Screening and Evaluation of Potential Efflux Pump Inhibitors with a Seaweed Compound Diphenylmethane-Scaffold against Drug-Resistant Escherichia coli
Abstract
1. Introduction
2. Results and Discussion
2.1. Molecular Docking
2.2. IC50 Assay for Antibiotics and DPE, and DPT and BPA against Drug-Resistant E. coli
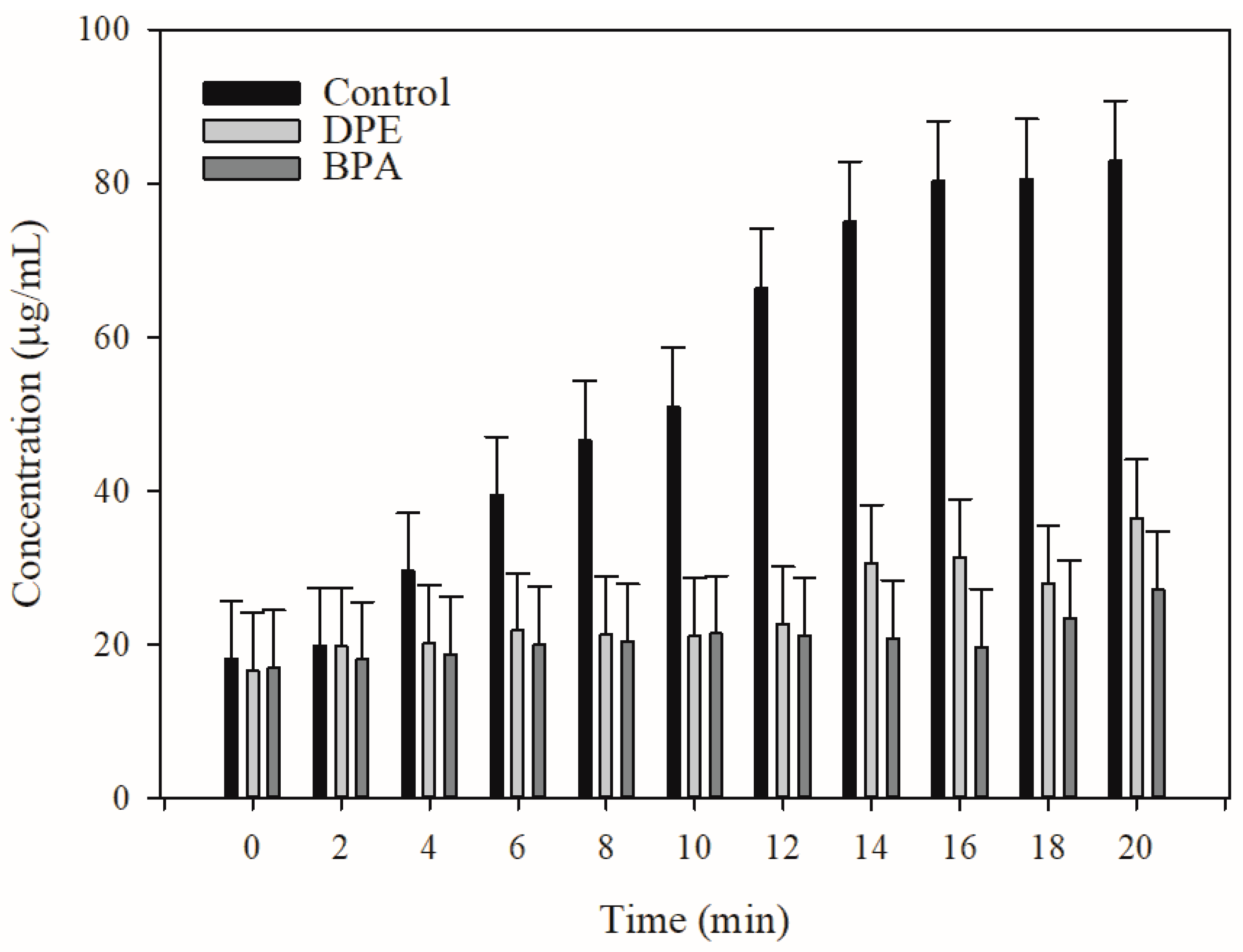
2.3. Pump Efflux Efficiency Reduced by DPE and BPA
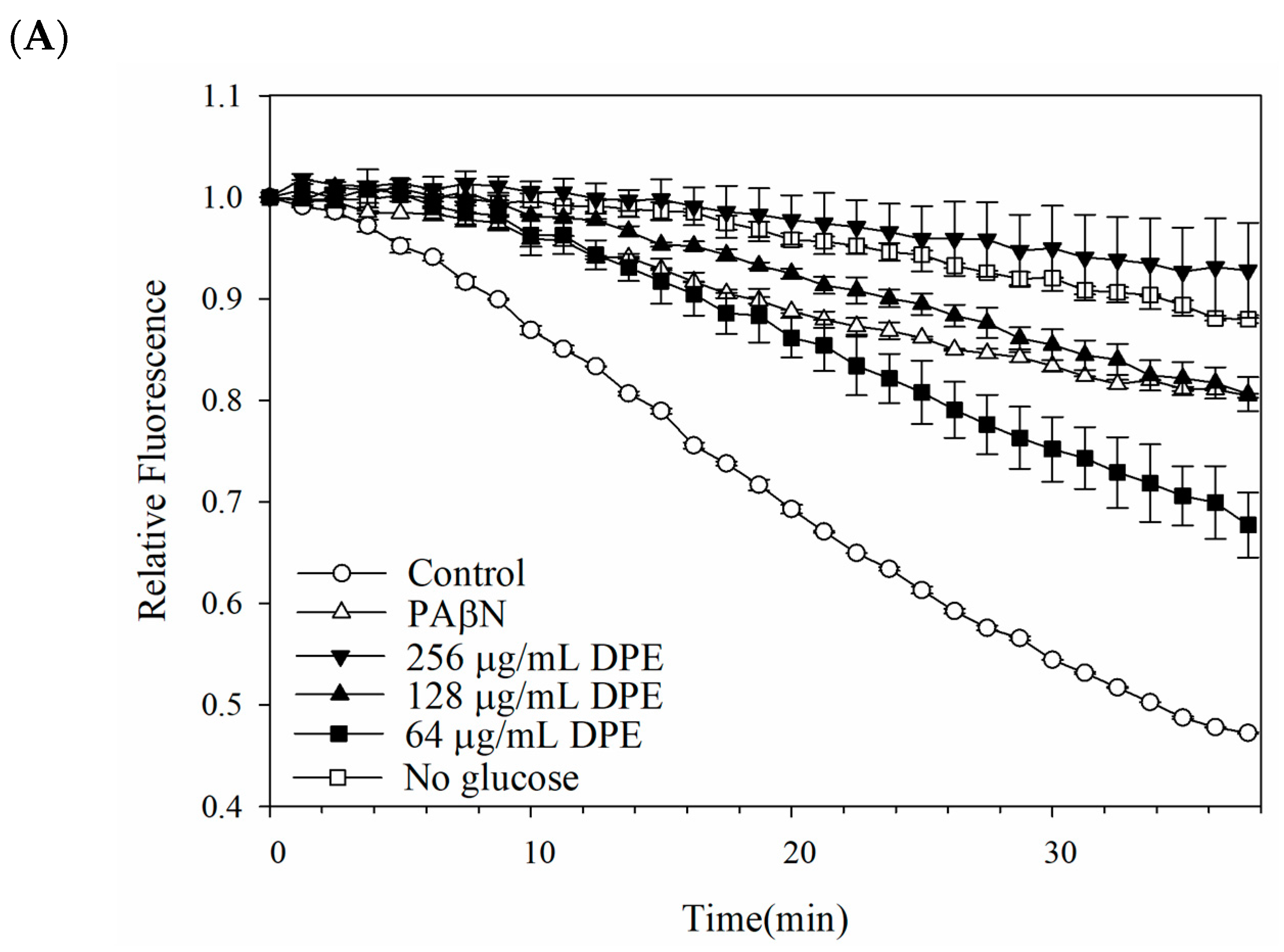
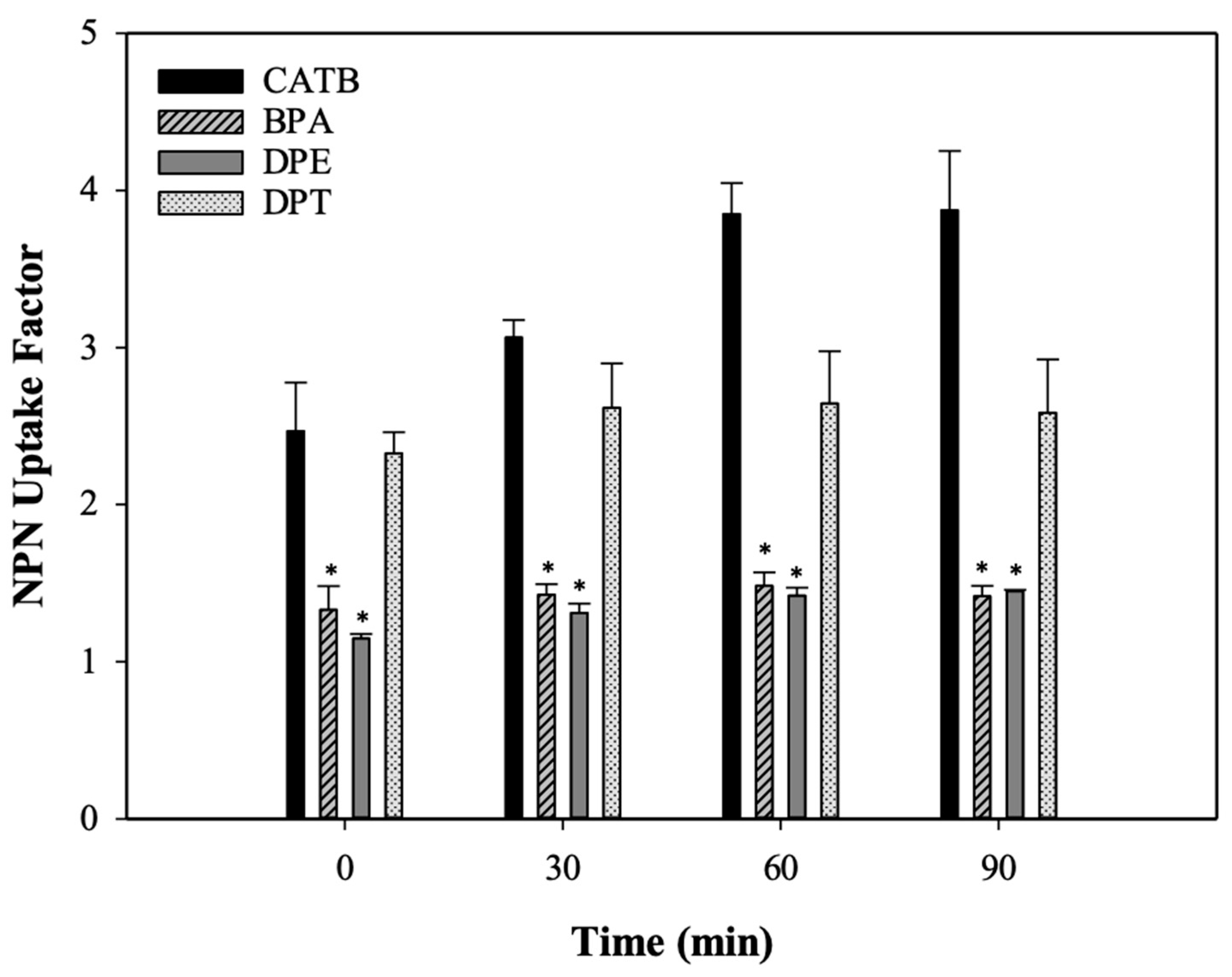
2.4. Outer Membrane Permeability of Kam3-AcrB
2.5. Post-Antibiotic Effect of DPE and BPA Coupled with Erythromycin on E. coli Kam3
2.6. Cytotoxicity Test of DPE and BPA
3. Materials and Methods
3.1. Bacterial Strains, Constructs, Media and Chemicals
3.2. Molecular Docking
3.3. MIC and IC50 Determination
3.4. Modulation Assays
3.5. Drug Accumulation Assay
3.6. Efflux Inhibition Assay
3.7. Monitoring Drug Efflux Using MALDI-TOF Mass Spectrometry
3.8. Post-Antibiotic Effect Assay
3.9. Membrane Integrity Assay
3.10. Cytotoxicity Assay
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 12 December 2022).
- Naghavi, M. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 6, 629–655. [Google Scholar] [CrossRef]
- Cooper, M.A.; Shlaes, D. Fix the antibiotics pipeline. Nature 2011, 472, 32. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H.; Pagès, J.M. Broad-specificity efflux pumps and their role in multidrug resistance of Gram-negative bacteria. Fems Microbiol. Rev. 2012, 36, 340–363. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, S.; Klinger-Strobel, M.; Bohnert, J.A.; Wendler, S.; Rödel, J.; Pletz, M.W.; Löffler, B.; Tuchscherr, L. Clinically Approved Drugs Inhibit the Multidrug NorA Efflux Pump and Reduce Biofilm Formation. Front. Microbiol. 2019, 10, 2762. [Google Scholar] [CrossRef] [PubMed]
- Neyfakh, A.A.; Borsch, C.M.; Kaatz, G.W. Fluoroquinolone Resistance Protein Nora of Staphylococcus aureus Is a Multidrug Efflux Transporter. Antimicrob. Agents Chemother. 1993, 37, 128–129. [Google Scholar] [CrossRef] [PubMed]
- Zwama, M.; Yamaguchi, A. Molecular mechanisms of AcrB-mediated multidrug export. Res. Microbiol. 2018, 169, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Bera, A.; Roy, R.K.; Joshi, P.; Patra, N. Machine learning-guided discovery of AcrB and MexB efflux pump inhibitors. J. Phys. Chem. B 2024, 128, 648–663. [Google Scholar] [CrossRef] [PubMed]
- Compagne, N.; Vieira Da Cruz, A.; Muller, R.T.; Hartkoorn, R.C.; Flipo, M.; Pos, K.M. Update on the Discovery of Efflux Pump Inhibitors against Critical Priority Gram-Negative Bacteria. Antibiotics 2023, 12, 180. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.J.; Huang, Y.J.; Lin, H.J.; Chang, C.J.; Hsu, P.H.; Ooi, G.X.; Huang, M.Y.; Lin, H.V. Phenolic compound ethyl 3,4-dihydroxybenzoate retards drug efflux and potentiates antibiotic activity. Antibiotics 2022, 11, 497. [Google Scholar] [CrossRef]
- Schillaci, D.; Spanò, V.; Parrino, B.; Carbone, A.; Montalbano, A.; Barraja, P.; Diana, P.; Cirrincione, G.; Cascioferro, S. Pharmaceutical Approaches to Target Antibiotic Resistance Mechanisms. J. Med. Chem. 2017, 60, 8268–8297. [Google Scholar] [CrossRef]
- Zuo, Z.; Weng, J.; Wang, W. Insights into the inhibitory mechanism of D13-9001 to the multidrug transporter AcrB through molecular dynamics simulations. J. Phys. Chem. B 2016, 120, 2145–2154. [Google Scholar] [CrossRef] [PubMed]
- Dias, K.J.S.D.; Miranda, G.M.; Bessa, J.R.; De Araujo, A.C.J.; Freitas, P.R.; De Almeida, R.S.; Paulo, C.L.R.; Neto, J.B.D.; Coutinho, H.D.M.; Ribeiro, J. Terpenes as bacterial efflux pump inhibitors: A systematic review. Front. Pharmacol. 2022, 13, 953982. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ge, X. Role of berberine as a potential efflux pump inhibitor against MdfA from Escherichia coli: In vitro and in silico studies. Microbiol. Spectr. 2023, 11, e0332422. [Google Scholar] [CrossRef] [PubMed]
- Opperman, T.J.; Nguyen, S. Recent advances toward a molecular mechanism of efflux pump inhibition. Front. Microbiol. 2015, 6, 421. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.K.; Troy, D.J. Seaweed Sustainability: Food and Non-Food Applications; Elsevier/Academic Press: Amsterdam, The Netherlands; Boston, MA, USA, 2015; 470p. [Google Scholar]
- Lu, W.J.; Lin, H.J.; Hsu, P.H.; Lai, M.; Chiu, J.Y.; Lin, H.T.V. Brown and Red Seaweeds Serve as Potential Efflux Pump Inhibitors for Drug-Resistant. Evid.-Based Compl. Alt. 2019, 2019, 1836982. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.J.; Hsu, P.H.; Chang, C.J.; Su, C.K.; Huang, Y.J.; Lin, H.J.; Lai, M.; Ooi, G.X.; Dai, J.Y.; Lin, H.V. Identified seaweed compound diphenylmethane serves as an efflux pump inhibitor in drug-resistant Escherichia coli. Antibiotics 2021, 10, 1378. [Google Scholar] [CrossRef] [PubMed]
- Reading, E.; Ahdash, Z.; Fais, C.; Ricci, V.; Wang-Kan, X.; Grimsey, E.; Stone, J.; Malloci, G.; Lau, A.M.; Findlay, H.; et al. Perturbed structural dynamics underlie inhibition and altered efflux of the multidrug resistance pump AcrB. Nat. Commun. 2020, 11, 5565. [Google Scholar] [CrossRef] [PubMed]
- Tam, H.K.; Foong, W.E.; Oswald, C.; Herrmann, A.; Zeng, H.; Pos, K.M. Allosteric drug transport mechanism of multidrug transporter AcrB. Nat. Commun. 2021, 12, 3889. [Google Scholar] [CrossRef] [PubMed]
- Prasch, S.; Bucar, F. Plant derived inhibitors of bacterial efflux pumps: An update. Phytochem. Rev. 2015, 14, 961–974. [Google Scholar] [CrossRef]
- Khoshnood, S.; Heidary, M.; Hashemi, A.; Shahi, F.; Saki, M.; Kouhsari, E.; Eslami, G.; Goudarzi, H. Involvement of the AcrAB Efflux Pump in Ciprofloxacin Resistance in Clinical Klebsiella pneumoniae Isolates. Infect. Disord. Drug Targets 2021, 21, 564–571. [Google Scholar] [CrossRef]
- Tambat, R.; Mahey, N.; Chandal, N.; Verma, D.K.; Jangra, M.; Thakur, K.G.; Nandanwar, H. A Microbe-Derived Efflux Pump Inhibitor of the Resistance-Nodulation-Cell Division Protein Restores Antibiotic Susceptibility in Escherichia coli and Pseudomonas aeruginosa. Acs Infect. Dis. 2022, 8, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.; Fernandes, L.; Costa, S.S.; Cannalire, R.; Manfronf, G.; Tabarrin, O.; Couto, I.; Sabatini, S.; Viveiros, M. Mode of action of the 2-phenylquinoline efflux inhibitor PQQ4R against Escherichia coli. Peerj 2017, 5, e3168. [Google Scholar] [CrossRef] [PubMed]
- Opperman, T.J.; Kwasny, S.M.; Kim, H.S.; Nguyen, S.T.; Houseweart, C.; D’Souza, S.; Walker, G.C.; Peet, N.P.; Nikaido, H.; Bowlin, T.L. Characterization of a Novel Pyranopyridine Inhibitor of the AcrAB Efflux Pump of Escherichia coli. Antimicrob. Agents Chemother. 2014, 58, 722–733. [Google Scholar] [CrossRef]
- Hanberger, H.; Nilsson, L.E.; Kihlstrom, E.; Maller, R. Postantibiotic Effect of Beta-Lactam Antibiotics on Escherichia coli Evaluated by Bioluminescence Assay of Bacterial Atp. Antimicrob. Agents Chemother. 1990, 34, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Tambat, R.; Jangra, M.; Mahey, N.; Chandal, N.; Kaur, M.; Chaudhary, S.; Verma, D.K.; Thakur, K.G.; Raje, M.; Jachak, S.; et al. Microbe-Derived Indole Metabolite Demonstrates Potent Multidrug Efflux Pump Inhibition in Staphylococcus aureus. Front. Microbiol. 2019, 10, 2153. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, N.J.; Hewitt, P. Phase I and II enzyme characterization of two sources of HepG2 cell lines. Xenobiotica 2004, 34, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, T.B.; Santos, G.F.; Ventura, S.C.; Hiruma-Lima, C.A.; Gaivao, I.O.M.; Maistro, E.L. Cytotoxic and genotoxic potential of geraniol in peripheral blood mononuclear cells and human hepatoma cell line (HepG2). Genet. Mol. Res. 2017, 16, gmr16039777. [Google Scholar] [CrossRef] [PubMed]
- Chandal, N.; Tambat, R.; Kalia, R.; Kumar, G.; Mahey, N.; Jachak, S.; Nandanwar, H. Efflux pump inhibitory potential of indole derivatives as an arsenal against overexpressing Staphylococcus aureus. Microbiol. Spectr. 2023, 11, e04876-22. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Kodama, K.; Shiota, S.; Mine, T.; Kataoka, A.; Mizushima, T.; Tsuchiya, T. NorM, a putative multidrug efflux protein, of Vibrio parahaemolyticus and its homolog in Escherichia coli. Antimicrob. Agents Chemother. 1998, 42, 1778–1782. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. Software News and Update AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Eicher, T.; Cha, H.J.; Seeger, M.A.; Brandstätter, L.; El-Delik, J.; Bohnert, J.A.; Kern, W.V.; Verrey, F.; Grütter, M.G.; Diederichs, K.; et al. Transport of drugs by the multidrug transporter AcrB involves an access and a deep binding pocket that are separated by a switch-loop. Proc. Natl. Acad. Sci. USA 2012, 109, 5687–5692. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.J.; Hsu, P.H.; Lin, H.T.V. A Novel Cooperative Metallo-β-Lactamase Fold Metallohydrolase from Pathogen Exhibits β-Lactam Antibiotic-Degrading Activities. Antimicrob. Agents Chemother. 2021, 65, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Soothill, J.S.; Ward, R.; Girling, A.J. The IC50—An Exactly Defined Measure of Antibiotic-Sensitivity. J. Antimicrob. Chemother. 1992, 29, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Machado, L.; Spengler, G.; Evaristo, M.; Handzlik, J.; Molnar, J.; Viveiros, M.; Kiec-Kononowicz, K.; Amaral, L. Biological activity of twenty-three hydantoin derivatives on intrinsic efflux pump system of Salmonella enterica serovar Enteritidis NCTC 13349. In Vivo 2011, 25, 769–772. [Google Scholar] [PubMed]
- Lu, W.J.; Lin, H.J.; Janganan, T.K.; Li, C.Y.; Chin, W.C.; Bavro, V.N.; Lin, H.T.V. ATP-Binding Cassette Transporter VcaM from is Dependent on the Outer Membrane Factor Family for Its Function. Int. J. Mol. Sci. 2018, 19, 1000. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.J.; Lin, H.J.; Hsu, P.H.; Lin, H.T.V. Determination of drug efflux pump efficiency in drug-resistant bacteria using MALDI-TOF MS. Antibiotics 2020, 9, 639. [Google Scholar] [CrossRef] [PubMed]
- Stubbings, W.J.; Bostock, J.M.; Ingham, E.; Chopra, I. Assessment of a microplate method for determining the post-antibiotic effect in Staphylococcus aureus and Escherichia coli. J. Antimicrob. Chemoth 2004, 54, 139–143. [Google Scholar] [CrossRef]
- Helander, I.M.; Mattila-Sandholm, T. Fluorometric assessment of Gram-negative bacterial permeabilization. J. Appl. Microbiol. 2000, 88, 213–219. [Google Scholar] [CrossRef] [PubMed]
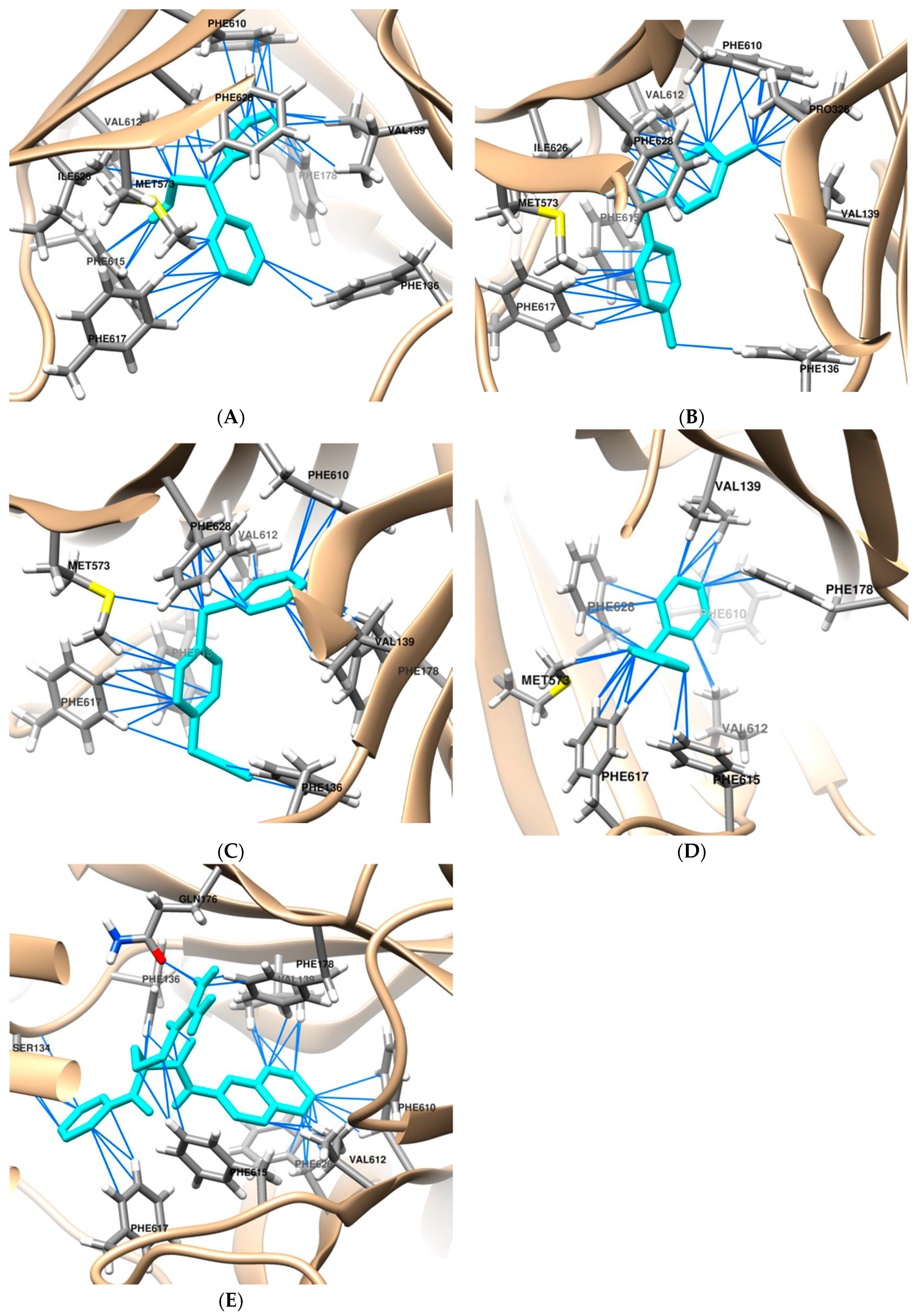



| Docking Parameters | Molecular Docking Results | ||||
|---|---|---|---|---|---|
| PAβN | DPM | DPE | DPT | BPA | |
| Binding free energy (kcal/mol) | −8.8 | −8.1 | −8.2 | −8.2 | −8.5 |
| Number of pseudobond (<4 Å) | 42 | 32 | 42 | 43 | 34 |
| Number of van der Waals contact (<4 Å) | 10 | 8 | 10 | 10 | 8 |
| Number of contact residues 2 | - | 7 | 8 | 7 | 7 |
| Compound | Interaction Residues a |
|---|---|
| PAβN | Ser134 c (2.1 Å), Phe136, Val139, Gln,176 c (2.1 Å), PHE 178 Phe610, Val612, Phe615 b, Phe617, Phe628 b |
| DPM | Val139, Phe178 b, Met573, Phe610, Val612, Phe615 b, Phe617, Phe628 b |
| DPE | Phe136, Val139, Phe178 b, Met573, Phe610, Val612, Phe615 b, Phe617, Ile626, Phe628 b |
| DPT | Phe136, Val139, Pro326, Met573, Phe610, Val612, Phe615 b, Phe617, Ile626, Phe628 b |
| BPA | Phe136, Val139, Met573, Phe610, Val612, Phe615 b, Phe617, Phe628 |
| IC50 (μg/mL) | ||||
|---|---|---|---|---|
| Compound | Antibiotics | Alone | Combination (Compound/Antibiotic) | MF |
| DPE | Erythromycin | 125 | 16/31.25 | 4 |
| Ciprofloxacin | 0.015 | 128/0.0075 | 2 | |
| Clarithromycin | 87.5 | 128/21.88 | 2 | |
| Tetracycline | 0.39 | 128/0.2 | 2 | |
| DPT | Erythromycin | 125 | 4/62.5 | 2 |
| Ciprofloxacin | 0.015 | 8/0.0075 | 2 | |
| Clarithromycin | 87.5 | 4/43.75 | 2 | |
| Tetracycline | 0.39 | 8/0.2 | 2 | |
| BPA | Erythromycin | 125 | 32/31.25 | 4 |
| Ciprofloxacin | 0.015 | 32/0.0075 | 2 | |
| Clarithromycin | 87.5 | 8/21.88 | 2 | |
| Tetracycline | 0.39 | 2/0.2 | 2 | |
| DPM | Erythromycin | 125 | 7.81/62.5 | 2 |
| Ciprofloxacin | 0.015 | 250/0.0075 | 2 | |
| Clarithromycin | 87.5 | 250/43.75 | 2 | |
| Tetracycline | 0.39 | 500/0.098 | 2 | |
| PAβN | Erythromycin | 125 | 10/3.91 | 32 |
| Ciprofloxacin | 0.015 | 2.5/0.0038 | 4 | |
| Clarithromycin | 87.5 | 1.25/10.94 | 8 | |
| Tetracycline | 0.39 | 0.63/0.049 | 8 | |
| Compound | Concentration | RFF ± SD |
|---|---|---|
| Control | none | 0.54 ± 0.06 d |
| PAβN | 20 µg/mL | 1.26 ± 0.03 c |
| DPE | 128 µg/mL | 2.47 ± 1.02 ab |
| 64 µg/mL | 1.32 ± 0.14 c | |
| 32 µg/mL | 1.11 ± 0.00 c | |
| DPT | 32 µg/mL | 0.49 ± 0.01 d |
| BPA | 64 µg/mL | 3.47 ± 0.27 a |
| 32 µg/mL | 1.97 ± 0.23 bc | |
| 16 µg/mL | 1.19 ± 0.03 c |
| Regimen | Mean PAE (h) ± SD | ||
|---|---|---|---|
| 1/2 IC50 | 2 IC50 | 6 IC50 | |
| Erythromycin | 0.61 ± 0.01 | 1.00 ± 0.33 | 1.10 ± 0.38 |
| Erythromycin + DPE | 1.09 ± 0.32 | 1.13 ± 0.35 | 1.44 ± 0.2 |
| Erythromycin + BPA | 1.08 ± 0.37 | 1.21 ± 0.58 | 1.39 ± 0.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, W.-J.; Lian, Y.-W.; Chang, C.-J.; Lin, H.-J.; Huang, C.-Y.; Hsu, P.-H.; Lin, H.-T. Screening and Evaluation of Potential Efflux Pump Inhibitors with a Seaweed Compound Diphenylmethane-Scaffold against Drug-Resistant Escherichia coli. Antibiotics 2024, 13, 628. https://doi.org/10.3390/antibiotics13070628
Lu W-J, Lian Y-W, Chang C-J, Lin H-J, Huang C-Y, Hsu P-H, Lin H-T. Screening and Evaluation of Potential Efflux Pump Inhibitors with a Seaweed Compound Diphenylmethane-Scaffold against Drug-Resistant Escherichia coli. Antibiotics. 2024; 13(7):628. https://doi.org/10.3390/antibiotics13070628
Chicago/Turabian StyleLu, Wen-Jung, Yu-Wei Lian, Chun-Ju Chang, Hsuan-Ju Lin, Chian-Yun Huang, Pang-Hung Hsu, and Hong-Ting Lin. 2024. "Screening and Evaluation of Potential Efflux Pump Inhibitors with a Seaweed Compound Diphenylmethane-Scaffold against Drug-Resistant Escherichia coli" Antibiotics 13, no. 7: 628. https://doi.org/10.3390/antibiotics13070628
APA StyleLu, W.-J., Lian, Y.-W., Chang, C.-J., Lin, H.-J., Huang, C.-Y., Hsu, P.-H., & Lin, H.-T. (2024). Screening and Evaluation of Potential Efflux Pump Inhibitors with a Seaweed Compound Diphenylmethane-Scaffold against Drug-Resistant Escherichia coli. Antibiotics, 13(7), 628. https://doi.org/10.3390/antibiotics13070628






