The Impact of Surgical Bowel Preparation on the Microbiome in Colon and Rectal Surgery
Abstract
:1. Introduction
2. Bowel Preparation
2.1. Evolution of Bowel Preparation
2.2. Mechanical Bowel Preparation Controversy
3. Microbiome
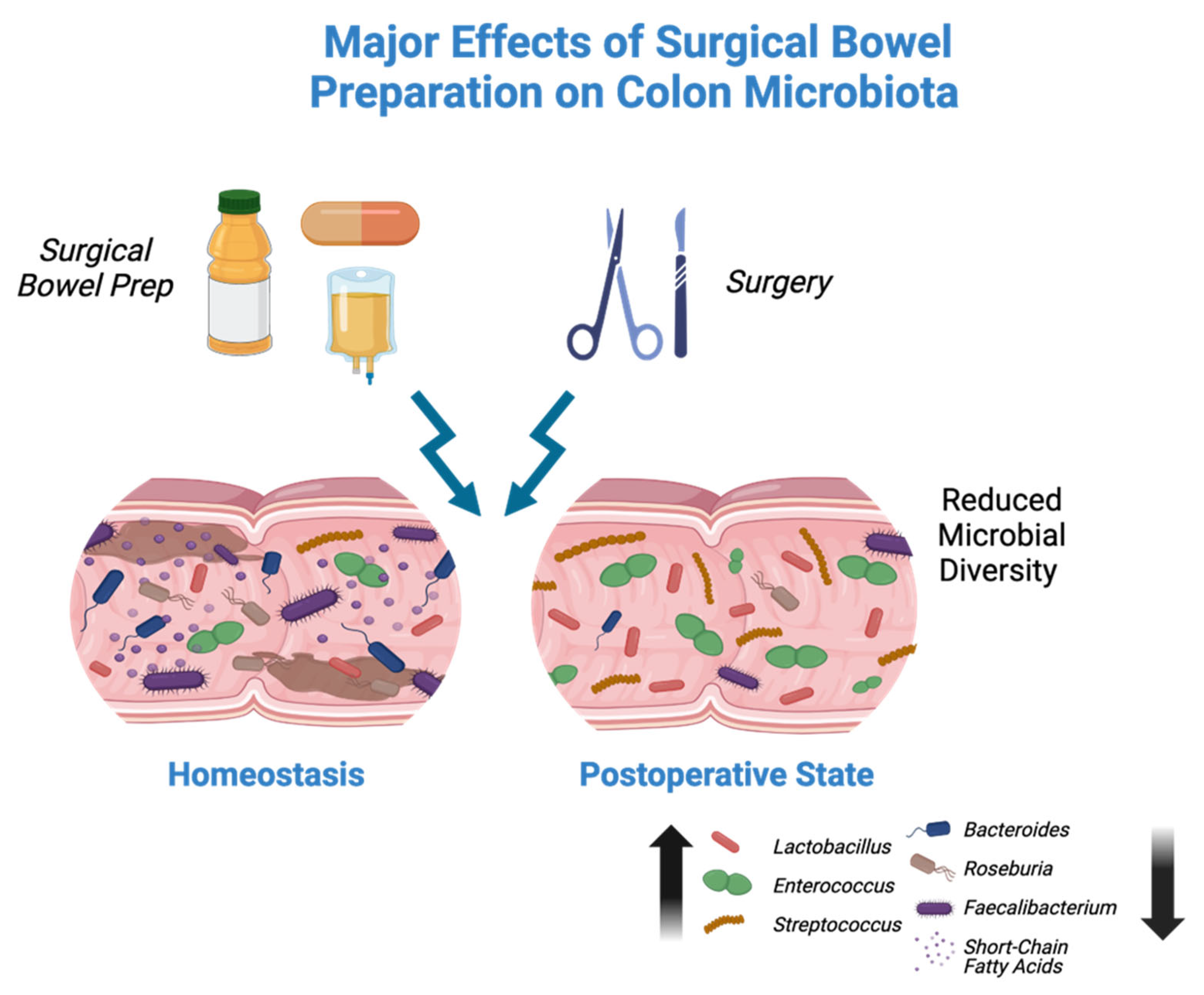
4. Impact of Bowel Preparation on the Microbiome
5. Role of Probiotics in Colorectal Surgery
6. Clinical Implications and Areas of Future Study
7. Limitations
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ASCRS | American Society of Colon and Rectal Surgeons |
| ERAS | Enhanced Recovery After Surgery |
| IBD | Inflammatory bowel disease |
| MBP | Mechanical bowel preparation |
| NSQIP | National Surgical Quality Improvement Program |
| OA | Oral antibiotic |
| SBP | Surgical bowel preparation |
| SSI | Surgical site infection |
References
- Petrou, N.A.; Kontovounisios, C. The Use of Mechanical Bowel Preparation and Oral Antibiotic Prophylaxis in Elective Colorectal Surgery: A Call for Change in Practice. Cancers 2022, 14, 5990. [Google Scholar] [CrossRef] [PubMed]
- Young, H.; Knepper, B.; Moore, E.E.; Johnson, J.L.; Mehler, P.; Price, C.S. Surgical Site Infection after Colon Surgery: National Healthcare Safety Network Risk Factors and Modeled Rates Compared with Published Risk Factors and Rates. J. Am. Coll. Surg. 2012, 214, 852–859. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Guidelines for the Prevention of Surgical Site Infection, 2nd ed.; World Health Organization: Geneva, Switzerland, 2018; Available online: https://iris.who.int/handle/10665/277399 (accessed on 14 May 2024).
- Ban, K.A.; Minei, J.P.; Laronga, C.; Harbrecht, B.G.; Jensen, E.H.; Fry, D.E.; Itani, K.M.; E Dellinger, P.; Ko, C.Y.; Duane, T.M. American College of Surgeons and Surgical Infection Society: Surgical Site Infection Guidelines, 2016 Update. J. Am. Coll. Surg. 2017, 224, 59–74. [Google Scholar] [CrossRef]
- Migaly, J.; Bafford, A.C.; Francone, T.D.; Gaertner, W.B.; Eskicioglu, C.; Bordeianou, L.; Feingold, D.L.; Steele, S.R. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Use of Bowel Preparation in Elective Colon and Rectal Surgery. Dis Colon Rectum. 2019, 62, 3–8, Correction in Dis. Colon. Rectum. 2019, 62, e436. [Google Scholar] [CrossRef]
- Alverdy, J.C.; Hyman, N.; Gilbert, J.; Luo, J.N.; Krezalek, M. Preparing the Bowel for Surgery: Learning from the Past and Planning for the Future. J. Am. Coll. Surg. 2017, 225, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Russ, A.J.; Casillas, M.A. Gut Microbiota and Colorectal Surgery: Impact on Postoperative Complications. Clin. Colon. Rectal Surg. 2016, 29, 253–257. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103, Correction in Microbiome 2020, 8, 119. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Shobar, R.M.; Velineni, S.; Keshavarzian, A.; Swanson, G.; DeMeo, M.T.; E Melson, J.; Losurdo, J.; Engen, P.A.; Sun, Y.; Koenig, L.; et al. The Effects of Bowel Preparation on Microbiota-Related Metrics Differ in Health and in Inflammatory Bowel Disease and for the Mucosal and Luminal Microbiota Compartments. Clin. Transl. Gastroenterol. 2016, 7, e143. [Google Scholar] [CrossRef]
- Drago, L.; Toscano, M.; De Grandi, R.; Casini, V.; Pace, F. Persisting changes of intestinal microbiota after bowel lavage and colonoscopy. Eur. J. Gastroenterol. Hepatol. 2016, 28, 532–537. [Google Scholar] [CrossRef]
- Harrell, L.; Wang, Y.; Antonopoulos, D.; Young, V.; Lichtenstein, L.; Huang, Y.; Hanauer, S.; Chang, E. Standard Colonic Lavage Alters the Natural State of Mucosal-Associated Microbiota in the Human Colon. PLoS ONE 2012, 7, e32545. [Google Scholar] [CrossRef] [PubMed]
- Nalluri-Butz, H.; Bobel, M.C.; Nugent, J.; Boatman, S.; Emanuelson, R.; Melton-Meaux, G.; Madoff, R.D.; Jahansouz, C.; Staley, C.; Gaertner, W.B. A pilot study demonstrating the impact of surgical bowel preparation on intestinal microbiota composition following colon and rectal surgery. Sci. Rep. 2022, 12, 10559. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.E.; Quietmeyer, C.M. Bowel preparation: Current status. Clin. Colon. Rectal Surg. 2009, 22, 14–20. [Google Scholar] [CrossRef]
- Poth, E.J. Historical development of intestinal antisepsis. World J. Surg. 1982, 6, 153–159. [Google Scholar] [CrossRef]
- Kumar, A.S.; Kelleher, D.C.; Sigle, G.W. Bowel Preparation before Elective Surgery. Clin. Colon. Rectal. Surg. 2013, 26, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Keighley, M.R. A clinical and physiological evaluation of bowel preparation for elective colorectal surgery. World J. Surg. 1982, 6, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.R.; Santa Ana, C.A.; Morawski, S.G.; Fordtran, J.S. Development of a lavage solution associated with minimal water and electrolyte absorption or secretion. Gastroenterology 1980, 78, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Nichols, R.L.; Smith, J.W.; Garcia, R.Y.; Waterman, R.S.; Holmes, J.W. Current practices of preoperative bowel preparation among North American colorectal surgeons. Clin. Infect. Dis. 1997, 24, 609–619. [Google Scholar] [CrossRef]
- Zmora, O.; Wexner, S.D.; Hajjar, L.; Park, T.; Efron, J.E.; Nogueras, J.J.; Weiss, E.G. Trends in Preparation for Colorectal Surgery: Survey of the Members of the American Society of Colon and Rectal Surgeons. Am. Surg. 2003, 69, 150–154. [Google Scholar] [CrossRef]
- Rosenberg, I.L.; Graham, N.G.; De Dombal, F.T.; Goligher, J.C. Preparation of the intestine in patients undergoing major large-bowel surgery, mainly for neoplasms of the colon and rectum. Br. J. Surg. 1971, 58, 266–269. [Google Scholar] [CrossRef]
- Cohn, I., Jr. Kanamycin for bowel sterilization. Ann. N. Y. Acad. Sci. 1958, 76, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Bowel preparation for surgery. Lancet 1978, 2, 1132–1133.
- Nichols, R.L.; Condon, R.E. Antibiotic preparation of the colon: Failure of commonly used regimens. Surg. Clin. N. Am. 1971, 51, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Sellwood, R.A.; Burn, J.I.; Waterworth, P.M.; Welbourn, R.B. A second clinical trial to compare two methods for preoperative preparation of the large bowel. Br. J. Surg. 1969, 56, 610–612. [Google Scholar] [CrossRef] [PubMed]
- Nichols, R.L.; Broido, P.; Condon, R.E.; Gorbach, S.L.; Nyhus, L.M. Effect of preoperative neomycin-erythromycin intestinal preparation on the incidence of infectious complications following colon surgery. Ann. Surg. 1973, 178, 453–462. [Google Scholar] [CrossRef]
- Clarke, J.S.; Condon, R.E.; Bartlett, J.G.; Gorbach, S.L.; Nichols, R.L.; Ochi, S. Preoperative oral antibiotics reduce septic complications of colon operations: Results of prospective, randomized, double-blind clinical study. Ann. Surg. 1977, 186, 251–259. [Google Scholar] [CrossRef]
- Ju, Y.U.; Min, B.W. A Review of Bowel Preparation Before Colorectal Surgery. Ann. Coloproctol. 2021, 37, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Platell, C.; Hall, J. What is the role of mechanical bowel preparation in patients undergoing colorectal surgery? Dis. Colon. Rectum. 1998, 41, 875–883. [Google Scholar] [CrossRef]
- Young Tabusso, F.; Celis Zapata, J.; Berrospi Espinoza, F.; Payet Meza, E.; Ruiz Figueroa, E. Preparación mecánica en cirugía electiva colo-rectal. Costumbre o necesidad? [Mechanical preparation in elective colorectal surgery, a usual practice or a necessity?]. Rev. Gastroenterol. Peru 2002, 22, 152–158. [Google Scholar]
- Burke, P.; Mealy, K.; Gillen, P.; Joyce, W.; Traynor, O.; Hyland, J. Requirement for bowel preparation in colorectal surgery. Br. J. Surg. 1994, 81, 907–910. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.C., Jr.; Batista, J.; Sirimarco, M.T.; Guimarães, A.S.; Levy, C.E. Prospective randomized trial of mechanical bowel preparation in patients undergoing elective colorectal surgery. Br. J. Surg. 1994, 81, 1673–1676. [Google Scholar] [CrossRef]
- Bucher, P.; Mermillod, B.; Gervaz, P.; Morel, P. Mechanical bowel preparation for elective colorectal surgery: A meta-analysis. Arch. Surg. 2004, 139, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Güenaga, K.F.; Matos, D.; Wille-Jørgensen, P. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst. Rev. 2011, 2011, CD001544. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, U.O.; Scott, M.J.; Schwenk, W.; Demartines, N.; Roulin, D.; Francis, N.; McNaught, C.E.; MacFie, J.; Liberman, A.S.; Soop, M.; et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS(®)) Society recommendations. World J. Surg. 2013, 37, 259–284. [Google Scholar] [CrossRef] [PubMed]
- NICE. Surgical Site Infections: Prevention and Treatment; National Institute for Health and Care Excellence (NICE): London, UK, 2019; Available online: https://www.nice.org.uk/guidance/ng125/resources/surgical-site-infections-prevention-and-treatment-pdf-66141660564421 (accessed on 14 May 2024).
- Ohge, H.; Mayumi, T.; Haji, S.; Kitagawa, Y.; Kobayashi, M.; Kobayashi, M.; Mizuguchi, T.; Mohri, Y.; Sakamoto, F.; Shimizu, J.; et al. The Japan Society for Surgical Infection: Guidelines for the prevention, detection, and management of gastroenterological surgical site infection, 2018. Surg. Today 2021, 51, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Konishi, T.; Watanabe, T.; Kishimoto, J.; Nagawa, H. Elective colon and rectal surgery differ in risk factors for wound infection: Results of prospective surveillance. Ann. Surg. 2006, 244, 758–763. [Google Scholar] [CrossRef]
- Singh, S.S.; Shinde, R.K. Minimally Invasive Gastrointestinal Surgery: A Review. Cureus 2023, 15, e48864. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abd El Aziz, M.A.; Grass, F.; Calini, G.; Behm, K.T.; D’angelo, A.-L.; Kelley, S.R.; Mathis, K.L.; Larson, D.W.M. Oral Antibiotics Bowel Preparation Without Mechanical Preparation for Minimally Invasive Colorectal Surgeries: Current Practice and Future Prospects. Dis Colon. Rectum. 2022, 65, e897–e906. [Google Scholar] [CrossRef]
- Koskenvuo, L.; Lunkka, P.; Varpe, P.; Hyöty, M.; Satokari, R.; Haapamäki, C.; Lepistö, A.; Sallinen, V. Mechanical bowel preparation and oral antibiotics versus mechanical bowel preparation only prior rectal surgery (MOBILE2): A multicentre, double-blinded, randomised controlled trial—Study protocol. BMJ Open 2021, 11, e051269. [Google Scholar] [CrossRef]
- Lee, J.Y.; Tsolis, R.M.; Bäumler, A.J. The microbiome and gut homeostasis. Science 2022, 377, eabp9960. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.; Garges, S.; Giovanni, M.; McInnes, P.; Wang, L.; Schloss, J.A.; Bonazzi, V.; McEwen, J.E.; Wetterstrand, K.A.; Deal, C.; et al. The NIH human microbiome project. Genome Res. 2009, 19, 317–2323. [Google Scholar]
- Morowitz, M.J.; Babrowski, T.; Carlisle, E.M.; Olivas, A.; Romanowski, K.S.; Seal, J.B.; Liu, D.C.; Alverdy, J.C. The Human Microbiome and Surgical Disease. Ann. Surg. 2011, 253, 1094–1101. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Mahurkar, A.; Rahnavard, G.; Crabtree, J.; Orvis, J.; Hall, A.B.; Brady, A.; Creasy, H.H.; McCracken, C.; Giglio, M.G.; et al. Strains, functions and dynamics in the expanded Human Microbiome Project. Nature 2017, 550, 61–66, Correction in Nature 2017, 551, 256. [Google Scholar] [CrossRef] [PubMed]
- Tropini, C.; Earle, K.A.; Huang, K.C.; Sonnenburg, J.L. The Gut Microbiome: Connecting Spatial Organization to Function. Cell Host Microbe 2017, 21, 433–442. [Google Scholar] [CrossRef]
- Sundin, O.H.; Mendoza-Ladd, A.; Zeng, M.; Diaz-Arévalo, D.; Morales, E.; Fagan, B.M.; Ordoñez, J.; Velez, P.; Antony, N.; McCallum, R.W. The human jejunum has an endogenous microbiota that differs from those in the oral cavity and colon. BMC Microbiol. 2017, 17, 160. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Louis, P.; Duncan, S.H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Amabebe, E.; Robert, F.O.; Agbalalah, T.; Orubu, E.S.F. Microbial dysbiosis-induced obesity: Role of gut microbiota in homoeostasis of energy metabolism. Br. J. Nutr. 2020, 123, 1127–1137. [Google Scholar] [CrossRef]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Guigoz, Y.; Doré, J.; Schiffrin, E.J. The inflammatory status of old age can be nurtured from the intestinal environment. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 13–20. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Mulders, R.J.; de Git, K.C.G.; Schéle, E.; Dickson, S.L.; Sanz, Y.; Adan, R.A.H. Microbiota in obesity: Interactions with enteroendocrine, immune and central nervous systems. Obes. Rev. 2018, 19, 435–451. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ran, X.; Li, B.; Li, Y.; He, D.; Huang, B.; Fu, S.; Liu, J.; Wang, W. Sodium Butyrate Inhibits Inflammation and Maintains Epithelium Barrier Integrity in a TNBS-induced Inflammatory Bowel Disease Mice Model. EBioMedicine 2018, 30, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Kelly, C.J.; Battista, K.D.; Schaefer, R.; Lanis, J.M.; Alexeev, E.E.; Wang, R.X.; Onyiah, J.C.; Kominsky, D.J.; Colgan, S.P. Microbial-Derived Butyrate Promotes Epithelial Barrier Function through IL-10 Receptor-Dependent Repression of Claudin-2. J. Immunol. 2017, 199, 2976–2984. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Leoni, G.; Quiros, M.; Wu, H.; Desai, C.; Nishio, H.; Jones, R.M.; Nusrat, A.; Neish, A.S. The microenvironment of injured murine gut elicits a local pro-restitutive microbiota. Nat. Microbiol. 2016, 1, 15021. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, Z.E.; DiBaise, J.K.; Isern, N.G.; Hoyt, D.W.; Marcus, A.K.; Kang, D.-W.; Crowell, M.D.; E Rittmann, B.; Krajmalnik-Brown, R. Distinctive microbiomes and metabolites linked with weight loss after gastric bypass, but not gastric banding. ISME J. 2017, 11, 2047–2058. [Google Scholar] [CrossRef]
- Żak-Gołąb, A.; Kocełak, P.; Aptekorz, M.; Zientara, M.; Juszczyk, Ł.; Martirosian, G.; Chudek, J.; Olszanecka-Glinianowicz, M. Gut Microbiota, Microinflammation, Metabolic Profile, and Zonulin Concentration in Obese and Normal Weight Subjects. Int. J. Endocrinol. 2013, 2013, 674106. [Google Scholar] [CrossRef]
- Sapone, A.; de Magistris, L.; Pietzak, M.; Clemente, M.G.; Tripathi, A.; Cucca, F.; Lampis, R.; Kryszak, D.; Cartenì, M.; Generoso, M.; et al. Zonulin Upregulation Is Associated with Increased Gut Permeability in Subjects with Type 1 Diabetes and Their Relatives. Diabetes 2006, 55, 1443–1449. [Google Scholar] [CrossRef]
- Sturgeon, C.; Fasano, A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers 2016, 4, e1251384. [Google Scholar] [CrossRef]
- Goldblum, S.E.; Rai, U.; Tripathi, A.; Thakar, M.; De Leo, L.; Di Toro, N.; Not, T.; Ramachandran, R.; Puche, A.C.; Hollenberg, M.D.; et al. The active Zot domain (aa 288-293) increases ZO-1 and myosin 1C serine/threonine phosphorylation, alters interaction between ZO-1 and its binding partners, and induces tight junction disassembly through proteinase activated receptor 2 activation. FASEB J. 2011, 25, 144–158. [Google Scholar] [CrossRef]
- Ott, B.; Skurk, T.; Hastreiter, L.; Lagkouvardos, I.; Fischer, S.; Büttner, J.; Kellerer, T.; Clavel, T.; Rychlik, M.; Haller, D.; et al. Effect of caloric restriction on gut permeability, inflammation markers, and fecal microbiota in obese women. Sci. Rep. 2017, 7, 11955. [Google Scholar] [CrossRef] [PubMed]
- Olsson, A.; Gustavsen, S.; Langkilde, A.R.; Hansen, T.; Sellebjerg, F.; Søndergaard, H.B.; Oturai, A. Circulating levels of tight junction proteins in multiple sclerosis: Association with inflammation and disease activity before and after disease modifying therapy. Mult. Scler. Relat. Disord. 2021, 54, 103136. [Google Scholar] [CrossRef] [PubMed]
- Manuzak, J.A.; Zevin, A.S.; Cheu, R.; Richardson, B.; Modesitt, J.; Hensley-McBain, T.; Miller, C.; Gustin, A.T.; Coronado, E.; Gott, T.; et al. Antibiotic-induced microbiome perturbations are associated with significant alterations to colonic mucosal immunity in rhesus macaques. Mucosal Immunol. 2020, 13, 471–480, Correction in Mucosal Immunol. 2020, 13, 558. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; O’Quinn, D.B.; Silberger, D.J.; Schoeb, T.R.; Fouser, L.; Ouyang, W.; Hatton, R.D.; Weaver, C.T. Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity 2012, 37, 1061–1075. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Friedrich, C.; Hagemann, S.C.; Korte, W.H.; Goharani, N.; Cording, S.; Eberl, G.; Sparwasser, T.; Lochner, M. Regulatory T cells promote a protective Th17-associated immune response to intestinal bacterial infection with C. rodentium. Mucosal Immunol. 2014, 7, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.C.; Tan, X.-Y.; Luxenberg, D.P.; Karim, R.; Dunussi-Joannopoulos, K.; Collins, M.; Fouser, L.A. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006, 203, 2271–2279. [Google Scholar] [CrossRef] [PubMed]
- Faith, J.J.; Guruge, J.L.; Charbonneau, M.; Subramanian, S.; Seedorf, H.; Goodman, A.L.; Clemente, J.C.; Knight, R.; Heath, A.C.; Leibel, R.L.; et al. The Long-Term Stability of the Human Gut Microbiota. Science 2013, 341, 1237439. [Google Scholar] [CrossRef] [PubMed]
- Gaines, S.; Shao, C.; Hyman, N.; Alverdy, J.C. Gut microbiome influences on anastomotic leak and recurrence rates following colorectal cancer surgery. Br. J. Surg. 2018, 105, e131–e141. [Google Scholar] [CrossRef] [PubMed]
- Dethlefsen, L.; Relman, D.A. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4554–4561. [Google Scholar] [CrossRef]
- Dethlefsen, L.; Huse, S.; Sogin, M.L.; Relman, D.A. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008, 6, e280. [Google Scholar] [CrossRef]
- Jakobsson, H.E.; Jernberg, C.; Andersson, A.F.; Sjölund-Karlsson, M.; Jansson, J.K.; Engstrand, L. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS ONE 2010, 5, e9836. [Google Scholar] [CrossRef] [PubMed]
- Hartman, A.L.; Lough, D.M.; Barupal, D.K.; Fiehn, O.; Fishbein, T.; Zasloff, M.; Eisen, J.A. Human gut microbiome adopts an alternative state following small bowel transplantation. Proc. Natl. Acad. Sci. USA 2009, 106, 17187–17192. [Google Scholar] [CrossRef]
- Croswell, A.; Amir, E.; Teggatz, P.; Barman, M.; Salzman, N.H. Prolonged Impact of Antibiotics on Intestinal Microbial Ecology and Susceptibility to Enteric Salmonella Infection. Infect. Immun. 2009, 77, 2741–2753. [Google Scholar] [CrossRef]
- Fang, X.; Vázquez-Baeza, Y.; Elijah, E.; Vargas, F.; Ackermann, G.; Humphrey, G.; Lau, R.; Weldon, K.C.; Sanders, J.G.; Panitchpakdi, M.; et al. Gastrointestinal Surgery for Inflammatory Bowel Disease Persistently Lowers Microbiome and Metabolome Diversity. Inflamm Bowel Dis. 2021, 27, 603–616, Correction in Inflamm. Bowel Dis. 2021, 27, 1368. [Google Scholar] [CrossRef] [PubMed]
- Ferrie, S.; Webster, A.; Wu, B.; Tan, C.; Carey, S. Gastrointestinal surgery and the gut microbiome: A systematic literature review. Eur. J. Clin. Nutr. 2021, 75, 12–25. [Google Scholar] [CrossRef]
- Pineda, C.E.; Shelton, A.A.; Hernandez-Boussard, T.; Morton, J.M.; Welton, M.L. Mechanical bowel preparation in intestinal surgery: A meta-analysis and review of the literature. J. Gastrointest. Surg. 2008, 12, 2037–2044. [Google Scholar] [CrossRef]
- Dahabreh, I.J.; Steele, D.W.; Shah, N.; Trikalinos, T.A. Oral mechanical bowel preparation for colorectal surgery: Systematic review and meta-analysis. Dis. Colon. Rectum. 2015, 58, 698–707. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.L.; Allison, G.E.; Grimpen, F.; Pavli, P. Impact of colonoscopy bowel preparation on intestinal microbiota. PLoS ONE 2013, 8, e62815. [Google Scholar] [CrossRef]
- Gorkiewicz, G.; Thallinger, G.G.; Trajanoski, S.; Lackner, S.; Stocker, G.; Hinterleitner, T.; Gülly, C.; Högenauer, C. Alterations in the Colonic Microbiota in Response to Osmotic Diarrhea. PLoS ONE 2013, 8, e55817. [Google Scholar] [CrossRef]
- Jalanka, J.; Salonen, A.; Salojärvi, J.; Ritari, J.; Immonen, O.; Marciani, L.; Gowland, P.; Hoad, C.; Garsed, K.; Lam, C.; et al. Effects of bowel cleansing on the intestinal microbiota. Gut 2015, 64, 1562–1568. [Google Scholar] [CrossRef]
- Drago, L.; Valentina, C.; Fabio, P. Gut microbiota, dysbiosis and colon lavage. Dig. Liver. Dis. 2019, 51, 1209–1213. [Google Scholar] [CrossRef]
- McHeyzer-Williams, M. Local sentries for class switching. Nat. Immunol. 2007, 8, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; De Angelis, M.; De Pasquale, I.; Ndagijimana, M.; Vernocchi, P.; Ricciuti, P.; Gagliardi, F.; Laghi, L.; Crecchio, C.; Guerzoni, M.E.; et al. Duodenal and faecal microbiota of celiac children: Molecular, phenotype and metabolome characterization. BMC Microbiol. 2011, 11, 219. [Google Scholar] [CrossRef] [PubMed]
- Pace, N.R.; Olsen, G.J.; Woese, C.R. Ribosomal RNA phylogeny and the primary lines of evolutionary descent. Cell 1986, 45, 325–326. [Google Scholar] [CrossRef] [PubMed]
- Carroll, I.M.; Ringel-Kulka, T.; Ferrier, L.; Wu, M.C.; Siddle, J.P.; Bueno, L.; Ringel, Y. Fecal protease activity is associated with compositional alterations in the intestinal microbiota. PLoS ONE 2013, 8, e78017. [Google Scholar] [CrossRef]
- Carroll, I.M.; Maharshak, N. Enteric bacterial proteases in inflammatory bowel disease-pathophysiology and clinical implications. World J. Gastroenterol. 2013, 19, 7531–7543. [Google Scholar] [CrossRef]
- Persson, J.E.; Viana, P.; Persson, M.; Relvas, J.H.; Danielski, L.G. Perioperative or Postoperative Probiotics Reduce Treatment-Related Complications in Adult Colorectal Cancer Patients Undergoing Surgery: A Systematic Review and Meta-analysis. J. Gastrointest. Cancer 2024, 55, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Veziant, J.; Bonnet, M.; Occean, B.V.; Dziri, C.; Pereira, B.; Slim, K. Probiotics/Synbiotics to Reduce Infectious Complications after Colorectal Surgery: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2022, 14, 3066. [Google Scholar] [CrossRef]
- Liu, Z.H.; Huang, M.-J.; Zhang, X.-W.; Wang, L.; Huang, N.-Q.; Peng, H.; Lan, P.; Peng, J.S.; Yang, Z.; Xia, Y.; et al. The effects of perioperative probiotic treatment on serum zonulin concentration and subsequent postoperative infectious complications after colorectal cancer surgery: A double-center and double-blind randomized clinical trial. Am. J. Clin. Nutr. 2013, 97, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.W.; Du, P.; Gao, J.; Yang, B.R.; Fang, W.J.; Ying, C.M. Preoperative probiotics decrease postoperative infectious complications of colorectal cancer. Am. J. Med. Sci. 2012, 343, 199–205. [Google Scholar] [CrossRef]
- Pitsillides, L.; Pellino, G.; Tekkis, P.; Kontovounisios, C. The Effect of Perioperative Administration of Probiotics on Colorectal Cancer Surgery Outcomes. Nutrients 2021, 13, 1451. [Google Scholar] [CrossRef] [PubMed]
- Paine, H.; Jones, F.; Kinross, J. Preparing the Bowel (Microbiome) for Surgery: Surgical Bioresilience. Clin. Colon. Rectal. Surg. 2023, 36, 138–145. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kwa, W.T.; Sundarajoo, S.; Toh, K.Y.; Lee, J. Application of emerging technologies for gut microbiome research. Singap. Med. J. 2023, 64, 45–53. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roume, H.; Mondot, S.; Saliou, A.; Le Fresne-Languille, S.; Doré, J. Multicenter evaluation of gut microbiome profiling by next-generation sequencing reveals major biases in partial-length metabarcoding approach. Sci. Rep. 2023, 13, 22593. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weaver, L.; Troester, A.; Jahansouz, C. The Impact of Surgical Bowel Preparation on the Microbiome in Colon and Rectal Surgery. Antibiotics 2024, 13, 580. https://doi.org/10.3390/antibiotics13070580
Weaver L, Troester A, Jahansouz C. The Impact of Surgical Bowel Preparation on the Microbiome in Colon and Rectal Surgery. Antibiotics. 2024; 13(7):580. https://doi.org/10.3390/antibiotics13070580
Chicago/Turabian StyleWeaver, Lauren, Alexander Troester, and Cyrus Jahansouz. 2024. "The Impact of Surgical Bowel Preparation on the Microbiome in Colon and Rectal Surgery" Antibiotics 13, no. 7: 580. https://doi.org/10.3390/antibiotics13070580




