Coatings Based on Essential Oils for Combating Antibiotic Resistance
Abstract
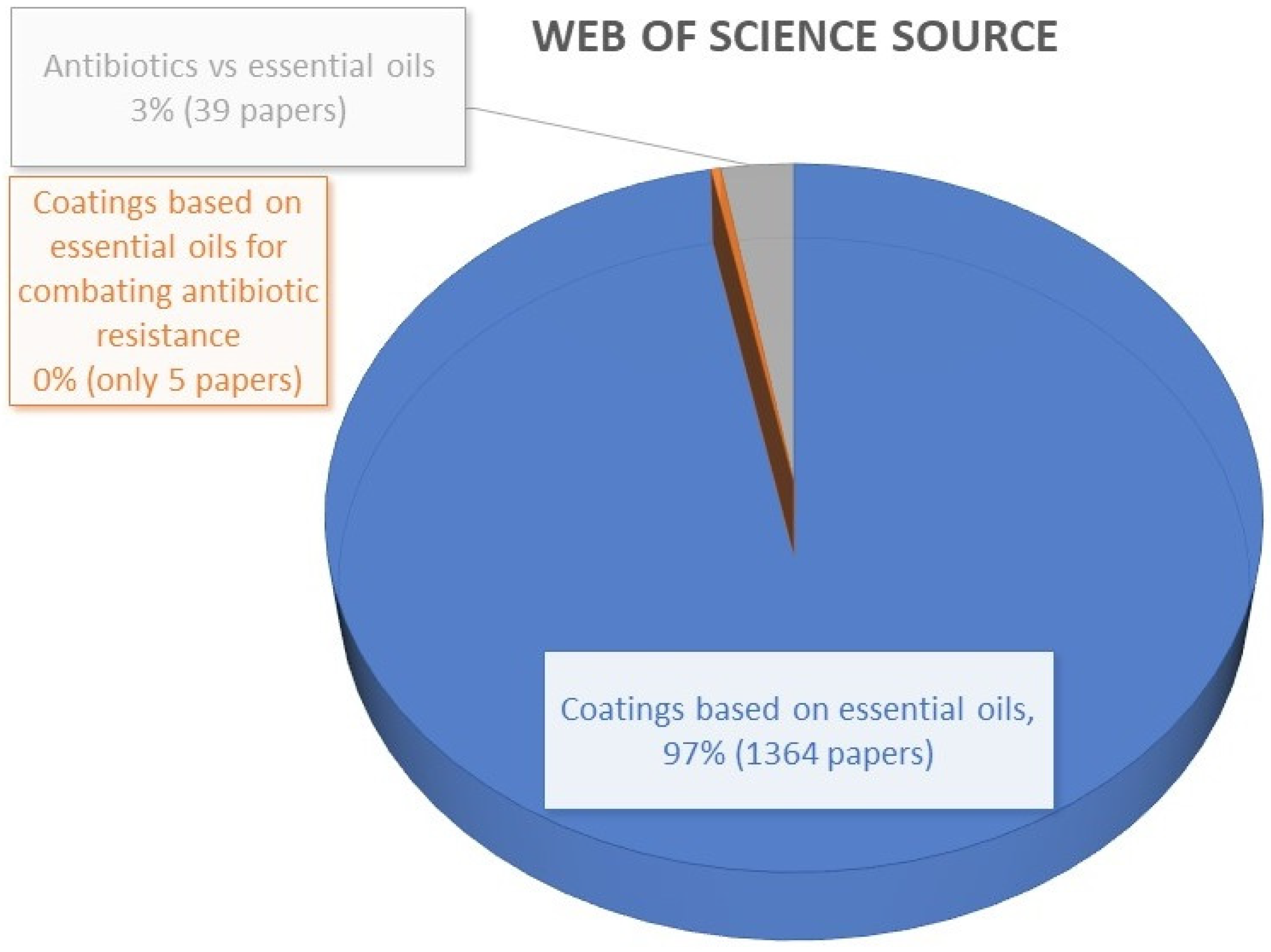
:1. Introduction to the Topic and Justification of the Review Need
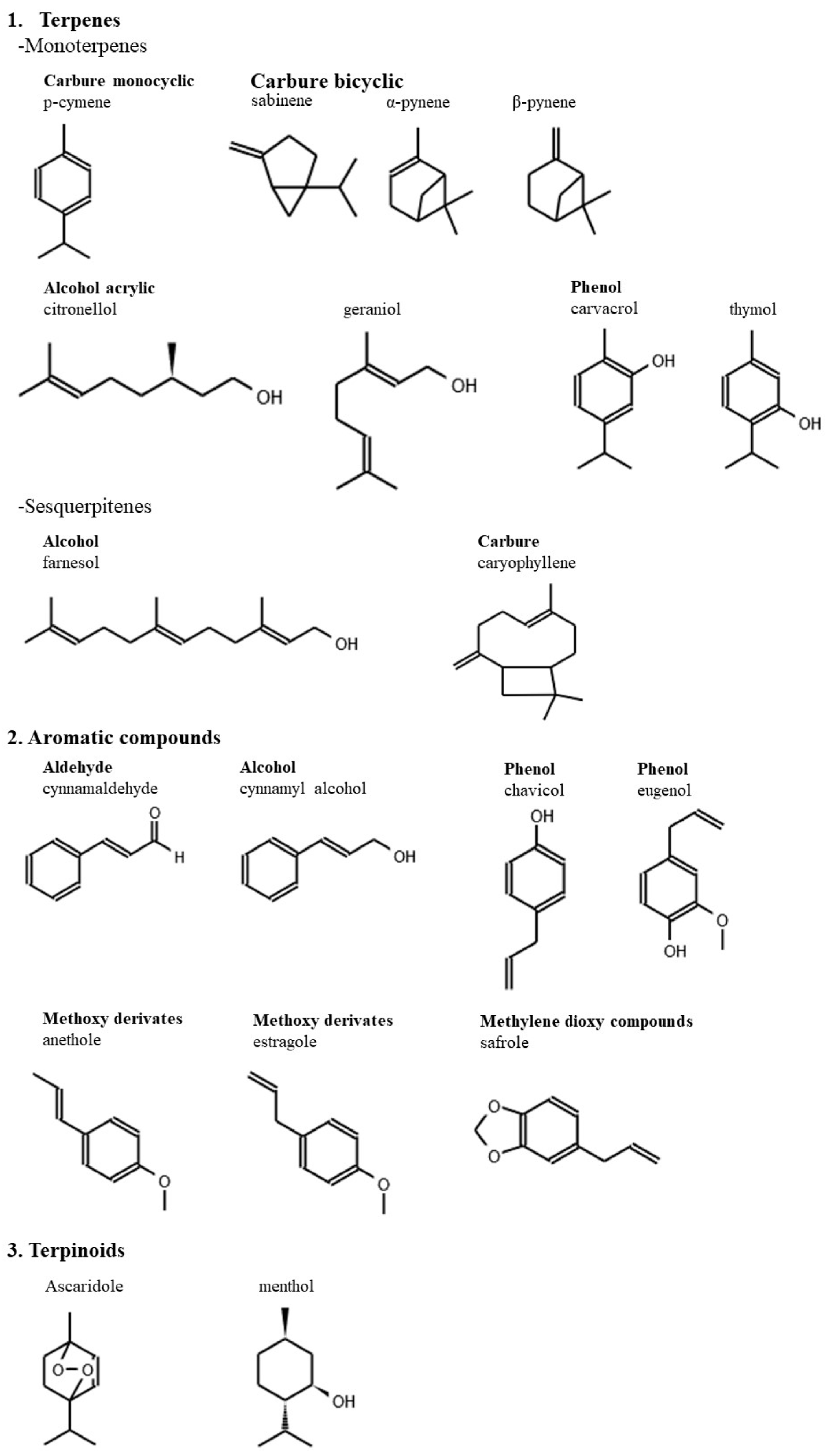
2. Chemical Composition of EOs
3. Biochemical Properties of EOs
3.1. Antimicrobial Activity
3.2. Anti-Inflammatory Effects
3.3. Antioxidant Properties
4. Methods of EO Extraction
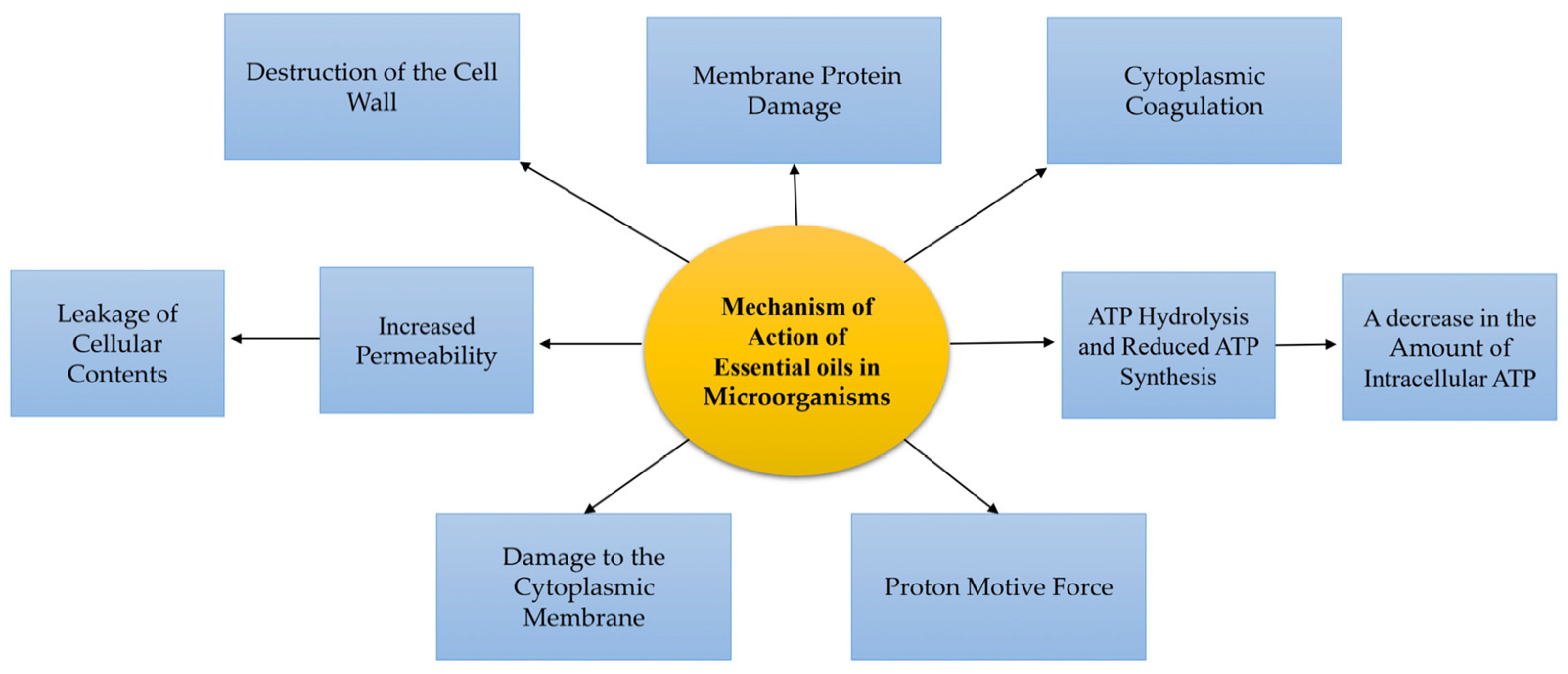
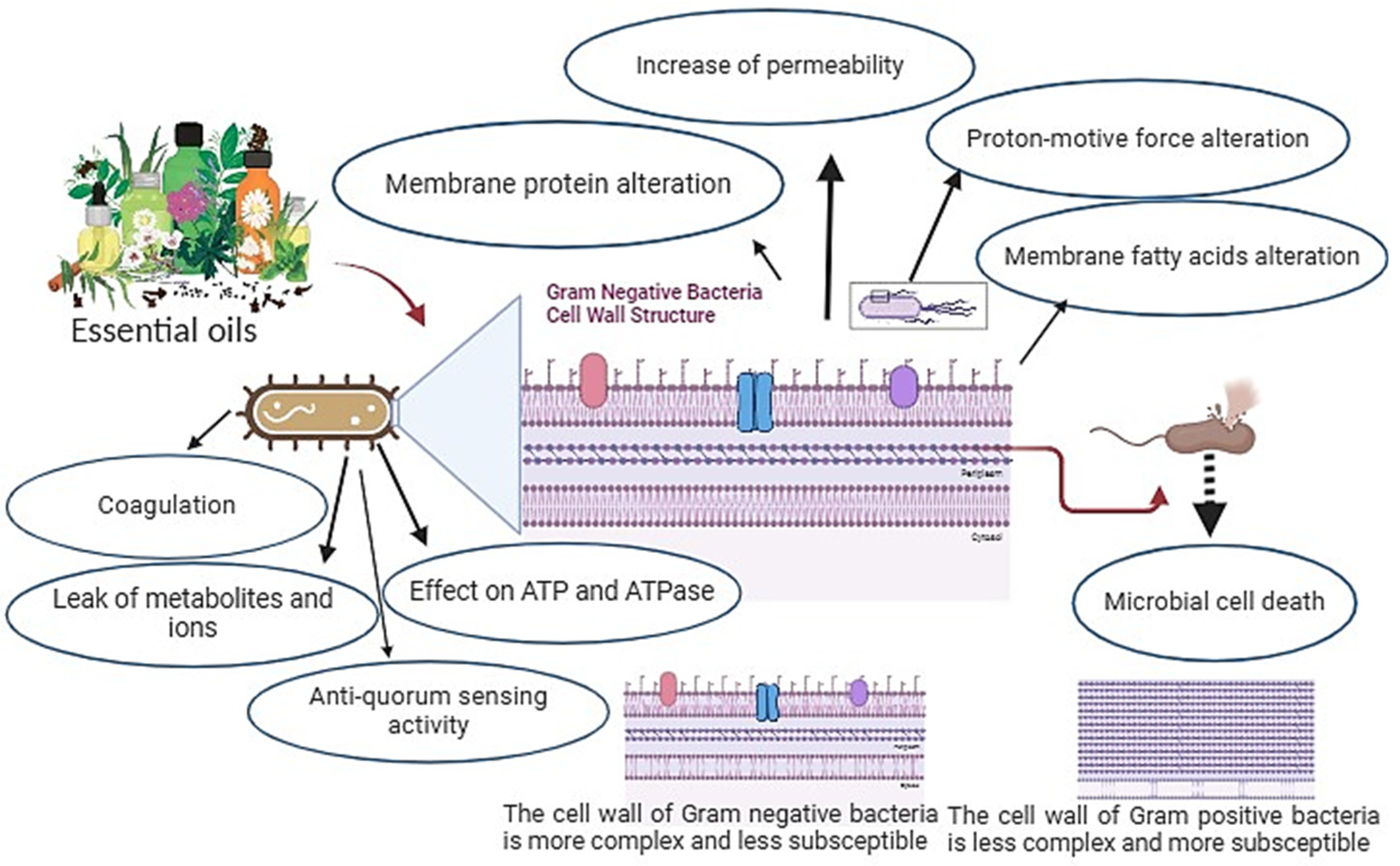
5. Mechanisms to Combat Bacterial Microorganisms
5.1. Mechanisms of Action at the Cellular Level
5.2. Enzyme Inhibition and Modulation
6. EOs vs. Antibiotics
7. Advanced Coating Solutions for Medical Devices
7.1. Challenges in Developing Effective Coatings
7.2. EO Formulations for Coatings
7.3. Various Approaches for Integrating Essential Oils into Coatings
8. Evaluation of EO Coatings
- (i)
- Testing antimicrobial efficacy
- (ii)
- Assessing biocompatibility and cytotoxicity
- (iii)
- Long-term stability and durability studies
9. Applications of Essential Oil Coatings
10. Applications of EO-Based Coatings
10.1. Medical Devices and Implants
10.2. Wound Dressings and Bandages
10.3. Food Packaging and Preservation
11. Future Directions and Challenges
11.1. Potential for Combination Therapies
11.2. Limitations and Potential Drawbacks
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klobucar, K.; Brown, E.D. New potentiators of ineffective antibiotics: Targeting the Gram-negative outer membrane to overcome intrinsic resistance. Curr. Opin. Chem. Biol. 2022, 66, 102099. [Google Scholar] [CrossRef]
- Dutescu, I.A.; Hillier, S.A. Encouraging the development of new antibiotics: Are financial incentives the right way forward? A systematic review and case study. Infect. Drug Resist. 2021, 14, 415–434. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, S.; Tschudin-Sutter, S.; Egli, A.; Osthoff, M. Optimizing antibiotic therapies to reduce the risk of bacterial resistance. Eur. J. Intern. Med. 2022, 99, 7–12. [Google Scholar] [CrossRef]
- Pulingam, T.; Parumasivam, T.; Gazzali, A.M.; Sulaiman, A.M.; Chee, J.Y.; Lakshmanan, M.; Chin, C.F.; Sudesh, K. Antimicrobial resistance: Prevalence, economic burden, mechanisms of resistance and strategies to overcome. Eur. J. Pharm. Sci. 2022, 170, 106103. [Google Scholar] [CrossRef]
- Namivandi-Zangeneh, R.; Wong, E.H.; Boyer, C. Synthetic antimicrobial polymers in combination therapy: Tackling antibiotic resistance. ACS Infect. Dis. 2021, 7, 215–253. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Foderaro, T.; Kramer, L.; Markley, A.L.; Lee, J.; Traylor, M.J.; Fox, J.M. Evolution-guided biosynthesis of terpenoid inhibitors. ACS Synth. Biol. 2022, 11, 3015–3027. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.; Lucia, A. Essential oils and their individual components in cosmetic products. Cosmetics 2021, 8, 114. [Google Scholar] [CrossRef]
- Kaya, D.A.; Ghica, M.V.; Dănilă, E.; Öztürk, Ş.; Türkmen, M.; Albu Kaya, M.G.; Dinu-Pîrvu, C.-E. Selection of optimal operating conditions for extraction of Myrtus Communis L. essential oil by the steam distillation method. Molecules 2020, 25, 2399. [Google Scholar] [CrossRef] [PubMed]
- Kumar Pandey, V.; Shams, R.; Singh, R.; Dar, A.H.; Pandiselvam, R.; Rusu, A.V.; Trif, M. A comprehensive review on clove (Caryophyllus aromaticus L.) essential oil and its significance in the formulation of edible coatings for potential food applications. Front. Nutr. 2022, 9, 987674. [Google Scholar] [CrossRef]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential oils and their major components: An updated review on antimicrobial activities, mechanism of action and their potential application in the food industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.Y.; Cheng, T.H.; Wang, A.H.J. Structure, catalysis, and inhibition mechanism of prenyltransferase. IUBMB Life 2021, 73, 40–63. [Google Scholar] [CrossRef] [PubMed]
- Gayán, E.; Torres, J.A.; Paredes-Sabja, D. Hurdle approach to increase the microbial inactivation by high pressure processing: Effect of essential oils. Food Eng. Rev. 2012, 4, 141–148. [Google Scholar] [CrossRef]
- Oldfield, E.; Lin, F.Y. Terpene biosynthesis: Modularity rules. Angew. Chem. Int. Ed. 2012, 51, 1124–1137. [Google Scholar] [CrossRef] [PubMed]
- Baser, K.H.C.; Buchbauer, G. Handbook of Essential Oils: SCIENCE, Technology, and Applications; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Dembitsky, V.M. Highly Oxygenated Cyclobutane Ring in Biomolecules: Insights into Structure and Activity. Oxygen 2024, 4, 181–235. [Google Scholar] [CrossRef]
- Semitsoglou-Tsiapou, S.; Meador, T.B.; Peng, B.; Aluwihare, L. Photochemical (UV–vis/H2O2) degradation of carotenoids: Kinetics and molecular end products. Chemosphere 2022, 286, 131697. [Google Scholar] [CrossRef] [PubMed]
- Alicandri, E.; Paolacci, A.R.; Osadolor, S.; Sorgonà, A.; Badiani, M.; Ciaffi, M. On the evolution and functional diversity of terpene synthases in the Pinus species: A review. J. Mol. Evol. 2020, 88, 253–283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, X.; Zhang, Y.; Wang, F.; Zhang, C.; Li, X. Development of isopentenyl phosphate kinases and their application in terpenoid biosynthesis. Biotechnol. Adv. 2023, 64, 108124. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Wang, M.; Khan, I.M.; Xu, J.; Peng, C.; Wang, Z. Preparation, characterization, and antibiofilm activity of cinnamic acid conjugated hydroxypropyl chitosan derivatives. Int. J. Biol. Macromol. 2021, 189, 657–667. [Google Scholar] [CrossRef]
- Silva, A.; Silva, V.; Igrejas, G.; Aires, A.; Falco, V.; Valentão, P.; Poeta, P. Phenolic compounds classification and their distribution in winemaking by-products. Eur. Food Res. Technol. 2023, 249, 207–239. [Google Scholar] [CrossRef]
- Marchiosi, R.; dos Santos, W.D.; Constantin, R.P.; de Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Mota, T.R.; de Oliveira, D.M.; Foletto-Felipe, M.d.P.; Abrahão, J. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Bayala, B.; Coulibaly, A.Y.; Djigma, F.W.; Nagalo, B.M.; Baron, S.; Figueredo, G.; Lobaccaro, J.-M.A.; Simpore, J. Chemical composition, antioxidant, anti-inflammatory and antiproliferative activities of the essential oil of Cymbopogon nardus, a plant used in traditional medicine. Biomol. Concepts 2020, 11, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Ortega, L.A.J.; Gonçalves, S.; Heredia, J.B.; Pereira, M.d.L.G.; Romano, A.; Shin, H.-S.; Patra, J.K. Anti-obesogenic effects of plant natural products: A focus on Korean traditional foods. Trends Food Sci. Technol. 2024, 148, 104470. [Google Scholar] [CrossRef]
- Wang, Q.; Mei, J.; Cao, J.; Xie, J. Effects of Melissa officinalis L. essential oil in comparison with anaesthetics on gill tissue damage, liver metabolism and immune parameters in sea bass (Lateolabrax maculatus) during simulated live transport. Biology 2021, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Martínez, F.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef] [PubMed]
- Baptista-Silva, S.; Borges, S.; Ramos, O.L.; Pintado, M.; Sarmento, B. The progress of essential oils as potential therapeutic agents: A review. J. Essent. Oil Res. 2020, 32, 279–295. [Google Scholar] [CrossRef]
- Dupuis, V.; Cerbu, C.; Witkowski, L.; Potarniche, A.-V.; Timar, M.C.; Żychska, M.; Sabliov, C.M. Nanodelivery of essential oils as efficient tools against antimicrobial resistance: A review of the type and physical-chemical properties of the delivery systems and applications. Drug Deliv. 2022, 29, 1007–1024. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Filho, J.G.d.; Duarte, L.G.; Silva, Y.B.; Milan, E.P.; Santos, H.V.; Moura, T.C.; Bandini, V.P.; Vitolano, L.E.S.; Nobre, J.J.; Moreira, C.T. Novel approach for improving papaya fruit storage with carnauba wax nanoemulsion in combination with Syzigium aromaticum and Mentha spicata essential oils. Coatings 2023, 13, 847. [Google Scholar] [CrossRef]
- Dontje, A.E.; Schuiling-Veninga, C.C.; van Hunsel, F.P.; Ekhart, C.; Demirci, F.; Woerdenbag, H.J. The Therapeutic Potential of Essential Oils in Managing Inflammatory Skin Conditions: A Scoping Review. Pharmaceuticals 2024, 17, 571. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, L.; Wang, S.; Gao, Y.; Jin, F. Molecular mechanism of the anti-inflammatory effects of plant essential oils: A systematic review. J. Ethnopharmacol. 2023, 301, 115829. [Google Scholar] [CrossRef]
- Diniz do Nascimento, L.; Barbosa de Moraes, A.A.; Santana da Costa, K.; Pereira Galúcio, J.M.; Taube, P.S.; Leal Costa, C.M.; Neves Cruz, J.; de Aguiar Andrade, E.H.; Guerreiro de Faria, L.J. Bioactive natural compounds and antioxidant activity of essential oils from spice plants: New findings and potential applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef]
- Kong, A.S.-Y.; Maran, S.; Yap, P.S.-X.; Lim, S.-H.E.; Yang, S.-K.; Cheng, W.-H.; Tan, Y.-H.; Lai, K.-S. Anti-and Pro-oxidant properties of essential oils against antimicrobial resistance. Antioxidants 2022, 11, 1819. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Henika, P.R.; Mandrell, R.E. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J. Food Prot. 2002, 65, 1545–1560. [Google Scholar] [CrossRef]
- European Pharmacopoeia. European Directorate for the Quality of Medicines & Healthcare. Available online: https://www.edqm.eu/en/ (accessed on 25 June 2024).
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.A.; Casabianca, H.; El Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.P.; Devkota, H.P.; Nigam, M.; Adetunji, C.O.; Srivastava, N.; Saklani, S.; Shukla, I.; Azmi, L.; Shariati, M.A.; Coutinho, H.D.M. Combination of essential oils in dairy products: A review of their functions and potential benefits. LWT 2020, 133, 110116. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils—Present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef]
- Ni, Z.-J.; Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.-G.; Hu, F.; Wei, Z.-J. Recent updates on the chemistry, bioactivities, mode of action, and industrial applications of plant essential oils. Trends Food Sci. Technol. 2021, 110, 78–89. [Google Scholar] [CrossRef]
- Maurya, A.; Prasad, J.; Das, S.; Dwivedy, A.K. Essential oils and their application in food safety. Front. Sustain. Food Syst. 2021, 5, 653420. [Google Scholar] [CrossRef]
- Stefanakis, M.K.; Touloupakis, E.; Anastasopoulos, E.; Ghanotakis, D.; Katerinopoulos, H.E.; Makridis, P. Antibacterial activity of essential oils from plants of the genus Origanum. Food Control 2013, 34, 539–546. [Google Scholar] [CrossRef]
- Tzora, A.; Giannenas, I.; Karamoutsios, A.; Papaioannou, N.; Papanastasiou, D.; Bonos, E.; Skoufos, S.; Bartzanas, T.; Skoufos, I. Effects of oregano, attapulgite, benzoic acid and their blend on chicken performance, intestinal microbiology and intestinal morphology. J. Poult. Sci. 2017, 54, 218–227. [Google Scholar] [CrossRef]
- Maharaj, S.; McGaw, D. Mathematical model for the removal of essential oil constituents during steam distillation extraction. Processes 2020, 8, 400. [Google Scholar] [CrossRef]
- Machado, C.A.; Oliveira, F.O.; de Andrade, M.A.; Hodel, K.V.S.; Lepikson, H.; Machado, B.A.S. Steam distillation for essential oil extraction: An evaluation of technological advances based on an analysis of patent documents. Sustainability 2022, 14, 7119. [Google Scholar] [CrossRef]
- Kant, R.; Kumar, A. Review on essential oil extraction from aromatic and medicinal plants: Techniques, performance and economic analysis. Sustain. Chem. Pharm. 2022, 30, 100829. [Google Scholar] [CrossRef]
- Mihai, A.D.; Chircov, C.; Grumezescu, A.M.; Holban, A.M. Magnetite nanoparticles and essential oils systems for advanced antibacterial therapies. Int. J. Mol. Sci. 2020, 21, 7355. [Google Scholar] [CrossRef] [PubMed]
- Myers, T.G.; Valverde, A.; Aguareles, M.; Calvo-Schwarzwalder, M.; Font, F. Modelling mass transfer from a packed bed by fluid extraction. Int. J. Heat Mass Transf. 2022, 188, 122562. [Google Scholar] [CrossRef]
- Moradi, S.; Fazlali, A.; Hamedi, H. Microwave-assisted hydro-distillation of essential oil from rosemary: Comparison with traditional distillation. Avicenna J. Med. Biotechnol. 2018, 10, 22. [Google Scholar]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Thilakarathna, R.; Siow, L.F.; Tang, T.-K.; Lee, Y.Y. A review on application of ultrasound and ultrasound assisted technology for seed oil extraction. J. Food Sci. Technol. 2023, 60, 1222–1236. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Prado-Gonjal, J.; Morán, E. Microwave assisted hydrothermal synthesis of nanoparticles. arXiv 2022, arXiv:2203.02394. [Google Scholar]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef]
- Bolouri, P.; Salami, R.; Kouhi, S.; Kordi, M.; Asgari Lajayer, B.; Hadian, J.; Astatkie, T. Applications of essential oils and plant extracts in different industries. Molecules 2022, 27, 8999. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Bassolé, I.H.N.; Juliani, H.R. Essential oils in combination and their antimicrobial properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [PubMed]
- Sikkema, J.; de Bont, J.A.; Poolman, B. Interactions of cyclic hydrocarbons with biological membranes. J. Biol. Chem. 1994, 269, 8022–8028. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, C.W.; Duarte, L.G.; Pedrino, I.C.; Mitsuyuki, M.C.; Junior, S.B.; Ferreira, M.D. Effect of carnauba wax nanoemulsion associated with Syzygium aromaticum and Mentha piperita essential oils as an alternative to extend lychee post-harvest shelf life. Sustain. Food Technol. 2024, 2, 426–436. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (tea tree) oil: A review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Chinemerem Nwobodo, D.; Ugwu, M.C.; Oliseloke Anie, C.; Al-Ouqaili, M.T.; Chinedu Ikem, J.; Victor Chigozie, U.; Saki, M. Antibiotic resistance: The challenges and some emerging strategies for tackling a global menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef]
- Gajdács, M.; Urbán, E.; Stájer, A.; Baráth, Z. Antimicrobial resistance in the context of the sustainable development goals: A brief review. Eur. J. Investig. Health Psychol. Educ. 2021, 11, 71–82. [Google Scholar] [CrossRef]
- Lupia, C.; Castagna, F.; Bava, R.; Naturale, M.D.; Zicarelli, L.; Marrelli, M.; Statti, G.; Tilocca, B.; Roncada, P.; Britti, D. Use of Essential Oils to Counteract the Phenomena of Antimicrobial Resistance in Livestock Species. Antibiotics 2024, 13, 163. [Google Scholar] [CrossRef]
- Van, L.T.; Hagiu, I.; Popovici, A.; Marinescu, F.; Gheorghe, I.; Curutiu, C.; Ditu, L.M.; Holban, A.-M.; Sesan, T.E.; Lazar, V. Antimicrobial Efficiency of Some Essential Oils in Antibiotic-Resistant Pseudomonas aeruginosa Isolates. Plants 2022, 11, 2003. [Google Scholar] [CrossRef] [PubMed]
- Kiymaci, M.; Savluk, M.; GÜMÜŞTAŞ, M.; Uvey, M.; Unal, N. Antibacterial and antibiofilm activity of Melaleuca alternifolia (tea tree) essential oil against colistin resistant Salmonella enterica serotypes isolated from poultry environmental specimens. J. Res. Pharm. 2023, 27, 508–518. [Google Scholar]
- Das, S.; Vishakha, K.; Banerjee, S.; Nag, D.; Ganguli, A. Exploring the antibacterial, antibiofilm, and antivirulence activities of tea tree oil-containing nanoemulsion against carbapenem-resistant Serratia marcescens associated infections. Biofouling 2022, 38, 100–117. [Google Scholar] [CrossRef] [PubMed]
- Boren, K.; Crown, A.; Carlson, R. Multidrug and pan-antibiotic resistance—The role of antimicrobial and synergistic essential oils: A review. Nat. Prod. Commun. 2020, 15, 1934578X20962595. [Google Scholar] [CrossRef]
- Nuță, D.C.; Limban, C.; Chiriță, C.; Chifiriuc, M.C.; Costea, T.; Ioniță, P.; Nicolau, I.; Zarafu, I. Contribution of essential oils to the fight against microbial biofilms—A review. Processes 2021, 9, 537. [Google Scholar] [CrossRef]
- Lambert, R.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Zamuner, C.F.C.; Marin, V.R.; Dilarri, G.; Hypolito, G.B.; Sass, D.C.; Ferreira, H. Oregano essential oil and its main components Thymol and Carvacrol as alternatives to control citrus canker. Front. Agron. 2023, 5, 1148969. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef] [PubMed]
- Sienkiewicz, M.; Łysakowska, M.; Denys, P.; Kowalczyk, E. The antimicrobial activity of thyme essential oil against multidrug resistant clinical bacterial strains. Microb. Drug Resist. 2012, 18, 137–148. [Google Scholar] [CrossRef]
- Unlu, M.; Ergene, E.; Unlu, G.V.; Zeytinoglu, H.S.; Vural, N. Composition, antimicrobial activity and in vitro cytotoxicity of essential oil from Cinnamomum zeylanicum Blume (Lauraceae). Food Chem. Toxicol. 2010, 48, 3274–3280. [Google Scholar] [CrossRef]
- Mulyaningsih, S.; Sporer, F.; Zimmermann, S.; Reichling, J.; Wink, M. Synergistic properties of the terpenoids aromadendrene and 1,8-cineole from the essential oil of Eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomedicine 2010, 17, 1061–1066. [Google Scholar] [CrossRef]
- Hendry, E.; Worthington, T.; Conway, B.R.; Lambert, P. Antimicrobial efficacy of eucalyptus oil and 1, 8-cineole alone and in combination with chlorhexidine digluconate against microorganisms grown in planktonic and biofilm cultures. J. Antimicrob. Chemother. 2009, 64, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Al-Wabel, N.A.; Shams, S.; Ahamad, A.; Khan, S.A.; Anwar, F. Essential oils used in aromatherapy: A systemic review. Asian Pac. J. Trop. Biomed. 2015, 5, 601–611. [Google Scholar] [CrossRef]
- Carrol, D.H.; Chassagne, F.; Dettweiler, M.; Quave, C.L. Antibacterial activity of plant species used for oral health against Porphyromonas gingivalis. PLoS ONE 2020, 15, e0239316. [Google Scholar] [CrossRef] [PubMed]
- Bungau, A.F.; Radu, A.-F.; Bungau, S.G.; Vesa, C.M.; Tit, D.M.; Purza, A.L.; Endres, L.M. Emerging Insights into the Applicability of Essential Oils in the Management of Acne Vulgaris. Molecules 2023, 28, 6395. [Google Scholar] [CrossRef]
- Romero-Montero, A.; Melgoza-Ramírez, L.J.; Ruíz-Aguirre, J.A.; Chávez-Santoscoy, A.; Magaña, J.J.; Cortés, H.; Leyva-Gómez, G.; Del Prado-Audelo, M.L. Essential-Oils-Loaded Biopolymeric Nanoparticles as Strategies for Microbial and Biofilm Control: A Current Status. Int. J. Mol. Sci. 2023, 25, 82. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-L.; Lang, D.-Q.; Wu, C.; Chen, Q.-C.; Lin, S.-X.; Li, X.-Y.; Liu, Q.; Jiang, C.-P.; Shen, C.-Y. Chemical Composition and Antibacterial and Antiulcerative Colitis Activities of Essential Oil from Pruni Semen. J. Agric. Food Chem. 2024, 72, 1096–1113. [Google Scholar] [CrossRef]
- Chen, T.; Kong, Q.; Kuang, X.; Zhou, J.; Wang, H.; Zhou, L.; Yang, H.; Feng, S.; Ding, C. Chemical composition of Litsea pungens essential oil and its potential antioxidant and antimicrobial activities. Molecules 2023, 28, 6835. [Google Scholar] [CrossRef] [PubMed]
- Iacovelli, F.; Romeo, A.; Lattanzio, P.; Ammendola, S.; Battistoni, A.; La Frazia, S.; Vindigni, G.; Unida, V.; Biocca, S.; Gaziano, R. Deciphering the Broad Antimicrobial Activity of Melaleuca alternifolia Tea Tree Oil by Combining Experimental and Computational Investigations. Int. J. Mol. Sci. 2023, 24, 12432. [Google Scholar] [CrossRef]
- Mary Mawumenyo Mamattah, K.; Kusiwaa Adomako, A.; Nketia Mensah, C.; Borquaye, L.S. Chemical Characterization, Antioxidant, Antimicrobial, and Antibiofilm Activities of Essential Oils of Plumeria alba (Forget-Me-Not). Biochem. Res. Int. 2023, 2023, 1040478. [Google Scholar] [CrossRef]
- Giamarellou, H.; Galani, L.; Karavasilis, T.; Ioannidis, K.; Karaiskos, I. Antimicrobial stewardship in the hospital setting: A narrative review. Antibiotics 2023, 12, 1557. [Google Scholar] [CrossRef] [PubMed]
- Al-Mijalli, S.H.; Mrabti, H.N.; El Hachlafi, N.; El Kamili, T.; Elbouzidi, A.; Abdallah, E.M.; Flouchi, R.; Assaggaf, H.; Qasem, A.; Zengin, G. Integrated analysis of antimicrobial, antioxidant, and phytochemical properties of Cinnamomum verum: A comprehensive In vitro and In silico study. Biochem. Syst. Ecol. 2023, 110, 104700. [Google Scholar] [CrossRef]
- Munive Nuñez, K.V.; Abreu, A.C.d.S.; Almeida, J.M.d.; Gonçalves, J.L.; Bonsaglia, É.C.R.; dos Santos, M.V.; Silva, N.C.C. Antimicrobial Activity of Selected Essential Oils against Staphylococcus aureus from Bovine Mastitis. Dairy 2024, 5, 54–65. [Google Scholar] [CrossRef]
- Iseppi, R.; Mariani, M.; Condò, C.; Sabia, C.; Messi, P. Essential oils: A natural weapon against antibiotic-resistant bacteria responsible for nosocomial infections. Antibiotics 2021, 10, 417. [Google Scholar] [CrossRef] [PubMed]
- Sahal, G.; Woerdenbag, H.J.; Hinrichs, W.L.; Visser, A.; Tepper, P.G.; Quax, W.J.; van der Mei, H.C.; Bilkay, I.S. Antifungal and biofilm inhibitory effect of Cymbopogon citratus (lemongrass) essential oil on biofilm forming by Candida tropicalis isolates; an in vitro study. J. Ethnopharmacol. 2020, 246, 112188. [Google Scholar] [CrossRef] [PubMed]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Effects of Melaleuca alternifolia (tea tree) essential oil and the major monoterpene component terpinen-4-ol on the development of single-and multistep antibiotic resistance and antimicrobial susceptibility. Antimicrob. Agents Chemother. 2012, 56, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Nova, B.G.V.; dos Santos Silva, L.; da Silva Andrade, M.; de Santana, A.V.S.; da Silva, L.C.T.; Sá, G.C.; Zafred, I.F.; de Aguiar Moreira, P.H.; Monteiro, C.A.; da Silva, L.C.N. The essential oil of Melaleuca alternifolia incorporated into hydrogel induces antimicrobial and anti-inflammatory effects on infected wounds by Staphylococcus aureus. Biomed. Pharmacother. 2024, 173, 116389. [Google Scholar]
- Tomičić, Z.; Tomičić, R.; Kocić Tanackov, S.; Raspor, P. Essential Oils as Antimicrobial and Anti-Adhesion Agents against Bacteria Salmonella Typhimurium and Staphylococcus Aureus, and Yeasts Candida Albicans and Saccharomyces Cerevisiae. 2022. Available online: http://oa.fins.uns.ac.rs/handle/123456789/198 (accessed on 25 June 2024).
- Rossi, C.; Chaves-López, C.; Serio, A.; Casaccia, M.; Maggio, F.; Paparella, A. Effectiveness and mechanisms of essential oils for biofilm control on food-contact surfaces: An updated review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2172–2191. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.F.; Balbinot Filho, C.A.; Borges, C.D. Essential oils as natural antimicrobials for application in edible coatings for minimally processed apple and melon: A review on antimicrobial activity and characteristics of food models. Food Packag. Shelf Life 2022, 31, 100781. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, H.; Rhim, J.-W.; Cao, J.; Jiang, W. Effective strategies of sustained release and retention enhancement of essential oils in active food packaging films/coatings. Food Chem. 2022, 367, 130671. [Google Scholar] [CrossRef]
- Negut, I.; Bita, B.; Groza, A. Polymeric Coatings and Antimicrobial Peptides as Efficient Systems for Treating Implantable Medical Devices Associated-Infections. Polymers 2022, 14, 1611. [Google Scholar] [CrossRef] [PubMed]
- Zikeli, F.; Jusic, J.; Palocci, C.; Mugnozza, G.S.; Romagnoli, M. Spray Coating of Wood with Nanoparticles from Lignin and Polylactic Glycolic Acid Loaded with Thyme Essential Oils. Polymers 2024, 16, 947. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Xu, P.; Xiang, H.; Wen, M.; Ye, X.; Chu, C.; Tong, S. Comprehensive two-dimensional countercurrent chromatography× gas chromatography characterization of Artemisia argyi essential oil. Anal. Chim. Acta 2023, 1237, 340614. [Google Scholar] [CrossRef] [PubMed]
- Kazlauskaite, J.A.; Matulyte, I.; Marksa, M.; Lelesius, R.; Pavilonis, A.; Bernatoniene, J. Application of Antiviral, Antioxidant and Antibacterial Glycyrrhiza glabra L., Trifolium pratense L. Extracts and Myristica fragrans Houtt. Essential Oil in Microcapsules. Pharmaceutics 2023, 15, 464. [Google Scholar] [CrossRef] [PubMed]
- Colovic, G. Original Brand Name Manufacturing. J. Text. Sci. Eng. 2016, 6, 260. [Google Scholar] [CrossRef]
- Wang, X.; Ben Ahmed, N.; Alvarez, G.S.; Tuttolomondo, M.V.; Hélary, C.; Desimone, M.F.; Coradin, T. Sol-gel encapsulation of biomolecules and cells for medicinal applications. Curr. Top. Med. Chem. 2015, 15, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Loh, X.J. Layer-by-layer assemblies for antibacterial applications. Biomater. Sci. 2015, 3, 1505–1518. [Google Scholar] [CrossRef] [PubMed]
- Motelica, L.; Ficai, D.; Oprea, O.-C.; Ficai, A.; Ene, V.-L.; Vasile, B.-S.; Andronescu, E.; Holban, A.-M. Antibacterial biodegradable films based on alginate with silver nanoparticles and lemongrass essential oil–innovative packaging for cheese. Nanomaterials 2021, 11, 2377. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols–a review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A fascinating method for the preparation of ultrathin fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef]
- Hidouri, S.; Jafari, R.; Momen, G. Development of a polydimethylsiloxane–Eucalyptus essential oil antibacterial coating. J. Coat. Technol. Res. 2024, 21, 747–760. [Google Scholar] [CrossRef]
- Li, Y.; Tang, C.; He, Q. Effect of orange (Citrus sinensis L.) peel essential oil on characteristics of blend films based on chitosan and fish skin gelatin. Food Biosci. 2021, 41, 100927. [Google Scholar] [CrossRef]
- Li, X.-L.; Shen, Y.; Hu, F.; Zhang, X.-X.; Thakur, K.; Rengasamy, K.R.; Khan, M.R.; Busquets, R.; Wei, Z.-J. Fortification of polysaccharide-based packaging films and coatings with essential oils: A review of their preparation and use in meat preservation. Int. J. Biol. Macromol. 2023, 242, 124767. [Google Scholar] [CrossRef]
- Özbek, H.; Öztürk, M.; Öztürk, A.; Ceylan, E.; Yener, Z. Determination of lethal doses of volatile and fixed oils of several plants. East. J. Med. 2004, 9, 4–6. [Google Scholar]
- Raymond, F.; Souleymanou, A.; Jacqueline, N.O.T.; Damien, E.; Henri, A.Z.P.; Fabrice, F.B.; Kumar, S. Antifungal potential of lemongrass essential oil as affected by plant age and Arbuscular Mycorrizal fungi inoculation. J. Biotechnol. Biochem. 2020, 6, 9–16. [Google Scholar]
- Rajkowska, K.; Kunicka-Styczyńska, A.; Maroszyńska, M.; Dąbrowska, M. The effect of thyme and tea tree oils on morphology and metabolism of Candida albicans. Acta Biochim. Pol. 2014, 61, 305–310. [Google Scholar] [CrossRef]
- Yan, C.; Kim, S.-R.; Ruiz, D.R.; Farmer, J.R. Microencapsulation for food applications: A review. ACS Appl. Bio Mater. 2022, 5, 5497–5512. [Google Scholar] [CrossRef]
- Zhang, X.; Ismail, B.B.; Cheng, H.; Jin, T.Z.; Qian, M.; Arabi, S.A.; Liu, D.; Guo, M. Emerging chitosan-essential oil films and coatings for food preservation-A review of advances and applications. Carbohydr. Polym. 2021, 273, 118616. [Google Scholar] [CrossRef]
- EN1040; Chemical Disinfectants and Antiseptics—Quantitative Suspension Test for the Evaluation of Basic Bactericidal Activity of Chemical Disinfectants and Antiseptics—Test Method and Requirements (Phase 1). European Committee for Standardization: Brussels, Belgium, 2005.
- JIS Z 2801; Antimicrobial Products—Test for Antimicrobial Activity and Efficacy. Japanese Standards Association: Tokyo, Japan, 2000.
- Govindan, R.; Chackaravarthi, G.; Ramachandran, G.; Chelliah, C.K.; Muthuchamy, M.; Quero, F.; Mothana, R.A.; Noman, O.M.; Siddiqui, N.A.; Li, W.-J. Effective removal of biofilm formation in Acinetobacter baumannii using chitosan nanoparticles loaded plant essential oils. J. King Saud Univ.-Sci. 2022, 34, 101845. [Google Scholar] [CrossRef]
- French, G. Bactericidal agents in the treatment of MRSA infections—The potential role of daptomycin. J. Antimicrob. Chemother. 2006, 58, 1107–1117. [Google Scholar] [CrossRef]
- Sakoulas, G.; Moellering Jr, R.C.; Eliopoulos, G.M. Adaptation of methicillin-resistant Staphylococcus aureus in the face of vancomycin therapy. Clin. Infect. Dis. 2006, 42, S40–S50. [Google Scholar] [CrossRef] [PubMed]
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for cell viability assays. Food Front. 2020, 1, 332–349. [Google Scholar] [CrossRef]
- Goel, N.; Hashmi, Z.; Khan, N.; Ahmad, R.; Khan, W.H. Recent Strategies to Combat Multidrug Resistance. In Non-Traditional Approaches to Combat Antimicrobial Drug Resistance; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–27. [Google Scholar]
- Anyanwu, M.U.; Nwobi, O.C.; Okpala, C.O.R.; Ezeonu, I.M. Mobile tigecycline resistance: An emerging health catastrophe requiring urgent one health global intervention. Front. Microbiol. 2022, 13, 808744. [Google Scholar] [CrossRef] [PubMed]
- Ugurel, E.; Turgut-Balik, D. Synergistic combination of carvedilol, amlodipine, amitriptyline, and antibiotics as an alternative treatment approach for the susceptible and multidrug-resistant A. baumannii infections via drug repurposing. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Chivandi, E.; Dangarembizi, R.; Nyakudya, T.T.; Erlwanger, K.H. Use of essential oils as a preservative of meat. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier: Amsterdam, The Netherlands, 2016; pp. 85–91. [Google Scholar]
- Mann, R.; Holmes, A.; McNeilly, O.; Cavaliere, R.; Sotiriou, G.A.; Rice, S.A.; Gunawan, C. Evolution of biofilm-forming pathogenic bacteria in the presence of nanoparticles and antibiotic: Adaptation phenomena and cross-resistance. J. Nanobiotechnol. 2021, 19, 291. [Google Scholar] [CrossRef] [PubMed]
- Pourkhosravani, E.; Dehghan Nayeri, F.; Mohammadi Bazargani, M. Decoding antibacterial and antibiofilm properties of cinnamon and cardamom essential oils: A combined molecular docking and experimental study. AMB Express 2021, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Ricciardelli, A.; Casillo, A.; Corsaro, M.M.; Tutino, M.L.; Parrilli, E.; van der Mei, H.C. Pentadecanal and pentadecanoic acid coatings reduce biofilm formation of Staphylococcus epidermidis on PDMS. Pathog. Dis. 2020, 78, ftaa012. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, V.A.; Melo, R.S.; Pereira, A.M.G.; Azevedo, Á.M.A.; Matos, M.N.C.; Cavalcante, R.M.B.; Rocha, R.R.; de Queiroz Albuquerque, V.; Guerrero, J.A.P.; Junior, F.E.A.C. Essential oils as an innovative approach against biofilm of multidrug-resistant Staphylococcus aureus. In Bacterial Biofilms; IntechOpen: London, UK, 2020; p. 20. [Google Scholar]
- Puiu, R.A.; Bîrcă, A.C.; Grumezescu, V.; Duta, L.; Oprea, O.C.; Holban, A.M.; Hudiță, A.; Gălățeanu, B.; Balaure, P.C.; Grumezescu, A.M. Multifunctional Polymeric Biodegradable and Biocompatible Coatings Based on Silver Nanoparticles: A Comparative In Vitro Study on Their Cytotoxicity towards Cancer and Normal Cell Lines of Cytostatic Drugs versus Essential-Oil-Loaded Nanoparticles and on Their Antimicrobial and Antibiofilm Activities. Pharmaceutics 2023, 15, 1882. [Google Scholar] [CrossRef]
- Chircov, C.; Miclea, I.I.; Grumezescu, V.; Grumezescu, A.M. Essential oils for bone repair and regeneration—Mechanisms and applications. Materials 2021, 14, 1867. [Google Scholar] [CrossRef]
- Gherasim, O.; Popescu, R.C.; Grumezescu, V.; Mogoșanu, G.D.; Mogoantă, L.; Iordache, F.; Holban, A.M.; Vasile, B.Ș.; Bîrcă, A.C.; Oprea, O.-C. MAPLE coatings embedded with essential oil-conjugated magnetite for anti-biofilm applications. Materials 2021, 14, 1612. [Google Scholar] [CrossRef]
- Anghel, A.G.; Grumezescu, A.M.; Chirea, M.; Grumezescu, V.; Socol, G.; Iordache, F.; Oprea, A.E.; Anghel, I.; Holban, A.M. MAPLE fabricated Fe3O4@ Cinnamomum verum antimicrobial surfaces for improved gastrostomy tubes. Molecules 2014, 19, 8981–8994. [Google Scholar] [CrossRef]
- Grumezescu, A.M.; Chifiriuc, M.C.; Saviuc, C.; Grumezescu, V.; Hristu, R.; Mihaiescu, D.E.; Stanciu, G.A.; Andronescu, E. Hybrid nanomaterial for stabilizing the antibiofilm activity of Eugenia carryophyllata essential oil. IEEE Trans. Nanobiosci. 2012, 11, 360–365. [Google Scholar] [CrossRef]
- Chifiriuc, C.; Grumezescu, V.; Grumezescu, A.M.; Saviuc, C.; Lazăr, V.; Andronescu, E. Hybrid magnetite nanoparticles/Rosmarinus officinalis essential oil nanobiosystem with antibiofilm activity. Nanoscale Res. Lett. 2012, 7, 209. [Google Scholar] [CrossRef]
- Kus, K.J.; Ruiz, E.S. Wound dressings–a practical review. Curr. Dermatol. Rep. 2020, 9, 298–308. [Google Scholar] [CrossRef]
- Smith, R.; Russo, J.; Fiegel, J.; Brogden, N. Antibiotic delivery strategies to treat skin infections when innate antimicrobial defense fails. Antibiotics 2020, 9, 56. [Google Scholar] [CrossRef]
- Stoica, A.E.; Chircov, C.; Grumezescu, A.M. Nanomaterials for wound dressings: An up-to-date overview. Molecules 2020, 25, 2699. [Google Scholar] [CrossRef]
- Zhang, W.; Goksen, G.; Zhou, Y.; Yang, J.; Khan, M.R.; Ahmad, N.; Fei, T. Application of a Chitosan–Cinnamon Essential Oil Composite Coating in Inhibiting Postharvest Apple Diseases. Foods 2023, 12, 3518. [Google Scholar] [CrossRef]
- Huang, X.; Lao, Y.; Pan, Y.; Chen, Y.; Zhao, H.; Gong, L.; Xie, N.; Mo, C.-H. Synergistic antimicrobial effectiveness of plant essential oil and its application in seafood preservation: A review. Molecules 2021, 26, 307. [Google Scholar] [CrossRef]
- Larsson, D.; Flach, C.-F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef]
- Langeveld, W.T.; Veldhuizen, E.J.; Burt, S.A. Synergy between essential oil components and antibiotics: A review. Crit. Rev. Microbiol. 2014, 40, 76–94. [Google Scholar] [CrossRef]
- Owen, L.; Laird, K. Synchronous application of antibiotics and essential oils: Dual mechanisms of action as a potential solution to antibiotic resistance. Crit. Rev. Microbiol. 2018, 44, 414–435. [Google Scholar] [CrossRef] [PubMed]
- Iseppi, R.; Condò, C.; Messi, P. Synergistic Inhibition of Methicillin-Resistant Staphylococcus aureus (MRSA) by Melaleuca alternifolia Chell (Tea Tree) and Eucalyptus globulus Labill. Essential Oils in Association with Oxacillin. Antibiotics 2023, 12, 846. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.; Fatima, J.; Shakil, S.; Rizvi, S.M.D.; Kamal, M.A. Antibiotic resistance and extended spectrum beta-lactamases: Types, epidemiology and treatment. Saudi J. Biol. Sci. 2015, 22, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Valente, J.; Zuzarte, M.; Gonçalves, M.; Lopes, M.; Cavaleiro, C.; Salgueiro, L.; Cruz, M. Antifungal, antioxidant and anti-inflammatory activities of Oenanthe crocata L. essential oil. Food Chem. Toxicol. 2013, 62, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.F.; Mee, B.J.; Riley, T.V. Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrob. Agents Chemother. 2002, 46, 1914–1920. [Google Scholar] [CrossRef] [PubMed]
- Barbarossa, A.; Sblano, S.; Rosato, A.; Carrieri, A.; Corbo, F.; Clodoveo, M.L.; Fracchiolla, G.; Carocci, A. Synergistic action of Cinnamomum verum essential oil with sertraline. Antibiotics 2022, 11, 1617. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hamid, M.I.; El-Tarabili, R.M.; Bahnass, M.M.; Alshahrani, M.A.; Saif, A.; Alwutayd, K.M.; Safhi, F.A.; Mansour, A.T.; Alblwi, N.A.N.; Ghoneim, M.M. Partnering essential oils with antibiotics: Proven therapies against bovine Staphylococcus aureus mastitis. Front. Cell. Infect. Microbiol. 2023, 13, 1265027. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.-H.; He, H.-L.; Wu, S.-B.; Dong, C.-L.; Lu, S.-Y.; Shan, T.-J.; Fang, L.-X.; Liao, X.-P.; Liu, Y.-H.; Sun, J. Rapid screening of essential oils as substances which enhance antibiotic activity using a modified well diffusion method. Antibiotics 2021, 10, 463. [Google Scholar] [CrossRef] [PubMed]
- Tisserand, R.; Young, R. Essential Oil Safety: A Guide for Health Care Professionals; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Schnaubelt, K. The Healing Intelligence of Essential Oils: The Science of Advanced Aromatherapy; Simon and Schuster: New York, NY, USA, 2011. [Google Scholar]
- Bhardwaj, S.; Rashmi; Parcha, V. Effect of seasonal variation on chemical composition and physicochemical properties of Hedychium spicatum rhizomes essential oil. J. Essent. Oil Bear. Plants 2019, 22, 1593–1600. [Google Scholar] [CrossRef]
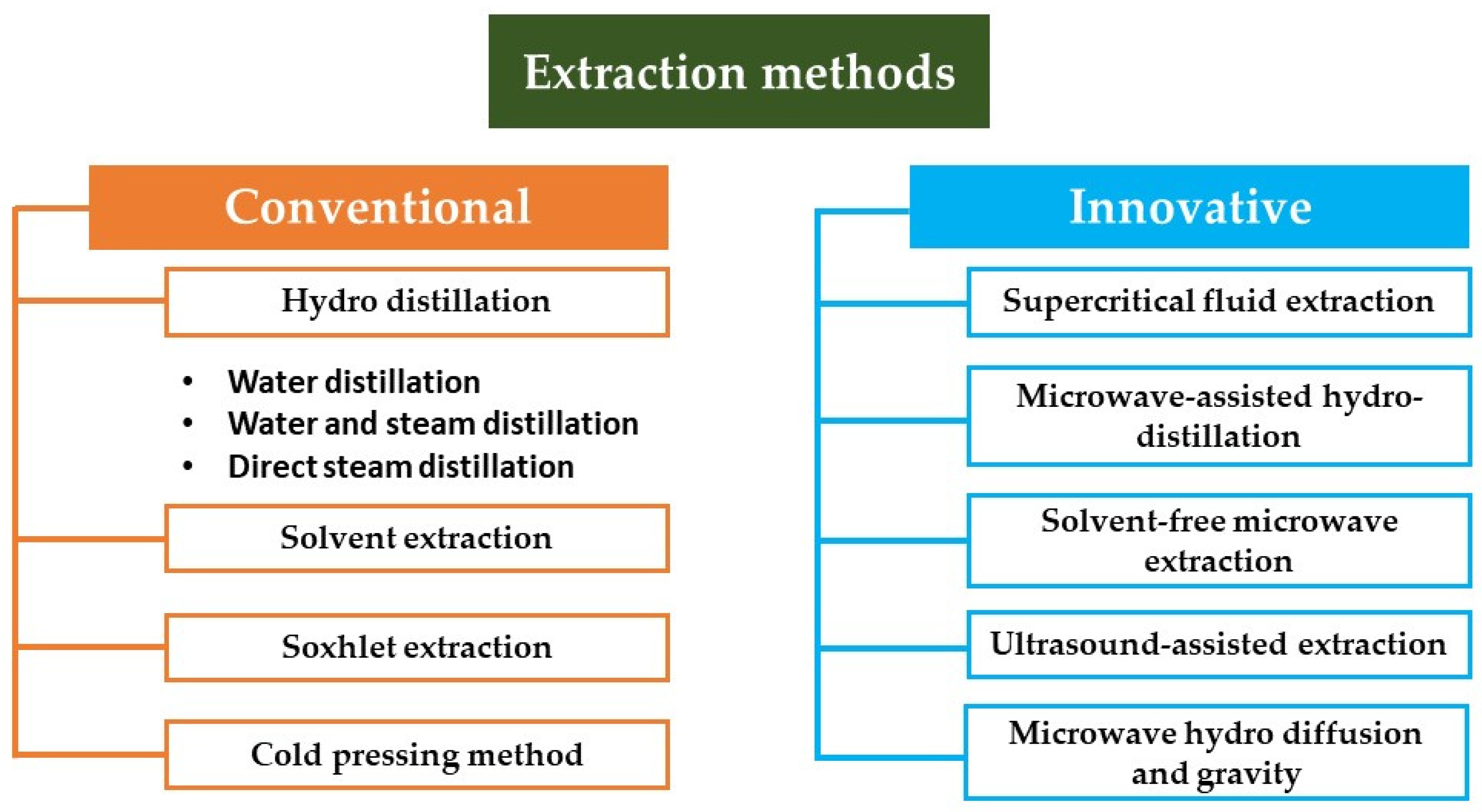

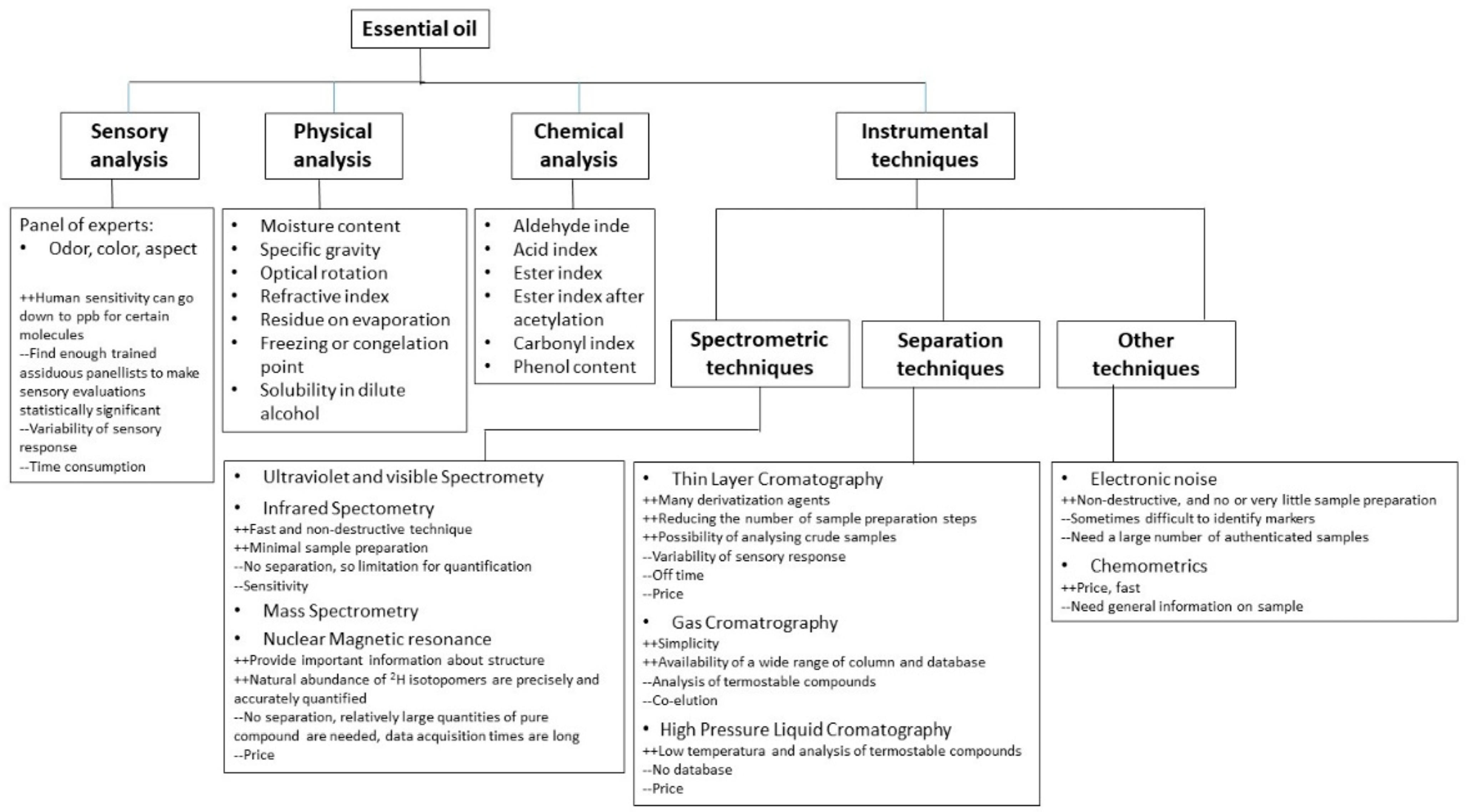
| Extraction Method | Principle | Advantages | Disadvantages | Ref. | ||
|---|---|---|---|---|---|---|
| Conventional | Hydro distillation | Water distillation | The plant material is fully submerged in water that is then brought to a boil. It is crucial to continuously stir the plant material during the boiling process to prevent clumps of denser material from settling at the bottom of the container, where they can thermally degrade. | - Allows for the processing of finely powdered plant material or parts which, if exposed to live steam, would otherwise clump together, creating barriers that prevent steam penetration | - Complete extraction is not possible | [43] |
| Water and steam distillation | The steam can be generated either in a satellite boiler or within the still, although separated from the plant material. The used equipment is similar to that used in the water distillation technique, but the plant material is placed above the boiling water on a perforated grid | - High oil production - More energy efficient than water distillation, as it is a faster process. | - Because of the low pressure of the steam, oils with a high boiling range necessitate a greater steam quantity, therefore requiring many distillation hours. | [8] | ||
| Direct steam distillation | The plant material is distilled with steam generated outside the still, in a satellite steam generator (boiler). | - The amount of steam can be controlled - The plant material is not boiled above 100 °C, therefore avoiding thermal degradation - Large-scale oil production | This method requires more capital expenditure than the other two processes. | [44] | ||
| Solvent extraction | Uses solvents such as hexane or ethanol to dissolve essential oils from plant materials. The solution is then evaporated to leave behind the oils. | - Efficient for extracting oils from delicate materials that cannot withstand heat | - Possible solvent residue in the oils, which may require further purification - Use of chemicals makes it less eco-friendly - Long extraction time and high solvent consumption | [45] | ||
| Soxhlet extraction | - Uses a Soxhlet extractor where the solvent continuously cycles through the plant material to extract oils. | - Can extract oils completely - Good for small quantities of material - Efficient and thorough extraction | - Requires significant amounts of solvent -Time-consuming - Risk of thermal degradation of oils | [9] | ||
| Cold pressing | Also known as expression or mechanical extraction, this method is primarily used for citrus essential oils such as orange, lemon, and lime. The rind of the fruit is mechanically pressed to release the oil. | - No heat is involved, which preserves the integrity of the oil. - Simpler and more natural process | - Limited to materials that easily release their oils (e.g., citrus peels) - Lower yields compared to methods that use heat or solvents | [46] | ||
| Innovative | Supercritical fluid extraction | Uses supercritical fluids, such as CO2, as solvents under high pressure and temperature to extract essential oils. The fluid penetrates the material and dissolves the volatile compounds, which are then collected after the pressure is reduced. | - No solvent residue - High purity and quality of oils - Efficient extraction of delicate compounds | - High initial cost of equipment - Requires precise control of temperature and pressure | [47] | |
| Microwave-assisted hydro distillation | Combines microwave heating with traditional hydro distillation. The microwave energy heats the water and plant material rapidly to release essential oils. | - Faster than traditional distillation - Reduced energy consumption | - Potential degradation of heat-sensitive compounds - Scale-up can be challenging | [48] | ||
| Solvent-free microwave extraction | Utilizes microwave energy to directly heat the plant material without the use of water or any solvents, causing the cell walls to rupture and release essential oils. | - Low solvent use - Faster process than conventional distillation - Reduced thermal degradation of compounds | - Relatively new technique with limited data on large-scale application - Equipment and process control can be complex | [49] | ||
| Ultrasound-assisted extraction | Uses ultrasonic waves to create micro-bubbles in a liquid medium. These bubbles collapse and create intense local pressure, disrupting cell walls and releasing essential oils. | - Low temperature process - Can be combined with other methods - Energy efficient | - May require longer extraction times - Scale-up requires careful optimization | [50] | ||
| Microwave hydro diffusion and gravity | Applies microwave energy to vaporize water within the plant material. The vapor rises, capturing essential oils, and then condenses due to gravity in a collector. | - Low solvent use Faster process than conventional distillation - Reduced thermal degradation of compounds | - Relatively new technique with limited data on large-scale application - Equipment and process control can be complex | [51] | ||
| EOs | Antibiotics | |
|---|---|---|
| Mechanism of action | Typically contain multiple bioactive compounds that disrupt bacterial cell membranes, inhibit enzymes, and interfere with pathogen metabolism. For example, thymol and carvacrol, found in thyme oil, are known to damage cell membranes and inhibit bacterial growth [54]. | Usually target specific bacterial functions or structures, such as cell wall synthesis (e.g., beta-lactams), protein synthesis (e.g., tetracyclines), DNA replication (e.g., fluoroquinolones), or metabolic pathways (e.g., sulfonamides) [68]. |
| Spectrum of activity | Often have broad-spectrum activity due to their multi-compound nature. For example, tea tree oil has been shown to be effective against a wide range of bacteria, including Gram-positive and Gram-negative strains [69]. | Can be broad-spectrum (e.g., tetracyclines) or narrow-spectrum (e.g., penicillin). Broad-spectrum antibiotics can target a wide range of bacteria, while narrow-spectrum antibiotics are effective against specific types [70]. |
| Resistance development | The complex mixture of compounds in EOs makes it more challenging for bacteria to develop resistance. Additionally, EOs can disrupt bacterial communication (quorum sensing), which is essential for resistance mechanisms [71]. | Overuse and misuse of antibiotics have led to widespread antibiotic resistance. Bacteria can develop resistance through various mechanisms, such as producing enzymes that degrade the antibiotic (e.g., beta-lactamases) or altering the antibiotic’s target site [54]. |
| Safety and side effects | Generally regarded as safe when used appropriately, but can cause allergic reactions and skin irritation in some individuals. High concentrations or improper use can lead to toxicity [71]. | While effective, they can cause side effects such as gastrointestinal disturbances, allergic reactions, and, in some cases, more severe adverse effects such as organ toxicity. Long-term use can disrupt the natural microbiota, leading to secondary infections such as Clostridium difficile colitis [71]. |
| Application and use | Used in various forms, such as topical applications, inhalation, or incorporated into products such as creams and lotions. Their versatility makes them suitable for both therapeutic and preventive measures [69]. | Administered orally, intravenously, or topically, depending on the type and severity of infection. Their use is strictly regulated and requires medical supervision to prevent misuse and resistance development [54]. |
| Studied Essential Oil vs. Antibiotic | Key Finding | Reference |
|---|---|---|
| Lavender oil vs. amoxicillin in treating bacterial infections | Lavender oil showed significant antibacterial activity, comparable to Amoxicillin in certain bacterial strains. This study evaluates the antimicrobial activity of lavender oil, particularly against Staphylococcus aureus, showcasing its potential as a complementary agent to traditional antibiotics. | [71] |
| Comparative study of cinnamon oil and ciprofloxacin | Cinnamon oil exhibited potent antibacterial effects, sometimes exceeding those of Ciprofloxacin, especially against E. coli. Focusing on the antimicrobial activity of cinnamon oil, this study highlights its efficacy against a spectrum of pathogens, including comparisons with traditional antibiotics. | [72] |
| Tea tree oil’s effectiveness vs. methicillin against MRSA infections | Tea tree oil was highly effective against MRSA strains, offering a viable alternative when conventional antibiotics such as Methicillin failed. This paper discusses the synergistic effects of tea tree oil when used alongside conventional antibiotics to combat MRSA infections, illustrating the oil’s potential in overcoming antibiotic resistance. | [73] |
| Eucalyptus oil versus vancomycin on Staphylococcus aureus | Eucalyptus oil showed potential as an antibiotic alternative, with strong antibacterial properties comparable to Vancomycin. Providing insight into the antibacterial properties of eucalyptus oil, particularly against MRSA and E. coli, this study supports the potential use of eucalyptus oil as an antibiotic alternative. | [74] |
| The role of peppermint oil vs. clarithromycin in treating Helicobacter pylori infections | Peppermint oil demonstrated a promising inhibitory effect on H. pylori, suggesting a potential role in treatment modalities. While focused broadly on the use of essential oils in aromatherapy, this review mentions the antimicrobial effects of peppermint oil, including potential applications against H. pylori. | [75] |
| Approach | Essential Oils Used | Application Surface | Integration Method | Key Benefits |
|---|---|---|---|---|
| Microencapsulation [98] | Lavender, Tea Tree | Textiles, Medical Devices | Encapsulation of essential oil in a polymer matrix | Controlled release, Enhanced durability, Protection of volatile compounds |
| Sol-Gel Process [99] | Thyme, Cinnamon | Glass, Ceramics | Dispersion of essential oils in a sol-gel to form a thin film | Antimicrobial properties, Chemical stability, Transparency |
| Layer-by-Layer (LbL) Assembly [100] | Peppermint, Eucalyptus | Fabrics, Packaging | Sequential deposition of polymers and essential oils | Customizable release profiles, Thin coating, Versatile application |
| Nanocomposite Coatings [101] | Citronella, Lemongrass | Outdoor Equipment, Walls | Incorporation into nanoscale fillers distributed in a polymer matrix | Improved mechanical properties, Slow release of essential oils, Mosquito repellent |
| Plasma Polymerization [102] | Geranium | Resistance to antibiotics | Plasma-induced grafting of essential oil molecules onto surfaces | Durable bonding, Uniform coating, Suitable for sensitive materials |
| Electrospraying/Electrospinning [103] | Clove, Oregano | Filtration Systems, Wound Dressings | Formation of fibers or particles loaded with essential oils via electrostatic forces | High surface area, Efficient use of essential oils, Applicable to complex shapes |
| Dip Coating [104] | Eucalyptus, Lavender | Food Packaging, Cutlery | Immersion of the object into an essential oil-infused solution | Antimicrobial properties, Simple implementation, Biodegradability |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Visan, A.I.; Negut, I. Coatings Based on Essential Oils for Combating Antibiotic Resistance. Antibiotics 2024, 13, 625. https://doi.org/10.3390/antibiotics13070625
Visan AI, Negut I. Coatings Based on Essential Oils for Combating Antibiotic Resistance. Antibiotics. 2024; 13(7):625. https://doi.org/10.3390/antibiotics13070625
Chicago/Turabian StyleVisan, Anita Ioana, and Irina Negut. 2024. "Coatings Based on Essential Oils for Combating Antibiotic Resistance" Antibiotics 13, no. 7: 625. https://doi.org/10.3390/antibiotics13070625
APA StyleVisan, A. I., & Negut, I. (2024). Coatings Based on Essential Oils for Combating Antibiotic Resistance. Antibiotics, 13(7), 625. https://doi.org/10.3390/antibiotics13070625







