. The Treatment Efficacy Regarding Isolate Status and Type of the Applied Treatments
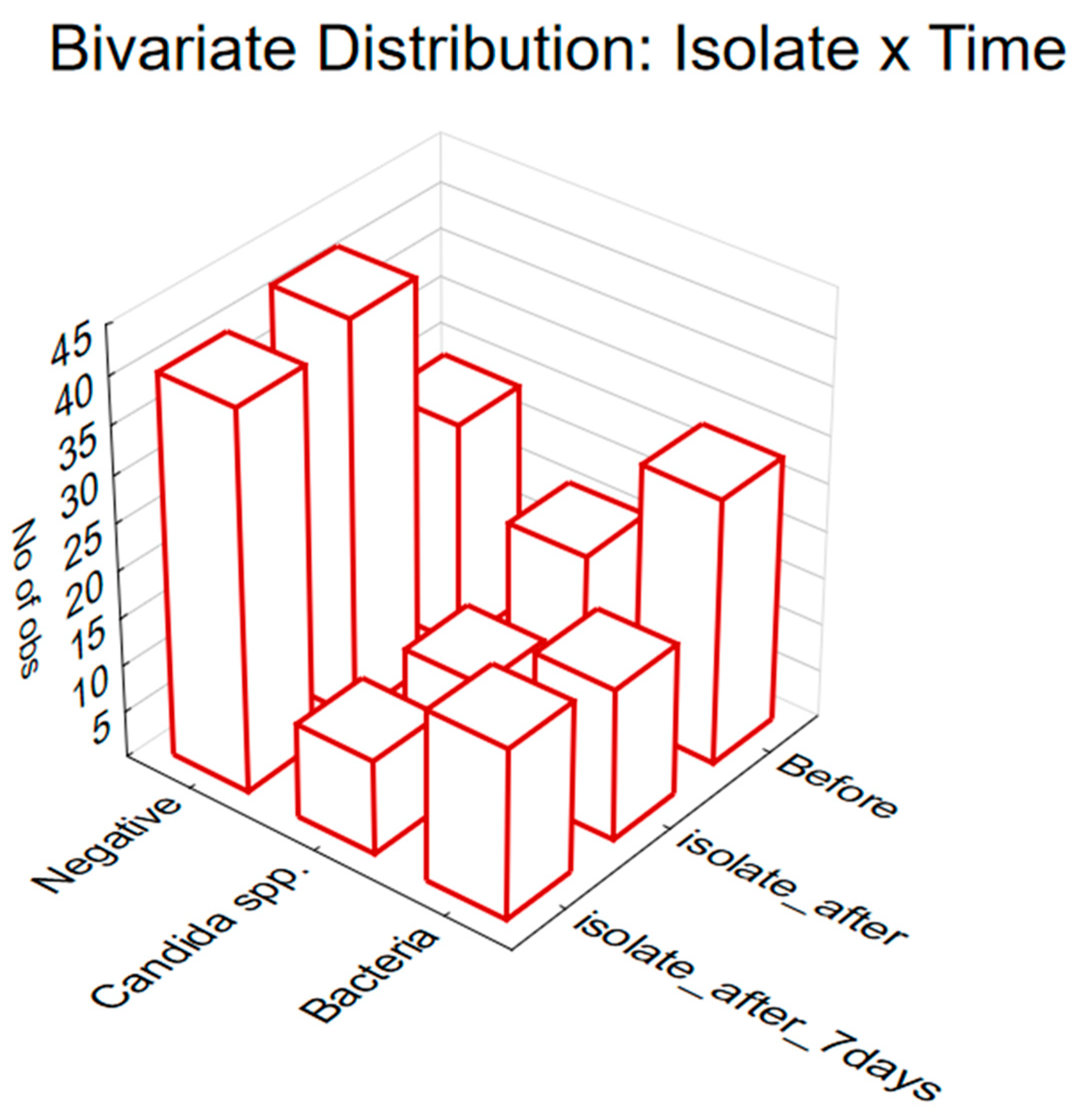
2.3. The Treatment Efficacy Regarding Isolate Status and Observed Time Point
3. Discussion
4. Materials and Methods
4.1. Development of Intramammary EO-Based Formulation (Phyto-Bomat)
4.2. Therapeutic Protocol
4.3. Isolation and Identification of the Pathogens and In Vitro Studies of EO Antimicrobial Activity
4.4. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krukowski, H.; Saba, L. Bovine mycotic mastitis. Folia Vet 2003, 47, 3–7. [Google Scholar]
- Benić, M.; Maćešić, N.; Cvetnić, L.; Habrun, B.; Cvetnić, Ž.; Turk, R.; Đuričić, D.; Lojkić, M.; Dobranić, V.; Valpotić, H. Bovine mastitis: A persistent and evolving problem requiring novel approaches for its control-a review. Vet. Arh. 2018, 88, 535–557. [Google Scholar] [CrossRef]
- Caneschi, A.; Bardhi, A.; Barbarossa, A.; Zaghini, A. Plant Essential Oils as a Tool in the Control of Bovine Mastitis: An Update. Molecules 2023, 28, 3425. [Google Scholar] [CrossRef] [PubMed]
- Cvetnić, L.; Samardžija, M.; Habrun, B.; Kompes, G.; Benić, M. Microbiological monitoring of mastitis pathogens in the control of udder health in dairy cows. Slov. Vet. Res. 2016, 53, 131–140. [Google Scholar]
- Huilca-Ibarra, M.P.; Vasco-Julio, D.; Ledesma, Y.; Guerrero-Freire, S.; Zurita, J.; Castillejo, P.; Barceló Blasco, F.; Yanez, L.; Changoluisa, D.; Echeverría, G. High prevalence of prototheca Bovis infection in dairy cattle with chronic mastitis in Ecuador. Vet. Sci. 2022, 9, 659. [Google Scholar] [CrossRef]
- Milanov, D.; Prunic, B.; Velhner, M.; Bojkovski, J. Diagnosis of yeast mastitis in dairy cows. Lucr. Stiintifice Med. Vet. 2014, 47, 56–64. [Google Scholar]
- Zaragoza, C.S.; Olivares, R.A.C.; Watty, A.E.D.; de la Peña Moctezuma, A.; Tanaca, L.V. Yeasts isolation from bovine mammary glands under different mastitis status in the Mexican High Plateu. Rev. Iberoam. Micol. 2011, 28, 79–82. [Google Scholar] [CrossRef]
- Pachauri, S.; Varshney, P.; Dash, S.; Gupta, M. Involvement of fungal species in bovine mastitis in and around Mathura India. Vet World 2013, 6, 393–395. [Google Scholar] [CrossRef]
- Zhou, Y.; Ren, Y.; Fan, C.; Shao, H.; Zhang, Z.; Mao, W.; Wei, C.; Ni, H.; Zhu, Z.; Hou, X. Survey of mycotic mastitis in dairy cows from Heilongjiang Province, China. Trop. Anim. Health Prod. 2013, 45, 1709–1714. [Google Scholar] [CrossRef]
- Du, J.; Wang, X.; Luo, H.; Wang, Y.; Liu, X.; Zhou, X. Epidemiological investigation of non-albicans Candida species recovered from mycotic mastitis of cows in Yinchuan, Ningxia of China. BMC Vet. Res. 2018, 14, 251. [Google Scholar] [CrossRef]
- Moretti, A.; Pasquali, P.; Mencaroni, G.; Boncio, L.; Fioretti, D.P. Relationship between cell counts in bovine milk and the presence of mastitis pathogens (yeasts and bacteria). J. Vet. Med. Ser. B 1998, 45, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Benbelkacem, I.; Selles, S.M.A.; Aissi, M.; Khaldi, F.; Ghazi, K. In vitro assessment of antifungal and antistaphylococcal activities of Cinnamomum aromaticum essential oil against subclinical mastitis pathogens. Veterinaria 2019, 68, 31–37. [Google Scholar]
- Morales-Ubaldo, A.L.; Rivero-Perez, N.; Valladares-Carranza, B.; Velázquez-Ordoñez, V.; Delgadillo-Ruiz, L.; Zaragoza-Bastida, A. Bovine mastitis, a worldwide impact disease: Prevalence, antimicrobial resistance, and viable alternative approaches. Vet. Anim. Sci. 2023, 21, 100306. [Google Scholar] [CrossRef] [PubMed]
- Al-Said, A.A.; Marwa, A.; Elfadadny, A.; Bantun, F.; Batiha, G.E.-S.; Conte-Junior, C.A. In vitro utility of zinc oxide nanoparticles and antifungal drugs for the treatment of mycotic mastitis in dairy cows in Egypt. Preprint 2022. [Google Scholar] [CrossRef]
- Ksouri, S.; Djebir, S.; Bentorki, A.; Gouri, A.; Hadef, Y.; Benakhla, A. Antifungal activity of essential oils extract from Origanum floribundum Munby, Rosmarinus officinalis L. and Thymus ciliatus Desf. against Candida albicans isolated from bovine clinical mastitis. J. Mycol. Médicale 2017, 27, 245–249. [Google Scholar] [CrossRef]
- Barreiros, Y.; de Meneses, A.C.; Alves, J.L.F.; Mumbach, G.D.; Ferreira, F.A.; Machado, R.A.F.; Bolzan, A.; de Araujo, P.H.H. Xanthan gum-based film-forming suspension containing essential oils: Production and in vitro antimicrobial activity evaluation against mastitis-causing microorganisms. LWT 2022, 153, 112470. [Google Scholar] [CrossRef]
- Tomanić, D.Z.; Stanojević, J.B.; Galić, I.M.; Ružić, Z.N.; Kukurić, T.B.; Tešin, N.B.; Prpa, B.P.; Kovačević, Z.R. Review of trends in essential oils as alternatives to antibiotics in bovine mastitis treatment. Zb. Matice Srp. Prir. Nauk. 2022, 142, 47–60. [Google Scholar] [CrossRef]
- Neculai-Valeanu, A.S.; Ariton, A.M.; Mădescu, B.M.; Rîmbu, C.M.; Creangă, Ş. Nanomaterials and essential oils as candidates for developing novel treatment options for bovine mastitis. Animals 2021, 11, 1625. [Google Scholar] [CrossRef]
- Bellache, M.; Torres-Pagan, N.; Verdeguer, M.; Benfekih, L.A.; Vicente, O.; Sestras, R.E.; Sestras, A.F.; Boscaiu, M. Essential oils of three aromatic plant species as natural herbicides for environmentally friendly agriculture. Sustainability 2022, 14, 3596. [Google Scholar] [CrossRef]
- Cho, T.J.; Park, S.M.; Yu, H.; Seo, G.H.; Kim, H.W.; Kim, S.A.; Rhee, M.S. Recent advances in the application of antibacterial complexes using essential oils. Molecules 2020, 25, 1752. [Google Scholar] [CrossRef]
- Budri, P.E.; Silva, N.C.; Bonsaglia, E.C.; Júnior, A.F.; Júnior, J.A.; Doyama, J.T.; Gonçalves, J.L.; Santos, M.V.d.; Fitzgerald-Hughes, D.; Rall, V.L. Effect of essential oils of Syzygium aromaticum and Cinnamomum zeylanicum and their major components on biofilm production in Staphylococcus aureus strains isolated from milk of cows with mastitis. J. Dairy Sci. 2015, 98, 5899–5904. [Google Scholar] [CrossRef] [PubMed]
- Pașca, C.; Mărghitaș, L.; Dezmirean, D.; Bobiș, O.; Bonta, V.; Chirilă, F.; Matei, I.; Fiț, N. Medicinal plants based products tested on pathogens isolated from mastitis milk. Molecules 2017, 22, 1473. [Google Scholar] [CrossRef] [PubMed]
- Cerioli, M.F.; Moliva, M.V.; Cariddi, L.N.; Reinoso, E.B. Effect of the essential oil of Minthostachys verticillata (Griseb.) epling and limonene on biofilm production in pathogens causing bovine mastitis. Front. Vet. Sci. 2018, 5, 146. [Google Scholar] [CrossRef] [PubMed]
- Szweda, P.; Zalewska, M.; Pilch, J.; Kot, B.; Milewski, S. Essential oils as potential anti-staphylococcal agents. Acta Vet.-Beogr. 2018, 68, 95–107. [Google Scholar]
- Kovačević, Z.; Kladar, N.; Čabarkapa, I.; Radinović, M.; Maletić, M.; Erdeljan, M.; Božin, B. New perspective of Origanum vulg L. and Satureja montana L. essential oils as bovine mastitis treatment alternatives. Antibiotics 2021, 10, 1460. [Google Scholar] [CrossRef] [PubMed]
- Kovačević, Z.; Radinović, M.; Čabarkapa, I.; Kladar, N.; Božin, B. Natural agents against bovine mastitis pathogens. Antibiotics 2021, 10, 205. [Google Scholar] [CrossRef] [PubMed]
- Tomanić, D.; Božin, B.; Kladar, N.; Stanojević, J.; Čabarkapa, I.; Stilinović, N.; Apić, J.; Božić, D.D.; Kovačević, Z. Environmental bovine mastitis pathogens: Prevalence, antimicrobial susceptibility, and sensitivity to Thymus vulgaris L., Thymus serpyllum L., and Origanum vulgare L. essential oils. Antibiotics 2022, 11, 1077. [Google Scholar] [CrossRef] [PubMed]
- Tomanić, D.; Božin, B.; Čabarkapa, I.; Kladar, N.; Radinović, M.; Maletić, M.; Kovačević, Z. Chemical composition, antioxidant and antibacterial activity of two different essential oils against mastitis associated pathogens. Acta Vet. 2022, 72, 45–58. [Google Scholar] [CrossRef]
- Pereira, A.M.S.; de Castro Franca, S.; Fachin, A.L.; Bertoni, B.W.; Pina, E.S.; da Silva Coppede, J. Phytotherapic Pharmaceutical Combination of Lippia salviifolia and Lippia sidoides, Phytotherapic Pharmaceutical Composition, Process for Preparing a Phytotherapic Pharmaceutical Composition and Veterinary Uses Thereof. U.S. Patent 9,295,706, 29 March 2016. [Google Scholar]
- Ucella-Filho, J.G.M.; Ferreira, N.S.; Alves, M.R.; Ignacchiti, M.D.C.; Júnior, A.F.D.; Resende, J.A. Evaluation of natural products as therapeutic alternatives for bovine mastitis and implications for future research. S. Afr. J. Bot. 2024, 167, 310–321. [Google Scholar] [CrossRef]
- Malinowski, E.; Lassa, H.; Klossowska, A.; Markiewicz, H.; Kaczmarowski, M.; Smulski, S. Relationship between mastitis agents and somatic cell count in foremilk samples. Bull.-Vet. Inst. Pulawy 2006, 50, 349. [Google Scholar]
- Abd El-Razik, K.A.; Abdelrahman, K.A.; Abd El-Moez, S.I.; Danial, E.N. New approach in diagnosis and treatment of bovine mycotic mastitis in Egypt. Afr. J. Microbiol. Res. 2011, 5, 5725–5732. [Google Scholar] [CrossRef]
- Tesfaye, B.; Matios, L.; Getachew, T.; Tafesse, K.; Abebe, O.; Letebrihan, Y.; Mekdes, T.; Tilaye, D. Study on bovine mastitis with isolation of bacterial and fungal causal agents and assessing antimicrobial resistance patterns of isolated Staphylococcus species in and around Sebeta town, Ethiopia. Afr. J. Microbiol. Res. 2019, 13, 23–32. [Google Scholar] [CrossRef]
- Krukowski, H.; Tietze, M.; Majewski, T.; Różański, P. Survey of yeast mastitis in dairy herds of small-type farms in the Lublin region, Poland. Mycopathologia 2001, 150, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Wawron, W.; Bochniarz, M.; Piech, T. Yeast mastitis in dairy cows in the middle-eastern part of Poland. Bull.-Vet. Inst. Pulawy 2010, 54, 201–204. [Google Scholar]
- Bakr, E.M.; El-Tawab, A.E.-k.M.A.; Elshemey, T.M.; Abd-Elrhman, A.H. Diagnostic and Therapeutic Studies on Mycotic Mastitis in Cattle. Alex. J. Vet. Sci. 2015, 46, 138–145. [Google Scholar] [CrossRef]
- Aljaafari, M.N.; AlAli, A.O.; Baqais, L.; Alqubaisy, M.; AlAli, M.; Molouki, A.; Ong-Abdullah, J.; Abushelaibi, A.; Lai, K.-S.; Lim, S.-H.E. An overview of the potential therapeutic applications of essential oils. Molecules 2021, 26, 628. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; De Feo, V. Essential oils and antifungal activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Khan, A.; Akhtar, F.; Yousuf, S.; Xess, I.; Khan, L.; Manzoor, N. Fungicidal activity of thymol and carvacrol by disrupting ergosterol biosynthesis and membrane integrity against Candida. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 41–50. [Google Scholar] [CrossRef]
- Mugnaini, L.; Nardoni, S.; Pinto, L.; Pistelli, L.; Leonardi, M.; Pisseri, F.; Mancianti, F. In vitro and in vivo antifungal activity of some essential oils against feline isolates of Microsporum canis. J. Mycol. Medicale 2012, 22, 179–184. [Google Scholar] [CrossRef]
- Ebani, V.V.; Nardoni, S.; Bertelloni, F.; Giovanelli, S.; Rocchigiani, G.; Pistelli, L.; Mancianti, F. Antibacterial and antifungal activity of essential oils against some pathogenic bacteria and yeasts shed from poultry. Flavour Fragr. J. 2016, 31, 302–309. [Google Scholar] [CrossRef]
- Hu, F.; Tu, X.-F.; Thakur, K.; Hu, F.; Li, X.-L.; Zhang, Y.-S.; Zhang, J.-G.; Wei, Z.-J. Comparison of antifungal activity of essential oils from different plants against three fungi. Food Chem. Toxicol. 2019, 134, 110821. [Google Scholar] [CrossRef]
- Mutlu-Ingok, A.; Devecioglu, D.; Dikmetas, D.N.; Karbancioglu-Guler, F.; Capanoglu, E. Antibacterial, antifungal, antimycotoxigenic, and antioxidant activities of essential oils: An updated review. Molecules 2020, 25, 4711. [Google Scholar] [CrossRef]
- Abd Rashed, A.; Rathi, D.-N.G.; Ahmad Nasir, N.A.H.; Abd Rahman, A.Z. Antifungal properties of essential oils and their compounds for application in skin fungal infections: Conventional and nonconventional approaches. Molecules 2021, 26, 1093. [Google Scholar] [CrossRef]
- Tortorano, A.M.; Prigitano, A.; Dho, G.; Piccinini, R.; Dapra, V.; Viviani, M.A. In vitro activity of conventional antifungal drugs and natural essences against the yeast-like alga Prototheca. J. Antimicrob. Chemother. 2008, 61, 1312–1314. [Google Scholar] [CrossRef][Green Version]
- Nardoni, S.; Pisseri, F.; Pistelli, L.; Najar, B.; Luini, M.; Mancianti, F. In vitro activity of 30 essential oils against bovine clinical isolates of Prototheca zopfii and Prototheca blaschkeae. Vet. Sci. 2018, 5, 45. [Google Scholar] [CrossRef]
- Corona-Gómez, L.; Hernández-Andrade, L.; Mendoza-Elvira, S.; Suazo, F.M.; Ricardo-González, D.I.; Quintanar-Guerrero, D. In vitro antimicrobial effect of essential tea tree oil (Melaleuca alternifolia), thymol, and carvacrol on microorganisms isolated from cases of bovine clinical mastitis. Int. J. Vet. Sci. Med. 2022, 10, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Raheel, I.A.; Hassan, W.H.; Salam, H.S.; Abed, A.H.; Salem, S.S. Antifungal Effect of Some Natural Substances on Fluconazole-resistant Candida species Recovered from Mastitis. J. Adv. Vet. Res. 2023, 13, 1583–1587. [Google Scholar]
- Schroder, T.; Gaskin, S.; Ross, K.; Whiley, H. Antifungal activity of essential oils against fungi isolated from air. Int. J. Occup. Environ. Health 2017, 23, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Shao, Y.-L.; Tang, Y.-J.; Zhou, W.-W. Antifungal activity of essential oil compounds (geraniol and citral) and inhibitory mechanisms on grain pathogens (Aspergillus flavus and Aspergillus ochraceus). Molecules 2018, 23, 2108. [Google Scholar] [CrossRef]
- Ashraf, A.; Fatma, I.; Tamer, F.; Wedad, A. Antimicrobial activity of some essential oils against S. Aureus and Candida Albicans with mastitic relevance. Behna Vet. Med. J. 2015, 28, 281–288. [Google Scholar]
- da Silva Rocha, A.R.F.; da Costa Santos, J.V.; Moura, R.H.; Lago, E.C.; de Lima, F.L. Prospecting of anti-Candida bioactive in Origanum vulgare L. artisanal essential oil. Res. Soc. Dev. 2021, 10, e220101421758. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F. Antibacterial and antifungal activities of essential oils. In Lipids and Essential Oils as Antimicrobial Agents; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 255–306. [Google Scholar]
- Bozin, B.; Mimica-Dukic, N.; Simin, N.; Anackov, G. Characterization of the volatile composition of essential oils of some Lamiaceae spices and the antimicrobial and antioxidant activities of the entire oils. J. Agric. Food Chem. 2006, 54, 1822–1828. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, M.; Glamočlija, J.; Ferreira, I.C.; Calhelha, R.C.; Fernandes, Â.; Marković, T.; Marković, D.; Giweli, A.; Soković, M. Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L., Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. essential oils. Ind. Crops Prod. 2014, 52, 183–190. [Google Scholar] [CrossRef]
- Gavaric, N.; Mozina, S.S.; Kladar, N.; Bozin, B. Chemical profile, antioxidant and antibacterial activity of thyme and oregano essential oils, thymol and carvacrol and their possible synergism. J. Essent. Oil Bear. Plants 2015, 18, 1013–1021. [Google Scholar] [CrossRef]
- Lemos, M.F.; Lemos, M.F.; Pacheco, H.P.; Guimarães, A.C.; Fronza, M.; Endringer, D.C.; Scherer, R. Seasonal variation affects the composition and antibacterial and antioxidant activities of Thymus vulgaris. Ind. Crops Prod. 2017, 95, 543–548. [Google Scholar] [CrossRef]
- Kovačević, Z.; Tomanić, D.; Čabarkapa, I.; Šarić, L.; Stanojević, J.; Bijelić, K.; Galić, I.; Ružić, Z.; Erdeljan, M.; Kladar, N. Chemical Composition, Antimicrobial Activity, and Withdrawal Period of Essential Oil-Based Pharmaceutical Formulation in Bovine Mastitis Treatment. Int. J. Environ. Res. Public Health 2022, 19, 16643. [Google Scholar] [CrossRef] [PubMed]
- Tomanić, D.; Kladar, N.; Radinović, M.; Stančić, I.; Erdeljan, M.; Stanojević, J.; Galić, I.; Bijelić, K.; Kovačević, Z. Intramammary Ethno-Veterinary Formulation in Bovine Mastitis Treatment for Optimization of Antibiotic Use. Pathogens 2023, 12, 259. [Google Scholar] [CrossRef]
- Tamminen, L.-M.; Emanuelson, U.; Blanco-Penedo, I. Systematic review of phytotherapeutic treatments for different farm animals under European conditions. Front. Vet. Sci. 2018, 5, 140. [Google Scholar] [CrossRef] [PubMed]
- Hase, P.; Digraskar, S.; Ravikanth, K.; Dandale, M.; Maini, S. Management of subclinical mastitis with mastilep gel and herbal spray (AV/AMS/15). Int. J. Pharm. Pharmacol 2013, 4, 64–67. [Google Scholar]
- Cho, B.-W.; Cha, C.-N.; Lee, S.-M.; Kim, M.-J.; Park, J.-Y.; Yoo, C.-Y.; Son, S.-E.; Kim, S.; Lee, H.-J. Therapeutic effect of oregano essential oil on subclinical bovine mastitis caused by Staphylococcus aureus and Escherichia coli. Korean J. Vet. Res. 2015, 55, 253–257. [Google Scholar] [CrossRef]
- Mullen, K.; Lee, A.; Lyman, R.; Mason, S.; Washburn, S.; Anderson, K. An in vitro assessment of the antibacterial activity of plant-derived oils. J. Dairy Sci. 2014, 97, 5587–5591. [Google Scholar] [CrossRef] [PubMed]
- Pinedo, P.; Karreman, H.; Bothe, H.; Velez, J.; Risco, C. Efficacy of a botanical preparation for the intramammary treatment of clinical mastitis on an organic dairy farm. Can. Vet. J. 2013, 54, 479. [Google Scholar] [CrossRef] [PubMed]
- Ebani, V.V.; Mancianti, F. Use of essential oils in veterinary medicine to combat bacterial and fungal infections. Vet. Sci. 2020, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Rosato, A.; Vitali, C.; Gallo, D.; Balenzano, L.; Mallamaci, R. The inhibition of Candida species by selected essential oils and their synergism with amphotericin B. Phytomedicine 2008, 15, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Rosato, A.; Vitali, C.; Piarulli, M.; Mazzotta, M.; Argentieri, M.P.; Mallamaci, R. In vitro synergic efficacy of the combination of Nystatin with the essential oils of Origanum vulgare and Pelargonium graveolens against some Candida species. Phytomedicine 2009, 16, 972–975. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Rolta, R.; Dev, K.; Sourirajan, A. Synergistic potential of essential oils with antibiotics to combat fungal pathogens: Present status and future perspectives. Phytother. Res. 2021, 35, 6089–6100. [Google Scholar] [CrossRef] [PubMed]
- Scalas, D.; Mandras, N.; Roana, J.; Tardugno, R.; Cuffini, A.M.; Ghisetti, V.; Benvenuti, S.; Tullio, V. Use of Pinus sylvestris L.(Pinaceae), Origanum vulgare L. (Lamiaceae), and Thymus vulgaris L. (Lamiaceae) essential oils and their main components to enhance itraconazole activity against azole susceptible/not-susceptible Cryptococcus neoformans strains. BMC Complement. Altern. Med. 2018, 18, 143. [Google Scholar] [CrossRef]
- Carbone, C.; Teixeira, M.d.C.; Sousa, M.d.C.; Martins-Gomes, C.; Silva, A.M.; Souto, E.M.B.; Musumeci, T. Clotrimazole-loaded mediterranean essential oils NLC: A synergic treatment of Candida skin infections. Pharmaceutics 2019, 11, 231. [Google Scholar] [CrossRef] [PubMed]
- Willcox, J.K.; Ash, S.L.; Catignani, G.L. Antioxidants and prevention of chronic disease. Crit. Rev. Food Sci. Nutr. 2004, 44, 275–295. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
- Langeveld, W.T.; Veldhuizen, E.J.; Burt, S.A. Synergy between essential oil components and antibiotics: A review. Crit. Rev. Microbiol. 2014, 40, 76–94. [Google Scholar] [CrossRef] [PubMed]
- ALIMS. Summary of Product Characteristics-RILEXINE 200 LC. Available online: https://www.alims.gov.rs/doc_file/lekovi_veterina/smpc/323-01-00317-19-001.pdf (accessed on 10th October 2023.).
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters; Version V.13.11. June 2023. Available online: https://www.eucast.org/mic_and_zone_distributions_and_ecoffs (accessed on 23rd June 2023).
- The European Committee on Antimicrobial Susceptibility Testing. Media Preparation for EUCAST Disk Diffusion Testing and for Determination of MIC Values by the Broth Microdilution Method; V.6.0. January 2020. Available online: http://www.eucast.org (accessed on 23rd June 2023).
- Garcia, L. Synergism testing: Broth microdilution checkerboard and broth macrodilution methods. In Clinical Microbiology Procedures Handbook, 3rd ed.; Leber, A.L., Ed.; American Society of Microbiology: Washington, DC, USA; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Hu, Z.Q.; Zhao, W.H.; Asano, N.; Yoda, Y.; Hara, Y.; Shimamura, T. Epigallocatechin gallate synergistically enhances the activity of carbapenems against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2002, 46, 558–560. [Google Scholar] [CrossRef] [PubMed]

| Microorganisms | TV (mg/mL) | TS (mg/mL) | OV (mg/mL) | SM (mg/mL) | ||||
|---|---|---|---|---|---|---|---|---|
| Bacteria | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
| Streptococcus spp. | 3.12–6.25 | 6.25–12.5 | 0.39–3.12 | 0.78–12.5 | 3.12–6.25 | 6.25–12.5 | 0.39–6.25 | 0.78->12.5 |
| Staphylococcus spp. | 6.25 | 12.5 | 1.56–3.12 | 3.12–6.25 | 3.12 | 6.25 | 6.25 | 12.5 |
| Enterobacterales | 1.56–3.12 | 3.12–6.25 | 1.56–3.12 | 3.12–6.25 | 0.78–3.12 | 1.56–6.25 | 3.12 | 6.25 |
| Yeasts | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC |
| C. albicans | 1.56–3.12 | 3.12–6.25 | 0.78–3.12 | 3.12–12.5 | 1.56–3.12 | 3.12–6.25 | 1.56–3.12 | 3.12–6.25 |
| C. glabrata | 1.56–6.25 | 3.12–12.5 | 0.78–3.12 | 1.56–6.25 | 0.78–1.56 | 1.56–3.12 | 3.12–6.25 | 6.25–12.5 |
| C. parapsilosis | 6.25 | 12.5 | 1.56 | 3.12 | 1.56 | 3.12 | 6.25 | 12.5 |
| C. krusei | 3.12 | 6.25 | 1.56 | 3.12 | 1.56 | 6.25 | 6.25 | 12.5 |
| Candida albicans ATCC 24433 | 0.78 | 1.56 | 0.39 | 0.78 | 0.39 | 0.78 | 0.78 | 1.56 |
| C. krusei ATCC 6258 | 1.56 | 3.12 | 0.78 | 3.12 | 0.78 | 3.12 | 1.56 | 3.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomanić, D.; Božić, D.D.; Kladar, N.; Samardžija, M.; Apić, J.; Baljak, J.; Kovačević, Z. Clinical Evidence on Expansion of Essential Oil-Based Formulation’s Pharmacological Activity in Bovine Mastitis Treatment: Antifungal Potential as Added Value. Antibiotics 2024, 13, 575. https://doi.org/10.3390/antibiotics13070575
Tomanić D, Božić DD, Kladar N, Samardžija M, Apić J, Baljak J, Kovačević Z. Clinical Evidence on Expansion of Essential Oil-Based Formulation’s Pharmacological Activity in Bovine Mastitis Treatment: Antifungal Potential as Added Value. Antibiotics. 2024; 13(7):575. https://doi.org/10.3390/antibiotics13070575
Chicago/Turabian StyleTomanić, Dragana, Dragana D. Božić, Nebojša Kladar, Marko Samardžija, Jelena Apić, Jovan Baljak, and Zorana Kovačević. 2024. "Clinical Evidence on Expansion of Essential Oil-Based Formulation’s Pharmacological Activity in Bovine Mastitis Treatment: Antifungal Potential as Added Value" Antibiotics 13, no. 7: 575. https://doi.org/10.3390/antibiotics13070575
APA StyleTomanić, D., Božić, D. D., Kladar, N., Samardžija, M., Apić, J., Baljak, J., & Kovačević, Z. (2024). Clinical Evidence on Expansion of Essential Oil-Based Formulation’s Pharmacological Activity in Bovine Mastitis Treatment: Antifungal Potential as Added Value. Antibiotics, 13(7), 575. https://doi.org/10.3390/antibiotics13070575







