Isavuconazole Pharmacokinetics in Critically Ill Patients: Relationship with Clinical Effectiveness and Patient Safety
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Isavuconazole Pharmacokinetics
2.3. Definitions
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
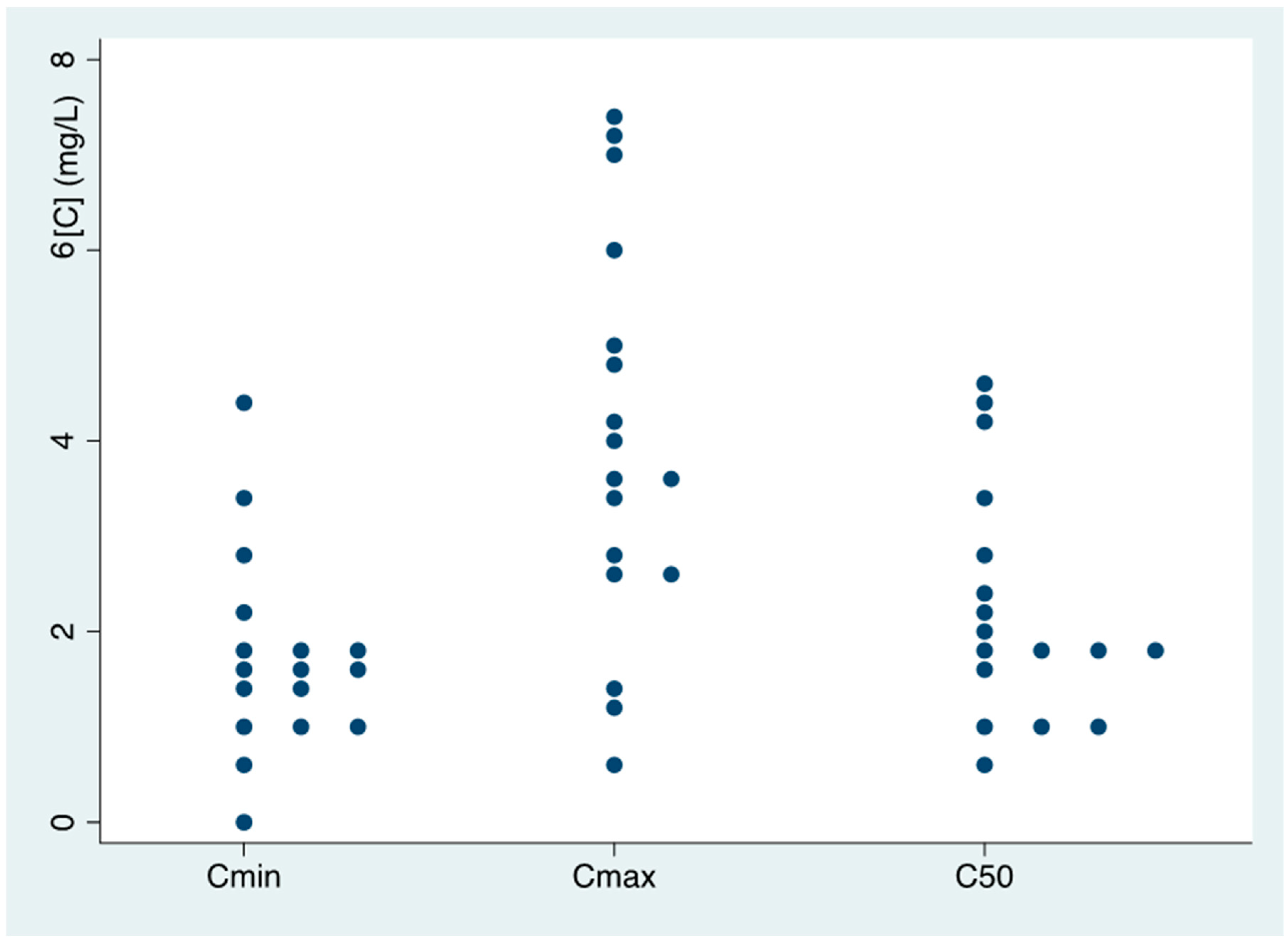
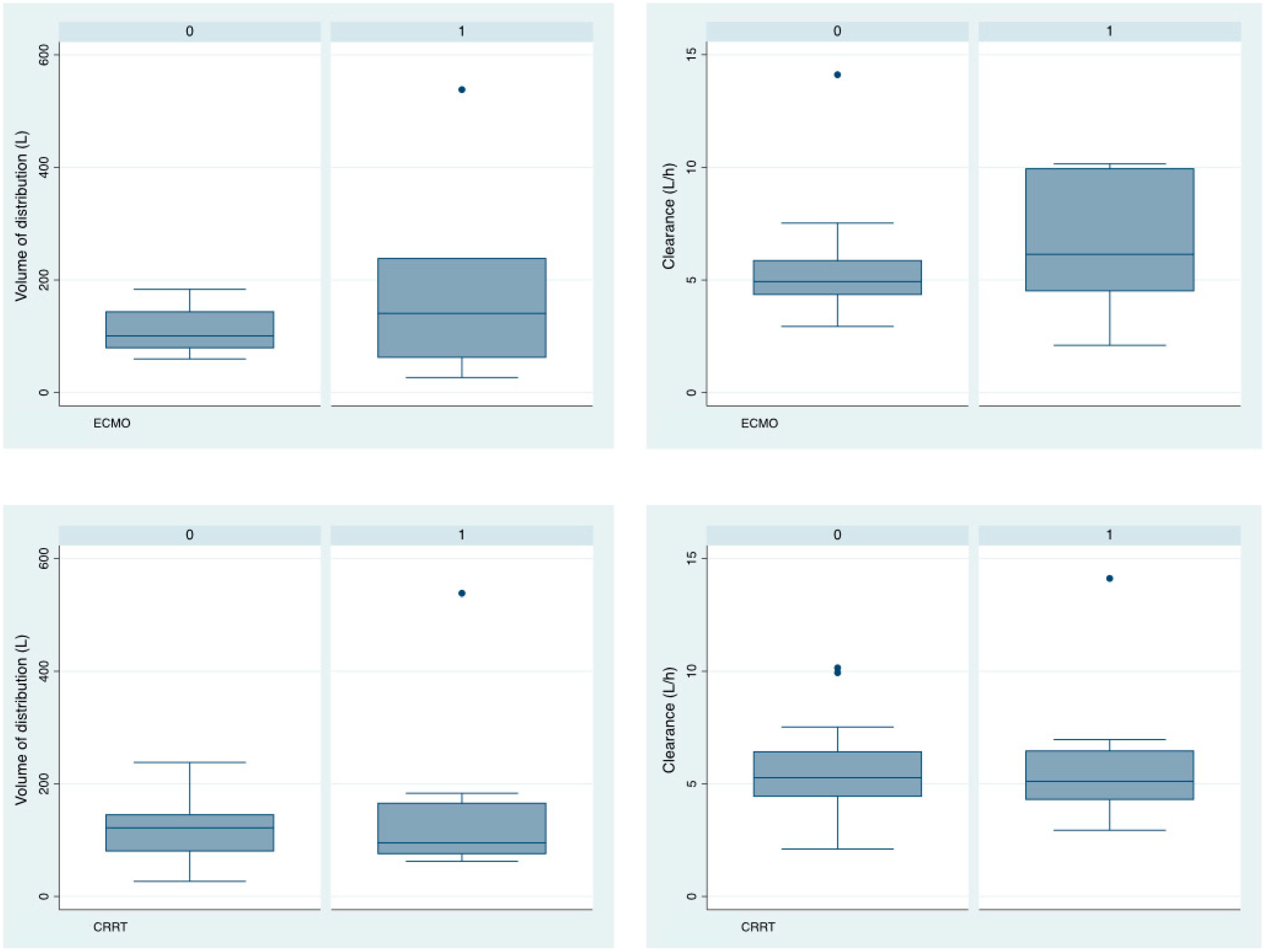
3.2. Isavuconazole Pharmacokinetics
3.3. Relationship between Isavuconazole Pk and Clinical Parameters
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suberviola Cañas, B.; Jáuregui, R.; Ballesteros, M.A.; Leizaola, O.; González-Castro, A.; Castellanos-Ortega, A. Efectos Del Retraso y La Inadecuación Del Tratamiento Antibiótico En La Supervivencia de Los Pacientes En Shock Séptico. Med. Intensiv. 2015, 39, 459–466. [Google Scholar] [CrossRef]
- Sulaiman, H.; Roberts, J.A.; Abdul-Aziz, M.H. Pharmacokinetics and Pharmacodynamics of Beta-Lactam Antibiotics in Critically Ill Patients. Farm. Hosp. 2022, 46, 182–190. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Alffenaar, J.W.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.A.; Pea, F.; Sjovall, F.; et al. Antimicrobial Therapeutic Drug Monitoring in Critically Ill Adult Patients: A Position Paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef]
- Ruiz, J.; Gordon, M.; Villarreal, E.; Peruccioni, M.; Marqués, M.R.; Poveda-Andrés, J.L.; Castellanos-Ortega, Á.; Ramirez, P. Impact of Voriconazole Plasma Concentrations on Treatment Response in Critically Ill Patients. J. Clin. Pharm. Ther. 2019, 44, 572–578. [Google Scholar] [CrossRef]
- Stott, K.E.; Hope, W.W. Therapeutic Drug Monitoring for Invasive Mould Infections and Disease: Pharmacokinetic and Pharmacodynamic Considerations. J. Antimicrob. Chemother. 2017, 72, i12–i18. [Google Scholar] [CrossRef] [PubMed]
- McCreary, E.K.; Davis, M.R.; Narayanan, N.; Andes, D.R.; Cattaneo, D.; Christian, R.; Lewis, R.E.; Watt, K.M.; Wiederhold, N.P.; Johnson, M.D. Utility of Triazole Antifungal Therapeutic Drug Monitoring: Insights from the Society of Infectious Diseases Pharmacists: Endorsed by the Mycoses Study Group Education and Research Consortium. Pharmacotherapy 2023, 43, 1043–1050. [Google Scholar] [CrossRef]
- Martin-Loeches, I.; Antonelli, M.; Cuenca-Estrella, M.; Dimopoulos, G.; Einav, S.; De Waele, J.J.; Garnacho-Montero, J.; Kanj, S.S.; Machado, F.R.; Montravers, P.; et al. ESICM/ESCMID Task Force on Practical Management of Invasive Candidiasis in Critically Ill Patients. Intensive Care Med. 2019, 45, 789–805. [Google Scholar] [CrossRef]
- Boonstra, J.M.; Märtson, A.G.; Sandaradura, I.; Kosterink, J.G.W.; van der Werf, T.S.; Marriott, D.J.E.; Zijlstra, J.G.; Touw, D.J.; Alffenaar, J.W.C. Optimization of Fluconazole Dosing for the Prevention and Treatment of Invasive Candidiasis Based on the Pharmacokinetics of Fluconazole in Critically Ill Patients. Antimicrob. Agents Chemother. 2021, 65, 10–128. [Google Scholar] [CrossRef]
- Maertens, J.A.; Raad, I.I.; Marr, K.A.; Patterson, T.F.; Kontoyiannis, D.P.; Cornely, O.A.; Bow, E.J.; Rahav, G.; Neofytos, D.; Aoun, M.; et al. Isavuconazole versus Voriconazole for Primary Treatment of Invasive Mould Disease Caused by Aspergillus and Other Filamentous Fungi (SECURE): A Phase 3, Randomised-Controlled, Non-Inferiority Trial. Lancet 2016, 387, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Kovanda, L.; Kowalski, D.; Lu, Q.; Townsend, R.; Bonate, P.L. Population Pharmacokinetics of Isavuconazole from Phase 1 and Phase 3 (SECURE) Trials in Adults and Target Attainment in Patients with Invasive Infections Due to Aspergillus and Other Filamentous Fungi. Antimicrob. Agents Chemother. 2016, 60, 5483–5491. [Google Scholar] [CrossRef]
- Risum, M.; Vestergaard, M.B.; Weinreich, U.M.; Helleberg, M.; Vissing, N.H.; Jørgensen, R. Therapeutic Drug Monitoring of Isavuconazole: Serum Concentration Variability and Success Rates for Reaching Target in Comparison with Voriconazole. Antibiotics 2021, 10, 487. [Google Scholar] [CrossRef] [PubMed]
- Mertens, B.; Elkayal, O.; Dreesen, E.; Wauters, J.; Meersseman, P.; Debaveye, Y.; Degezelle, K.; Vermeersch, P.; Gijsen, M.; Spriet, I. Isavuconazole Exposure in Critically Ill Patients Treated with Extracorporeal Membrane Oxygenation: Two Case Reports and a Narrative Literature Review. Antibiotics 2023, 12, 1085. [Google Scholar] [CrossRef] [PubMed]
- Höhl, R.; Bertram, R.; Kinzig, M.; Haarmeyer, G.S.; Baumgärtel, M.; Geise, A.; Muschner, D.; Prosch, D.; Reger, M.; Naumann, H.T.; et al. Isavuconazole Therapeutic Drug Monitoring in Critically Ill ICU Patients: A Monocentric Retrospective Analysis. Mycoses 2022, 65, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Bertram, R.; Naumann, H.T.; Bartsch, V.; Hitzl, W.; Kinzig, M.; Haarmeyer, G.S.; Baumgärtel, M.; Geise, A.; Muschner, D.; Nentwich, J.; et al. Clinical and Demographic Factors Affecting Trough Levels of Isavuconazole in Critically Ill Patients with or without COVID-19. Mycoses 2023, 66, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Jansen, A.M.E.; Mertens, B.; Spriet, I.; Verweij, P.E.; Schouten, J.; Wauters, J.; Debaveye, Y.; ter Heine, R.; Brüggemann, R.J.M. Population Pharmacokinetics of Total and Unbound Isavuconazole in Critically Ill Patients: Implications for Adaptive Dosing Strategies. Clin. Pharmacokinet. 2023, 62, 1701–1711. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ramos, J.; Gras-Martín, L.; Ramírez, P. Antimicrobial Pharmacokinetics and Pharmacodynamics in Critical Care: Adjusting the Dose in Extracorporeal Circulation and to Prevent the Genesis of Multiresistant Bacteria. Antibiotics 2023, 12, 475. [Google Scholar] [CrossRef]
- Lyster, H.; Shekar, K.; Watt, K.; Reed, A.; Roberts, J.A.; Abdul-Aziz, M.H. Antifungal Dosing in Critically Ill Patients on Extracorporeal Membrane Oxygenation. Clin. Pharmacokinet. 2023, 62, 931–942. [Google Scholar] [CrossRef]
- Zurl, C.; Waller, M.; Schwameis, F.; Muhr, T.; Bauer, N.; Zollner-Schwetz, I.; Valentin, T.; Meinitzer, A.; Ullrich, E.; Wunsch, S.; et al. Isavuconazole Treatment in a Mixed Patient Cohort with Invasive Fungal Infections: Outcome, Tolerability and Clinical Implications of Isavuconazole Plasma Concentrations. J. Fungi 2020, 6, 90. [Google Scholar] [CrossRef]
- Miller, M.; Kludjian, G.; Mohrien, K.; Morita, K. Decreased Isavuconazole Trough Concentrations in the Treatment of Invasive Aspergillosis in an Adult Patient Receiving Extracorporeal Membrane Oxygenation Support. Am. J. Health Pharm. 2022, 79, 1245–1249. [Google Scholar] [CrossRef]
- Perez, L.; Corne, P.; Pasquier, G.; Konecki, C.; Sadek, M.; Le Bihan, C.; Klouche, K.; Mathieu, O.; Reynes, J.; Cazaubon, Y. Population Pharmacokinetics of Isavuconazole in Critical Care Patients with COVID-19-Associated Pulmonary Aspergillosis and Monte Carlo Simulations of High Off-Label Doses. J. Fungi 2023, 9, 211. [Google Scholar] [CrossRef]
- Mueller, S.C.; Karasch, I.; Lakner, J.; Wacke, R. Validation of an Isavuconazole High-Performance Liquid Chromatography Assay in Plasma for Routine Therapeutic Drug Monitoring Applications. Ther. Drug Monit. 2018, 40, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Furfaro, E.; Signori, A.; Di Grazia, C.; Dominietto, A.; Raiola, A.M.; Aquino, S.; Ghiggi, C.; Ghiso, A.; Ungaro, R.; Angelucci, E.; et al. Serial Monitoring of Isavuconazole Blood Levels during Prolonged Antifungal Therapy. J. Antimicrob. Chemother. 2019, 74, 2341–2346. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.V.; Kovanda, L.L.; Hope, W.W.; Andes, D.; Mouton, J.W.; Kowalski, D.L.; Townsend, R.W.; Mujais, S.; Bonate, P.L. Exposure-Response Relationships for Isavuconazole in Patients with Invasive Aspergillosis and Other Filamentous Fungi. Antimicrob. Agents Chemother. 2017, 61, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chan Kwong, A.H.X.P.; Calvier, E.A.M.; Fabre, D.; Gattacceca, F.; Khier, S. Prior Information for Population Pharmacokinetic and Pharmacokinetic/Pharmacodynamic Analysis: Overview and Guidance with a Focus on the NONMEM PRIOR Subroutine. J. Pharmacokinet. Pharmacodyn. 2020, 47, 431–446. [Google Scholar] [CrossRef] [PubMed]
- Kriegl, L.; Hatzl, S.; Zurl, C.; Reisinger, A.C.; Schilcher, G.; Eller, P.; Gringschl, Y.; Muhr, T.; Meinitzer, A.; Prattes, J.; et al. Isavuconazole Plasma Concentrations in Critically Ill Patients during Extracorporeal Membrane Oxygenation. J. Antimicrob. Chemother. 2022, 77, 2500–2505. [Google Scholar] [CrossRef] [PubMed]
- Koehler, P.; Bassetti, M.; Chakrabarti, A.; Chen, S.C.A.; Colombo, A.L.; Hoenigl, M.; Klimko, N.; Lass-Flörl, C.; Oladele, R.O.; Vinh, D.C.; et al. Defining and Managing COVID-19-Associated Pulmonary Aspergillosis: The 2020 ECMM/ISHAM Consensus Criteria for Research and Clinical Guidance. Lancet Infect. Dis. 2021, 21, e149–e162. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Flörl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and Management of Aspergillus Diseases: Executive Summary of the 2017 ESCMID-ECMM-ERS Guideline. Clin. Microbiol. Infect. 2018, 24, e1–e38. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, P.; Garnacho-Montero, J. Invasive Aspergillosis in Critically Ill Patients. Rev. Iberoam. Micol. 2018, 35, 210–216. [Google Scholar] [CrossRef]
- Alqarihi, A.; Kontoyiannis, D.P.; Ibrahim, A.S. Mucormycosis in 2023: An Update on Pathogenesis and Management. Front. Cell. Infect. Microbiol. 2023, 13, 1254919. [Google Scholar] [CrossRef]
- Kanj, S.S.; Omrani, A.S.; Al-Abdely, H.M.; Subhi, A.; El Fakih, R.; Abosoudah, I.; Kanj, H.; Dimopoulos, G. Survival Outcome of Empirical Antifungal Therapy and the Value of Early Initiation: A Review of the Last Decade. J. Fungi 2022, 8, 1146. [Google Scholar] [CrossRef]
- McCarthy, M.W.; Moriyama, B.; Petraitiene, R.; Walsh, T.J.; Petraitis, V. Clinical Pharmacokinetics and Pharmacodynamics of Isavuconazole. Clin. Pharmacokinet. 2018, 57, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events (CTCAE) v6.0; National Cancer Institute: Bethesda, MD, USA, 2020.
- Gómez-López, A. Antifungal Therapeutic Drug Monitoring: Focus on Drugs without a Clear Recommendation. Clin. Microbiol. Infect. 2020, 26, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Andes, D.; Kovanda, L.; Desai, A.; Kitt, T.; Zhao, M.; Walsh, T.J. Isavuconazole Concentration in Real-World Practice: Consistency with Results from Clinical Trials. Antimicrob. Agents Chemother. 2018, 62, 10–128. [Google Scholar] [CrossRef] [PubMed]
- Kaindl, T.; Andes, D.; Engelhardt, M.; Saulay, M.; Larger, P.; Groll, A.H. Variability and Exposure-Response Relationships of Isavuconazole Plasma Concentrations in the Phase 3 SECURE Trial of Patients with Invasive Mould Diseases. J. Antimicrob. Chemother. 2019, 74, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Mikulska, M.; Melchio, M.; Signori, A.; Ullah, N.; Miletich, F.; Sepulcri, C.; Limongelli, A.; Giacobbe, D.; Balletto, E.; Russo, C.; et al. Lower Blood Levels of Isavuconazole in Critically Ill Patients Compared with Other Populations: Possible Need for Therapeutic Drug Monitoring. J. Antimicrob. Chemother. 2024, 79, 37. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Venkataramanan, R.; Rivosecchi, R.M.; Tang, C.; Marini, R.V.; Shields, R.K.; Clancy, C.J.; Nguyen, M.H. Population Pharmacokinetics of Intravenous Isavuconazole in Solid-Organ Transplant Recipients. Antimicrob. Agents Chemother. 2020, 64, 1–13. [Google Scholar] [CrossRef]
- Hatzl, S.; Kriegl, L.; Posch, F.; Schilcher, G.; Eller, P.; Reisinger, A.; Grinschgl, Y.; Muhr, T.; Meinitzer, A.; Hoenigl, M.; et al. Early Attainment of Isavuconazole Target Concentration Using an Increased Loading Dose in Critically Ill Patients with Extracorporeal Membrane Oxygenation. J. Antimicrob. Chemother. 2023, 78, 2902–2908. [Google Scholar] [CrossRef]
| Total (n = 24) | |
|---|---|
| Cause of ICU Admission | |
| Septic shock (n, %) | 3 (12.5) |
| Cardiogenic shock (n, %) | 4 (16.7) |
| Acute respiratory failure (n, %) | 15 (62.5) |
| Acute stroke (n, %) | 2 (8.3) |
| Co-morbidities | |
| Haematological disease | 2 (8.3) |
| Diabetes mellitus, n (%) | 5 (20.8) |
| HIV, n (%) | 1 (4.17) |
| Corticosteroid therapy, n (%) | 3 (12.5) |
| Immunosuppression, n (%) Liver disease, n (%) | 4 (16.7) 0 (0.0) |
| Age (years) (mean, SD) | 53.2 (16.4) |
| Male (n, %) | 17 (70.8) |
| Weight (kg) (mean, SD) | 75.1 (12.6) |
| Height (cm) (mean, SD) | 169.7 (0.1) |
| Body mass index (kg/m2) (mean, SD) | 26.03 (3.77) |
| SAPS3 score (median, IQR) | 63 (57–71.5) |
| Mechanical ventilation (n, %) | 23 (95.8) |
| CRRT (n, %) | 8 (33.3) |
| ECMO (n, %) | 7 (29.1) |
| Length of ICU stay (days) (mean, SD) | 47.3 (39.1) |
| ICU mortality (n, %) | 14 (58.3) |
| Biological parameters | |
| Serum creatinine (median, IQR) | 0.61 (0.47–0.79) |
| Albumin (g/L) (mean, SD) | 2.4 (0.4) |
| AST (U/L) (median, IQR) | 60 (28–70) |
| ALT (U/L) (median, IQR) | 51.5 (22.102) |
| GGT (U/L) (median, IQR) | 270 (211–309) |
| Total bilirubin (μmol/L) (mean, SD) | 1.1 (1.2) |
| Mycological diagnosis * | |
| Aspergillus galactomannan antigen in BAL (median, IQR) | 0.122 (0.038–0.169) |
| Aspergillus galactomannan antigen in serum (median, IQR) | 0.045 (0.013–0.428) |
| 1,3-beta-d-glucan (pg/mL) (median, IQR) | 7.3 (4.3–37.9) |
| Parameter (Units) | Mean (SE) | %RSE | CI95% | Shrinkage (%) | %CV |
|---|---|---|---|---|---|
| Volume of distribution (L) | 122 (20.7) | 17 | 81.43–162.57 | 16.6 | 67 |
| Clearance (L/h) | 5.36 (0.576) | 35.7 | 4.23–6.49 | 9.6 | 45.3 |
| AUC (mg·h/L) | 37.31 (48.5) | - | - | - | - |
| Clinical Response (n = 6) | Non-Clinical Response (n = 5) | p | Microbiological Response (n = 8) | Non-Microbiological Response (n = 3) | p | ECMO (n = 7) | noECMO noCRRT (n = 11) | p | CRRT (n = 8) | noECMO noCRRT (n = 11) | p | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cmin (mean (SD)) | 1.85 (1.22) | 1.45 (0.52) | 0.558 | 1.66 (1.09) | 1.8 (0.57) | 0.871 | 1.57 (0.63) | 1.92 (0.21) | 0.524 | 1.70 (0.40) | 1.92 (0.21) | 0.609 |
| <1 mg/L (n, %) | 1 (16.7) | - | - | 1 (12.5) | 0 (0.0) | - | 2 (28.6) | 1 (9.0) | - | 1 (12.5) | 1 (9.0) | - |
| >5 mg/L (n, %) | - | - | - | - | - | - | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Cerezuela, M.; Maya Gallegos, C.; Marqués-Miñana, M.R.; Broch Porcar, M.J.; Cruz-Sánchez, A.; Mateo-Pardo, J.C.; Peris Ribera, J.E.; Gimeno, R.; Castellanos-Ortega, Á.; Poveda Andrés, J.L.; et al. Isavuconazole Pharmacokinetics in Critically Ill Patients: Relationship with Clinical Effectiveness and Patient Safety. Antibiotics 2024, 13, 706. https://doi.org/10.3390/antibiotics13080706
Martín-Cerezuela M, Maya Gallegos C, Marqués-Miñana MR, Broch Porcar MJ, Cruz-Sánchez A, Mateo-Pardo JC, Peris Ribera JE, Gimeno R, Castellanos-Ortega Á, Poveda Andrés JL, et al. Isavuconazole Pharmacokinetics in Critically Ill Patients: Relationship with Clinical Effectiveness and Patient Safety. Antibiotics. 2024; 13(8):706. https://doi.org/10.3390/antibiotics13080706
Chicago/Turabian StyleMartín-Cerezuela, María, Cristina Maya Gallegos, María Remedios Marqués-Miñana, María Jesús Broch Porcar, Andrés Cruz-Sánchez, Juan Carlos Mateo-Pardo, José Esteban Peris Ribera, Ricardo Gimeno, Álvaro Castellanos-Ortega, José Luis Poveda Andrés, and et al. 2024. "Isavuconazole Pharmacokinetics in Critically Ill Patients: Relationship with Clinical Effectiveness and Patient Safety" Antibiotics 13, no. 8: 706. https://doi.org/10.3390/antibiotics13080706
APA StyleMartín-Cerezuela, M., Maya Gallegos, C., Marqués-Miñana, M. R., Broch Porcar, M. J., Cruz-Sánchez, A., Mateo-Pardo, J. C., Peris Ribera, J. E., Gimeno, R., Castellanos-Ortega, Á., Poveda Andrés, J. L., & Ramírez Galleymore, P. (2024). Isavuconazole Pharmacokinetics in Critically Ill Patients: Relationship with Clinical Effectiveness and Patient Safety. Antibiotics, 13(8), 706. https://doi.org/10.3390/antibiotics13080706






