Development and ELISA Characterization of Antibodies against the Colistin, Vancomycin, Daptomycin, and Meropenem: A Therapeutic Drug Monitoring Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
General Methods and Instruments
2.2. Chemicals and Immunochemicals
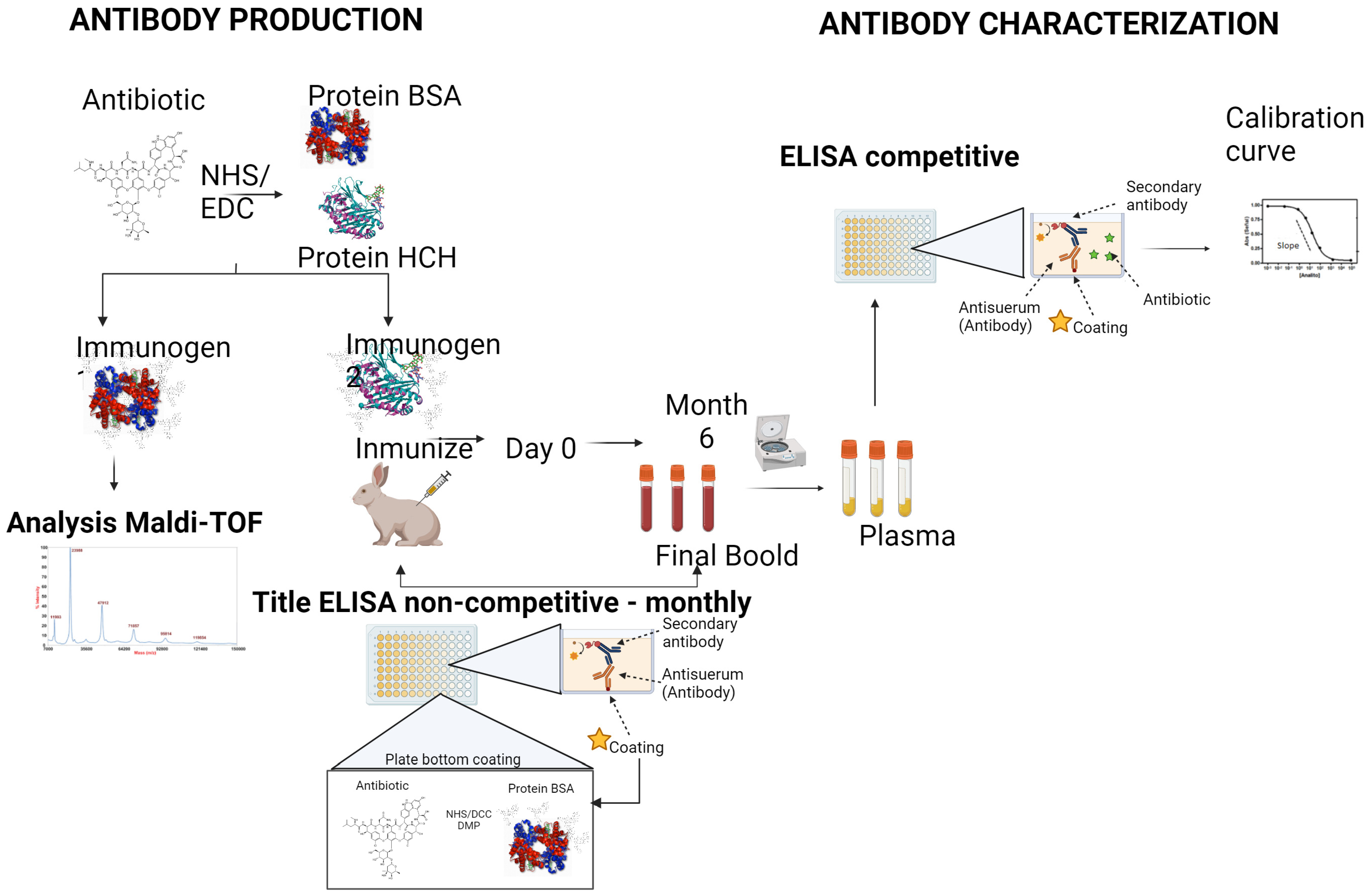
2.3. Antibody Development Preparation of Bioconjugates
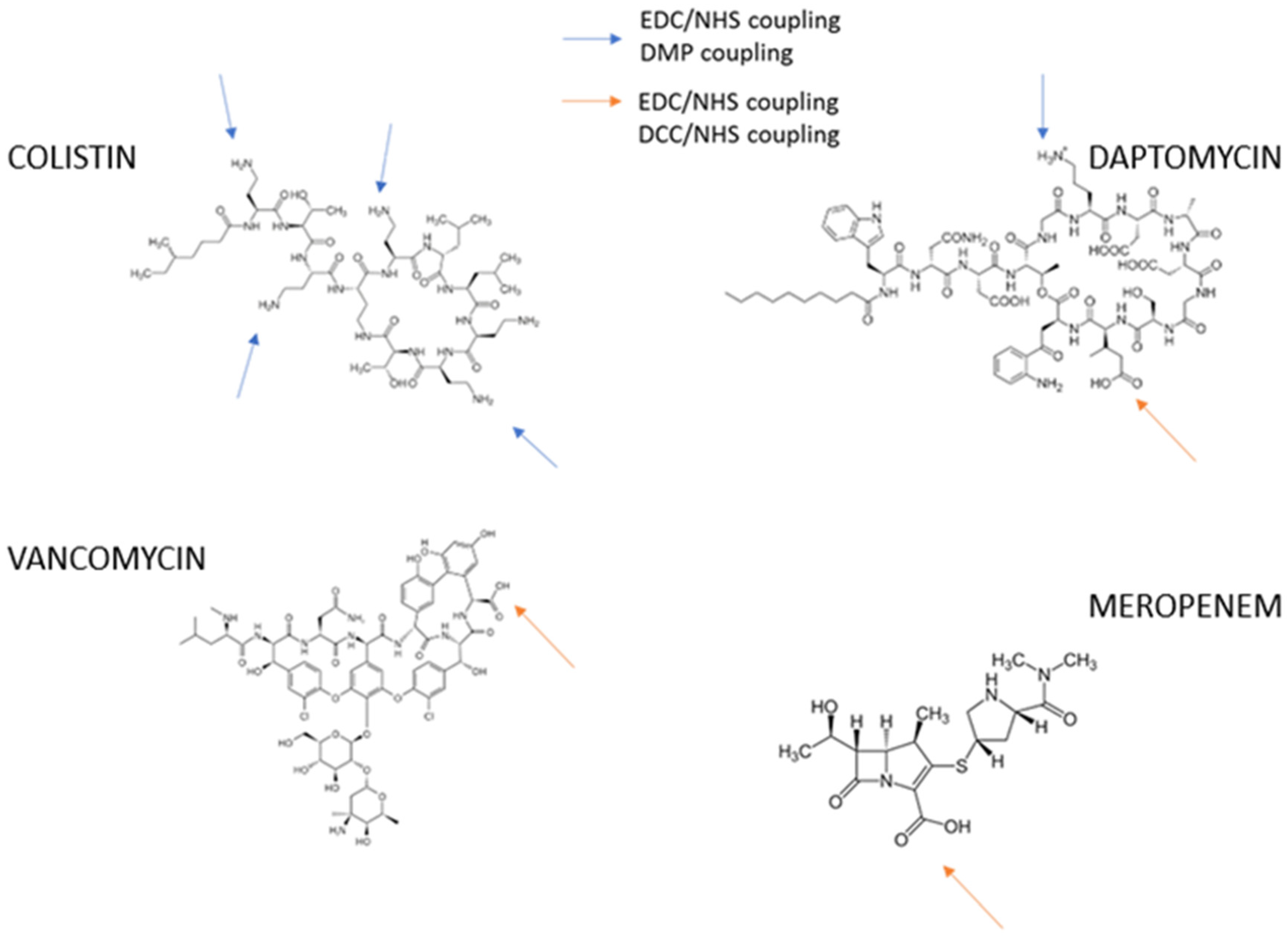
2.3.1. Immunogens
2.3.2. Coating Antigens
2.3.3. Preparation of Coating Antigens
DCC/NHS
Dimethyl Pimelimidate (DMP)
Hapten Density Analysis
2.4. Polyclonal Antibodies
2.4.1. Antibody Titer
2.4.2. Two-Dimensional Titration Assays
2.4.3. Competitive Assay
3. Results
3.1. Preparation of Immunogens
3.1.1. Preparation of Coating Antigens: Active Ester DCC/NHS and DMP Bioconjugates
3.1.2. Characterization by MALDI-TOF MS
3.2. Characterization of Polyclonal Antibodies against Antibiotic
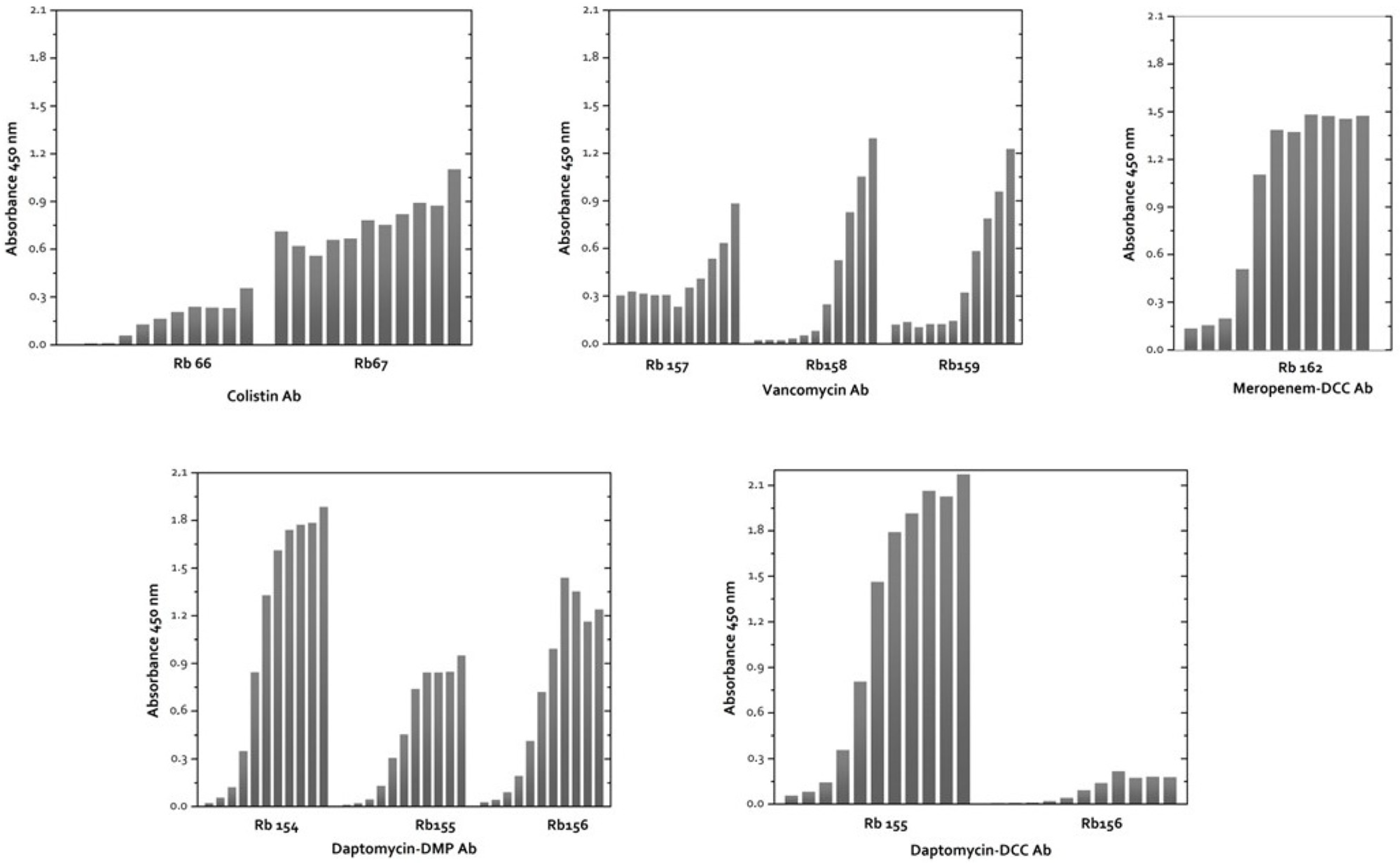
3.2.1. Antibody Production and Antibody Titer
3.2.2. ELISA Development
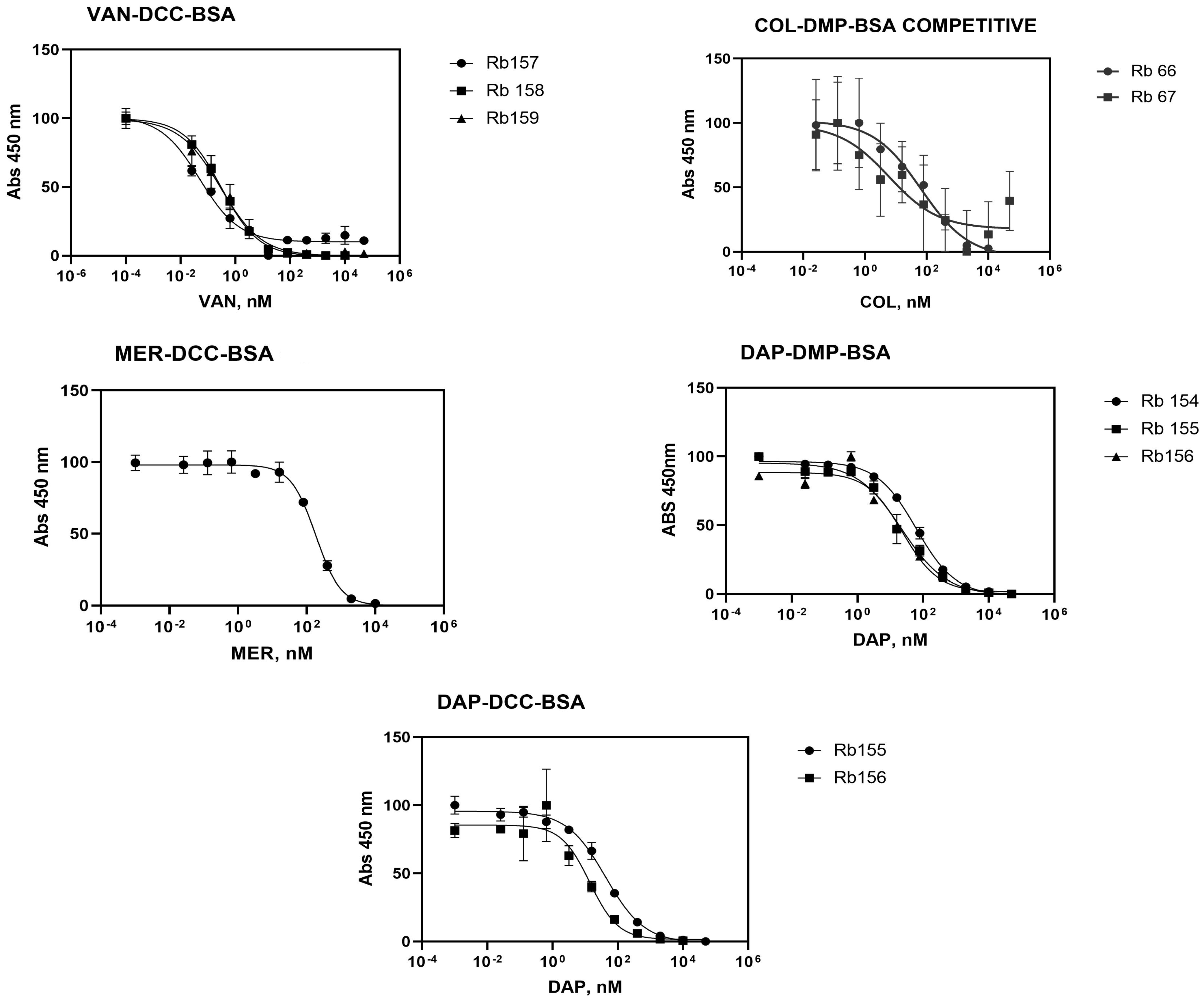
3.2.3. Indirect Competitive ELISA Immunoassay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aljeldah, M.M. Antimicrobial Resistance and Its Spread Is a Global Threat. Antibiotics 2022, 11, 1082. [Google Scholar] [CrossRef]
- Dadgostar, P. Antimicrobial resistance: Implications and costs. Infect. Drug Resist. 2019, 12, 3903. [Google Scholar] [CrossRef]
- World Health Organization. New report calls for urgent action to avert antimicrobial resistance crisis. Jt. News Release 2019, 29. [Google Scholar]
- World Health Organization. Antimicrobial resistance. Wkly. Epidemiol. Rec. = Relev. Épidémiologique Hebd. 2000, 75, 336. [Google Scholar]
- Marston, H.D.; Dixon, D.M.; Knisely, J.M.; Palmore, T.N.; Fauci, A.S. Antimicrobial resistance. JAMA 2016, 316, 1193–1204. [Google Scholar] [CrossRef]
- Acar, J.; Rostel, B. Antimicrobial resistance: An overview. Rev. Sci. Tech. Off. Int. Epizoot. 2001, 20, 797–810. [Google Scholar] [CrossRef]
- Gajdács, M.; Urbán, E.; Stájer, A.; Baráth, Z. Antimicrobial resistance in the context of the sustainable development goals: A brief review. Eur. J. Investig. Health Psychol. Educ. 2021, 11, 71–82. [Google Scholar] [CrossRef]
- Garzón, V.; Bustos, R.-H.; Pinacho, D.G. Personalized medicine for antibiotics: The role of nanobiosensors in therapeutic drug monitoring. J. Pers. Med. 2020, 10, 147. [Google Scholar] [CrossRef]
- Hart, C.; Kariuki, S. Antimicrobial resistance in developing countries. BMJ 1998, 317, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-S.; Lee, M.-H. Overview of therapeutic drug monitoring. Korean J. Intern. Med. 2009, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ghiculescu, R. Therapeutic drug monitoring: Which drugs, why, when and how to do it. Aust. Prescr. 2008, 31, 42–44. [Google Scholar] [CrossRef]
- George, R.; Haywood, A.; Khan, S.; Radovanovic, M.; Simmonds, J.; Norris, R. Enhancement and suppression of ionization in drug analysis using HPLC-MS/MS in support of therapeutic drug monitoring: A review of current knowledge of its minimization and assessment. Ther. Drug Monit. 2018, 40, 1–8. [Google Scholar] [PubMed]
- Legrand, T.; Chhun, S.; Rey, E.; Blanchet, B.; Zahar, J.-R.; Lanternier, F.; Pons, G.; Jullien, V. Simultaneous determination of three carbapenem antibiotics in plasma by HPLC with ultraviolet detection. J. Chromatogr. B 2008, 875, 551–556. [Google Scholar] [CrossRef] [PubMed]
- van Nuland, M.; Venekamp, N.; de Vries, N.; de Jong, K.A.; Rosing, H.; Beijnen, J.H. Development and validation of an UPLC-MS/MS method for the therapeutic drug monitoring of oral anti-hormonal drugs in oncology. J. Chromatogr. B 2019, 1106, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Drabińska, N.; Hewett, K.; White, P.; Avison, M.B.; Persad, R.; Ratcliffe, N.M.; de Lacy Costello, B. Application of a solid-phase microextraction-gas chromatography-mass spectrometry/metal oxide sensor system for detection of antibiotic susceptibility in urinary tract infection-causing Escherichia coli—A proof of principle study. Adv. Med. Sci. 2022, 67, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chianella, I.; Guerreiro, A.; Moczko, E.; Caygill, J.S.; Piletska, E.V.; De Vargas Sansalvador, I.M.P.; Whitcombe, M.J.; Piletsky, S.A. Direct Replacement of Antibodies with Molecularly Imprinted Polymer Nanoparticles in ELISA Development of a Novel Assay for Vancomycin. Anal. Chem. 2013, 85, 8462–8468. [Google Scholar] [CrossRef] [PubMed]
- Odekerken, J.C.; Logister, D.M.; Assabre, L.; Arts, J.J.; Walenkamp, G.H.; Welting, T.J. ELISA-based detection of gentamicin and vancomycin in protein-containing samples. SpringerPlus 2015, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Reder-Christ, K.; Bendas, G. Biosensor applications in the field of antibiotic research—A review of recent developments. Sensors 2011, 11, 9450–9466. [Google Scholar] [CrossRef] [PubMed]
- González Pinacho, D. Analytical Strategies Based on the Design of Class Immunoreactives for the Control of Quinolones Residues in Milk; Universidad de Barcelona: Barcelona, Spain, 2015. [Google Scholar]
- Bustos, R.H.; Zapata, C.; Esteban, E.; García, J.-C.; Jáuregui, E.; Jaimes, D. Label-free quantification of anti-TNF-α in patients treated with adalimumab using an optical biosensor. Sensors 2018, 18, 691. [Google Scholar] [CrossRef]
- Zangar, R.C.; Daly, D.S.; White, A.M. ELISA microarray technology as a high-throughput system for cancer biomarker validation. Expert Rev. Proteom. 2006, 3, 37–44. [Google Scholar] [CrossRef]
- Pinho, A.; Fortuna, A.; Falcão, A.; Santos, A.; Seiça, R.; Estevens, C.; Veiga, F.; Ribeiro, A. Comparison of ELISA and HPLC-MS methods for the determination of exenatide in biological and biotechnology-based formulation matrices. J. Pharm. Anal. 2019, 9, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Adrian, J.; Font, H.; Diserens, J.-M.; Sanchez-Baeza, F.; Marco, M.-P. Generation of broad specificity antibodies for sulfonamide antibiotics and development of an enzyme-linked immunosorbent assay (ELISA) for the analysis of milk samples. J. Agric. Food Chem. 2009, 57, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Hnasko, R. Elisa; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Li, Y.; Ji, B.; Chen, W.; Liu, L.; Xu, C.; Peng, C.; Wang, L. Production of new class-specific polyclonal antibody for determination of fluoroquinolones antibiotics by indirect competitive ELISA. Food Agric. Immunol. 2008, 19, 251–264. [Google Scholar] [CrossRef]
- Pinacho, D.G.; Sanchez-Baeza, F.; Marco, M.-P. Molecular modeling assisted hapten design to produce broad selectivity antibodies for fluoroquinolone antibiotics. Anal. Chem. 2012, 84, 4527–4534. [Google Scholar] [CrossRef] [PubMed]
- Huet, A.-C.; Charlier, C.; Tittlemier, S.A.; Singh, G.; Benrejeb, S.; Delahaut, P. Simultaneous determination of (fluoro) quinolone antibiotics in kidney, marine products, eggs, and muscle by enzyme-linked immunosorbent assay (ELISA). J. Agric. Food Chem. 2006, 54, 2822–2827. [Google Scholar] [CrossRef]
- Mahmoudi, R.; Norian, R.; Pajohi-Alamoti, M. Antibiotic residues in Iranian honey by ELISA. Int. J. Food Prop. 2014, 17, 2367–2373. [Google Scholar] [CrossRef]
- Burkin, M.A.; Galvidis, I.A.; Surovoy, Y.A.; Plyushchenko, I.V.; Rodin, I.A.; Tsarenko, S.V. Development of ELISA formats for polymyxin B monitoring in serum of critically ill patients. J. Pharm. Biomed. Anal. 2021, 204, 114275. [Google Scholar] [CrossRef] [PubMed]
- Farrell, C.D.; Rowell, F.J.; Cumming, R.H. A rapid fluorescence ELISA for ceftazidime. Anal. Proc. Incl. Anal. Commun. 1995, 32, 205–206. [Google Scholar] [CrossRef]
- Gaudin, V.; Hédou, C.; Rault, A.; Verdon, E.; Soumet, C. Evaluation of three ELISA kits for the screening of colistin residue in porcine and poultry muscle according to the European guideline for the validation of screening methods. Food Addit. Contam. Part A 2020, 37, 1651–1666. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Saita, T.; Oka, A.; Kataoka, H.; Shin, M. Development of a Sensitive Enzyme-linked Immunosorbent Assay for Daptomycin. Yakugaku Zasshi 2021, 141, 427–431. [Google Scholar] [CrossRef]
- Salvador, J.-P.; Vasylieva, N.; Gonzalez-Garcia, I.; Jin, M.; Caster, R.; Siegel, J.B.; Hammock, B.D. Nanobody-Based Lateral Flow Immunoassay for the Rapid Detection of Aflatoxin B1 in Almond Milk. ACS Food Sci. Technol. 2022, 2, 1276–1282. [Google Scholar] [CrossRef]
- Soto, D.; Silva, C.; Andresen, M.; Soto, N.; Wong, K.-Y.; Andresen, M. Therapeutic monitoring of antibiotics: New methodologies: Biosensors. Rev. Méd. Chile 2015, 143, 1050–1057. [Google Scholar] [CrossRef][Green Version]
- Mascini, M.; Tombelli, S. Biosensors for biomarkers in medical diagnostics. Biomarkers 2008, 13, 637–657. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, D.; Lai, C.; Zeng, G.; Qin, L.; Zhang, C.; Yi, H.; Li, B.; Deng, R.; Liu, S. Recent advances in sensors for tetracycline antibiotics and their applications. TrAC Trends Anal. Chem. 2018, 109, 260–274. [Google Scholar] [CrossRef]
- Korposh, S.; Chianella, I.; Guerreiro, A.; Caygill, S.; Piletsky, S.; James, S.W.; Tatam, R.P. Selective vancomycin detection using optical fibre long period gratings functionalised with molecularly imprinted polymer nanoparticles. Analyst 2014, 139, 2229–2236. [Google Scholar] [CrossRef] [PubMed]
- Stanković, V.; Đurđić, S.; Ognjanović, M.; Antić, B.; Kalcher, K.; Mutić, J.; Stanković, D.M. Anti-human albumin monoclonal antibody immobilized on EDC-NHS functionalized carboxylic graphene/AuNPs composite as promising electrochemical HSA immunosensor. J. Electroanal. Chem. 2020, 860, 113928. [Google Scholar] [CrossRef]
- Font, H.; Adrian, J.; Galve, R.; Estévez, M.-C.; Castellari, M.; Gratacós-Cubarsí, M.; Sánchez-Baeza, F.; Marco, M.-P. Immunochemical assays for direct sulfonamide antibiotic detection in milk and hair samples using antibody derivatized magnetic nanoparticles. J. Agric. Food Chem. 2008, 56, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.M.; Martínez, M.I.; Suárez, A.M.; Herranz, C.; Casaus, P.; Cintas, L.M.; Rodríguez, J.M.; Hernández, P.E. Generation of polyclonal antibodies of predetermined specificity against pediocin PA-1. Appl. Environ. Microbiol. 1998, 64, 4536–4545. [Google Scholar] [CrossRef] [PubMed]
- Adrian, J.; Pinacho, D.G.; Granier, B.; Diserens, J.-M.; Sánchez-Baeza, F.; Marco, M. A multianalyte ELISA for immunochemical screening of sulfonamide, fluoroquinolone and ß-lactam antibiotics in milk samples using class-selective bioreceptors. Anal. Bioanal. Chem. 2008, 391, 1703–1712. [Google Scholar] [CrossRef]
- Narain, R. Chemistry of Bioconjugates: Synthesis, Characterization, and Biomedical Applications. In Chemistry of Bioconjugates; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Sunasee, R.; Narain, R. Covalent and noncovalent bioconjugation strategies. In Chemistry of Bioconjugates; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 1–75. [Google Scholar]
- Colom, G.; Salvador, J.-P.; Acosta, G.; Albericio, F.; Royo, M.; Marco, M.-P. Competitive ELISA for N-terminal pro-brain natriuretic peptide (NT-proBNP) determination in human plasma. Analyst 2020, 145, 6719–6727. [Google Scholar] [CrossRef]
- Lemus, R.; Karol, M.H. Conjugation of haptens. In Allergy Methods Protocols; Humana Press: Totowa, NJ, USA, 2008; Volume 138, pp. 167–182. [Google Scholar]
- Brinkley, M. A brief survey of methods for preparing protein conjugates with dyes, haptens and crosslinking reagents. Bioconjugate Chem. 1992, 3, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Kevin Park, B.; Kitteringham, N.R. Drug-protein conjugation and its immunological consequences. Drug Metab. Rev. 1990, 22, 87–144. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Yin, Y.; Lu, L.; Ding, H.; Wang, L.; Yu, T.; Zhu, J.-J.; Zheng, X.-D.; Zhang, Y.-Z. Preparation of high-affinity rabbit monoclonal antibodies for ciprofloxacin and development of an indirect competitive ELISA for residues in milk. J. Zhejiang Univ. Sci. B 2010, 11, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Fouquet, T.; Sato, H. Molecular characterization of high molecular weight polyesters by matrix-assisted laser desorption/ionization high-resolution time-of-flight mass spectrometry combined with on-plate alkaline degradation and mass defect analysis. J. Am. Soc. Mass Spectrom. 2018, 30, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Meetani, M.A.; Voorhees, K.J. MALDI mass spectrometry analysis of high molecular weight proteins from whole bacterial cells: Pretreatment of samples with surfactants. J. Am. Soc. Mass Spectrom. 2005, 16, 1422–1426. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Maghsoudlou, P. Enzyme-linked immunosorbent assay (ELISA): The basics. Br. J. Hosp. Med. 2016, 77, C98–C101. [Google Scholar] [CrossRef] [PubMed]
- Hernández Albors, A. Development of Immunochemical Techniques for the Detection of Cardiac Biomarkers; Universidad de Barcelona: Barcelona, Spain, 2017. [Google Scholar]
- Hermanson, G.T. Bioconjugate Techniques; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Xu, C.; Han, Q.; Dong, S.; Liu, X.; Liu, X. Establishment of an ultrasensitive indirect competitive time-resolved fluoroimmunoassay for vancomycin determination. Food Agric. Immunol. 2019, 30, 862–877. [Google Scholar] [CrossRef]
- Cheng, X.; Ma, J.; Su, J. An overview of analytical methodologies for determination of vancomycin in human plasma. Molecules 2022, 27, 7319. [Google Scholar] [CrossRef]
- Kell, A.J.; Stewart, G.; Ryan, S.; Peytavi, R.; Boissinot, M.; Huletsky, A.; Bergeron, M.G.; Simard, B. Vancomycin-modified nanoparticles for efficient targeting and preconcentration of Gram-positive and Gram-negative bacteria. ACS Nano 2008, 2, 1777–1788. [Google Scholar] [CrossRef]
- Hu, K.; Huang, X.; Jiang, Y.; Qiu, J.; Fang, W.; Yang, X. Influence of hapten density on immunogenicity for anti-ciprofloxacin antibody production in mice. BioScience Trends 2012, 6, 52–56. [Google Scholar] [CrossRef][Green Version]
- Li, Q.; Rodriguez, L.G.; Farnsworth, D.F.; Gildersleeve, J.C. Effects of hapten density on the induced antibody repertoire. ChemBioChem 2010, 11, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Trier, N.H.; Hansen, P.; Houen, G. Production and characterization of peptide antibodies. Methods 2012, 56, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Immunoglobulin-binding protein-based affinity chromatography in bispecific antibody purification: Functions beyond product capture. Protein Expr. Purif. 2021, 188, 105976. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. A brief introduction of IgG-like bispecific antibody purification: Methods for removing product-related impurities. Protein Expr. Purif. 2019, 155, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Pinho, A.; Rocha, M.; Alves, G.; Falcao, A.; Fortuna, A. Development and validation of an HPLC-FLD technique for colistin quantification and its plasma monitoring in hospitalized patients. Anal. Methods 2018, 10, 389–396. [Google Scholar] [CrossRef]
- Kameda, K.; Ikawa, K.; Ikeda, K.; Morikawa, N.; Nakashima, A.; Ohge, H.; Sueda, T. HPLC method for measuring meropenem and biapenem concentrations in human peritoneal fluid and bile: Application to comparative pharmacokinetic investigations. J. Chromatogr. Sci. 2010, 48, 406–411. [Google Scholar] [CrossRef]
- Verdier, M.-C.; Bentué-Ferrer, D.; Tribut, O.; Collet, N.; Revest, M.; Bellissant, E. Determination of daptomycin in human plasma by liquid chromatography-tandem mass spectrometry. Clinical application. Clin. Chem. Lab. Med. 2011, 49, 69–75. [Google Scholar] [CrossRef]
| Coupling Method | BioConjugate | MW (Conjugate) Da | Hapten Density (δ) |
|---|---|---|---|
| BSA | 66,432 | - | |
| EDC/NHS | COL-EDC-BSA | 82,769 | 14 |
| VAN-EDC-BSA | 71,195 | 3 | |
| DAP-EDC-BSA | No result | No result | |
| MER-EDC-BSA | 74,680 | 22 | |
| DMP | COL-DMP-BSA | 72,000 | 5 |
| DAP-DMP-BSA | 71,565 | 3 | |
| DCC/NHS | VAN-DCC-BSA | 71,714 | 4 |
| MER-DCC-BSA | 75,445 | 23 | |
| DAP-DCC-BSA | 91,116 | 15 |
| Antibiotic | Antibody | Antisera Dilution | Antigen Concentration (µg/mL) | Absorbance (450 nm) |
|---|---|---|---|---|
| Vancomycin (VAN-DCC-BSA) | Rb157 | 1/4000 | 2.5 | 0.8684 |
| Rb158 | 1/16,000 | 0.9435 | ||
| Rb159 | 1/4000 | 0.9801 | ||
| Colistin (COL-DMP-BSA) | Rb66 | 1/1000 | 2.5 | 0.9480 |
| Rb67 | 0.7506 | |||
| Meropenem (MER-DCC-BSA) | Rb162 | 1/4000 | 2.5 | 1.0649 |
| Daptomycin (DAP-DMP-BSA) | Rb154 | 1/20,000 | 0.5 | 1.0514 |
| Rb155 | 1/10,000 | 0.0625 | 0.9003 | |
| Rb156 | 0.01562 | 1.0426 | ||
| Daptomycin (DAP-DCC-BSA) | Rb154 | 1/64,000 | 5 | 1.0489 |
| Rb155 | 1/32,000 | 1.25 | 1.1917 | |
| Rb156 | 1/32,000 | 1.25 | 1.0569 |
| Antisera | Antigen | Amax | Amin | IC50 (nM) | Slope | LOD (nM) |
|---|---|---|---|---|---|---|
| Rb157 | VAN-DCC-BSA | 101.5 | 9.97 | 0.05 | −0.60 | 0.002 |
| Rb158 | 99.88 | −0.57 | 0.3 | −0.63 | 0.009 | |
| Rb159 | 99.35 | −0.22 | 0.29 | −0.57 | 0.006 | |
| Rb66 | COL-DMP-BSA | 146.9 | −58.2 | 7.56 | - | 0.003 |
| Rb67 | 216 | 4.62 | 8.75 | - | 0.002 | |
| Rb162 | MER-DCC-BSA | 1.46 | 0.14 | 183.6 | −1.19 | 23.9 |
| Rb154 | DAP-DMP-BSA | 96.34 | −1.17 | 61.75 | −0.72 | 1.568 |
| Rb155 | 95.29 | −0.85 | 22.27 | −0.63 | 0.257 | |
| Rb156 | 88.41 | 1.44 | 23.52 | −0.79 | 1.433 | |
| Rb154 | DAP-DCC-BSA | - | - | - | - | - |
| Rb155 | 95.63 | −0.09 | 42.52 | −0.72 | 0.937 | |
| Rb156 | 85.50 | 1.46 | 13.82 | −0.99 | 0.952 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garzon, V.; Salvador, J.-P.; Marco, M.-P.; G.-Pinacho, D.; Bustos, R.-H. Development and ELISA Characterization of Antibodies against the Colistin, Vancomycin, Daptomycin, and Meropenem: A Therapeutic Drug Monitoring Approach. Antibiotics 2024, 13, 600. https://doi.org/10.3390/antibiotics13070600
Garzon V, Salvador J-P, Marco M-P, G.-Pinacho D, Bustos R-H. Development and ELISA Characterization of Antibodies against the Colistin, Vancomycin, Daptomycin, and Meropenem: A Therapeutic Drug Monitoring Approach. Antibiotics. 2024; 13(7):600. https://doi.org/10.3390/antibiotics13070600
Chicago/Turabian StyleGarzon, Vivian, J.-Pablo Salvador, M.-Pilar Marco, Daniel G.-Pinacho, and Rosa-Helena Bustos. 2024. "Development and ELISA Characterization of Antibodies against the Colistin, Vancomycin, Daptomycin, and Meropenem: A Therapeutic Drug Monitoring Approach" Antibiotics 13, no. 7: 600. https://doi.org/10.3390/antibiotics13070600
APA StyleGarzon, V., Salvador, J.-P., Marco, M.-P., G.-Pinacho, D., & Bustos, R.-H. (2024). Development and ELISA Characterization of Antibodies against the Colistin, Vancomycin, Daptomycin, and Meropenem: A Therapeutic Drug Monitoring Approach. Antibiotics, 13(7), 600. https://doi.org/10.3390/antibiotics13070600








