A Novel Bacitracin-like Peptide from Mangrove-Isolated Bacillus paralicheniformis NNS4-3 against MRSA and Its Genomic Insights
Abstract
1. Introduction
2. Results
2.1. Exploration of Antimicrobial-Producing Bacterial Isolates from Mangrove Sediments
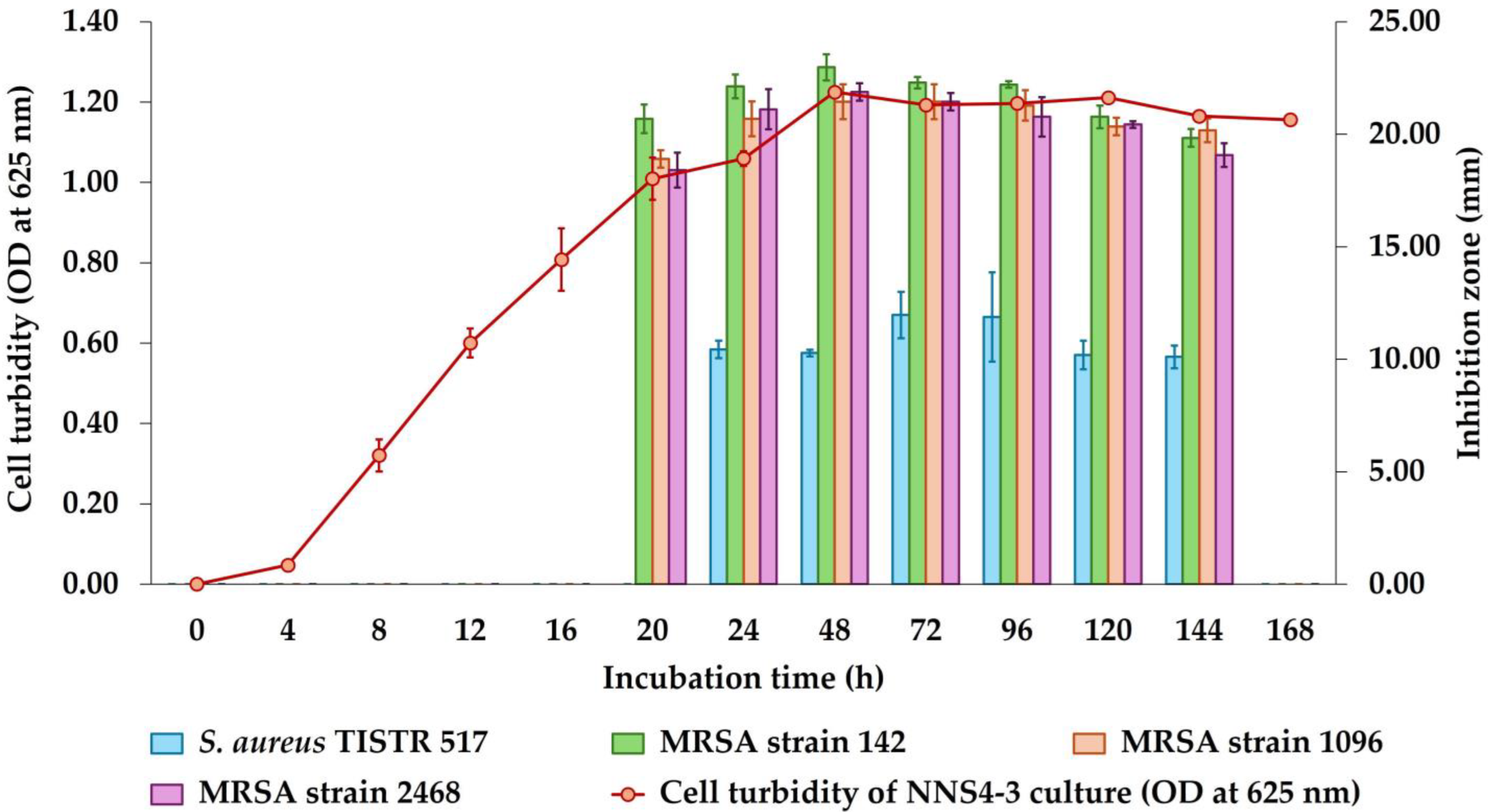
2.2. Kinetics of Antibacterial Component Production of NNS4-3
2.3. Purification of the Active Antibacterial Components
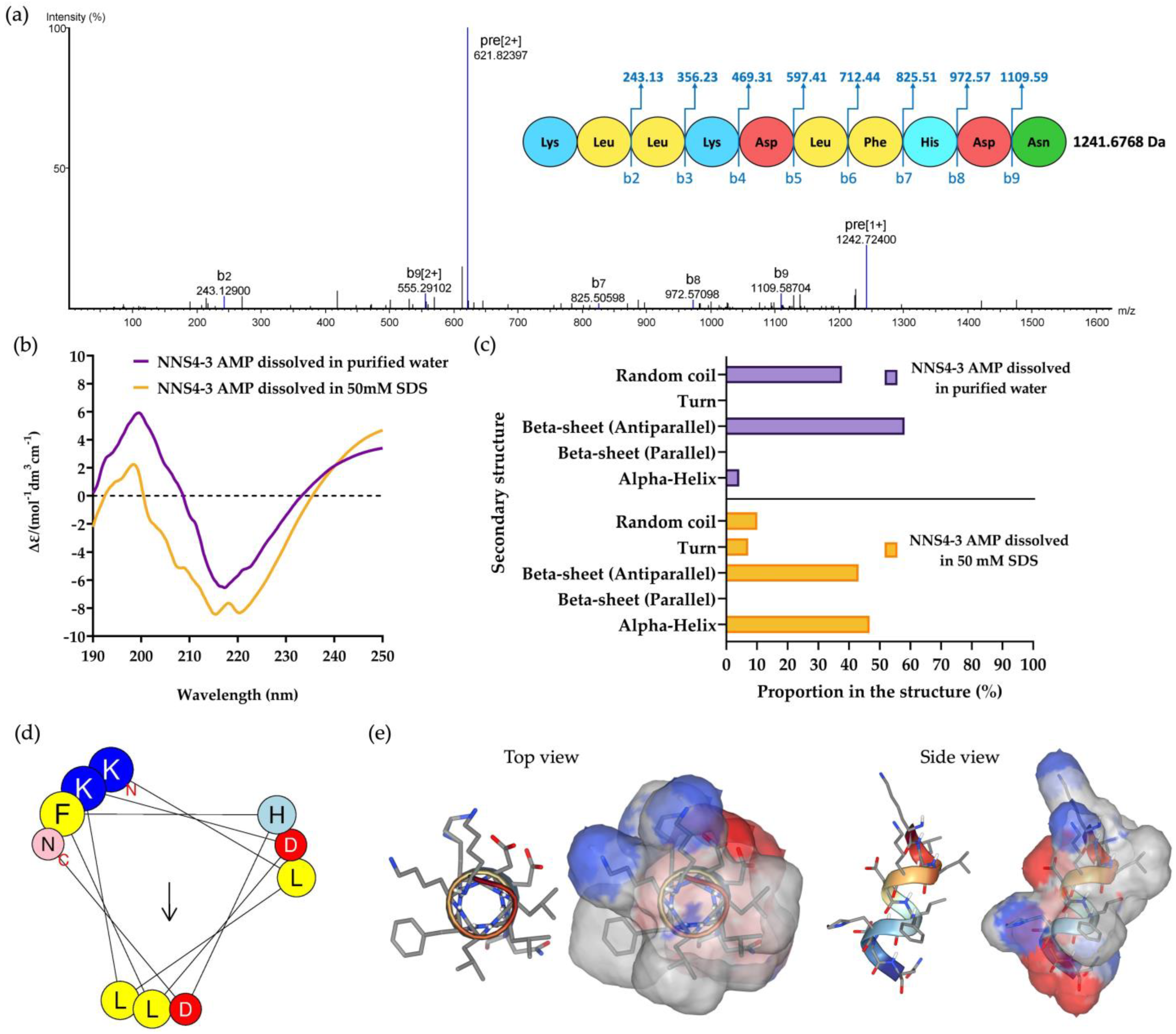
2.4. De Novo Amino Acid Sequence of the Purified AMP and Secondary Structure Determination
2.5. Determination of the Antimicrobial Activity of NNS4-3 AMP by the Microdilution Method
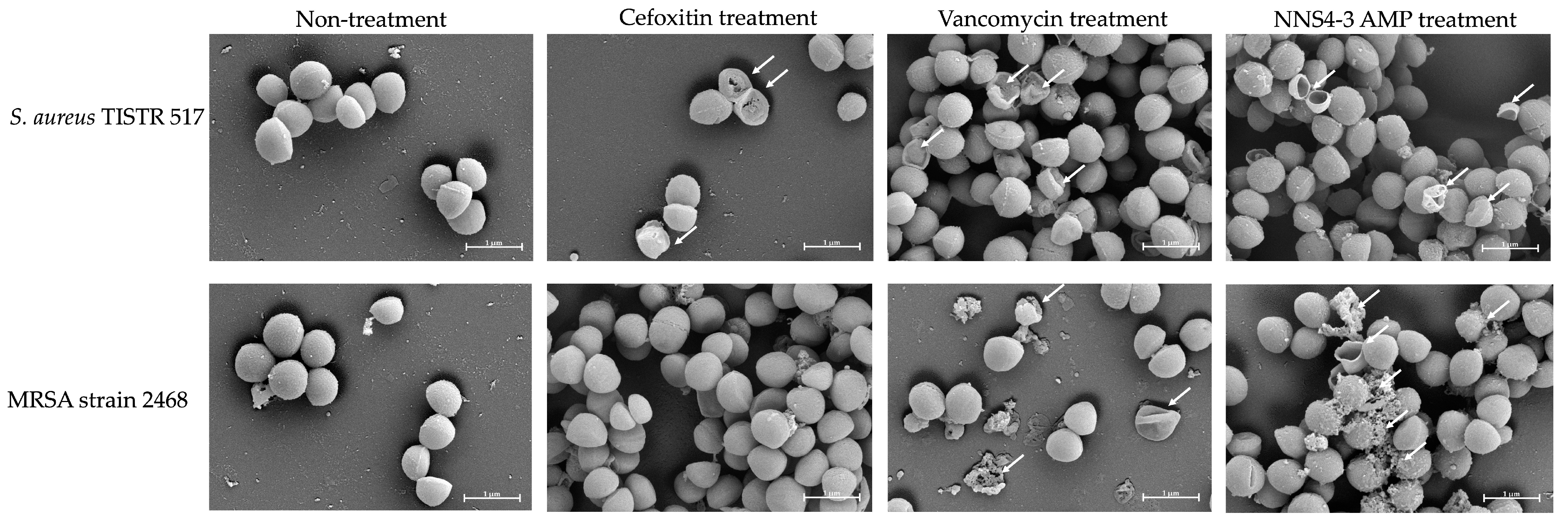
2.6. Scanning Electron Microscopic Studies
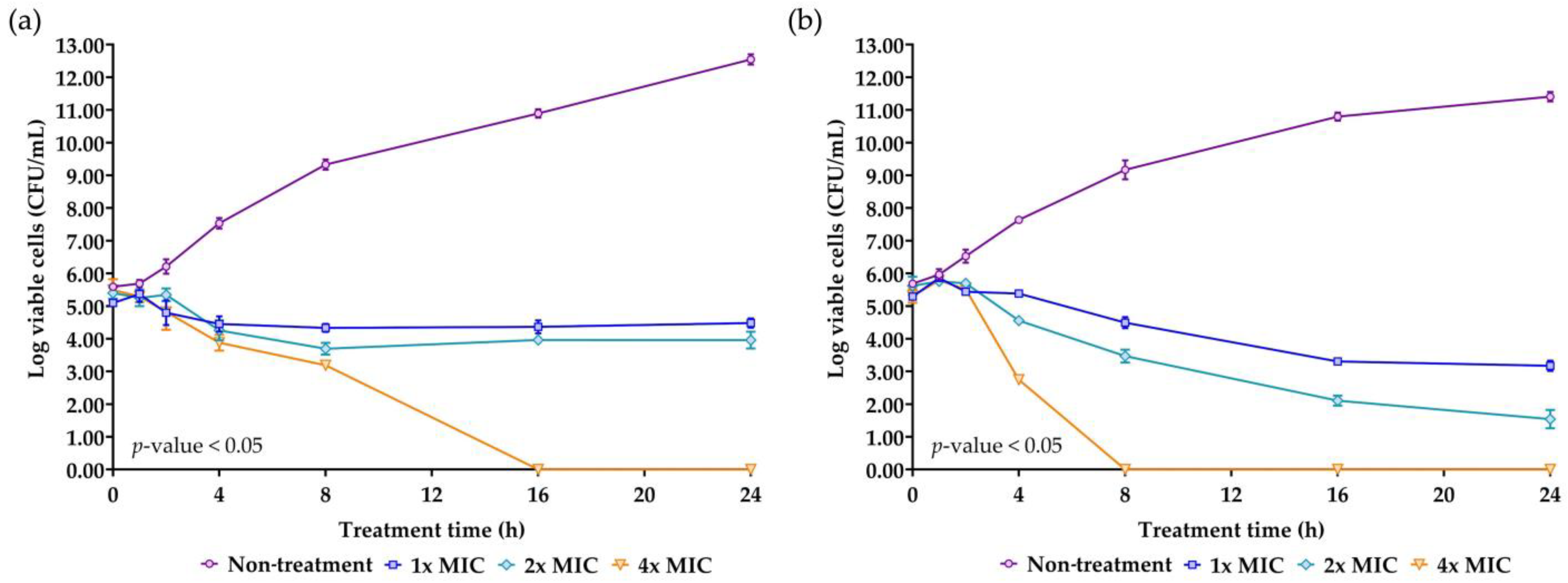
2.7. Killing Kinetics of the AMP Isolated from NNS4-3
2.8. Stability Determination of NNS4-3 AMP under Various Conditions: Temperature, Proteolytic Enzymes, Surfactants, and Acid-Base Treatment
2.9. Phenotypic Characterization of NNS4-3
2.10. Genome Insights for Coding Sequence Annotation and Genome-Based Phylogenetic Tree Construction
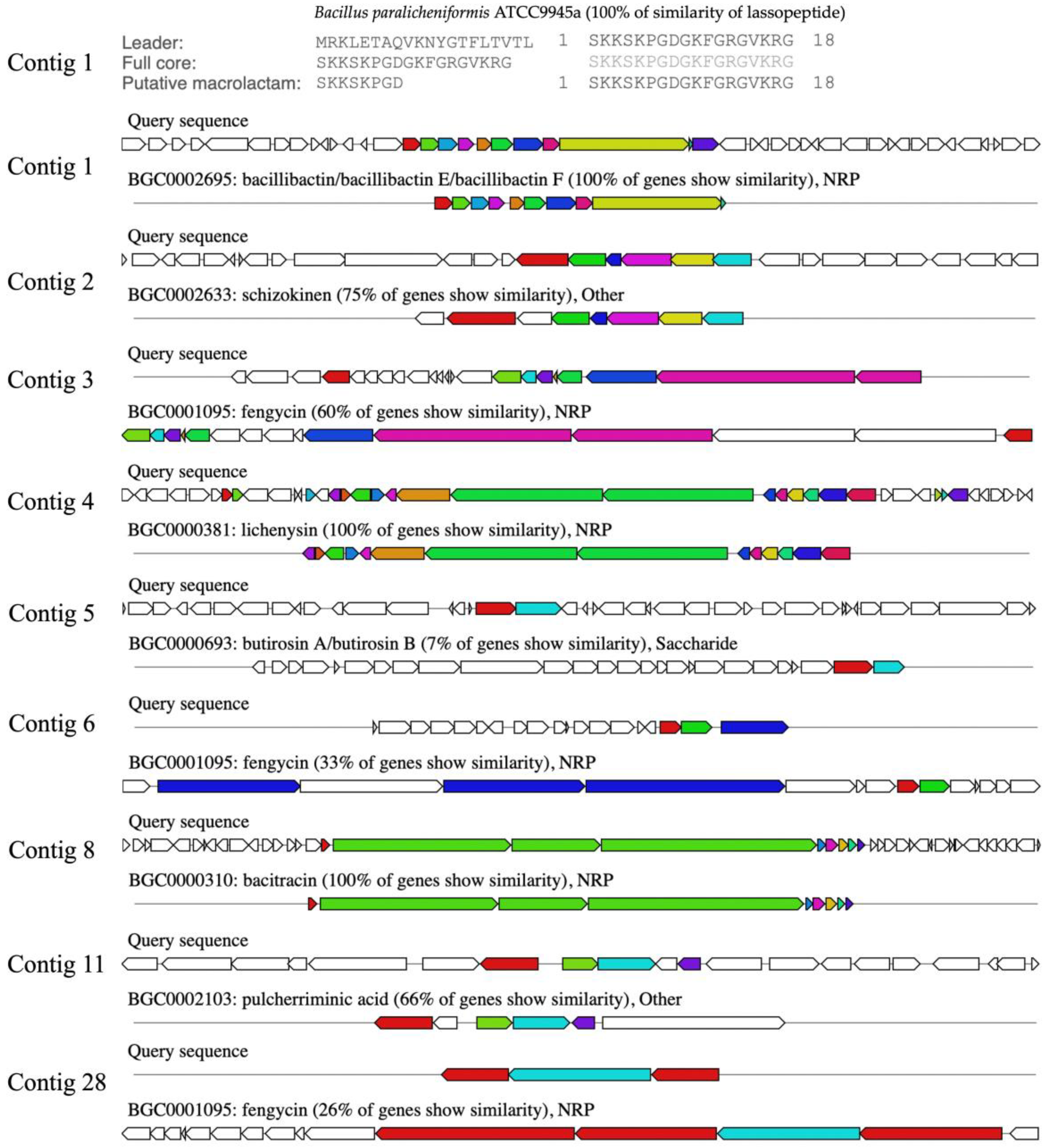
2.11. Comparison of Biosynthetic Gene Clusters of NNS4-3
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Bacterial Isolation
4.2. Screening of Antibacterial Activity by the Soft Agar Overlay Method against the MRSA Strain
4.3. Verification of Antibacterial Activity by the Agar Well Diffusion Method
4.4. Studies on the Production Kinetics of Antimicrobial Compounds
4.5. Purification of Anti-MRSA Components
4.6. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and Agar Overlay Assay
4.7. Peptide Sequencing by Mass Spectrometry and De Novo Peptide Sequencing
4.8. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of the Antimicrobial Compound
4.9. Scanning Electron Microscopy (SEM) of AMP-Treated Cells
4.10. Time-Kill Kinetics of the NNS4-3 AMP
4.11. Stability Studies of the NNS4-3 AMP
4.12. Bacterial Morphology Characterization
4.13. Whole Genome Sequencing and Bioinformatic Analysis
4.14. Antibiotic Susceptibility Test of NNS4-3
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- O’Neill, J. Review on Antimicrobial Resistance, Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Wellcome Trust and HM Government: London, UK, 2016; pp. 10–16.
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G., Jr. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef]
- Hartman, B.J.; Tomasz, A. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J. Bacteriol. 1984, 158, 513–516. [Google Scholar] [CrossRef]
- Dubos, R.J. Studies on a bactericidal agent extracted from a soil bacillus: I. Preparation of the agent. Its activity in vitro. J. Exp. Med. 1939, 70, 1–10. [Google Scholar]
- Dubos, R.J. Studies on a bactericidal agent extracted from a soil bacillus: II. Protective effect of the bactericidal agent against experimental pneumococcus infections in mice. J. Exp. Med. 1939, 70, 11–17. [Google Scholar] [CrossRef]
- Bin Hafeez, A.; Jiang, X.; Bergen, P.J.; Zhu, Y. Antimicrobial peptides: An update on classifications and databases. Int. J. Mol. Sci. 2021, 22, 11691. [Google Scholar] [CrossRef]
- Peschel, A.; Sahl, H.G. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat. Rev. Microbiol. 2006, 4, 529–536. [Google Scholar] [CrossRef]
- Ribeiro, R.; Pinto, E.; Fernandes, C.; Sousa, E. Marine cyclic peptides: Antimicrobial activity and synthetic strategies. Mar. Drugs 2022, 20, 397. [Google Scholar] [CrossRef]
- Wibowo, J.T.; Bayu, A.; Aryati, W.D.; Fernandes, C.; Yanuar, A.; Kijjoa, A.; Putra, M.Y. Secondary metabolites from marine-derived bacteria with antibiotic and antibiofilm activities against drug-resistant pathogens. Mar. Drugs 2023, 21, 50. [Google Scholar] [CrossRef]
- Waghu, F.H.; Joseph, S.; Ghawali, S.; Martis, E.A.; Madan, T.; Venkatesh, K.V.; Idicula-Thomas, S. Designing antibacterial peptides with enhanced killing kinetics. Front. Microbiol. 2018, 9, 325. [Google Scholar] [CrossRef]
- Hendlin, D.; Stapley, E.O.; Jackson, M.; Wallick, H.; Miller, A.K.; Wolf, F.J.; Miller, T.W.; Chaiet, L.; Kahan, F.M.; Foltz, E.L.; et al. Phosphonomycin, a new antibiotic produced by strains of streptomyces. Science 1969, 166, 122–123. [Google Scholar] [CrossRef]
- Donadio, S.; Sosio, M.; Stegmann, E.; Weber, T.; Wohlleben, W. Comparative analysis and insights into the evolution of gene clusters for glycopeptide antibiotic biosynthesis. Mol. Genet. Genom. 2005, 274, 40–50. [Google Scholar] [CrossRef]
- Torkar, K.G.; Bedenić, B. Antimicrobial susceptibility and characterization of metallo-β-lactamases, extended-spectrum β-lactamases, and carbapenemases of Bacillus cereus isolates. Microb. Pathog. 2018, 118, 140–145. [Google Scholar] [CrossRef]
- Podlesek, Z.; Comino, A.; Herzog-Velikonja, B.; Zgur-Bertok, D.; Komel, R.; Grabnar, M. Bacillus licheniformis bacitracin-resistance ABC transporter: Relationship to mammalian multidrug resistance. Mol. Microbiol. 1995, 16, 969–976. [Google Scholar] [CrossRef]
- Bernard, R.; El Ghachi, M.; Mengin-Lecreulx, D.; Chippaux, M.; Denizot, F. BcrC from Bacillus subtilis acts as an undecaprenyl pyrophosphate phosphatase in bacitracin resistance. J. Biol. Chem. 2005, 280, 28852–28857. [Google Scholar] [CrossRef]
- Harel, Y.M.; Bailone, A.; Bibi, E. Resistance to bacitracin as modulated by an Escherichia coli homologue of the bacitracin ABC transporter BcrC subunit from Bacillus licheniformis. J. Bacteriol. 1999, 181, 6176–6178. [Google Scholar] [CrossRef]
- Majumder, M.A.A.; Rahman, S.; Cohall, D.; Bharatha, A.; Singh, K.; Haque, M.; Gittens-St Hilaire, M. Antimicrobial Stewardship: Fighting antimicrobial resistance and protecting global public health. Infect. Drug Resist. 2020, 13, 4713–4738. [Google Scholar] [CrossRef]
- Hasanpour, A.H.; Sepidarkish, M.; Mollalo, A.; Ardekani, A.; Almukhtar, M.; Mechaal, A.; Hosseini, S.R.; Bayani, M.; Javanian, M.; Rostami, A. The global prevalence of methicillin-resistant Staphylococcus aureus colonization in residents of elderly care centers: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 2023, 12, 4. [Google Scholar] [CrossRef]
- Wu, X.; Wang, C.; He, L.; Xu, H.; Jing, C.; Chen, Y.; Lin, A.; Deng, J.; Cao, Q.; Deng, H.; et al. Antimicrobial resistance profile of methicillin-resistant Staphylococcus aureus isolates in children reported from the ISPED surveillance of bacterial resistance, 2016–2021. Front. Cell. Infect. Microbiol. 2023, 13, 1102779. [Google Scholar] [CrossRef]
- Palit, K.; Rath, S.; Chatterjee, S.; Das, S. Microbial diversity and ecological interactions of microorganisms in the mangrove ecosystem: Threats, vulnerability, and adaptations. Environ. Sci. Pollut. Res. Int. 2022, 29, 32467–32512. [Google Scholar] [CrossRef]
- Li, K.; Chen, S.; Pang, X.; Cai, J.; Zhang, X.; Liu, Y.; Zhu, Y.; Zhou, X. Natural products from mangrove sediments-derived microbes: Structural diversity, bioactivities, biosynthesis, and total synthesis. Eur. J. Med. Chem. 2022, 230, 114117. [Google Scholar] [CrossRef]
- Rajan, L.; Chakraborty, K.; Chakraborty, R.D. Pharmacological properties of some mangrove sediment-associated bacillus isolates. Arch. Microbiol. 2021, 203, 67–76. [Google Scholar] [CrossRef]
- Tran, C.; Cock, I.E.; Chen, X.; Feng, Y. Antimicrobial Bacillus: Metabolites and their mode of action. Antibiotics 2022, 11, 88. [Google Scholar] [CrossRef]
- Du, Y.; Ma, J.; Yin, Z.; Liu, K.; Yao, G.; Xu, W.; Fan, L.; Du, B.; Ding, Y.; Wang, C. Comparative genomic analysis of Bacillus paralicheniformis MDJK30 with its closely related species reveals an evolutionary relationship between B. paralicheniformis and B. licheniformis. BMC Genom. 2019, 20, 283. [Google Scholar] [CrossRef]
- Xu, J.; Qin, L.; Xu, X.; Shen, H.; Yang, X. Bacillus paralicheniformis RP01 enhances the expression of growth-related genes in cotton and promotes plant growth by altering microbiota inside and outside the root. Int. J. Mol. Sci. 2023, 24, 7227. [Google Scholar] [CrossRef]
- Rachinger, M.; Volland, S.; Meinhardt, F.; Daniel, R.; Liesegang, H. First Insights into the completely annotated genome sequence of Bacillus licheniformis strain 9945A. Genome Announc. 2013, 1, e00525-13. [Google Scholar] [CrossRef]
- Olajide, A.M.; Chen, S.; LaPointe, G. Markers to rapidly distinguish Bacillus paralicheniformis from the very close relative, Bacillus licheniformis. Front. Microbiol. 2021, 11, 596828. [Google Scholar] [CrossRef]
- Asif, M.; Li-Qun, Z.; Zeng, Q.; Atiq, M.; Ahmad, K.; Tariq, A.; Al-Ansari, N.; Blom, J.; Fenske, L.; Alodaini, H.A.; et al. Comprehensive genomic analysis of Bacillus paralicheniformis strain BP9, pan-genomic and genetic basis of biocontrol mechanism. Comput. Struct. Biotechnol. J. 2023, 21, 4647–4662. [Google Scholar] [CrossRef]
- Yeak, K.Y.C.; Perko, M.; Staring, G.; Fernandez-Ciruelos, B.M.; Wells, J.M.; Abee, T.; Wells-Bennik, M.H.J. Lichenysin Production by Bacillus licheniformis food isolates and toxicity to human cells. Front. Microbiol. 2022, 13, 831033. [Google Scholar] [CrossRef]
- Chen, X.H.; Koumoutsi, A.; Scholz, R.; Eisenreich, A.; Schneider, K.; Heinemeyer, I.; Morgenstern, B.; Voss, B.; Hess, W.R.; Reva, O.; et al. Comparative analysis of the complete genome sequence of the plant growth-promoting bacterium Bacillus amyloliquefaciens FZB42. Nat. Biotechnol. 2007, 25, 1007–1014. [Google Scholar] [CrossRef]
- Wu, S.; Zhong, J.; Huan, L. Genetics of subpeptin JM4-A and subpeptin JM4-B production by Bacillus subtilis JM4. Biochem. Biophys. Res. Commun. 2006, 344, 1147–1154. [Google Scholar] [CrossRef]
- Adimpong, D.B.; Sørensen, K.I.; Thorsen, L.; Stuer-Lauridsen, B.; Abdelgadir, W.S.; Nielsen, D.S.; Derkx, P.M.; Jespersen, L. Antimicrobial susceptibility of Bacillus strains isolated from primary starters for African traditional bread production and characterization of the bacitracin operon and bacitracin biosynthesis. Appl. Environ. Microbiol. 2012, 78, 7903–7914. [Google Scholar] [CrossRef]
- Pattnaik, P.; Kaushik, J.K.; Grover, S.; Batish, V.K. Purification and characterization of a bacteriocin-like compound (Lichenin) produced anaerobically by Bacillus licheniformis isolated from water buffalo. J. Appl. Microbiol. 2001, 91, 636–645. [Google Scholar] [CrossRef]
- Lundeen, R.A.; Chu, C.; Sander, M.; McNeill, K. Photooxidation of the antimicrobial, nonribosomal peptide bacitracin A by singlet oxygen under environmentally relevant conditions. Environ. Sci. Technol. 2016, 50, 8586–8595. [Google Scholar] [CrossRef]
- Jeong, D.W.; Lee, B.; Heo, S.; Oh, Y.; Heo, G.; Lee, J.H. Two genes involved in clindamycin resistance of Bacillus licheniformis and Bacillus paralicheniformis identified by comparative genomic analysis. PLoS ONE 2020, 15, e0231274. [Google Scholar] [CrossRef]
- Ngom, S.I.; Maski, S.; Rached, B.; Chouati, T.; Oliveira Correia, L.; Juste, C.; Meylheuc, T.; Henrissat, B.; El Fahime, E.; Amar, M.; et al. Exploring the hemicellulolytic properties and safety of Bacillus paralicheniformis as stepping stone in the use of new fibrolytic beneficial microbes. Sci. Rep. 2023, 13, 22785. [Google Scholar] [CrossRef]
- Agersø, Y.; Stuer-Lauridsen, B.; Bjerre, K.; Jensen, M.G.; Johansen, E.; Bennedsen, M.; Brockmann, E.; Nielsen, B. Antimicrobial susceptibility testing and tentative epidemiological cutoff values for five Bacillus species relevant for use as animal feed additives or for plant protection. Appl. Environ. Microbiol. 2018, 84, e01108-18. [Google Scholar] [CrossRef]
- Husna, K.B.E.; Won, M.H.; Jeong, M.I.; Oh, K.K.; Park, D.S. Characterization and genomic insight of surfactin-producing Bacillus velezensis and its biocontrol potential against pathogenic contamination in lettuce hydroponics. Environ. Sci. Pollut. Res. Int. 2023, 30, 121487–121500. [Google Scholar] [CrossRef]
- Dasagrandhi, C.; Pandith, A.; Imran, K. Sclerotiorin: A novel Azaphilone with demonstrated membrane targeting and DNA binding activity against methicillin-resistant Staphylococcus aureus. Microbiol. Biotechnol. Lett. 2020, 48, 429–438. [Google Scholar] [CrossRef]
- Yoshida, Y.; Matsuo, M.; Oogai, Y.; Kato, F.; Nakamura, N.; Sugai, M.; Komatsuzawa, H. Bacitracin sensing and resistance in Staphylococcus aureus. FEMS Microbiol. Lett. 2011, 320, 33–39. [Google Scholar] [CrossRef]
- Ahire, J.J.; Kashikar, M.S.; Lakshmi, S.G.; Madempudi, R. Identification and characterization of antimicrobial peptide produced by indigenously isolated Bacillus paralicheniformis UBBLi30 strain. 3 Biotech 2020, 10, 112. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, J.; Chen, Y. Alpha-helical cationic antimicrobial peptides: Relationships of structure and function. Protein Cell 2010, 1, 143–152. [Google Scholar] [CrossRef]
- Keil, B. Specificity of Proteolysis, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1992; pp. 43–227. [Google Scholar]
- Liu, K.; Yang, L.; Peng, X.; Gong, H.; Wang, J.; Lu, J.R.; Xu, H. Effects of conventional surfactants on the activity of designed antimicrobial peptide. Langmuir 2020, 36, 3531–3539. [Google Scholar] [CrossRef]
- Corrêa, J.A.F.; Evangelista, A.G.; de Melo Nazareth, T.; Luciano, F.B. Fundamentals on the molecular mechanism of action of antimicrobial peptides. Materialia 2019, 8, 100494. [Google Scholar] [CrossRef]
- Siewert, G.; Strominger, J.L. Bacitracin: An inhibitor of the dephosphorylation of lipid pyrophosphate, an intermediate in the biosynthesis of the peptidoglycan of bacterial cell walls. Proc. Natl. Acad. Sci. USA 1967, 57, 767–773. [Google Scholar] [CrossRef]
- Luo, Y.; Song, Y. Mechanism of antimicrobial peptides: Antimicrobial, anti-Inflammatory and antibiofilm activities. Int. J. Mol. Sci. 2021, 22, 11401. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial peptides: Classification, design, application and research progress in multiple fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Feijoo-Coronel, M.L.; Mendes, B.; Ramírez, D.; Peña-Varas, C.; de Los Monteros-Silva, N.Q.E.; Proaño-Bolaños, C.; de Oliveira, L.L.; Lívio, D.F.; da Silva, J.A.; da Silva, J.M.S.F.; et al. Antibacterial and antiviral properties of chenopodin-derived synthetic peptides. Antibiotics 2024, 13, 78. [Google Scholar] [CrossRef]
- Zhang, S.K.; Song, J.W.; Gong, F.; Li, S.B.; Chang, H.Y.; Xie, H.M.; Gao, H.W.; Tan, Y.X.; Ji, S.P. Design of an α-helical antimicrobial peptide with improved cell-selective and potent anti-biofilm activity. Sci. Rep. 2016, 6, 27394. [Google Scholar] [CrossRef]
- Zhang, M.; Ouyang, J.; Fu, L.; Xu, C.; Ge, Y.; Sun, S.; Li, X.; Lai, S.; Ke, H.; Yuan, B.; et al. Hydrophobicity determines the bacterial killing rate of α-helical antimicrobial peptides and influences the bacterial resistance development. J. Med. Chem. 2022, 65, 14701–14720. [Google Scholar] [CrossRef]
- Lee, J.; Lee, D.G. Concentration-dependent mechanism alteration of pleurocidin peptide in Escherichia coli. Curr. Microbiol. 2016, 72, 159–164. [Google Scholar] [CrossRef]
- Zhu, H.; Han, L.; Ni, Y.; Yu, Z.; Wang, D.; Zhou, J.; Li, B.; Zhang, W.; He, K. In vitro and in vivo antibacterial effects of nisin against Streptococcus suis. Probiotics Antimicrob. Proteins 2021, 13, 598–610. [Google Scholar] [CrossRef]
- Kashfi, R.; Kelsey, C.; Gang, D.J.; Call, D.R.; Gang, D.R. Metabolomic diversity and identification of antibacterial activities of bacteria isolated from marine sediments in Hawai’i and Puerto Rico. Front. Mol. Biosci. 2020, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Hockett, K.L.; Baltrus, D.A. Use of the soft-agar overlay technique to screen for bacterially produced inhibitory compounds. J. Vis. Exp. 2017, 119, e55064. [Google Scholar]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Regmi, S.; Choi, Y.S.; Choi, Y.H.; Kim, Y.K.; Cho, S.S.; Yoo, J.C.; Suh, J.W. Antimicrobial peptide from Bacillus subtilis CSB138: Characterization, killing kinetics, and synergistic potency. Int. Microbiol. 2017, 20, 43–53. [Google Scholar]
- Xu, C.; Fu, Y.; Liu, F.; Liu, Z.; Ma, J.; Jiang, R.; Song, C.; Jiang, Z.; Hou, J. Purification and antimicrobial mechanism of a novel bacteriocin produced by Lactobacillus rhamnosus 1.0320. LWT 2021, 137, 110338. [Google Scholar] [CrossRef]
- Songnaka, N.; Lertcanawanichakul, M.; Hutapea, A.M.; Krobthong, S.; Yingchutrakul, Y.; Atipairin, A. Purification and characterization of novel anti-MRSA peptides produced by Brevibacillus sp. SPR-20. Molecules 2022, 27, 8452. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the Expasy Server. In The Proteomics Protocols Handbook, 1st ed.; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Shi, J.; Chen, C.; Wang, D.; Tong, Z.; Wang, Z.; Liu, Y. Amphipathic peptide antibiotics with potent activity against multidrug-resistant pathogens. Pharmaceutics 2021, 13, 438. [Google Scholar] [CrossRef]
- Micsonai, A.; Wien, F.; Kernya, L.; Lee, Y.H.; Goto, Y.; Réfrégiers, M.; Kardos, J. Accurate secondary structure prediction and fold recognition for circular dichroism spectroscopy. Proc. Natl. Acad. Sci. USA 2015, 112, E3095–E3103. [Google Scholar] [CrossRef]
- Gautier, R.; Douguet, D.; Antonny, B.; Drin, G. HELIQUEST: A web server to screen sequences with specific alpha-helical properties. Bioinformatics 2008, 24, 2101–2102. [Google Scholar] [CrossRef] [PubMed]
- Rey, J.; Murail, S.; de Vries, S.; Derreumaux, P.; Tuffery, P. PEP-FOLD4: A pH-dependent force field for peptide structure prediction in aqueous solution. Nucleic Acids Res. 2023, 51, W432–W437. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; pp. 15–52. [Google Scholar]
- Uzair, B.; Menaa, F.; Khan, B.A.; Mohammad, F.V.; Ahmad, V.U.; Djeribi, R.; Menaa, B. Isolation, purification, structural elucidation and antimicrobial activities of kocumarin, a novel antibiotic isolated from actinobacterium Kocuria marina CMG S2 associated with the brown seaweed Pelvetia canaliculata. Microbiol. Res. 2018, 206, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, N.; Sapra, A. Gram Staining. [Updated 14 August 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK562156/ (accessed on 27 September 2023).
- Hussey, M.A.; Zayaitz, A. Endospore stain protocol. Am. Soc. Microbiol. 2007, 8, 1–11. [Google Scholar]
- Galaxy Community. The Galaxy platform for accessible, reproducible, and collaborative data analyses: 2024 update. Nucleic Acids Res. 2024, 52, gkae410. [Google Scholar]
- Babraham Bioinformatics: FastQC A Quality Control Tool for High Throughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 13 May 2024).
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Tseemann/Shovill: Faster SPAdes Assembly of Illumina Reads. Available online: https://github.com/tseemann/shovill (accessed on 23 March 2024).
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Parks, D.H.; Imelfort, M.; Skennerton, C.T.; Hugenholtz, P.; Tyson, G.W. CheckM: Assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015, 25, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2209. [Google Scholar] [CrossRef]
- Overbeek, R.; Olson, R.; Pusch, G.D.; Olsen, G.J.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Parrello, B.; Shukla, M.; et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 2014, 42, D206–D214. [Google Scholar]
- Blin, K.; Shaw, S.; Augustijn, H.E.; Reitz, Z.L.; Biermann, F.; Alanjary, M.; Fetter, A.; Terlouw, B.R.; Metcalf, W.W.; Helfrich, E.J.N.; et al. antiSMASH 7.0: New and improved predictions for detection, regulation, chemical structures and visualisation. Nucleic Acids Res. 2023, 51, W46–W50. [Google Scholar] [CrossRef] [PubMed]
- Terlouw, B.R.; Blin, K.; Navarro-Muñoz, J.C.; Avalon, N.E.; Chevrette, M.G.; Egbert, S.; Lee, S.; Meijer, D.; Recchia, M.J.J.; Reitz, Z.L.; et al. MIBiG 3.0: A community-driven effort to annotate experimentally validated biosynthetic gene clusters. Nucleic Acids Res. 2023, 51, D603–D610. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2016, 44, D67–D72. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Raphenya, A.R.; Alcock, B.; Waglechner, N.; Guo, P.; Tsang, K.K.; Lago, B.A.; Dave, B.M.; Pereira, S.; Sharma, A.N.; et al. CARD 2017: Expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res. 2017, 45, D566–D573. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.R.; Enns, E.; Marinier, E.; Mandal, A.; Herman, E.K.; Chen, C.Y.; Graham, M.; Van Domselaar, G.; Stothard, P. Proksee: In-depth characterization and visualization of bacterial genomes. Nucleic Acids Res. 2023, 51, W484–W492. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Göker, M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef]
- CLSI. M100 Performance Standards for Antimicrobial Susceptibility Testing, 13th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020; pp. 58–66. [Google Scholar]




| Isolates | Zone of Inhibition (mm ± SD; n = 3) | |||
|---|---|---|---|---|
| S. aureus TISTR 517 | MRSA Strain 142 | MRSA Strain 1096 | MRSA Strain 2468 | |
| NNS2-1 | 15.41 ± 1.20 | 19.47 ± 1.03 | 19.05 ± 1.83 | 19.98 ± 2.07 |
| NNS4-2 | 12.02 ± 0.64 | 12.19 ± 0.15 | 11.94 ± 0.53 | 13.12 ± 0.00 |
| NNS4-3 | 14.99 ± 0.59 | 23.20 ± 0.67 | 21.25 ± 0.15 | 22.94 ± 0.67 |
| NNS4-5-2 | 0.00 ± 0.00 | 20.49 ± 0.51 | 18.37 ± 0.96 | 20.07 ± 0.53 |
| Vancomycin (30 µg) | 23.37 ± 1.27 | 24.13 ± 1.27 | 24.38 ± 0.51 | 24.64 ± 1.02 |
| Cefoxitin (30 µg) | 35.87 ± 0.46 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Purification Procedure | Volume (mL) | Total Dried Weight (mg) | Activity (AU/mL) | Total Activity (AU) | Specific Activity (AU/mg) | Purification Factor | %Yield |
|---|---|---|---|---|---|---|---|
| Crude product | 984 | 843.30 | 20 | 19,680 | 23.34 | 1.00 | 100.00 |
| Precipitation | 47 | 132.40 | 80 | 3760 | 28.40 | 1.22 | 19.11 |
| RPC | 12 | 3.63 | 160 | 1920 | 528.63 | 22.65 | 9.76 |
| Compounds | Tested Strains | MIC (µg/mL) | MBC (µg/mL) |
|---|---|---|---|
| NNS4-3 AMP | S. aureus TISTR 517 | 16 | 64 |
| MRSA strain 142 | 1 | 4 | |
| MRSA strain 1096 | 1 | 4 | |
| MRSA strain 2468 | 1 | 4 | |
| Vancomycin | S. aureus TISTR 517 | 2 | 2 |
| MRSA strain 142 | 2 | 2 | |
| MRSA strain 1096 | 2 | 2 | |
| MRSA strain 2468 | 2 | 2 | |
| Cefoxitin | S. aureus TISTR 517 | 2 | 2 |
| MRSA strain 142 | >64 | ND | |
| MRSA strain 1096 | >64 | ND | |
| MRSA strain 2468 | >64 | ND |
| Conditions | % Residual Activity | ||
|---|---|---|---|
| 1 h | 6 h | 12 h | |
| Thermal stability | |||
| Non-treated sample | 100.00 ± 2.41 | 100.00 ± 0.57 | 100.00 ± 0.57 |
| Treated sample at 37 °C | 103.19 ± 3.68 | 100.00 ± 0.00 | 99.61 ± 6.44 |
| Treated sample at 60 °C | 101.82 ± 2.46 | 95.27 ± 4.31 | 90.47 ± 2.98 * |
| Treated sample at 80 °C | 97.52 ± 6.21 | 41.13 ± 35.62 * | 0.00 ± 0.00 * |
| Treated sample at 100 °C | 73.05 ± 1.57 * | 0.00 ± 0.00 * | 0.00 ± 0.00 * |
| Treated sample at 121 °C, 15 psi, 15 min | 61.83 ± 0.74 * | ||
| Treated sample at 121 °C, 15 psi, 30 min | 0.00 ± 0.00 * | ||
| Enzyme stability | |||
| Non-treated sample | 100.00 ± 1.52 | 100.00 ± 4.29 | 100.00 ± 2.62 |
| Sample + Proteinase K (1 mg/mL) | 98.66 ± 1.68 | 99.19 ± 1.07 | 99.07 ± 3.08 |
| Sample + Trypsin (1 mg/mL) | 95.57 ± 4.01 | 95.66 ± 2.62 | 93.81 ± 3.31 |
| Sample + α-chymotrypsin (1 mg/mL) | 96.65 ± 2.14 | 95.15 ± 6.64 | 97.06 ± 0.54 |
| Surfactant stability | |||
| Non-treated sample | 100.00 ± 6.20 | 100.00 ± 0.00 | 100.00 ± 3.74 |
| Sample + 1% Triton X-100 | 104.54 ± 4.96 | 102.27 + 2.93 | 103.40 ± 3.82 |
| Sample + 1% SDS | 87.00 ± 6.17 * | 86.69 ± 5.39 * | 86.25 ± 4.76 * |
| Effect of pH on the stability | |||
| Non-treated sample | 100.00 ± 6.68 | 100.00 ± 8.51 | 100.00 ± 6.89 |
| pH 1.2 | 98.12 ± 2.14 | 95.45 ± 0.79 | 94.14 ± 3.12 |
| pH 4.5 | 95.24 ± 3.29 | 99.64 ± 2.87 | 96.91 ± 2.99 |
| pH 6.8 | 98.87 ± 2.00 | 95.89 ± 5.00 | 92.54 ± 4.14 |
| pH 7.4 | 97.80 ± 1.32 | 94.82 ± 2.80 | 93.29 ± 1.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sermkaew, N.; Atipairin, A.; Wanganuttara, T.; Krobthong, S.; Aonbangkhen, C.; Yingchutrakul, Y.; Uchiyama, J.; Songnaka, N. A Novel Bacitracin-like Peptide from Mangrove-Isolated Bacillus paralicheniformis NNS4-3 against MRSA and Its Genomic Insights. Antibiotics 2024, 13, 716. https://doi.org/10.3390/antibiotics13080716
Sermkaew N, Atipairin A, Wanganuttara T, Krobthong S, Aonbangkhen C, Yingchutrakul Y, Uchiyama J, Songnaka N. A Novel Bacitracin-like Peptide from Mangrove-Isolated Bacillus paralicheniformis NNS4-3 against MRSA and Its Genomic Insights. Antibiotics. 2024; 13(8):716. https://doi.org/10.3390/antibiotics13080716
Chicago/Turabian StyleSermkaew, Namfa, Apichart Atipairin, Thamonwan Wanganuttara, Sucheewin Krobthong, Chanat Aonbangkhen, Yodying Yingchutrakul, Jumpei Uchiyama, and Nuttapon Songnaka. 2024. "A Novel Bacitracin-like Peptide from Mangrove-Isolated Bacillus paralicheniformis NNS4-3 against MRSA and Its Genomic Insights" Antibiotics 13, no. 8: 716. https://doi.org/10.3390/antibiotics13080716
APA StyleSermkaew, N., Atipairin, A., Wanganuttara, T., Krobthong, S., Aonbangkhen, C., Yingchutrakul, Y., Uchiyama, J., & Songnaka, N. (2024). A Novel Bacitracin-like Peptide from Mangrove-Isolated Bacillus paralicheniformis NNS4-3 against MRSA and Its Genomic Insights. Antibiotics, 13(8), 716. https://doi.org/10.3390/antibiotics13080716








