Synergistic Interactions between Selected β-Lactam Antibiotics and Cinnamic Acid and Its Chosen Derivatives
Abstract
:1. Introduction
2. Results and Discussion
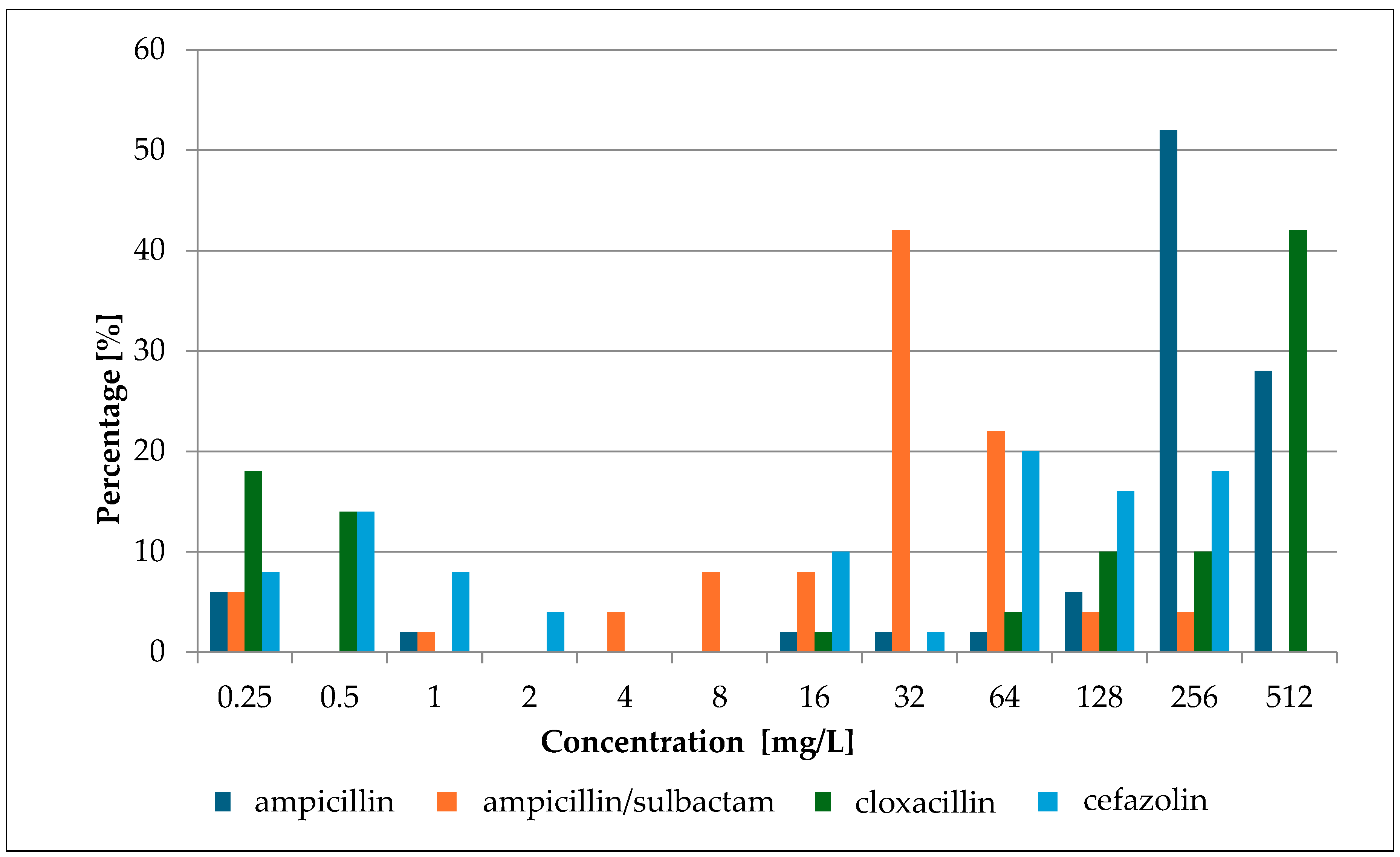
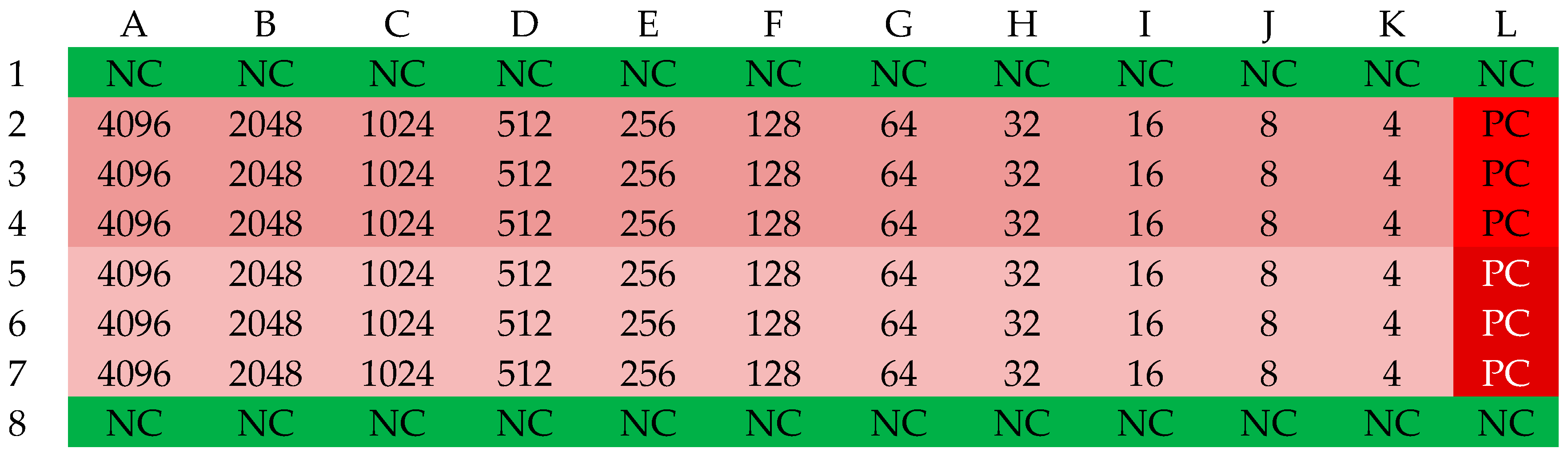
2.1. Minimum Inhibitory Concentration (MIC) of Selected Antibiotics
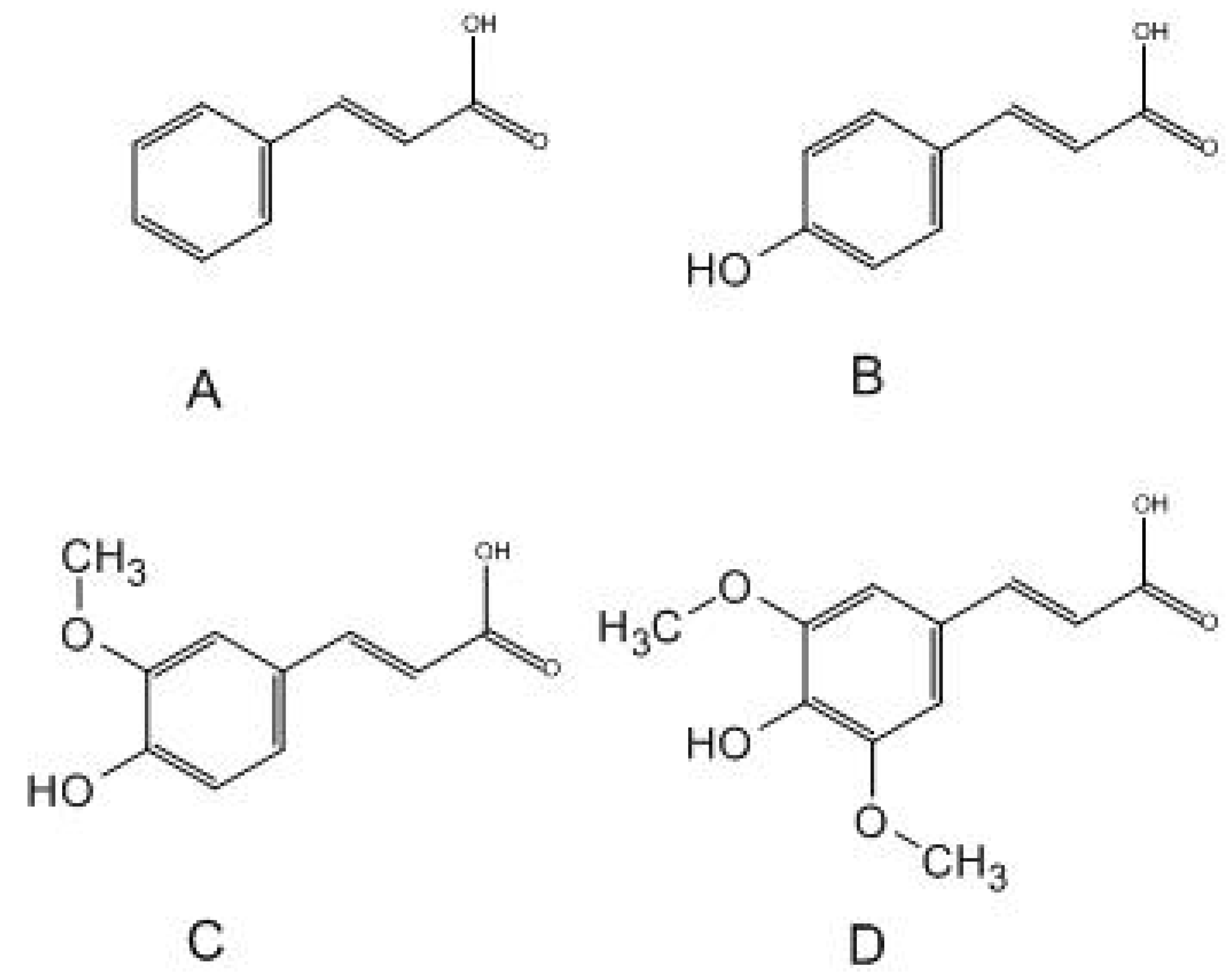
2.2. Minimum Inhibitory Concentration (MIC) of Cinnamic Acid and Derivatives
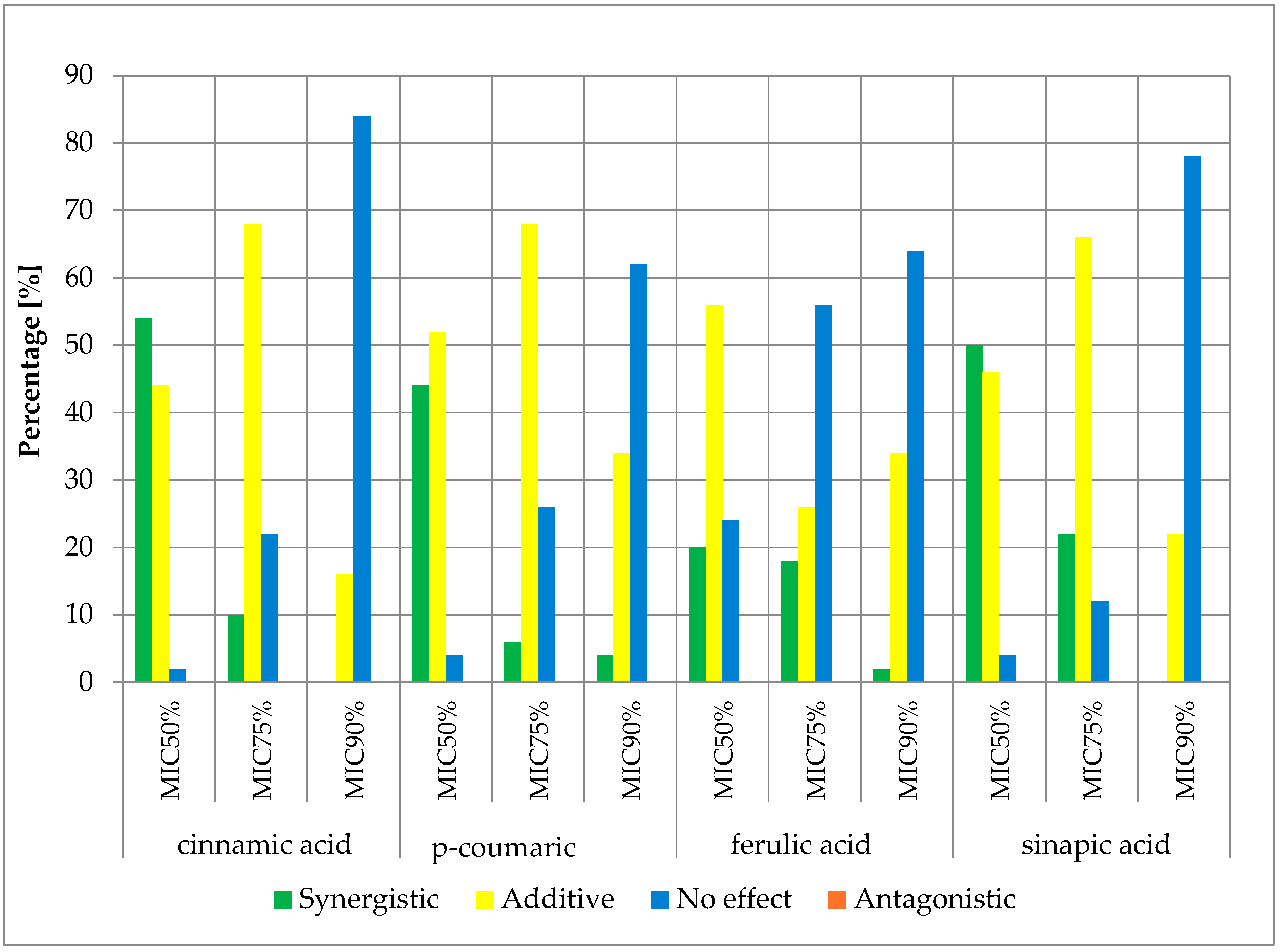
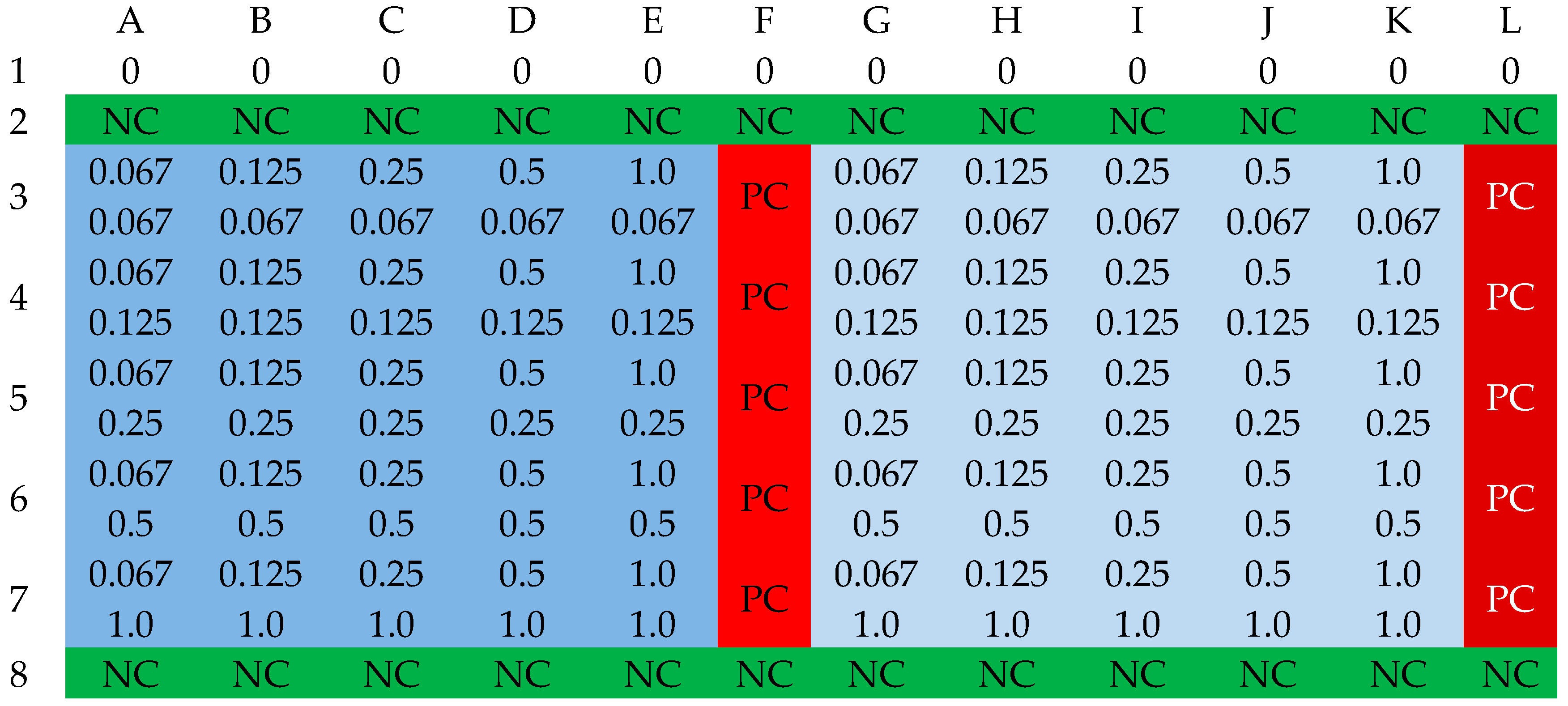
2.3. Fractional Inhibitory Concentration Index (FICi) of Selected Antibiotics and Cinnamic Acid and Derivatives
3. Experimental Section
3.1. Bacterial Strains
3.2. Determination of Antibiotic MIC Values
3.3. Determination of MIC Values of Selected Carboxylic Acids
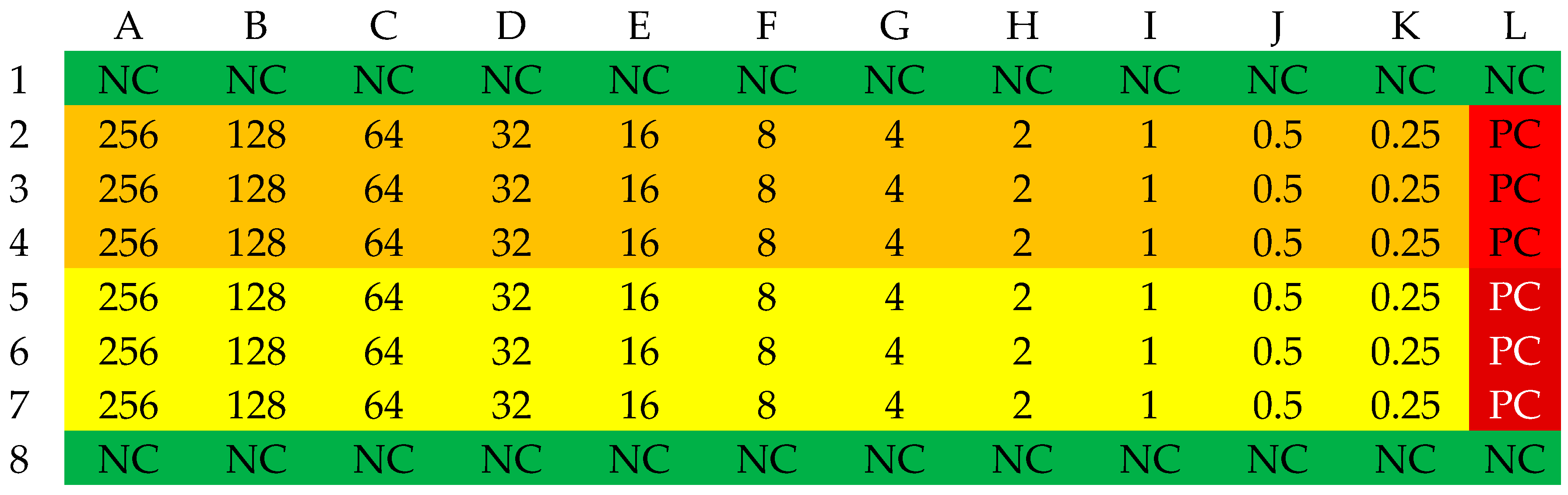
3.4. Determination of FIC Index
3.5. Interpretation of FIC Determination Results
- Synergy (FIC index ≤ 0.5);
- Additive effect (0.5 < FIC index ≤ 1);
- Lack of pharmacological effect (1 < FIC index ≤ 4);
- Antagonistic effect (FIC index > 4) [43].
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, M.M.; Horswill, A.R. Staphylococcus epidermidis-Skin friend or foe? PLoS Pathog. 2020, 16, e1009026. [Google Scholar] [CrossRef] [PubMed]
- Severn, M.M.; Horswill, A.R. Staphylococcus epidermidis and its dual lifestyle in skin health and infection. Nat. Rev. Microbiol. 2023, 21, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Argemi, X.; Hansmann, Y.; Prola, K.; Prévost, G. Coagulase-Negative Staphylococci Pathogenomics. Int. J. Mol. Sci. 2019, 20, 1215. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, C.; Sato, M.; Sato, C. Biofilm formation of Staphylococcus epidermidis imaged using atmospheric scanning electron microscopy. Anal. Bioanal. Chem. 2021, 413, 7549–7558. [Google Scholar] [CrossRef] [PubMed]
- Gunia-Krzyżak, A.; Słoczyńska, K.; Popiół, J.; Koczurkiewicz, P.; Marona, H.; Pękala, E. Cinnamic acid derivatives in cosmetics: Current use and future prospects. Int. J. Cosmet. Sci. 2018, 40, 356–366. [Google Scholar] [CrossRef]
- Guzman, J.D. Natural Cinnamic Acids, Synthetic Derivatives and Hybrids with Antimicrobial Activity. Molecules 2014, 19, 19292–19349. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Raouf, O.M.; El-Sayed, E.M.; Manie, M.F. Cinnamic Acid and Cinnamaldehyde Ameliorate Cisplatin-Induced Splenotoxicity in Rats. J. Biochem. Mol. Toxicol. 2015, 29, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhen, Y.; Zhu, Z.; Zhou, G.; Zheng, X. Cinnamic acid rescues behavioral deficits in a mouse model of traumatic brain injury by targeting miR-455-3p/HDAC2. Life Sci. 2019, 235, 116819. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, F.; Mascoti, L.; Sanchez, C.; Garibotto, F.; Giannini, F.; Kurina-Sanz, M.; Enriz, R. Structure-antifungal activity relationship of cinnamic acid derivatives. J. Agric. Food Chem. 2007, 55, 10635–10640. [Google Scholar] [CrossRef]
- de Morais, M.C.; de Oliveira Lima, E.; Perez-Castillo, Y.; de Sousa, D.P. Synthetic Cinnamides and Cinnamates: Antimicrobial Activity, Mechanism of Action, and In Silico Study. Molecules 2023, 28, 1918. [Google Scholar] [CrossRef]
- Carvalho, S.A.; da Silva, E.F.; de Souza, M.V.; Lourenço, M.C.; Vicente, F.R. Synthesis and antimycobacterial evaluation of new trans-cinnamic acid hydrazide derivatives. Bioorg. Med. Chem. Lett. 2008, 18, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.P.; Castillo, Y.P.; Monteiro, M.L.; Menezes, R.R.P.P.B.; Almeida, R.N.; Martins, A.M.C.; Sousa, D.P. Trypanocidal Mechanism of Action and in silico Studies of p-Coumaric Acid Derivatives. Int. J. Mol. Sci. 2019, 20, 5916. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.P.; Tomaz, D.C.; Ângelo de Souza, L.; Onofre, T.S.; Aquiles de Menezes, W.; Almeida-Silva, J.; Suarez-Fontes, A.M.; Rogéria de Almeida, M.; Manoel da Silva, A.; Bressan, G.C.; et al. Synthesis of cinnamic acid derivatives and leishmanicidal activity against Leishmania braziliensis. Eur. J. Med. Chem. 2019, 183, 111688. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Siddiqui, R.; Shah, M.R.; Khan, N.A. Gold Nanoparticle-Conjugated Cinnamic Acid Exhibits Antiacanthamoebic and Antibacterial Properties. Antimicrob. Agents Chemother. 2018, 62, e00630-18. [Google Scholar] [CrossRef] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 14.0. 2024. Available online: https://www.eucast.org (accessed on 1 January 2024).
- Ryu, H.; Mohayya, S.; Hong, T.; Modi, M.; Yang, J.; Abdul Azim, A.; Bhatt, P.J.; Brunetti, L.; Narayanan, N. Safety and Effectiveness of High-Dose Cefazolin in Patients With High Body Weight: A Retrospective Cohort Study. Open Forum. Infect. Dis. 2022, 9, ofac105. [Google Scholar] [CrossRef] [PubMed]
- David, M.Z.; Daum, R.S. Treatment of Staphylococcus aureus Infections. Curr. Top. Microbiol. Immunol. 2017, 409, 325–383. [Google Scholar] [CrossRef] [PubMed]
- Siriwong, S.; Teethaisong, Y.; Thumanu, K.; Dunkhunthod, B.; Eumkeb, G. The synergy and mode of action of quercetin plus amoxicillin against amoxicillin-resistant Staphylococcus epidermidis. BMC Pharmacol. Toxicol. 2016, 17, 39. [Google Scholar] [CrossRef]
- Ferraz, R.; Teixeira, V.; Rodrigues, D.; Fernandes, R.; Prudêncio, C.; Noronha, J.P.; Petrovski, Ž.; Branco, L.C. Antibacterial activity of Ionic Liquids based on ampicillin against resistant bacteria. RSC Adv. 2014, 4, 4301–4307. [Google Scholar] [CrossRef]
- Ba, X.; Raisen, C.L.; Restif, O.; Cavaco, L.M.; Vingsbo Lundberg, C.; Lee, J.Y.; Howden, B.P.; Bartels, M.D.; Strommenger, B.; Harrison, E.M.; et al. Cryptic susceptibility to penicillin/β-lactamase inhibitor combinations in emerging multidrug-resistant, hospital-adapted Staphylococcus epidermidis lineages. Nat. Commun. 2023, 14, 6479. [Google Scholar] [CrossRef]
- Putrawan, O.; Sri Rejeki, I.P. Ampicillin sulbactam resistance pattern as a first-line drug in children. Folia Medica Indones. 2016, 51, 187–189. [Google Scholar] [CrossRef]
- Stevoska, S.; Himmelbauer, F.; Stiftinger, J.; Stadler, C.; Gotterbarm, T.; Heyse, T.J.; Klasan, A. Significant Difference in Antimicrobial Resistance of Coagulase Negative Periprosthetic Joint Infection in Septic Revision Total Knee Arthroplasty between Two Major Orthopedic Centers. J. Arthroplast. 2022, 37, S306–S312. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, R.; Yousefimashouf, R.; Arabestani, M.R.; Sedighi, I.; Alikhani, M.Y. The issue beyond resistance: Methicillin-resistant Staphylococcus epidermidis biofilm formation is induced by subinhibitory concentrations of cloxacillin, cefazolin, and clindamycin. PLoS ONE 2022, 17, e0277287. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.M.; Dias, R.O.; Franco, O.L. Phenolic Compounds in Antimicrobial Therapy. J. Med. Food 2017, 20, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Auezova, L.; Najjar, A.; Kfoury, M.; Fourmentin, S.; Greige-Gerges, H. Antibacterial activity of free or encapsulated selected phenylpropanoids against Escherichia coli and Staphylococcus epidermidis. J. Appl. Microbiol. 2020, 128, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Taofiq, O.; Heleno, S.A.; Calhelha, R.C.; Fernandes, I.P.; Alves, M.J.; Barros, L.; González-Paramás, A.M.; Ferreira, I.C.F.R.; Barreiro, M.F. Phenolic acids, cinnamic acid, and ergosterol as cosmeceutical ingredients: Stabilization by microencapsulation to ensure sustained bioactivity. Microchem. J. 2019, 147, 469–477. [Google Scholar] [CrossRef]
- Letsididi, K.; Kekgabile, T.; Lou, Z.; Letsididi, R.; Mohammed, K.; Maguy, B. Antimicrobial and antibiofilm effects of trans-cinnamic acid nanoemulsion and its potential application on lettuce. LWT 2018, 94, 25–32. [Google Scholar] [CrossRef]
- Petrișor, G.; Motelica, L.; Ficai, D.; Ilie, C.-I.; Trușcǎ, R.D.; Surdu, V.-A.; Oprea, O.-C.; Mȋrț, A.-L.; Vasilievici, G.; Semenescu, A.; et al. Increasing Bioavailability of Trans-Ferulic Acid by Encapsulation in Functionalized Mesoporous Silica. Pharmaceutics 2023, 15, 660. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Shi, Y.; Zhang, C.; Cai, E.; Ge, X.; Xiang, Y.; Li, Y.; Zeng, B.; Shen, J. A Hybrid Hydrogel with Intrinsic Immunomodulatory Functionality for Treating Multidrug-Resistant Pseudomonas aeruginosa Infected Diabetic Foot Ulcers. ACS Mater. Lett. 2024, 6, 2533–2547. [Google Scholar] [CrossRef]
- Xiang, Y.; Pan, Z.; Qi, X.; Ge, X.; Xiang, J.; Xu, H.; Cai, E.; Lan, Y.; Chen, X.; Li, Y.; et al. A cuttlefish ink nanoparticle-reinforced biopolymer hydrogel with robust adhesive and immunomodulatory features for treating oral ulcers in diabetes. Bioact. Mater. 2024, 39, 562–581. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug. Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Engels, C.; Schieber, A. Sinapic Acid Derivatives in Defatted Oriental Mustard (Brassica juncea L.) Seed Meal Extracts Using UHPLC-DAD-ESI-MSn and Identification of Compounds with Antibacterial Activity. Eur. Food Res. Technol. 2012, 234, 535–542. [Google Scholar] [CrossRef]
- Prost, M.E.; Prost, R. Basic parameters of evaluation of the effectiveness of antibiotic therapy. OphthaTherapy 2017, 4, 233–236. [Google Scholar] [CrossRef]
- Wang, S.; Kang, O.-H.; Kwon, D.-Y. Trans-Cinnamaldehyde Exhibits Synergy with Conventional Antibiotic against Methicillin-Resistant Staphylococcus aureus. Int. J. Mol. Sci. 2021, 22, 2752. [Google Scholar] [CrossRef] [PubMed]
- Belmehdi, O.; Bouyahya, A.; Jekő, J.; Cziáky, Z.; Zengin, G.; Sotkó, G.; El Baaboua, A.; Senhaji, N.S.; Abrini, J. Synergistic Interaction between Propolis Extract, Essential Oils, and Antibiotics against Staphylococcus epidermidis and Methicillin-Resistant Staphylococcus aureus. Int. J. Second. Metab. 2021, 8, 195–213. [Google Scholar] [CrossRef]
- Ibrahim, M.K.; Mattar, Z.A.; Abdel-Khalek, H.H.; Azzam, Y.M. Evaluation of antibacterial efficacy of anise wastes against some multidrug resistant bacterial isolates. J. Radiat. Res. Appl. Sci. 2017, 10, 34–43. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Doble, M. Synergistic Interaction of Phenylpropanoids with Antibiotics against Bacteria. J. Med. Microbiol. 2010, 59, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Palaniappan, K.; Holley, R.A. Use of Natural Antimicrobials to Increase Antibiotic Susceptibility of Drug-Resistant Bacteria. Int. J. Food Microbiol. 2010, 140, 164–168. [Google Scholar] [CrossRef]
- Hałasa, R.; Bułakowska, A.; Sławiński, J.; Smoktunowicz, M.; Rapacka-Zdończyk, A.; Mizerska, U. Activity of Cinnamic Acid Derivatives with 4-Chloro-2-mercaptobenzenesulfonamide Moiety against Clinical HLAR and VRE Enterococcus spp. Antibiotics 2023, 12, 1691. [Google Scholar] [CrossRef] [PubMed]
- Wojtyczka, R.D.; Dziedzic, A.; Kępa, M.; Kubina, R.; Kabała-Dzik, A.; Mularz, T.; Idzik, D. Berberine Enhances the Antibacterial Activity of Selected Antibiotics against Coagulase-Negative Staphylococcus Strains In Vitro. Molecules 2014, 19, 6583–6596. [Google Scholar] [CrossRef]
- Miklasińska, M.; Kępa, M.; Wojtyczka, R.D.; Idzik, D.; Zdebik, A.; Orlewska, K.; Wąsik, T.J. Antibacterial Activity of Protocatechuic Acid Ethyl Ester on Staphylococcus aureus Clinical Strains Alone and in Combination with Antistaphylococcal Drugs. Molecules 2015, 20, 13536–13549. [Google Scholar] [CrossRef]
- Blanco-Blanco, J.; Bravo, M.; Simón, I.; Fernández-Llario, P.; Fajardo-Olivares, M.; Fernández-Calderón, M.C.; Cerrato, R. Synergistic Activity of Ingulados Bacteria with Antibiotics against Multidrug-Resistant Pathogens. Antibiotics 2024, 13, 200. [Google Scholar] [CrossRef] [PubMed]
- Aonofriesei, F. Effect of Methylpyrazoles and Coumarin Association on the Growth of Gram-Negative Bacteria. Arch. Microbiol. 2022, 204, 160. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zawiła, T.; Swolana, D.; Zawiła, M.; Wojtyczka, R.D. Synergistic Interactions between Selected β-Lactam Antibiotics and Cinnamic Acid and Its Chosen Derivatives. Antibiotics 2024, 13, 710. https://doi.org/10.3390/antibiotics13080710
Zawiła T, Swolana D, Zawiła M, Wojtyczka RD. Synergistic Interactions between Selected β-Lactam Antibiotics and Cinnamic Acid and Its Chosen Derivatives. Antibiotics. 2024; 13(8):710. https://doi.org/10.3390/antibiotics13080710
Chicago/Turabian StyleZawiła, Tomasz, Denis Swolana, Marta Zawiła, and Robert D. Wojtyczka. 2024. "Synergistic Interactions between Selected β-Lactam Antibiotics and Cinnamic Acid and Its Chosen Derivatives" Antibiotics 13, no. 8: 710. https://doi.org/10.3390/antibiotics13080710





