Differences in the Dwell Time of Peripherally Inserted Central Catheters between Patients with Catheter Colonization and Those Developing Central Line-Associated Bloodstream Infection: A Single Centre Retrospective Cohort Study
Abstract
1. Introduction
2. Results
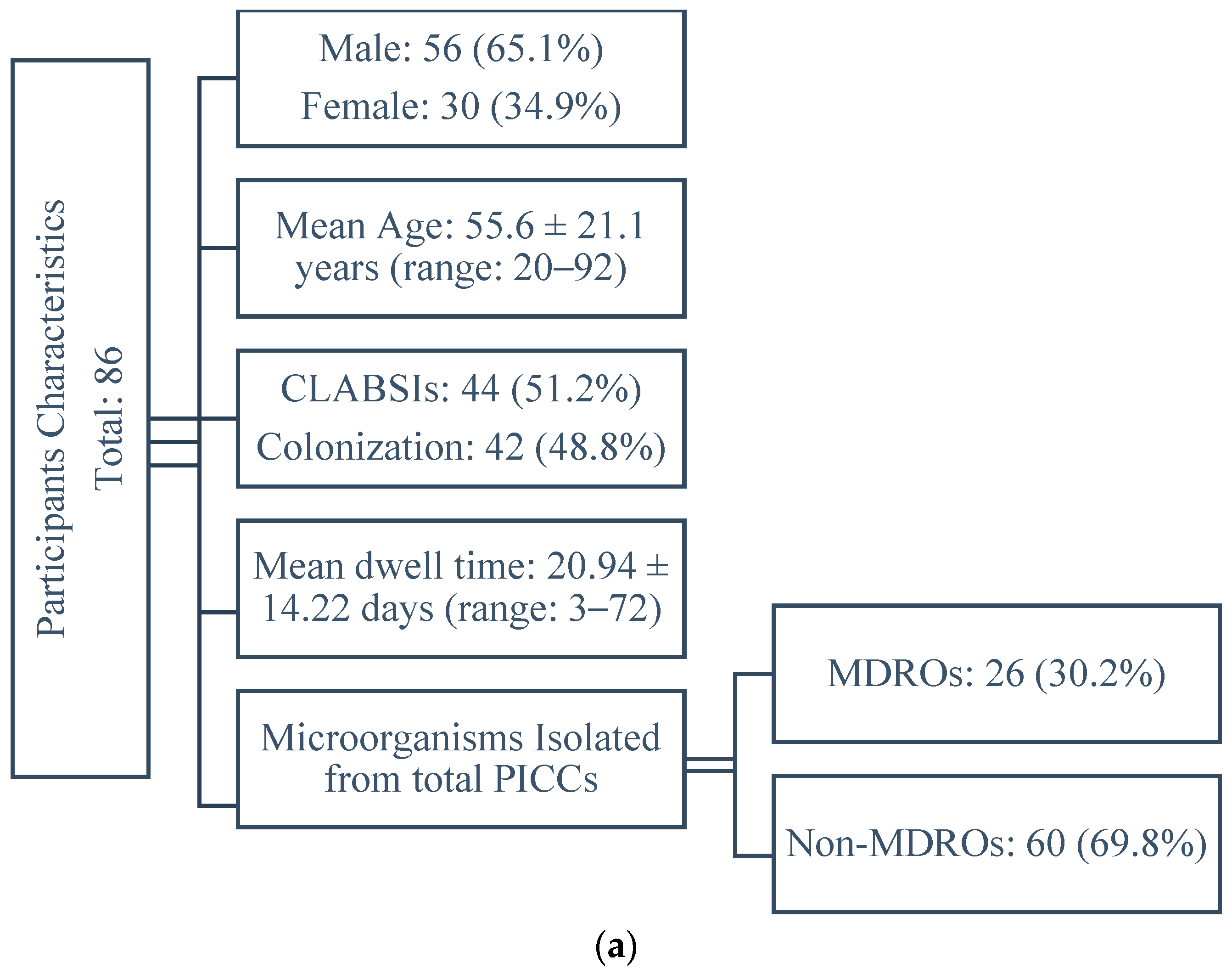
2.1. Participants Characteristics
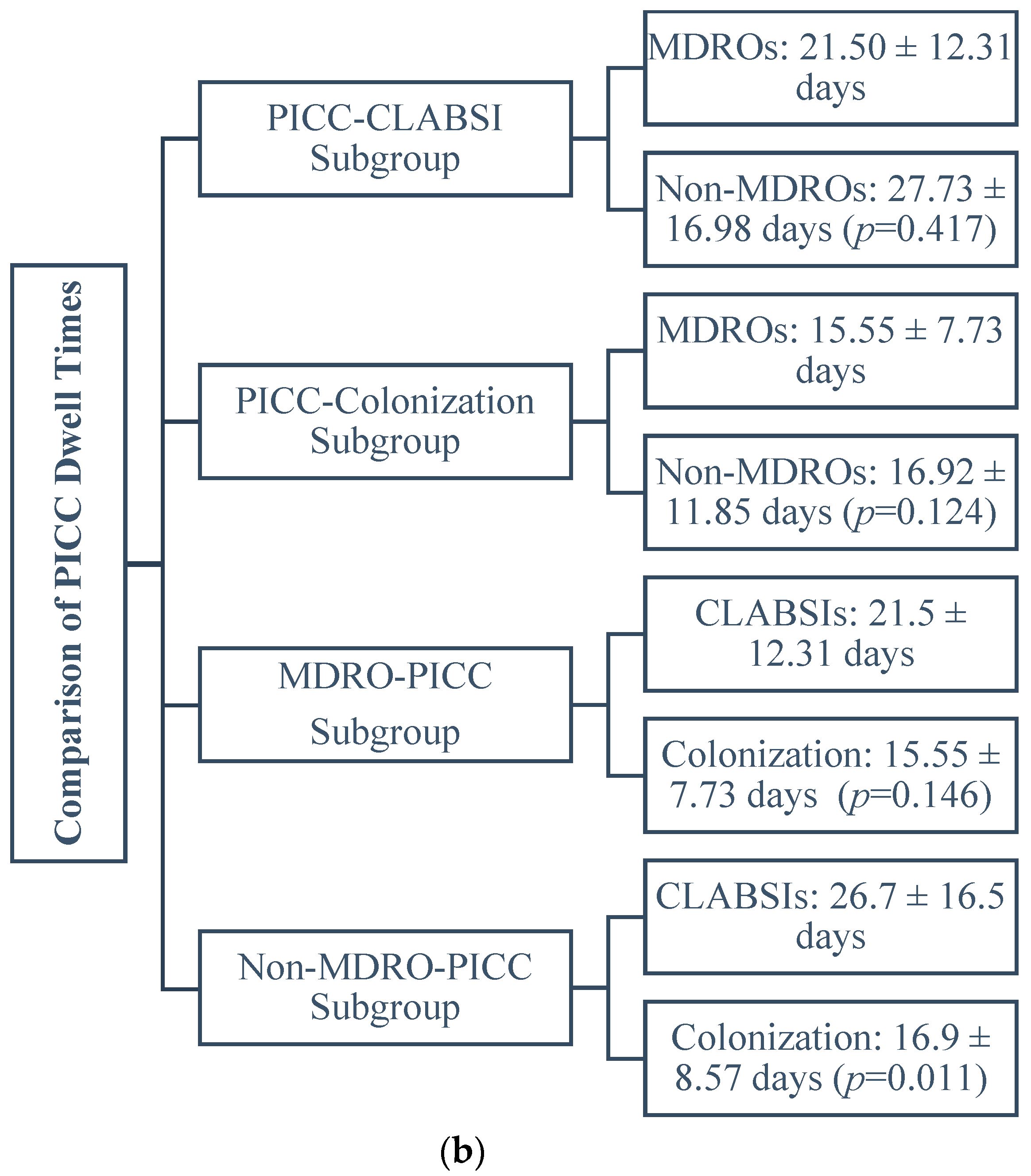
2.2. Comparison of the Dwell Times between PICCs Subgroups
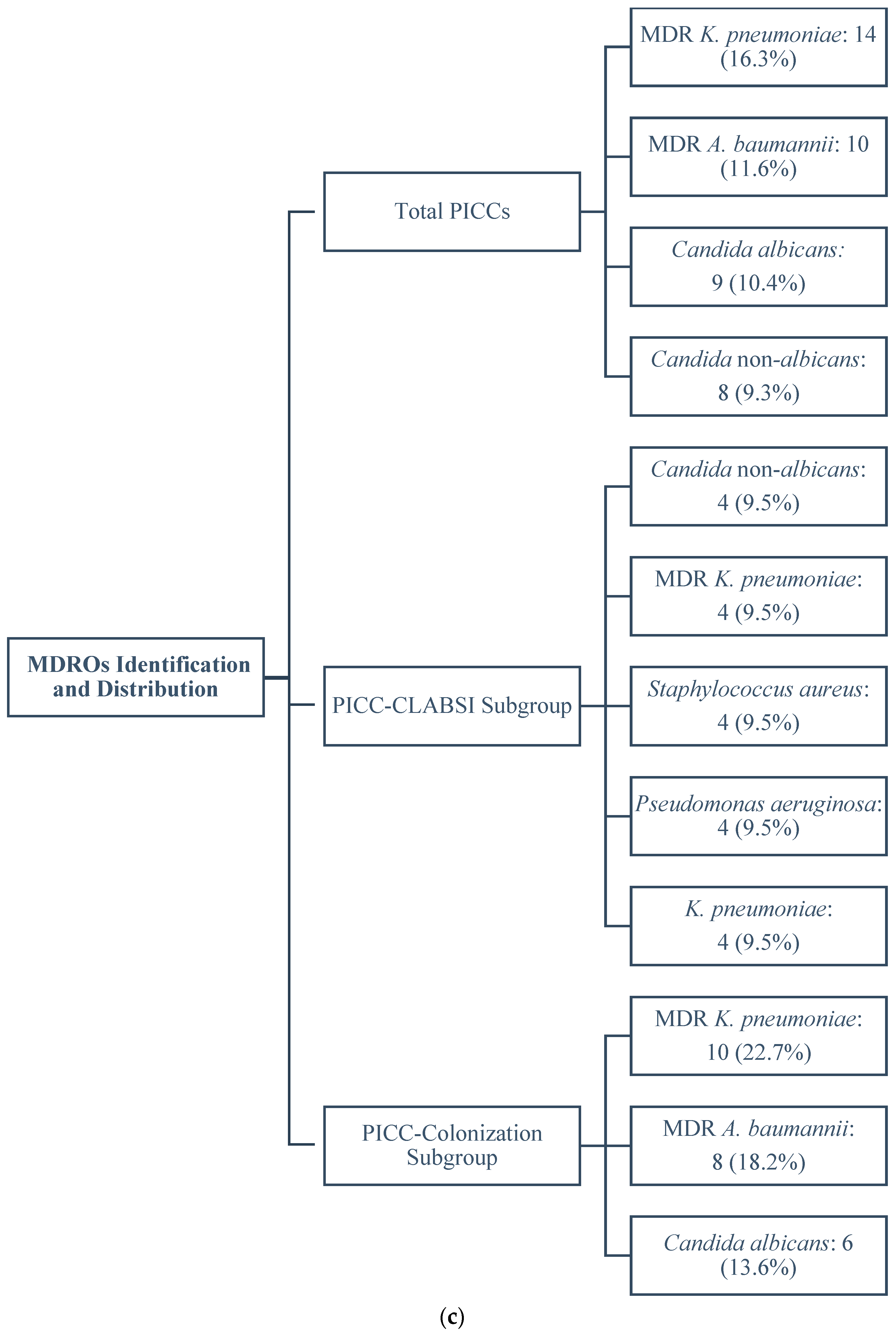
2.3. Identification and Distribution of MDROs
2.3.1. Total PICCs
2.3.2. PICC-CLABSI Subgroup
2.3.3. PICC-Colonization Subgroup
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Catheter Care
4.3. Culture Techniques
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Grady, N.P.; Alexander, M.; Dellinger, E.; Gerberding, J.L.; Heard, S.O.; Maki, D.G.; Masur, H.; McCormick, R.D.; Mermel, L.A.; Pearson, M.L.; et al. Guidelines for the prevention of intravascular catheter-related infections. Am. J. Infect. Control 2002, 30, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Mermel, L.A.; Farr, B.M.; Sherertz, R.J.; Raad, I.I.; O’Grady, N.; Harris, J.S.; Craven, D.E. Guidelines for the management of intravascular catheter-related infections. Clin. Infect. Dis. 2001, 32, 1249–1272. [Google Scholar] [CrossRef] [PubMed]
- Safdar, N.; Maki, D.G. Risk of catheter-related bloodstream infection with peripherally inserted central venous catheters used in hospitalized patients. Chest 2005, 128, 489–495. [Google Scholar] [CrossRef]
- Yamaguchi, R.S.; Noritomi, D.T.; Degaspare, N.V.; Muñoz, G.O.C.; Porto, A.P.M.; Costa, S.F.; Ranzani, O.T. Peripherally inserted central catheters are associated with lower risk of bloodstream infection compared with central venous catheters in paediatric intensive care patients: A propensity-adjusted analysis. Intensive Care Med. 2017, 43, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Pitiriga, V.; Bakalis, J.; Theodoridou, K.; Kanellopoulos, P.; Saroglou, G.; Tsakris, A. Lower risk of bloodstream infections for peripherally inserted central catheters compared to central venous catheters in critically ill patients. Antimicrob. Resist. Infect. Control 2022, 11, 137. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Ryder, M.A. Invited Review: Vascular access devices: Perspectives on designs, complications, and management. Nutr. Clin. Pract. 1993, 8, 145–152. [Google Scholar] [CrossRef]
- Johansson, E.; Hammarskjöld, F.; Lundberg, D.; Arnlind, H. Advantages and disadvantages of peripherally inserted central venous catheters (PICC) compared to other central venous lines: A systematic review of the literature. Taylor Fr. Online 2013, 52, 886–892. [Google Scholar] [CrossRef]
- Alshahrani, K.M.; Alhuwaishel, A.Z.; Alangari, N.M.; Asiri, M.A.; Al-Shahrani, N.A.; Alasmari, A.A.; Alzahrani, O.J.; Ayedh, A.Y.; Qitmah, M.M. Clinical Impacts and Risk Factors for Central Line-Associated Bloodstream Infection: A Systematic Review. Cureus 2023, 15, e40954. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baier, C.; Linke, L.; Eder, M.; Schwab, F.; Chaberny, I.F.; Vonberg, R.-P.; Ebadi, E. Incidence, risk factors and healthcare costs of central line-associated nosocomial bloodstream infections in hematologic and oncologic patients. PLoS ONE 2020, 15, e0227772. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jia, L.; Jiang, A. Pathology of catheter-related complications: What we need to know and what should be discovered. J. Int. Med. Res. 2022, 50, 3000605221127890. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cabrero, E.L.; Robledo, R.T.; Cuñado, A.C.; Sardelli, D.G.; López, C.H.; Formatger, D.G.; Perez, L.L.; López, C.E.; Moreno, A.T. Risk factors of catheter-associated bloodstream infection: Systematic review and meta-analysis. PLoS ONE 2023, 18, e0282290. [Google Scholar] [CrossRef]
- Sengupta, A.; Lehmann, C.; Diener-West, M.; Perl, T.M.; Milstone, A.M. Catheter duration and risk of CLA-BSI in neonates with PICCs. Pediatrics 2010, 125, 648–653. [Google Scholar] [CrossRef]
- Pitiriga, V.; Bakalis, J.; Kampos, E.; Kanellopoulos, P.; Saroglou, G.; Tsakris, A. Duration of central venous catheter placement and central line-associated bloodstream infections after the adoption of prevention bundles: A two-year retrospective study. Antimicrob. Resist. Infect. Control 2022, 11, 96. [Google Scholar] [CrossRef]
- Singhai, M.; Malik, A.; Shahid, M.; Malik, A.; Rawat, V. Colonization of peripheral intravascular catheters with biofilm producing microbes: Evaluation of risk factors. Niger. Med. J. 2012, 53, 37–41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cekin, Z.K.; Oncul, A.; Bayraktar, B. Bloodstream Infections Caused by Multidrug Resistant Bacteria: Clinical and Microbiological Features and Mortality. Sisli Etfal Hastan. Tip. Bul. 2023, 57, 416–425. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Litwin, A.; Fedorowicz, O.; Duszynska, W. Characteristics of Microbial Factors of Healthcare-Associated Infections Including Multidrug-Resistant Pathogens and Antibiotic Consumption at the University Intensive Care Unit in Poland in the Years 2011–2018. Int. J. Environ. Res. Public Health 2020, 17, 6943. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Milstone, A.M.; Reich, N.G.; Advani, S.; Yuan, G.; Bryant, K.; Coffin, S.E.; Huskins, W.C.; Livingston, R.; Saiman, L.; Smith, P.B.; et al. Catheter dwell time and CLABSIs in neonates with PICCs: A multicenter cohort study. Pediatrics 2013, 132, e1609–e1615. [Google Scholar] [CrossRef] [PubMed]
- Dünser, M.W.; Mayr, A.J.; Hinterberger, G.; Flörl, C.L.; Ulmer, H.; Schmid, S.; Friesenecker, B.; Lorenz, I.; Hasibeder, W.R. Central venous catheter colonization in critically ill patients: A prospective, randomized, controlled study comparing standard with two antiseptic-impregnated catheters. Anesth. Analg. 2005, 101, 1778–1784. [Google Scholar] [CrossRef] [PubMed]
- Pitiriga, V.C.; Bakalis, J.; Campos, E.; Kanellopoulos, P.; Sagris, K.; Saroglou, G.; Tsakris, A. Central Venous Catheters versus Peripherally Inserted Central Catheters: A Comparison of Indwelling Time Resulting in Colonization by Multidrug-Resistant Pathogens. Antibiotics 2024, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, N.P.; Alexander, M.; Burns, L.A.; Dellinger, E.P.; Garland, J.; Heard, S.O.; Lipsett, P.A.; Masur, H.; Mermel, L.A.; Pearson, M.L.; et al. Guidelines for the prevention of intravascular catheter-related infections. Am. J. Infect. Control 2011, 39, S1–S34. [Google Scholar] [CrossRef]
- Bouza, E.; Alvarado, N.; Alcala, L.; Perez, M.J.; Rincon, C.; Munoz, P. A randomized and prospective study of 3 procedures for the diagnosis of catheter-related bloodstream infection without catheter withdrawal. Clin. Infect. Dis. 2007, 44, 820–826. [Google Scholar] [CrossRef]
- Liao, W.-C.; Chung, W.-S.; Lo, Y.-C.; Shih, W.-H.; Chou, C.-H.; Chen, C.-Y.; Tu, C.-Y.; Ho, M.-W. Changing epidemiology and prognosis of nosocomial bloodstream infection: A single-center retrospective study in Taiwan. J. Microbiol. Immunol. Infect. 2021, 55, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Bouhrour, N.; Nibbering, P.H.; Bendali, F. Medical Device-Associated Biofilm Infections and Multidrug-Resistant Pathogens. Pathogens 2024, 13, 393. [Google Scholar] [CrossRef]
- Barrigah-Benissan, K.; Ory, J.; Simon, C.; Loubet, P.; Martin, A.; Beregi, J.-P.; Lavigne, J.-P.; Sotto, A.; Larcher, R. Clinical factors associated with peripherally inserted central catheters (PICC) related bloodstream infections: A single centre retrospective cohort. Antimicrob. Resist. Infect. Control 2023, 12, 5. [Google Scholar] [CrossRef]
- Tsalidou, M.; Stergiopoulou, T.; Bostanitis, I.; Nikaki, C.; Skoumpa, K.; Koutsoukou, T.; Papaioannidou, P. Surveillance of Antimicrobial Resistance and Multidrug Resistance Prevalence of Clinical Isolates in a Regional Hospital in Northern Greece. Antibiotics 2023, 12, 1595. [Google Scholar] [CrossRef]
- Karvouniaris, M.; Poulakou, G.; Tsiakos, K.; Chatzimichail, M.; Papamichalis, P.; Katsiaflaka, A.; Oikonomou, K.; Katsioulis, A.; Palli, E.; Komnos, A. ICU-Associated Gram-Negative Bloodstream Infection: Risk Factors Affecting the Outcome Following the Emergence of Colistin-Resistant Isolates in a Regional Greek Hospital. Antibiotics 2022, 11, 405. [Google Scholar] [CrossRef]
- Li, Y.; Ni, M. Regulation of biofilm formation in Klebsiella pneumoniae. Front. Microbiol. 2023, 14, 1238482. [Google Scholar] [CrossRef]
- Kreve, S.; Reis, A.C.D. Bacterial adhesion to biomaterials: What regulates this attachment? A review. Jpn. Dent. Sci. Rev. 2021, 57, 85–96. [Google Scholar] [CrossRef]
- Greene, C.; Vadlamudi, G.; Newton, D.; Foxman, B.; Xi, C. The influence of biofilm formation and multidrug resistance on environmental survival of clinical and environmental isolates of Acinetobacter baumannii. Am. J. Infect. Control 2016, 44, e65–e71. [Google Scholar] [CrossRef]
- Yang, C.H.; Su, P.W.; Moi, S.H.; Chuang, L.Y. Biofilm formation in Acinetobacter Baumannii: Genotype-phenotype correlation. Molecules 2019, 24, 1849. [Google Scholar] [CrossRef] [PubMed]
- Ceparano, M.; Baccolini, V.; Migliara, G.; Isonne, C.; Renzi, E.; Tufi, D.; De Vito, C.; De Giusti, M.; Trancassini, M.; Alessandri, F.; et al. Acinetobacter baumannii Isolates from COVID-19 patients in a hospital intensive care unit: Molecular typing and risk factors. Microorganisms 2022, 10, 722. [Google Scholar] [CrossRef]
- Revdiwala, S.; Rajdev, B.M.; Mulla, S. Characterization of bacterial etiologic agents of biofilm formation in medical devices in critical care setup. Crit. Care Res. Pract. 2012, 2012, 945805. [Google Scholar] [CrossRef]
- Shin, J.H.; Kee, S.J.; Shin, M.G.; Kim, S.H.; Shin, D.H.; Lee, S.K.; Suh, S.P.; Ryang, D.W. Biofilm production by isolates of Candida species recovered from nonneutropenic patients: Comparison of bloodstream isolates with isolates from other sources. J. Clin. Microbiol. 2002, 40, 1244–1248. [Google Scholar] [CrossRef]
- Michaelis, C.; Grohmann, E. Horizontal Gene Transfer of Antibiotic Resistance Genes in Biofilms. Antibiotics 2023, 12, 328. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, A.; Sun, J.; Liu, Y. Understanding bacterial biofilms: From definition to treatment strategies. Front. Cell. Infect. Microbiol. 2023, 13, 1137947. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cateau, E.; Rodier, M.H.; Imbert, C. In vitro efficacies of caspofungin or micafungin catheter lock solutions on Candida albicans biofilm growth. J. Antimicrob. Chemother. 2008, 62, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Letscher-Bru, V.; Herbrecht, R. Caspofungin: The first representative of a new antifungal class. J. Antimicrob. Chemother. 2003, 51, 513–521. [Google Scholar] [CrossRef]
- Morgan, J.; Meltzer, M.I.; Plikaytis, B.D.; Sofair, A.N.; Huie-White, S.; Wilcox, S.; Harrison, L.H.; Seaberg, E.C.; Hajjeh, R.A.; Teutsch, S.M. Excess mortality, hospital stay, and cost due to candidemia: A case-control study using data from population-based candidemia surveillance. Infect. Control Hosp. Epidemiol. 2005, 26, 540–547. [Google Scholar] [CrossRef]
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 2004, 39, 309–317. [Google Scholar] [CrossRef] [PubMed]
- de Grooth, H.; Timsit, J.-F.; Mermel, L.; Mimoz, O.; Buetti, N.; du Cheyron, D.; Straaten, H.O.-V.; Parienti, J.-J. Validity of surrogate endpoints assessing central venous catheter-related infection: Evidence from individual- and study-level analyses. Clin. Microbiol. Infect. 2019, 26, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Cavalheiro, M.; Teixeira, M.C. Candida biofilms: Threats, challenges, and promising strategies. Front. Med. 2018, 5, 28. [Google Scholar] [CrossRef]
- Maki, D.G.; Weise, C.E.; Sarafin, H.W. A semiquantitative culture method for identifying intravenous-catheter-related infection. N. Engl. J. Med. 1977, 296, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
| Patients’ Characteristics (n = 86) | N (%) |
|---|---|
| Respiratory disorders | 23 (26.7) |
| Trauma | 26 (30.2) |
| Diabetes mellitus | 20 (23.2) |
| Hypertension | 48 (55.8) |
| Cerebrovascular diseases | 40 (46.5) |
| Gastrointestinal disease | 19 (22) |
| Kidney disease | 18 (20.9) |
| Oncological Disorders | 20 (23.2) |
| Cardiovascular disease | 25 (29.0) |
| Immune deficiency/suppression | 45 (52.3) |
| During hospital stay | |
| ICU admission | 50 (58.1) |
| Total parenteral nutrition | 35 (40.7) |
| Mechanical ventilation | 31 (36.0) |
| Prolonged hospitalization (>1 month) | 45 (52.3) |
| Death | 15 (17.4) |
| Sepsis | 15 (17.4) |
| APACHE II at inclusion (mean ± SD) | 12.9 ± 7.5 |
| Microorganisms | Clabsi No (%) | Colonization No (%) |
|---|---|---|
| Fungi | ||
| Candida albicans | 3 (7.1) | 6 (13.6) |
| Candida non-albicans | 4 (9.5) | 4 (9.1) |
| Other fungi | 2 (4.8) | - |
| Gram-negatives | ||
| Enterobacter cloacae | 3 (7.1) | - |
| Klebsiella pneumoniae | 4 (9.5) | - |
| Pseudomonas aeruginosa | 4 (9.5) | 2 (4.5) |
| Proteus mirabilis | 1 (2.4) | 2 (4.5) |
| Serratia marcescens | 2 (4.8) | - |
| MDR Acinetobacter baumanni | 2 (4.8) | 8 (18.2) |
| MDR Klebsiella pneumoniae | 4 (9.5) | 10 (22.7) |
| MDR Pseudomonas aeruginosa | 2 (4.8) | - |
| Gram-positives | ||
| Staphylococcus aureus | 4 (9.5) | - |
| MRSA | - | 2 (4.5) |
| Staphylococcus haemolytic | 2 (4.8) | 2 (4.5) |
| Streptococcus salivarious | - | 2 (4.5) |
| Enterococcus faecalis | 2 (4.8) | - |
| Enterococcus faecium | - | 4 (9.1) |
| CnS | 3 (7.1) | 2 (4.5) |
| Total | 42 | 44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitiriga, V.C.; Campos, E.; Bakalis, J.; Saroglou, G.; Tsakris, A. Differences in the Dwell Time of Peripherally Inserted Central Catheters between Patients with Catheter Colonization and Those Developing Central Line-Associated Bloodstream Infection: A Single Centre Retrospective Cohort Study. Antibiotics 2024, 13, 632. https://doi.org/10.3390/antibiotics13070632
Pitiriga VC, Campos E, Bakalis J, Saroglou G, Tsakris A. Differences in the Dwell Time of Peripherally Inserted Central Catheters between Patients with Catheter Colonization and Those Developing Central Line-Associated Bloodstream Infection: A Single Centre Retrospective Cohort Study. Antibiotics. 2024; 13(7):632. https://doi.org/10.3390/antibiotics13070632
Chicago/Turabian StylePitiriga, Vassiliki C., Elsa Campos, John Bakalis, George Saroglou, and Athanasios Tsakris. 2024. "Differences in the Dwell Time of Peripherally Inserted Central Catheters between Patients with Catheter Colonization and Those Developing Central Line-Associated Bloodstream Infection: A Single Centre Retrospective Cohort Study" Antibiotics 13, no. 7: 632. https://doi.org/10.3390/antibiotics13070632
APA StylePitiriga, V. C., Campos, E., Bakalis, J., Saroglou, G., & Tsakris, A. (2024). Differences in the Dwell Time of Peripherally Inserted Central Catheters between Patients with Catheter Colonization and Those Developing Central Line-Associated Bloodstream Infection: A Single Centre Retrospective Cohort Study. Antibiotics, 13(7), 632. https://doi.org/10.3390/antibiotics13070632








