Therapeutic Potential of Mangosteen Pericarp Extract-Loaded Liposomes against Superficial Skin Infection Caused by Staphylococcus pseudintermedius in a Murine Model
Abstract
:1. Introduction
2. Results
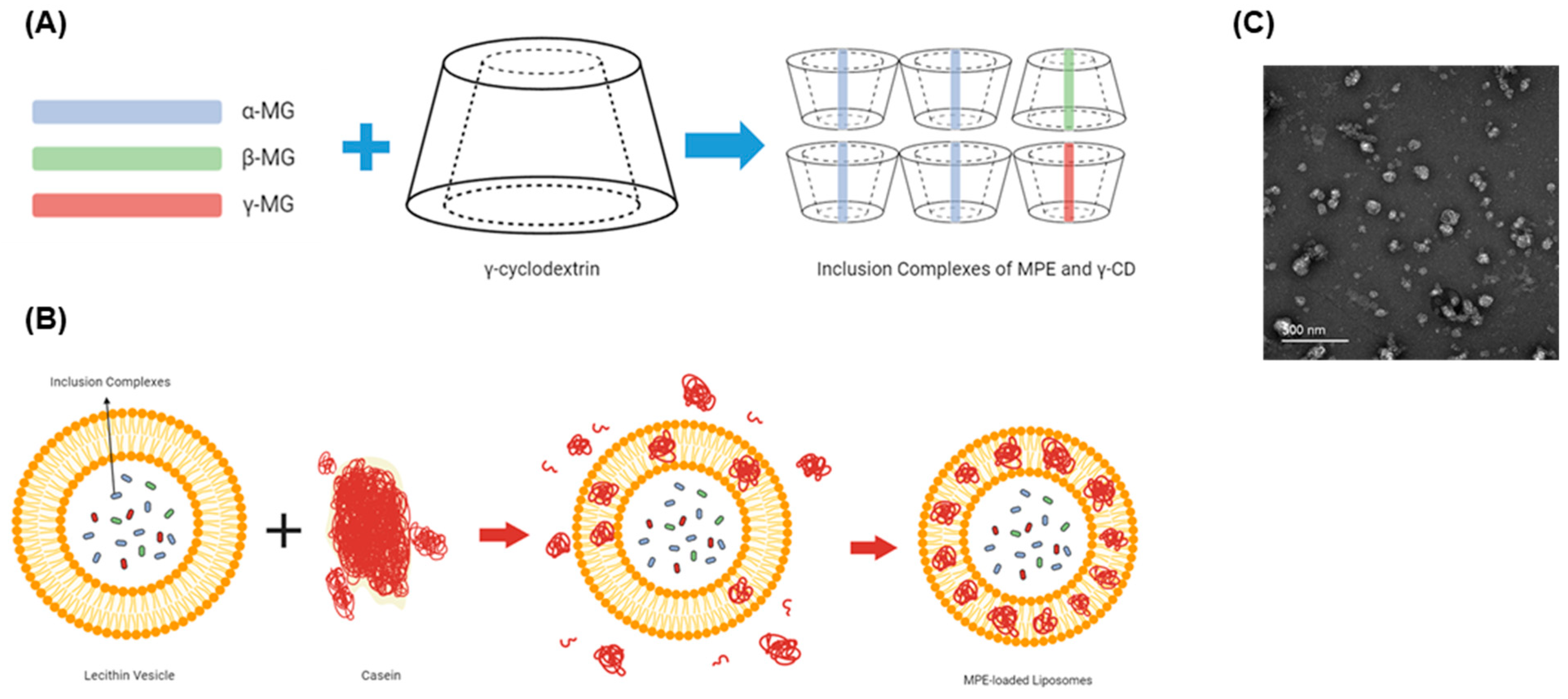
2.1. Characterization of MPE-Loaded Liposomes
2.2. Antibacterial Activity of MPE-Loaded Liposomes against Reference Bacterial Strains In Vitro
2.3. Antibacterial Activity of MPE-Loaded Liposomes against Clinical Isolates In Vitro
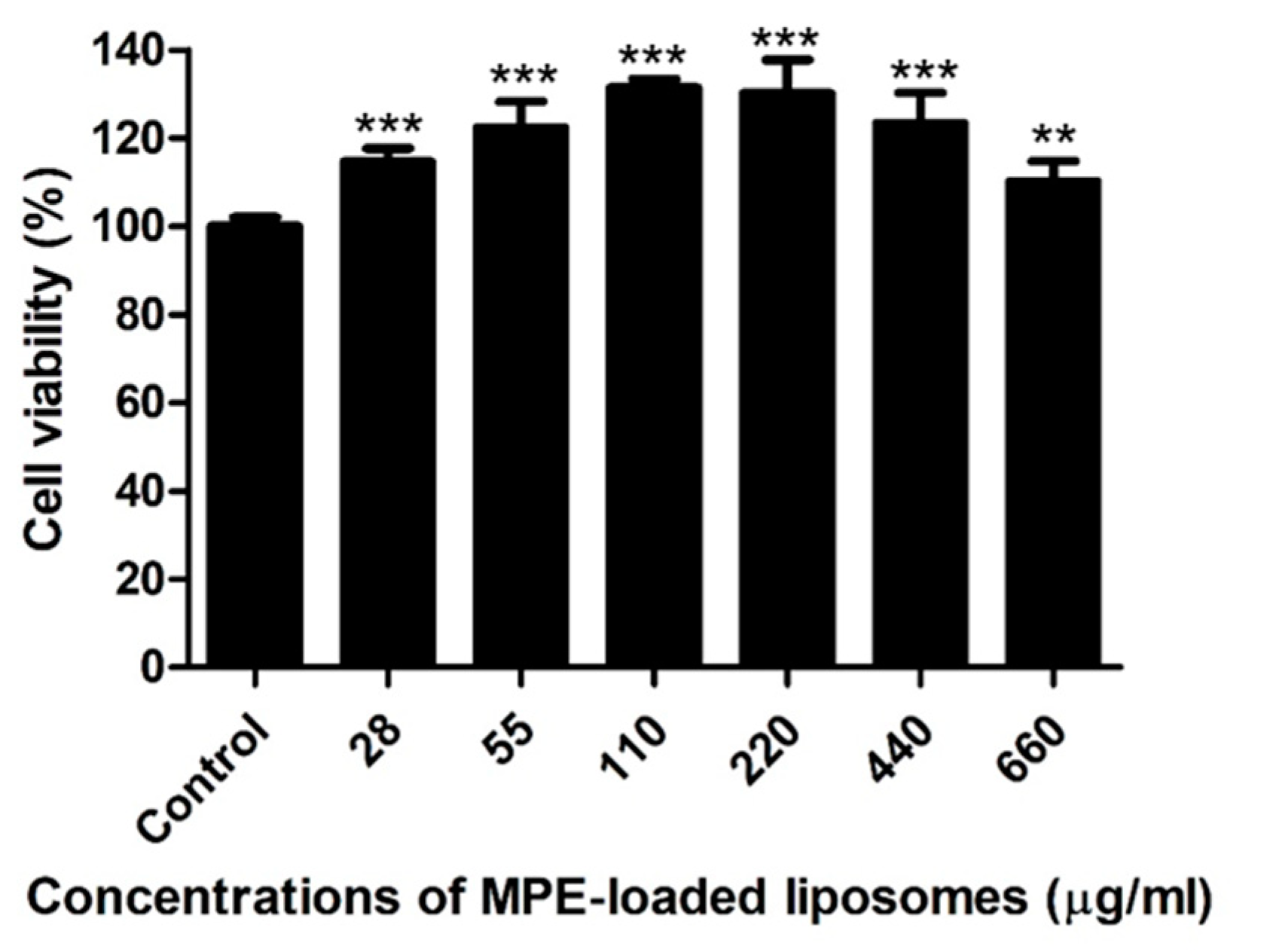
2.4. Host Cell Cytotoxicity of MPE-Loaded Liposomes In Vitro
2.5. Therapeutic Potential of MPE-Loaded Liposomes in a Murine Superficial Skin Infection Model Caused by S. pseudintermedius
3. Discussion
4. Materials and Methods
4.1. Preparation of MPE
4.2. Preparation of Inclusion Complex of MPE and γ-CD and MPE-Loaded Liposomes
4.3. Determination of α-MG Content in MPE and MPE-Loaded Liposomes
4.4. Bacterial Strains
4.5. Antimicrobial Susceptibility Testing
4.6. Cell Culture and Cytotoxicity Assay
4.7. Transmission Electron Microscopy Analysis
4.8. Mouse Skin Infection and Application of MPE-Loaded Liposomes
4.9. Histological Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baker, S.A.; Van-Balen, J.; Lu, B.; Hillier, A.; Hoet, A.E. Antimicrobial drug use in dogs prior to admission to a veterinary teaching hospital. J. Am. Vet. Med. Assoc. 2012, 241, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Hillier, A.; Lloyd, D.H.; Weese, J.S.; Blondeau, J.M.; Boothe, D.; Breitschwerdt, E.; Guardabassi, L.; Papich, M.G.; Rankin, S.; Turnidge, J.D.; et al. Guidelines for the diagnosis and antimicrobial therapy of canine superficial bacterial folliculitis (Antimicrobial Guidelines Working Group of the International Society for Companion Animal Infectious Diseases). Vet. Dermatol. 2014, 25, 163-e43. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.; Helbig, K. The complex diseases of Staphylococcus pseudintermedius in canines: Where to next? Vet. Sci. 2021, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Cavana, P.; Robino, P.; Stella, M.C.; Bellato, A.; Crosaz, O.; Fiora, S.R.; Nebbia, P. Staphylococci isolated from cats in Italy with superficial pyoderma and allergic dermatitis: Characterisation of isolates and their resistance to antimicrobials. Vet. Dermatol. 2023, 34, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Joosten, P.; Ceccarelli, D.; Odent, E.; Sarrazin, S.; Graveland, H.; Van Gompel, L. Antimicrobial usage and resistance in companion animals: A cross-sectional study in three European countries. Antibiotics 2020, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, A.J.; Harrison, E.M.; Stanczak-Mrozek, K.; Leggett, B.; Waller, A.; Holmes, M.A.; Lloyd, D.H.; Lindsay, J.A.; Loeffler, A. Genomic insights into the rapid emergence and evolution of MDR in Staphylococcus pseudintermedius. J. Antimicrob. Chemother. 2014, 70, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.T.; Lubbers, B.V.; Schwarz, S.; Watts, J.L. Applying definitions for multidrug resistance, extensive drug resistance and pandrug resistance to clinically significant livestock and companion animal bacterial pathogens. J. Antimicrob. Chemother. 2018, 73, 1460–1463. [Google Scholar] [CrossRef] [PubMed]
- Gunter, N.V.; Teh, S.S.; Lim, Y.M.; Mah, S.H. Natural xanthones and skin inflammatory diseases: Multitargeting mechanisms of action and potential application. Front. Pharmacol. 2020, 11, 594202. [Google Scholar] [CrossRef] [PubMed]
- Pedraza-Chaverri, J.; Cárdenas-Rodríguez, N.; Orozco-Ibarra, M.; Pérez-Rojas, J.M. Medicinal properties of mangosteen (Garcinia mangostana). Food Chem. Toxicol. 2008, 46, 3227–3239. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.Y.; Hashim, N.M.; Mariod, A.A.; Mohan, S.; Abdulla, M.A.; Abdelwahab, S.I.; Arbab, I.A. α-mangostin from Garcinia mangostana Linn: An updated review of its pharmacological properties. Arab. J. Chem. 2016, 9, 317–329. [Google Scholar] [CrossRef]
- Iinuma, M.; Tosa, H.; Tanaka, T.; Asai, F.; Kobayashi, Y.; Shimano, R.; Miyauchi, K. Antibacterial activity of xanthones from guttiferaeous plants against methicillin-resistant Staphylococcus aureus. J. Pharm. Pharmacol. 1996, 48, 861–865. [Google Scholar] [CrossRef]
- Sakagami, Y.; Iinuma, M.; Piyasena, K.G.; Dharmaratne, H.R. Antibacterial activity of alpha-mangostin against vancomycin resistant enterococci (VRE) and synergism with antibiotics. Phytomedicine 2005, 12, 203–208. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Falsetta, M.L.; Hwang, G.; Gonzalez-Begne, M.; Koo, H. Alpha-mangostin disrupts the development of Streptococcus mutans biofilms and facilitates its mechanical removal. PLoS ONE 2014, 9, e111312. [Google Scholar] [CrossRef] [PubMed]
- Felix, L.; Mishra, B.; Khader, R.; Ganesan, N.; Mylonakis, E. In vitro and in vivo bactericidal and antibiofilm efficacy of alpha-mangostin against Staphylococcus aureus persister cells. Front. Cell. Infect. Microbiol. 2022, 12, 898794. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.J.; Qiu, S.; Zou, H.; Lakshminarayanan, R.; Li, J.; Zhou, X.; Tang, C.; Saraswathi, P.; Verma, C.; Tan, D.T.H.; et al. Rapid bactericidal action of alpha-mangostin against MRSA as an outcome of membrane targeting. Biochim. Biophys. Acta 2013, 1828, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Lee, J.H.; Ko, S.Y.; Kim, N.; Kim, S.Y.; Lee, J.C. Antimicrobial activity of α-mangostin against Staphylococcus species from companion animals in vitro and therapeutic potential of α-mangostin in skin diseases caused by S. pseudintermedius. Front. Cell. Infect. Microbiol. 2023, 13, 1203663. [Google Scholar] [CrossRef]
- Wathoni, N.; Rusdin, A.; Motoyama, K.; Joni, I.M.; Lesmana, R.; Muchtaridi, M. Nanoparticle drug delivery systems for α-mangostin. Nanotechnol. Sci. Appl. 2020, 13, 23–36. [Google Scholar] [CrossRef]
- Rivero, B.; Garibay, I. Development and validation of a stability indicating HPLC method for the quantification of α-mangostin in dietary supplements. Nat. Prod. Commun. 2019, 14, 1–5. [Google Scholar] [CrossRef]
- Asasutjarit, R.; Larpmahawong, P.; Fuongfuchat, A.; Sareedenchai, V.; Veeranondha, S. Physicochemical properties and anti-Propionibacterium acnes activity of film forming solutions containing α-mangostin-rich extract. AAPS PharmSciTech 2014, 15, 306–316. [Google Scholar] [CrossRef]
- Mulia, K.; Putri, G.A.; Krisanti, E. Encapsulation of mangosteen extract in virgin coconut oil based nanoemulsions: Preparation and characterization for topical formulation. Mater. Sci. Forum 2018, 929, 234–242. [Google Scholar] [CrossRef]
- Suharyani, I.; Muchtaridi, M.; Mohammed, A.F.A.; Elamin, K.M.; Wathoni, N.; Abdassah, M. α-mangostin/γ-cyclodextrin inclusion complex: Formation and thermodynamic study. Polymers 2021, 13, 2890. [Google Scholar] [CrossRef] [PubMed]
- Le, N.T.T.; Cao, V.D.; Nguyen, T.N.Q.; Le, T.T.H.; Tran, T.T.; Thi, T.T.H. Soy lecithin-derived liposomal delivery systems: Surface modification and current applications. Int. J. Mole. Sci. 2019, 20, 4706. [Google Scholar] [CrossRef] [PubMed]
- Panja, S.; Khatua, D.K.; Halder, M. Effect of casein on pure lecithin liposome: Mixed biomacromolecular system for providing superior stabilization to hydrophobic molecules. Colloids Surf. B Biointerfaces 2019, 180, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Tadtong, S.; Viriyaroj, A.; Vorarat, S.; Nimkulrat, S.; Suksamrarn, S. Antityrosinase and antibacterial activities of mangosteen pericarp extract. J. Health Res. 2009, 23, 99–102. [Google Scholar]
- Rezeki, Y.A.; Hapidin, D.A.; Rachmawati, H.; Munir, M.M.; Khairurrijal, K. Formation of electrosprayed composite nanoparticles from polyvinylpyrrolidone/mangosteen pericarp extract. Adv. Powder Technol. 2020, 31, 1811–1824. [Google Scholar] [CrossRef]
- Sriboonyong, P.; Poommarin, P.; Sittiya, J.; Opanasopit, P.; Ngawhirunpat, T.; Patrojanasophon, P.; Pornpitchanarong, C. The utilization of mangosteen pericarp extract for anticoccidial drug replacement in broiler feed. Int. J. Vet. Sci. Med. 2022, 10, 90–99. [Google Scholar] [CrossRef]
- Dermawan, D.; Wathoni, N.; Muchtaridi, M. Host-guest interactions of α-mangostin with (α,β,γ)-cyclodextrins: Semi-empirical quantum mechanical methods of PM6 and PM7. J. Young Pharm. 2018, 11, 31–35. [Google Scholar] [CrossRef]
- Meylina, L.; Muchtaridi, M.; Joni, I.M.; Mohammed, A.F.A.; Wathoni, N. Nanoformulations of α-mangostin for cancer drug delivery system. Pharmaceutics 2021, 13, 1993. [Google Scholar] [CrossRef] [PubMed]
- Chin, G.S.; Todo, H.; Kadhum, R.; Hamid, A.; Sugibayashi, K. In vitro permeation and skin retention of α-mangostin proniosome. Chem. Pharm. Bull. 2016, 64, 1666–1673. [Google Scholar] [CrossRef]
- Yodhnu, S.; Sirikatitham, A.; Wattanapiromsakul, C. Validation of LC for the determination of α-mangostin in mangosteen peel extract: A tool for quality assessment of Garcinia mangostana L. J. Chromatogr. Sci. 2009, 47, 185–189. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2020. [Google Scholar]

| Bacteria | MIC (µg/mL) of α-MG * | MIC (µg/mL) of MPE-Loaded Liposomes | MBC (µg/mL) of MPE-Loaded Liposomes | |
|---|---|---|---|---|
| Broth Microdilution | Agar Dilution | |||
| S. aureus ATCC 29213 | 2 | 220 (4) ** | 880 (16) | 220 (4) |
| S. carprae KCTC 3583 | 2 | 110 (2) | 220 (4) | 110 (2) |
| S. epidermidis ATCC 12228 | 2 | 110 (2) | 110 (2) | 110 (2) |
| S. felis ATCC 49168 | 2 | 55(1) | 110 (2) | 55 (1) |
| S. intermedius KCTC 3344 | 2 | 110 (2) | 220 (4) | 110 (2) |
| S. pseudintermedius ATCC 49051 | 2 | 55 (1) | 110 (2) | 55 (1) |
| S. saprophyticus KCTC 3345 | 1 | 55 (1) | 110 (2) | 55 (1) |
| S. schleiferi ATCC 43808 | 2 | 55 (1) | 55 (1) | 55 (1) |
| E. coli ATCC 25922 | >64 | >3520 (>64) | >3520 (>64) | >3520 (>64) |
| P. aeruginosa ATCC 27853 | >64 | >3520 (>64) | >3520 (>64) | >3520 (>64) |
| Bacterial Isolates (No.) | No of Isolates with the Following MIC * (µg/mL) | MIC50 (µg/mL) | MIC90 (µg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 28 (0.5) ** | 55 (1) | 110 (2) | 220 (4) | 440 (8) | 880 (16) | >3520 (>64) | |||
| S. aureus (19) | 6 | 13 | 880 (16) | 880 (16) | |||||
| S. epidermidis (27) | 1 | 15 | 9 | 2 | 220 (4) | 440 (8) | |||
| S. felis (51) | 10 | 41 | 220 (4) | 220 (4) | |||||
| S. pseudintermedius (80) | 1 | 2 | 73 | 4 | 110 (2) | 110 (2) | |||
| S. schleiferi (64) | 23 | 41 | 220 (4) | 220 (4) | |||||
| E. coli (20) | 20 | >3520 (>64) | >3520 (>64) | ||||||
| P. aeruginosa (16) | 16 | >3520 (>64) | >3520 (>64) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-Y.; Park, S.-Y.; Lee, J.-H.; Kim, N.; Oh, H.-N.; Yoo, S.-Y.; Lee, D.-S.; Lee, J.-C. Therapeutic Potential of Mangosteen Pericarp Extract-Loaded Liposomes against Superficial Skin Infection Caused by Staphylococcus pseudintermedius in a Murine Model. Antibiotics 2024, 13, 612. https://doi.org/10.3390/antibiotics13070612
Kim S-Y, Park S-Y, Lee J-H, Kim N, Oh H-N, Yoo S-Y, Lee D-S, Lee J-C. Therapeutic Potential of Mangosteen Pericarp Extract-Loaded Liposomes against Superficial Skin Infection Caused by Staphylococcus pseudintermedius in a Murine Model. Antibiotics. 2024; 13(7):612. https://doi.org/10.3390/antibiotics13070612
Chicago/Turabian StyleKim, Seong-Yeop, Seong-Yong Park, Jung-Hwa Lee, Nayeong Kim, Ha-Na Oh, So-Young Yoo, Dae-Sung Lee, and Je-Chul Lee. 2024. "Therapeutic Potential of Mangosteen Pericarp Extract-Loaded Liposomes against Superficial Skin Infection Caused by Staphylococcus pseudintermedius in a Murine Model" Antibiotics 13, no. 7: 612. https://doi.org/10.3390/antibiotics13070612
APA StyleKim, S.-Y., Park, S.-Y., Lee, J.-H., Kim, N., Oh, H.-N., Yoo, S.-Y., Lee, D.-S., & Lee, J.-C. (2024). Therapeutic Potential of Mangosteen Pericarp Extract-Loaded Liposomes against Superficial Skin Infection Caused by Staphylococcus pseudintermedius in a Murine Model. Antibiotics, 13(7), 612. https://doi.org/10.3390/antibiotics13070612






