Investigating the Bactericidal Activity of an Ocular Solution Containing EDTA, Tris, and Polysorbate 80 and Its Impact on the In Vitro Efficacy of Neomycin Sulfate against Staphylococcus aureus: A Preliminary Study
Abstract
1. Introduction
2. Results
2.1. Effect of the Test Solution on Bacterial Growth
2.1.1. Staphylococcus aureus ATCC 6538
2.1.2. Pseudomonas aeruginosa ATCC 15442
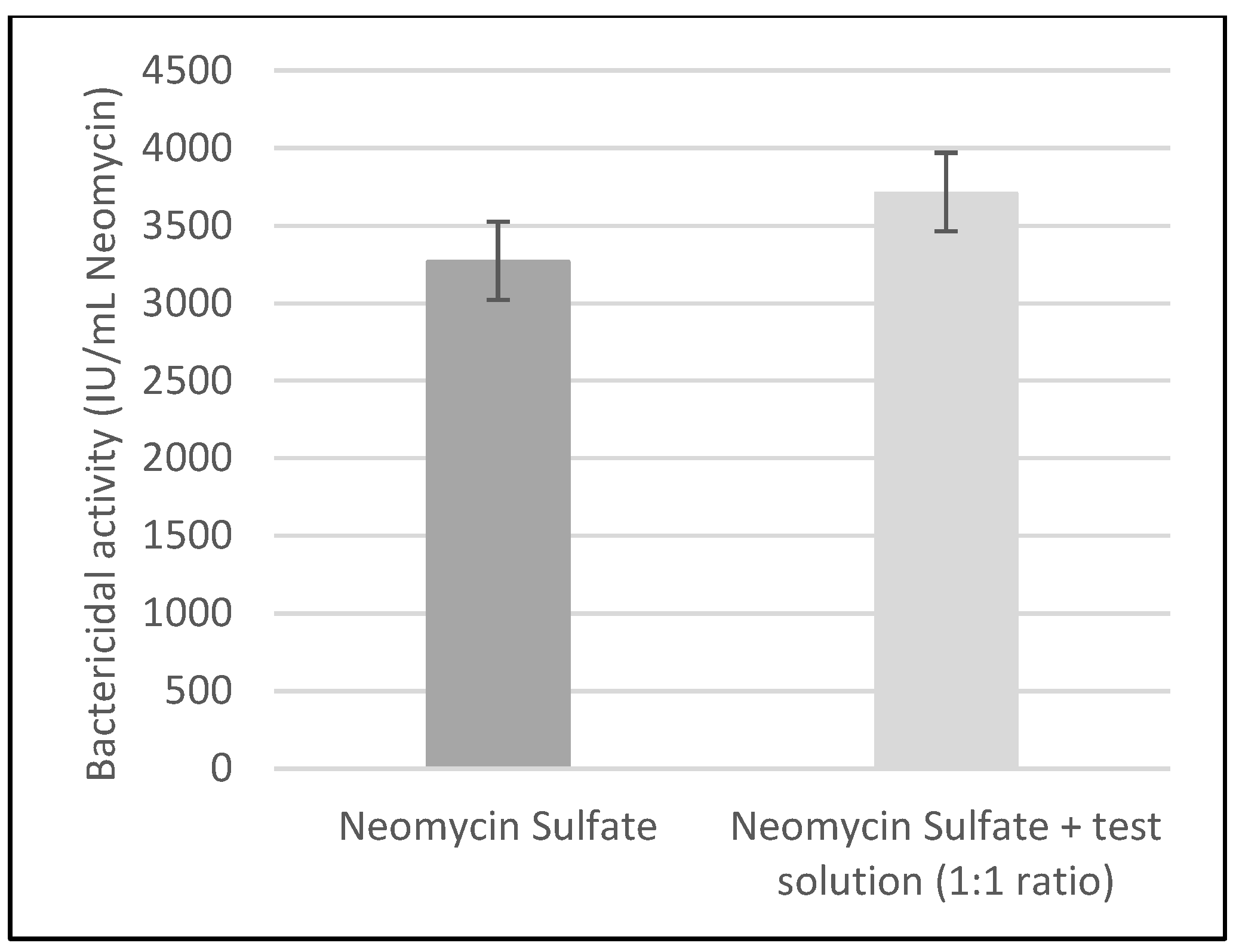
2.2. Effect of the Test Solution in Combination with Neomycin Sulfate on Bacterial Growth
3. Discussion
4. Materials and Methods
4.1. Test Product
4.2. Neomycin Sulfate Eye Drops
4.3. Bacterial Strains
4.4. Evaluation of the Bactericidal Activity of the Test Product
4.4.1. Preparation of the Test Product Concentrations
4.4.2. Preparation of the Bacterial Suspensions
4.4.3. Conduct of the Bactericidal Activity Trial
4.5. Evaluation of the Antimicrobial Activity of the Eye Drops and Test Product
4.5.1. Preparation of the Eye Drop Dilutions
4.5.2. Preparation of the Bacterial Suspensions
4.5.3. Conduct of the Eye Drop Antimicrobial Activity Trial
4.5.4. Conduct of the Potentiation of the Antimicrobial Activity of Neomycin Sulfate Eye Drops by the Test Solution Trial
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shah, S.; Wozniak, R.A.F. Staphylococcus aureus and Pseudomonas aeruginosa infectious keratitis: Key bacterial mechanisms that mediate pathogenesis and emerging therapeutics. Front. Cell. Infect. Microbiol. 2023, 13, 1250257. [Google Scholar] [CrossRef]
- Conly, J.; Johnston, B. Where are all the new antibiotics? The new antibiotic paradox. Can. J. Infect. Dis. Med. Microbiol. 2005, 16, 159–160. [Google Scholar] [CrossRef]
- Ling, L.L.; Schneider, T.; Peoples, A.J.; Spoering, A.L.; Engels, I.; Conlon, B.P.; Mueller, A.; Schäberle, T.F.; Hughes, D.E.; Epstein, S.; et al. A new antibiotic kills pathogens without detectable resistance. Nature 2015, 517, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Ekapopphan, D.; Srisutthakarn, A.; Moonarmart, W.; Buddhirongawatr, R.; Bangphoomi, N. Identification and antimicrobial susceptibility of microorganisms isolated from severe corneal ulcers of dogs in Thailand. J. Vet. Med. Sci. 2018, 80, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Tolar, E.L.; Hendrix, D.V.H.; Rohrbach, B.W.; Plummer, C.E.; Brooks, D.E.; Gelatt, K.N. Evaluation of clinical characteristics and bacterial isolates in dogs with bacterial keratitis: 97 cases (1993–2003). J. Am. Vet. Med. Assoc. 2006, 228, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Ollivier, F.J. Bacterial corneal diseases in dogs and cats. Clin. Tech. Small Anim. Pract. 2003, 18, 193–198. [Google Scholar] [CrossRef]
- Mauer, A.N.; Allbaugh, R.A.; Kreuder, A.J.; Sebbag, L. Impact of multi-drug resistance on clinical outcomes of dogs with corneal ulcers infected with Staphylococcus pseudintermedius. Front. Vet. Sci. 2022, 9, 1083294. [Google Scholar] [CrossRef]
- Hindley, K.E.; Groth, A.D.; King, M.; Graham, K.; Billson, F.M. Bacterial isolates, antimicrobial susceptibility, and clinical characteristics of bacterial keratitis in dogs presenting to referral practice in Australia. Vet. Ophthalmol. 2016, 19, 418–426. [Google Scholar] [CrossRef]
- Goldreich, J.E.; Franklin-Guild, R.J.; Ledbetter, E.C. Feline bacterial keratitis: Clinical features, bacterial isolates, and in vitro antimicrobial susceptibility patterns. Vet. Ophthalmol. 2020, 23, 90–96. [Google Scholar] [CrossRef]
- Joksimovic, M.; Ford, B.A.; Lazic, T.; Soldatovic, I.; Luzetsky, S.; Grozdanic, S. Antibiotic Recommendations for Treatment of Canine Stromal Corneal Ulcers. Vet. Sci. 2023, 10, 66. [Google Scholar] [CrossRef]
- Jinks, M.R.; Miller, E.J.; Diaz-Campos, D.; Mollenkopf, D.F.; Newbold, G.; Gemensky-Metzler, A.; Chandler, H.L. Using minimum inhibitory concentration values of common topical antibiotics to investigate emerging antibiotic resistance: A retrospective study of 134 dogs and 20 horses with ulcerative keratitis. Vet. Ophthalmol. 2020, 23, 806–813. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, L.; Li, J.; Li, J.; Cui, L.; Dong, J.; Meng, X.; Qian, C.; Wang, H. Antibiotic resistance, biofilm formation, and virulence factors of isolates of Staphylococcus pseudintermedius from healthy dogs and dogs with keratitis. Front. Vet. Sci. 2022, 9, 903633. [Google Scholar] [CrossRef]
- Hewitt, J.S.; Allbaugh, R.A.; Kenne, D.E.; Sebbag, L. Prevalence and Antibiotic Susceptibility of Bacterial Isolates from Dogs with Ulcerative Keratitis in Midwestern United States. Front. Vet. Sci. 2020, 7, 583965. [Google Scholar] [CrossRef] [PubMed]
- Suter, A.; Voelter, K.; Hartnack, S.; Spiess, B.M.; Pot, S.A. Septic keratitis in dogs, cats, and horses in Switzerland: Associated bacteria and antibiotic susceptibility. Vet. Ophthalmol. 2018, 21, 66–75. [Google Scholar] [CrossRef]
- Chalder, R.H.; Knott, T.; Rushton, J.O.; Nikolic-Pollard, D. Changes in antimicrobial resistance patterns of ocular surface bacteria isolated from horses in the UK: An eight-year surveillance study (2012–2019). Vet. Ophthalmol. 2020, 23, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Lewin, A.C.; Hicks, S.K.; Carter, R.T. A review of evidence-based management of infectious ocular surface disease in shelter-housed domestic cats. Vet. Ophthalmol. 2023, 26, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Goss, R.; Adams, V.J.; Heinrich, C.; Grundon, R.; Linn-Pearl, R.; Scurrell, E.; Hamzianpour, N. Progressive ulcerative keratitis in dogs in the United Kingdom: Microbial isolates, antimicrobial sensitivity, and resistance patterns. Vet. Ophthalmol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Hartley, C. Outcomes of treatments for keratomalacia in dogs and cats: A systematic review of the published literature including non-randomised controlled and non-controlled studies. J. Small Anim. Pract. 2021, 62, 840–849. [Google Scholar] [CrossRef]
- Guyonnet, A.; Desquilbet, L.; Faure, J.; Bourguet, A.; Donzel, E.; Chahory, S. Outcome of medical therapy for keratomalacia in dogs. J. Small Anim. Pract. 2020, 61, 253–258. [Google Scholar] [CrossRef]
- Singleton, P. Bacteria in Biology, Biotechnology, and Medicine, 6th ed.; John Wiley & Sons: Chichester, UK, 2004; ISBN 047009026X. [Google Scholar]
- Gupta, R.S. Origin of diderm (Gram-negative) bacteria: Antibiotic selection pressure rather than endosymbiosis likely led to the evolution of bacterial cells with two membranes. Antonie Van Leeuwenhoek 2011, 100, 171–182. [Google Scholar] [CrossRef]
- Leigue, L.; Montiani-Ferreira, F.; Moore, B.A. Antimicrobial susceptibility and minimal inhibitory concentration of Pseudomonas aeruginosa isolated from septic ocular surface disease in different animal species. Open Vet. J. 2016, 6, 215–222. [Google Scholar] [CrossRef] [PubMed]
- EMA; CVMP; CHMP. Categorisation of Antibiotics in the European Union: Answer to the Request from the European Commission for Updating the Scientific Advice on the Impact on Public Health and Animal Health of the Use of Antibiotics in Animals No. 682198. 2017. Available online: https://www.ema.europa.eu/en/documents/report/categorisation-antibiotics-european-union-answer-request-european-commission-updating-scientific_en.pdf (accessed on 28 September 2023).
- Banin, E.; Brady, K.M.; Greenberg, E.P. Chelator-induced dispersal and killing of Pseudomonas aeruginosa cells in a biofilm. Appl. Environ. Microbiol. 2006, 72, 2064–2069. [Google Scholar] [CrossRef] [PubMed]
- Wooley, R.E.; Jones, M.S.; Gilbert, J.P.; Shotts, E.B., Jr. In vitro effect of combinations of antimicrobial agents and EDTA-tromethamine on certain gram-positive bacteria. Am. J. Vet. Res. 1983, 44, 2167–2169. [Google Scholar] [PubMed]
- Wooley, R.E.; Jones, M.S.; Shotts, E.B. Uptake of antibiotics in gram-negative bacteria exposed to EDTA-Tris. Vet. Microbiol. 1984, 10, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Sparks, T.A.; Kemp, D.T.; Wooley, R.E.; Gibbs, P.S. Antimicrobial effect of combinations of EDTA-Tris and amikacin or neomycin on the microorganisms associated with otitis externa in dogs. Vet. Res. Commun. 1994, 18, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Farca, A.M.; Piromalli, G.; Maffei, F.; Re, G. Potentiating effect of EDTA-Tris on the activity of antibiotics against resistant bacteria associated with otitis, dermatitis and cystitis. J. Small Anim. Pract. 1997, 38, 243–245. [Google Scholar] [CrossRef]
- Boyd, M.; Santoro, D.; Gram, D. In vitro antimicrobial activity of topical otological antimicrobials and Tris-EDTA against resistant Staphylococcus pseudintermedius and Pseudomonas aeruginosa isolates from dogs. Vet. Dermatol. 2019, 30, 139-e40. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.Y.; Hickey, E.E.; Page, S.W.; Trott, D.J.; Hill, P.B. Biofilm production by pathogens associated with canine otitis externa, and the antibiofilm activity of ionophores and antimicrobial adjuvants. J. Vet. Pharmacol. Ther. 2019, 42, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Buckley, L.M.; McEwan, N.A.; Nuttall, T. Tris-EDTA significantly enhances antibiotic efficacy against multidrug-resistant Pseudomonas aeruginosa in vitro. Vet. Dermatol. 2013, 24, 519-e122. [Google Scholar] [CrossRef]
- Farca, A.M.; Nebbia, P.; Re, G. Potentiation of antibiotic activity by EDTA-tromethamine against three clinically isolated gram-positive resistant bacteria. An in vitro investigation. Vet. Res. Commun. 1994, 18, 1–6. [Google Scholar] [CrossRef]
- Finnegan, S.; Percival, S.L. EDTA: An Antimicrobial and Antibiofilm Agent for Use in Wound Care. Adv. Wound Care 2015, 4, 415–421. [Google Scholar] [CrossRef]
- Thoman, C.J. Ionophoric properties of polysorbate 80. J. Pharm. Sci. 1986, 75, 983–986. [Google Scholar] [CrossRef]
- Toutain-Kidd, C.M.; Kadivar, S.C.; Bramante, C.T.; Bobin, S.A.; Zegans, M.E. Polysorbate 80 inhibition of Pseudomonas aeruginosa biofilm formation and its cleavage by the secreted lipase LipA. Antimicrob. Agents Chemother. 2009, 53, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, A.M.; McClarty, B.M.; Robinson, C.; Spear, W.; Sanchez, M.; Sparkes, T.C.; Brooke, J.S. Polysorbate 80 and polymyxin B inhibit Stenotrophomonas maltophilia biofilm. Diagn. Microbiol. Infect. Dis. 2017, 87, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Mashima, K.; Miyazaki, M.; Hara, S.; Takata, T.; Kamimura, H.; Takagi, S.; Jimi, S. Inhibitory effects of polysorbate 80 on MRSA biofilm formed on different substrates including dermal tissue. Sci. Rep. 2019, 9, 3128. [Google Scholar] [CrossRef]
- European Council. European Pharmacopoeia, 10.0; European Directorate for the Quality of Medicines and Heatlhcare: Strasbourg, France, 2019; ISBN 978-3-7692-7453-0. [Google Scholar]
- Ramirez-Ronda, C.H.; Holmes, R.K.; Sanford, J.P. Effects of Divalent Cations on Binding of Aminoglycoside Antibiotics to Human Serum Proteins and to Bacteria. Antimicrob. Agents Chemother. 1975, 7, 239–245. [Google Scholar] [CrossRef]
- Hancock, R.E. Aminoglycoside uptake and mode of action-with special reference to streptomycin and gentamicin: I. Antagonists and mutants. J. Antimicrob. Chemother. 1981, 8, 249–276. [Google Scholar] [CrossRef] [PubMed]
- Gelatt, K.N.; Gilger, B.C.; Kern, T.J. Physiology of the Eye. In Veterinary Ophtalmology, 5th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 172–175. [Google Scholar]
- Sebbag, L.; Broadbent, V.L.; Kenne, D.E.; Perrin, A.L.; Mochel, J.P. Albumin in Tears Modulates Bacterial Susceptibility to Topical Antibiotics in Ophthalmology. Front. Med. 2021, 8, 663212. [Google Scholar] [CrossRef]
- Arad, D.; Deckel, R.; Pe’er, O.; Ross, M.; Sebbag, L.; Ofri, R. Is it necessary to wait several minutes between applications of different topical ophthalmic solutions? A preliminary study with tropicamide eye drops in healthy dogs. Vet. Ophthalmol. 2021, 24, 374–379. [Google Scholar] [CrossRef]
- Booth, J.H.; Benrimoj, S.I.; Nimmo, G.R. In vitro interactions of neomycin sulfate, bacitracin, and polymyxin B sulfate. Int. J. Dermatol. 1994, 33, 517–520. [Google Scholar] [CrossRef]
- Barter, L.S.; Watson, A.D.; Maddison, J.E. Owner compliance with short term antimicrobial medication in dogs. Aust. Vet. J. 1996, 74, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Grave, K.; Tanem, H. Compliance with short-term oral antibacterial drug treatment in dogs. J. Small Anim. Pract. 1999, 40, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, R.P.; Lassaline, M.E.; Holt, E.A. Owner compliance with instructions given for topical ophthalmic medications is associated with species and time between examinations. In Proceedings of the 53rd Annual Meeting of the American College of Veterinary Ophthalmologists, Palm Springs, CA, USA, 26–29 October 2022. [Google Scholar]
- Walter, H.; Verspohl, J.; Meißner, J.; Oltmanns, H.; Geks, A.K.; Busse, C. In Vitro Antimicrobial Activity of N-Acetylcysteine against Pathogens Most Commonly Associated with Infectious Keratitis in Dogs and Cats. Antibiotics 2023, 12, 559. [Google Scholar] [CrossRef] [PubMed]
- Suter, A.; Schmitt, S.; Hübschke, E.; Kowalska, M.; Hartnack, S.; Pot, S. The bactericidal effect of two photoactivated chromophore for keratitis-corneal crosslinking protocols (standard vs. accelerated) on bacterial isolates associated with infectious keratitis in companion animals. BMC Vet. Res. 2022, 18, 317. [Google Scholar] [CrossRef] [PubMed]
- Bonzano, C.; Di Zazzo, A.; Barabino, S.; Coco, G.; Traverso, C.E. Collagen Cross-Linking in the Management of Microbial Keratitis. Ocul. Immunol. Inflamm. 2019, 27, 507–512. [Google Scholar] [CrossRef]
- Marasini, S.; Dean, S.J.; Swift, S.; Perera, J.; Rupenthal, I.D.; Wang, T.; Read, H.; Craig, J.P. Preclinical confirmation of UVC efficacy in treating infectious keratitis. Ocul. Surf. 2022, 25, 76–86. [Google Scholar] [CrossRef]
- NF EN ISO 1040; Chemical Antiseptics and Disinfectants–Quantitative Suspension Tests for the Evaluation of the Basic Bactericidal Activity of Chemical Antiseptics and Disinfectants–Test Method and Requirements (Phase 1). AFNOR: La Plaine Saint-Denis, France, 2006.
| Tested Concentrations | ||||
|---|---|---|---|---|
| Initial Bacterial Suspension | 10% | 50% | 80% | |
| Number of viable microorganisms (CFU 1/mL) | 2.56 × 107 | >3.3 × 103 | >3.3 × 103 | >3.3 × 103 |
| Logarithmic reduction in the number of viable microorganisms at the trial solution concentration | N/A | <3.9 | <3.9 | <3.9 |
| Tested Concentrations | ||||
|---|---|---|---|---|
| Initial Bacterial Suspension | 10% | 50% | 80% | |
| Number of viable microorganisms (CFU/mL) | 2.92 × 107 | >3.3 × 103 | >3.3 × 103 | >3.3 × 103 |
| Logarithmic reduction in the number of viable microorganisms at the trial solution concentration | N/A | <4.0 | <4.0 | <4.0 |
| Activity Obtained (IU 1/mL Neomycin Sulfate) | 95% Confidence Interval (IU/mL Neomycin Sulfate) | |
|---|---|---|
| Neomycin Sulfate eye drops | 3267 | 3024–3527 |
| Neomycin Sulfate eye drops + test solution (1:1 ratio) | 3708 | 3554–3872 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amiriantz, S.; Hoummady, S.; Jarousse, E.; Roudeix, S.; Philippon, T. Investigating the Bactericidal Activity of an Ocular Solution Containing EDTA, Tris, and Polysorbate 80 and Its Impact on the In Vitro Efficacy of Neomycin Sulfate against Staphylococcus aureus: A Preliminary Study. Antibiotics 2024, 13, 611. https://doi.org/10.3390/antibiotics13070611
Amiriantz S, Hoummady S, Jarousse E, Roudeix S, Philippon T. Investigating the Bactericidal Activity of an Ocular Solution Containing EDTA, Tris, and Polysorbate 80 and Its Impact on the In Vitro Efficacy of Neomycin Sulfate against Staphylococcus aureus: A Preliminary Study. Antibiotics. 2024; 13(7):611. https://doi.org/10.3390/antibiotics13070611
Chicago/Turabian StyleAmiriantz, Sophie, Sara Hoummady, Elodie Jarousse, Séverine Roudeix, and Thomas Philippon. 2024. "Investigating the Bactericidal Activity of an Ocular Solution Containing EDTA, Tris, and Polysorbate 80 and Its Impact on the In Vitro Efficacy of Neomycin Sulfate against Staphylococcus aureus: A Preliminary Study" Antibiotics 13, no. 7: 611. https://doi.org/10.3390/antibiotics13070611
APA StyleAmiriantz, S., Hoummady, S., Jarousse, E., Roudeix, S., & Philippon, T. (2024). Investigating the Bactericidal Activity of an Ocular Solution Containing EDTA, Tris, and Polysorbate 80 and Its Impact on the In Vitro Efficacy of Neomycin Sulfate against Staphylococcus aureus: A Preliminary Study. Antibiotics, 13(7), 611. https://doi.org/10.3390/antibiotics13070611





