Rapid Reversal of Carbapenemase-Producing Pseudomonas aeruginosa Epidemiology from blaVIM- to blaNDM-harbouring Isolates in a Greek Tertiary Care Hospital
Abstract
:1. Introduction
2. Results
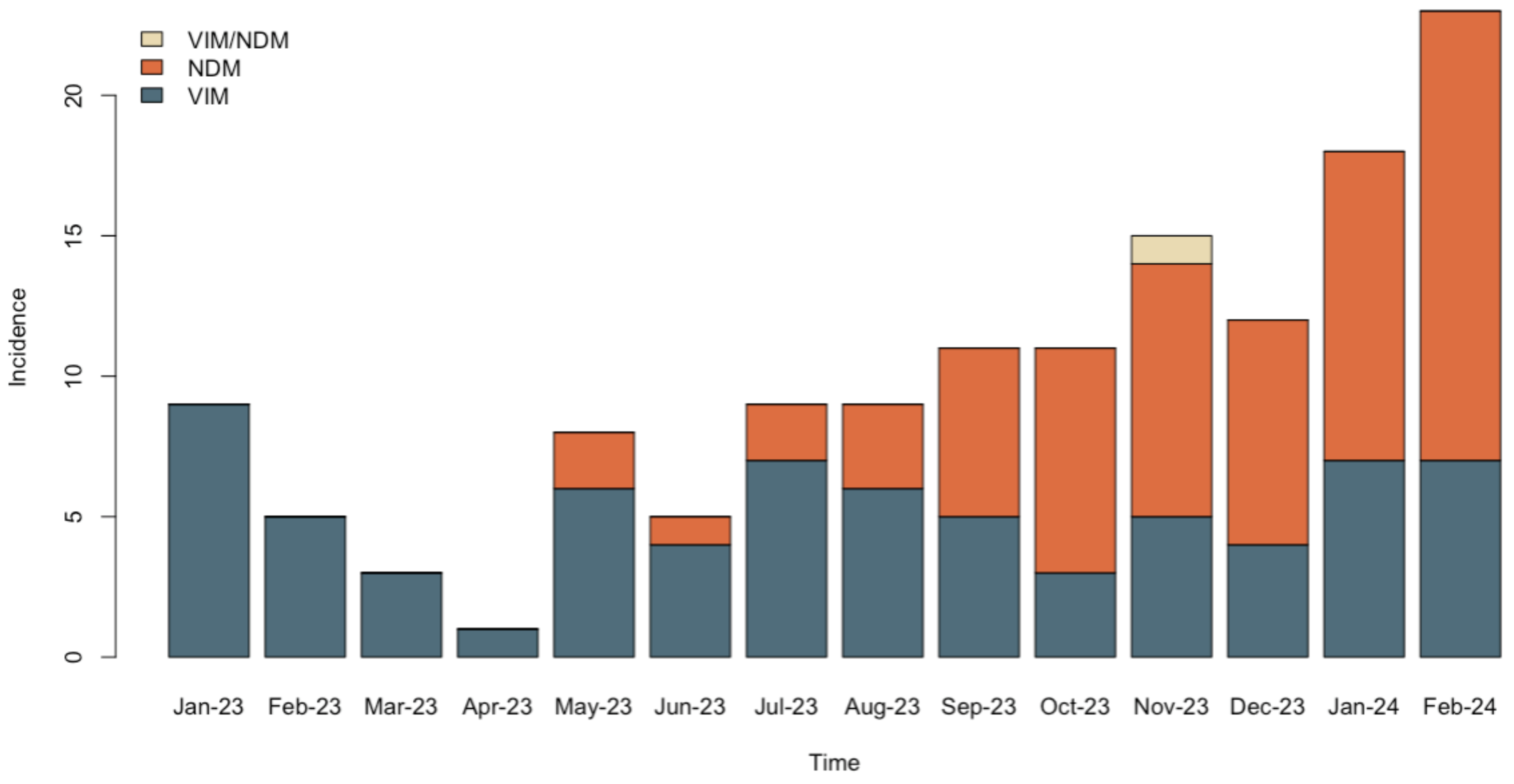
2.1. Carbapenem Resistant P. aeruginosa Isolates
2.2. Detection of Resistance Determinants by the NG-Test CARBA 5 and the Hybrispot Antimicrobial Resistance Direct Flow Chip (AMR)
2.3. Genomic Characterization of NDM-1-Producing Isolate 91845
2.4. MLST, Antimicrobial Resistance and Virulence Genes
2.5. Genomic Origin of the blandm Gene
3. Discussion
4. Materials and Methods
4.1. Hospital Setting and Patient Data
4.2. Study Sample
4.3. Bacterial Identification and Susceptibility Testing
4.4. NG-Test CARBA 5
4.5. Hybrispot Antimicrobial Resistance Direct Flow Chip (AMR)
4.6. Whole-Genome Sequencing and Bioinformatics Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Antimicrobial Resistance Surveillance in Europe 2023–2021 Data; European Centre for Disease Prevention and Control and World Health Organization: Stockholm, Sweden, 2023.
- Meletis, G.; Exindari, M.; Vavatsi, N.; Sofianou, D.; Diza, E. Mechanisms responsible for the emergence of carbapenem resistance in Pseudomonas aeruginosa. Hippokratia 2012, 16, 303–307. [Google Scholar]
- Partridge, S.R.; Kwong, S.M.; Firth, N.; Jensen, S.O. Mobile Genetic Elements Associated with Antimicrobial Resistance. Clin. Microbio. Rev. 2018, 31, e00088-17. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, J.P.; Montero, M.; Oliver, A.; Sorlí, L.; Luque, S.; Gómez-Zorrilla, S.; Benito, N.; Grau, S. Epidemiology and Treatment of Multidrug-Resistant and Extensively Drug-Resistant Pseudomonas aeruginosa Infections. Clin. Microbiol. Rev. 2019, 32, e00031-19. [Google Scholar] [CrossRef]
- Ismail, S.; Mahmoud, S. First detection of New Delhi metallo-β-lactamases variants (NDM-1, NDM-2) among Pseudomonas aeruginosa isolated from Iraqi hospitals. Iran. J. Microbiol. 2018, 10, 98–103. [Google Scholar] [PubMed]
- Giakkoupi, P.; Petrikkos, G.; Tzouvelekis, L.S.; Tsonas, S.; Legakis, N.J.; Vatopoulos, A.C.; WHONET Greece Study Group. Spread of integron-associated VIM-type metallo-beta-lactamase genes among imipenem-non-susceptible Pseudomonas aeruginosa strains in Greek hospitals. J. Clin. Microbiol. 2003, 41, 822–825. [Google Scholar] [CrossRef] [PubMed]
- Tsakris, A.; Poulou, A.; Kristo, I.; Pittaras, T.; Spanakis, N.; Pournaras, S.; Markou, F. Large dissemination of VIM-2-metallo-{beta}-lactamase-producing Pseudomonas aeruginosa strains causing health care-associated community-onset infections. J. Clin. Microbiol. 2009, 47, 3524–3529. [Google Scholar] [CrossRef] [PubMed]
- Meletis, G.; Vavatsi, N.; Exindari, M.; Protonotariou, E.; Sianou, E.; Haitoglou, C.; Sofianou, D.; Pournaras, S.; Diza, E. Accumulation of carbapenem resistance mechanisms in VIM-2-producing Pseudomonas aeruginosa under selective pressure. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Pappa, O.; Kefala, A.M.; Tryfinopoulou, K.; Dimitriou, M.; Kostoulas, K.; Dioli, C.; Moraitou, E.; Panopoulou, M.; Vogiatzakis, E.; Mavridou, A.; et al. Molecular Epidemiology of Multi-Drug Resistant Pseudomonas aeruginosa Isolates from Hospitalized Patients in Greece. Microorganisms 2020, 24, 1652. [Google Scholar] [CrossRef]
- Yong, D.; Toleman, M.A.; Giske, C.G.; Cho, H.S.; Sundman, K.; Lee, K.; Walsh, T.R. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 2009, 53, 5046–5054. [Google Scholar] [CrossRef] [PubMed]
- Cantόn, R.; Akόva, M.; Carmeli, Y.; Giske, C.G.; Glupczynski, Y.; Gniadkowski, M.; Livermore, D.M.; Miriagou, V.; Naas, T.; Rossolini, G.M.; et al. European Network on Carbapenemases. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin. Microbiol. Infect. 2012, 18, 413–431. [Google Scholar] [CrossRef]
- Johnson, A.P.; Woodford, N. Global spread of antibiotic resistance: The example of New Delhi metallo-beta-lactamase (NDM)-mediated carbapenem resistance. J. Med. Microbiol. 2013, 62, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Kumarasamy, K.; Toleman, M.; Walsh, T.; Bagaria, J.; Butt, F.; Balakrishnan, R.; Chaudhary, U.; Doumith, M.; Giske, C.; Irfan, S.; et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: A molecular, biological, and epidemiological study. Lancet Infect. Dis. 2010, 10, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Tychala, A.; Meletis, G.; Mantzana, P.; Kassomenaki, A.; Katsanou, C.; Daviti, A.; Kouroudi, L.; Skoura, L.; Protonotariou, E. Replacement of the Double Meropenem Disc Test with a Lateral. Flow Assay for the Detection of Carbapenemase-Producing Enterobacterales and Pseudomonas aeruginosa in Clinical Laboratory Practice. Antibiotics 2023, 12, 771. [Google Scholar] [CrossRef]
- Oliver, A.; Rojo-Molinero, E.; Arca-Suarez, J.; Beşli, Y.; Bogaerts, P.; Cantón, R.; Cimen, C.; Croughs, P.D.; Denis, O.; Giske, C.G.; et al. Pseudomonas aeruginosa antimicrobial susceptibility profiles, resistance mechanisms and international clonal lineages: Update from ESGARS-ESCMID/ISARPAE Group. Clin. Microbiol. Infect. 2024, 30, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Voulgari, E.; Gartzonika, C.; Vrioni, G.; Politi, L.; Priavali, E.; Levidiotou-Stefanou, S.; Tsakris, A. The Balkan region: NDM-1-producing Klebsiella pneumoniae ST11 clonal strain causing outbreaks in Greece. J. Antimicrob. Chemother. 2014, 69, 2091–2097. [Google Scholar] [CrossRef]
- Protonotariou, E.; Meletis, G.; Pilalas, D.; Mantzana, P.; Tychala, A.; Kotzamanidis, C.; Papadopoulou, D.; Papadopoulos, T.; Polemis, M.; Metallidis, S.; et al. Polyclonal Endemicity of Carbapenemase-Producing Klebsiella pneumoniae in ICUs of a Greek Tertiary Care Hospital. Antibiotics 2022, 11, 149. [Google Scholar] [CrossRef] [PubMed]
- Meletis, G.; Malousi, A.; Tychala, A.; Kassomenaki, A.; Vlachodimou, N.; Mantzana, P.; Metallidis, S.; Skoura, L.; Protonotariou, E. Probable Three-Species In Vivo Transfer of blaNDM-1 in a Single Patient in Greece: Occurrence of NDM-1-Producing Klebsiella pneumoniae, Proteus mirabilis, and Morganella morganii. Antibiotics 2023, 20, 1206. [Google Scholar] [CrossRef]
- Villegas, M.V.; Lolans, K.; Correa, A.; Kattan, J.N.; Lopez, J.A.; Quinn, J.P.; Colombian Nosocomial Resistance Study Group. First identification of Pseudomonas aeruginosa isolates producing a KPC-type carbapenem-hydrolyzing beta-lactamase. Antimicrob. Agents Chemother. 2007, 51, 1553–1555. [Google Scholar] [CrossRef]
- Watanabe, M.; Iyobe, S.; Inoue, M.; Mitsuhashi, S. Transferable imipenem resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1991, 35, 147–151. [Google Scholar] [CrossRef]
- Lauretti, L.; Riccio, M.L.; Mazzariol, A.; Cornaglia, G.; Amicosante, G.; Fontana, R.; Rossolini, G.M. Cloning and characterization of blaVIM, a new integron-borne metallo-beta-lactamase gene from a Pseudomonas aeruginosa clinical isolate. Antimicrob. Agents Chemother. 1999, 43, 1584–1590. [Google Scholar] [CrossRef]
- Toleman, M.A.; Simm, A.M.; Murphy, T.A.; Gales, A.C.; Biedenbach, D.J.; Jones, R.N.; Walsh, T.R. Molecular characterization of SPM-1, a novel metallo-beta-lactamase isolated in Latin America: Report from the SENTRY antimicrobial surveillance programme. J. Antimicrob. Chemother. 2002, 50, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.S.; Song, W.; Park, M.J.; Jeong, S.; Lee, N.; Jeong, S.H. Molecular Characterization of the First Emerged NDM-1-Producing Pseudomonas aeruginosa Isolates in South Korea. Microb. Drug Resist. 2021, 27, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Hammoudi Halat, D.; Ayoub Moubareck, C. The Intriguing Carbapenemases of Pseudomonas aeruginosa: Current Status, Genetic Profile, and Global Epidemiology. Yale J. Biol. Med. 2022, 22, 507–515. [Google Scholar]
- Jovcic, B.; Lepsanovic, Z.; Suljagic, V.; Rackov, G.; Begovic, J.; Topisirovic, L.; Kojic, M. Emergence of NDM-1 metallo-β-lactamase in Pseudomonas aeruginosa clinical isolates from Serbia. Antimicrob. Agents Chemother. 2011, 55, 3929–3931. [Google Scholar] [CrossRef] [PubMed]
- Khajuria, A.; Praharaj, A.K.; Kumar, M.; Grover, N. Emergence of NDM-1 in the Clinical Isolates of Pseudomonas aeruginosa in India. J. Clin. Diagn. Res. 2013, 7, 1328–1331. [Google Scholar] [CrossRef] [PubMed]
- Kulkova, N.; Babalova, M.; Sokolova, J.; Krcmery, V. First report of New Delhi metallo-β-lactamase-1-producing strains in Slovakia. Microb. Drug Resist. 2015, 21, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Janvier, F.; Jeannot, K.; Tessé, S.; Robert-Nicoud, M.; Delacour, H.; Rapp, C.; Mérens, A. Molecular characterization of blaNDM-1 in a sequence type 235 Pseudomonas aeruginosa isolate from France. Antimicrob Agents Chemother. 2013, 57, 3408–3411. [Google Scholar] [CrossRef] [PubMed]
- Carattoli, A.; Fortini, D.; Galetti, R.; Garcia-Fernandez, A.; Nardi, G.; Orazi, D.; Capone, A.; Majolino, I.; Proia, A.; Mariani, B.; et al. Isolation of NDM-1-producing Pseudomonas aeruginosa sequence type ST235 from a stem cell transplant patient in Italy, May 2013. Eur. Surveill. 2013, 18, 20633. [Google Scholar] [CrossRef]
- Loconsole, D.; Accogli, M.; Monaco, M.; Grosso, M.; De Robertis, A.L.; Morea, A.; Capozzi, L.; Del Sambro, L.; Simone, A.; De Letteriis, V.; et al. First detection of autochthonous extensively drug-resistant NDM-1 Pseudomonas aeruginosa ST235 from a patient with bloodstream infection in Italy, October 2019. Antimicrob. Resist. Infect. Control 2020, 9, 73. [Google Scholar] [CrossRef]
- Tsakris, A.; Pournaras, S.; Woodford, N.; Palepou, M.F.; Babini, G.S.; Douboyas, J.; Livermore, D.M. Outbreak of infections caused by Pseudomonas aeruginosa producing VIM-1 carbapenemase in Greece. J. Clin. Microbiol. 2000, 38, 1290–1292. [Google Scholar] [CrossRef]
- Mavroidi, A.; Tsakris, A.; Tzelepi, E.; Pournaras, S.; Loukova, V.; Tzouvelekis, L.S. Carbapenem-hydrolysing VIM-2 metallo- beta-lactamase in Pseudomonas aeruginosa from Greece. J. Antimicrob. Chemother. 2000, 46, 1041–1042. [Google Scholar] [CrossRef]
- Tsilipounidaki, K.; Gkountinoudis, C.; Florou, Z.; Fthenakis, G.; Miriagou, V.; Petinaki, E. First Detection and Molecular Characterization of Pseudomonas aeruginosa blaNDM-1 ST308 in Greece. Microorganisms 2023, 11, 2159. [Google Scholar] [CrossRef]
- Papadimitriou-Olivgeris, M.; Bartzavali, C.; Lambropoulou, A.; Solomou, A.; Tsiata, E.; Anastassiou, E.D.; Fligou, F.; Marangos, M.; Spiliopoulou, I.; Christofidou, M. Reversal of carbapenemase-producing Klebsiella pneumoniae epidemiology from blaKPC- to blaVIM-harbouring isolates in a Greek ICU after introduction of ceftazidime/avibactam. J. Antimicrob. Chemother. 2019, 1, 2051–2054. [Google Scholar] [CrossRef] [PubMed]
- López Montesinos, I.; Gómez-Zorrilla, S.; Palacios-Baena, Z.R.; Prim, N.; Echeverria-Esnal, D.; Gracia, M.P.; Montero, M.M.; Durán-Jordà, X.; Sendra, E.; Sorli, L.; et al. Aminoglycoside or Polymyxin Monotherapy for Treating Complicated Urinary Tract Infections Caused by Extensively Drug-Resistant Pseudomonas aeruginosa: A Propensity Score-Adjusted and Matched Cohort Study. Infect. Dis. Ther. 2022, 11, 335–350. [Google Scholar] [CrossRef] [PubMed]
- Samonis, G.; Maraki, S.; Karageorgopoulos, D.E.; Vouloumanou, E.K.; Falagas, M.E. Synergy of fosfomycin with carbapenems, colistin, netilmicin, and tigecycline against multidrug-resistant Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa clinical isolates. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 695–701. [Google Scholar] [CrossRef]
- Montero, M.; Horcajada, J.P.; Sorlí, L.; Alvarez-Lerma, F.; Grau, S.; Riu, M.; Sala, M.; Knobel, H. Effectiveness and safety of colistin for the treatment of multidrug-resistant Pseudomonas aeruginosa infections. Infection 2009, 37, 461–465. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Souvorov, A.; Agarwala, R.; Lipman, D.J. SKESA: Strategic k-mer extension for scrupulous assemblies. Genome Biol. 2018, 19, 153. [Google Scholar] [CrossRef]
- Bharat, A.; Petkau, A.; Avery, B.P.; Chen, J.C.; Folster, J.P.; Carson, C.A.; Kearney, A.; Nadon, C.; Mabon, P.; Thiessen, J.; et al. Correlation between Phenotypic and In Silico Detection of Antimicrobial Resistance in Salmonella enterica in Canada Using Staramr. Microorganisms 2022, 10, 292. [Google Scholar] [CrossRef]
- Larsen, M.V.; Cosentino, S.; Rasmussen, S.; Friis, C.; Hasman, H.; Marvig, R.L.; Jelsbak, L.; Sicheritz-Pontén, T.; Ussery, D.W.; Aarestrup, F.M.; et al. Multilocus sequence typing of total-genome-sequenced bacteria. J. Clin Microbiol. 2012, 50, 1355–1361. [Google Scholar] [CrossRef]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4. 0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef] [PubMed]
- Carattoli, A.; Zankari, E.; García-Fernández, A.; Voldby Larsen, M.; Lund, O.; Villa, L.; Møller Aarestrup, F.; Hasman, H. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef] [PubMed]
- Antipov, D.; Hartwick, N.; Shen, M.; Raiko, M.; Lapidus, A.; Pevzner, P.A. PlasmidSPAdes: Assembling plasmids from whole genome sequencing data. Bioinformatics 2016, 32, 3380–3387. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Shang, J.; Ji, Y.; Sun, Y. PLASMe: A tool to identify PLASMid contigs from short-read assemblies using transformer. Nucleic Acids Res. 2023, 51, 83. [Google Scholar] [CrossRef]
- Robertson, J.; Nash, J.H.E. MOB-suite: Software tools for clustering, reconstruction and typing of plasmids from draft assemblies. Microb. Genom. 2018, 4, 206. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.M.; Liljeberg, P.; Plosila, J.; Tenhunen, H. LastZ: An Ultra Optimized 3D Networks-on-Chip Architecture. In Proceedings of the 2011 14th Euromicro Conference on Digital System Design: Architectures, Methods and Tools, DSD 2011, Oulu, Finland, 31 August–2 September 2011; pp. 173–180. [Google Scholar]
- Grant, J.R.; Enns, E.; Marinier, E.; Mandal, A.; Herman, E.K.; Chen, C.Y.; Graham, M.; Van Domselaar, G.; Stothard, P. Proksee: In-depth characterization and visualization of bacterial genomes. Nucleic Acids Res. 2023, 51, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Alcock, B.P.; Raphenya, A.R.; Lau, T.T.Y.; Tsang, K.K.; Bouchard, M.; Edalatmand, A.; Huynh, W.; Nguyen, A.V.; Cheng, A.A.; Liu, S.; et al. CARD 2020: Antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2020, 48, 517–525. [Google Scholar] [CrossRef]
- Brown, C.L.; Mullet, J.; Hindi, F.; Stoll, J.E.; Gupta, S.; Choi, M.; Keenum, I.; Vikesland, P.; Pruden, A.; Zhang, L. MobileOG-db: A manually Curated Database of Protein Families Mediating the Life Cycle of Bacterial Mobile Genetic Elements. Appl. Environ. Microbiol. 2022, 88, 99122. [Google Scholar] [CrossRef]
- Jain, C.; Rodriguez-R, L.M.; Phillippy, A.M.; Konstantinidis, K.T.; Aluru, S. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat. Commun. 2018, 9, 5114. [Google Scholar] [CrossRef] [PubMed]

| Month/Year | Isolate ID | Date of Isolation | Carbapenemase | Specimen Type | Ward | Age (Years) | Patient Gender |
|---|---|---|---|---|---|---|---|
| January 2023 | 401 | 2 January 2023 | VIM | Rectal surveillance swab | ICU A | 99 | M |
| 371 | 2 January 2023 | VIM | Urine | NS | 68 | M | |
| 496 | 2 January 2023 | VIM | Urine | SURGB | 79 | M | |
| 2342 | 4 January 2023 | VIM | Urine | NB | 61 | F | |
| 11102 | 17 January 2023 | VIM | Blood | ICU A | 68 | F | |
| 12955 | 20 January 2023 | VIM | Blood | INT A | 83 | M | |
| 14753 | 23 January 2023 | VIM | Rectal surveillance swab | ICU A | 77 | F | |
| 15011 | 23 January 2023 | VIM | Sputum | INT B | 82 | M | |
| 20359 | 30 January 2023 | VIM | Blood | SURGB | 58 | M | |
| February 2023 | 34945 | 20 February 2023 | VIM | Urine | SURGC | 45 | F |
| 34955 | 20 February 2023 | VIM | BAL | ICU A | 72 | F | |
| 38980 | 24 February 2023 | VIM | Blood | NS | 81 | M | |
| 41488 | 28 February 2023 | VIM | Wound | EMERG | 70 | M | |
| 41273 | 28 February 2023 | VIM | Blood | EMERG | 87 | F | |
| March 2023 | 47005 | 8 March 2023 | VIM | BAL | SURGB | 41 | M |
| 47930 | 8 March 2023 | VIM | Urine | EMERG | 57 | M | |
| 52508 | 14 March 2023 | VIM | Urine | NB | 74 | F | |
| April 2023 | 84353 | 27 April 2023 | VIM | Blood | INT A | 87 | M |
| May 2023 | 86305 | 1 May 2023 | VIM | Blood | INT A | 65 | M |
| 91845 | 8 May 2023 | NDM | Blood | INT A | 85 | M | |
| 96242 | 15 May 2023 | VIM | BAL | ICU A | 64 | F | |
| 99556 | 19 May 2023 | VIM | Blood | CARD | 76 | M | |
| 100833 | 22 May 2023 | VIM | Bronchial secretions | ICU A | 70 | F | |
| 106450 | 30 May 2023 | NDM | Ocular swab | OPHT | 41 | F | |
| 106563 | 30 May 2023 | VIM | CVC | INT A | 58 | M | |
| 106902 | 31 May 2023 | VIM | Urine | INT B | 94 | F | |
| June 2023 | 111031 | 6 June 2023 | VIM | Blood | INT A | 85 | F |
| 110940 | 6 June 2023 | VIM | Blood | INT A | 89 | F | |
| 113902 | 11 June 2023 | VIM | Blood | BH | 66 | F | |
| 115521 | 13 June 2023 | NDM | Wound | NS | 57 | F | |
| 127808 | 30 June 2023 | VIM | Sputum | INT B | 57 | M | |
| July 2023 | 133885 | 1 July 2023 | VIM | Urine | INT A | 70 | M |
| 129750 | 4 July 2023 | NDM | Rectal surveillance swab | SURGB | 70 | M | |
| 129929 | 4 July 2023 | VIM | Rectal surveillance swab | ICU A | 65 | M | |
| 133820 | 10 July 2023 | VIM | Bronchial secretions | ICU A | 69 | M | |
| 136318 | 14 July 2023 | VIM | Bronchial secretions | ICU A | 61 | M | |
| 136808 | 14 July 2023 | VIM | Blood | INT B | 66 | M | |
| 138644 | 18 July 2023 | NDM | Blood | SURGB | 43 | M | |
| 142123 | 24 July 2023 | VIM | Urine | ICU A | 45 | M | |
| 144447 | 27 July 2023 | VIM | Sputum | INT B | 83 | F | |
| August 2023 | 147614 | 2 August 2023 | VIM | CVC | NS | 49 | M |
| 147288 | 2 August 2023 | VIM | BAL | SURGB | 50 | M | |
| 150146 | 7 August 2023 | VIM | Urine | EMERG | 78 | M | |
| 151234 | 8 August 2023 | VIM | Urine | NB | 82 | F | |
| 153153 | 11 August 2023 | ΝDM | Urine | INT A | 32 | F | |
| 153808 | 13 August 2023 | VIM | Tissue sample | INT A | 81 | M | |
| 159858 | 24 August 2023 | VIM | Urine | INT A | 76 | M | |
| 161810 | 28 August 2023 | ΝDM | ΒAL | ICU A | 70 | F | |
| 162377 | 29 August 2023 | ΝDM | Urine | INT A | 81 | F | |
| September 2023 | 164424 | 2 September 2023 | VIM | Urine | INT B | 77 | F |
| 165541 | 4 September 2023 | ΝDM | Blood | INT A | 76 | F | |
| 165760 | 5 September 2023 | VIM | Ocular swab | OPHT | 47 | M | |
| 168603 | 8 September 2023 | ΝDM | Blood | INT B | 85 | M | |
| 170177 | 12 September 2023 | VIM | Bronchial secretions | INT B | 61 | M | |
| 171353 | 13 September 2023 | VIM | Bronchial secretions | SURGB | 59 | M | |
| 172628 | 14 September 2023 | ΝDM | Urine | INT A | 84 | F | |
| 175558 | 19 September 2023 | ΝDM | Urine | INT A | 76 | M | |
| 177569 | 21 September 2023 | VIM | Urine | INT A | 61 | F | |
| 176948 | 21 September 2023 | ΝDM | Urine | INT A | 88 | F | |
| 176876 | 21 September 2023 | ΝDM | CVC | INT A | 86 | F | |
| October 2023 | 184504 | 2 October 2023 | ΝDM | BAL | ICU A | 67 | F |
| 184483 | 2 October 2023 | ΝDM | Urine | ICU A | 71 | M | |
| 186043 | 3 October 2023 | ΝDM | Blood | INT B | 77 | F | |
| 186274 | 4 October 2023 | VIM | Bronchial secretions | SURGB | 71 | M | |
| 188259 | 6 October 2023 | VIM | Wound | INT B | 50 | F | |
| 188453 | 6 October 2023 | ΝDM | Urine | INT A | 69 | M | |
| 188456 | 6 October 2023 | ΝDM | Urine | INT B | 86 | F | |
| 191530 | 11 October 2023 | ΝDM | Urine | ICU A | 48 | M | |
| 196558 | 18 October 2023 | VIM | Urine | ICU A | 55 | F | |
| 204219 | 30 October 2023 | ΝDM | Bronchial secretions | INT B | 88 | M | |
| 205032 | 31 October 2023 | ΝDM | Urine | INT A | 88 | F | |
| November 2023 | 206389 | 2 November 2023 | ΝDM | Wound | BH | 76 | M |
| 207607 | 4 November 2023 | ΝDM | Urine | INT A | 70 | F | |
| 207721 | 4 November 2023 | ΝDM | Urine | INT B | 73 | F | |
| 207883 | 5 November 2023 | ΝDM | Wound | NS | 33 | F | |
| 208276 | 6 November 2023 | VIM | Rectal surveillance swab | ICU A | 46 | M | |
| 208520 | 7 November 2023 | VIM | CVC | INT B | 87 | F | |
| 208299 | 11 November 2023 | VIM | Urine | INT A | 80 | F | |
| 209832 | 12 November 2023 | ΝDM | Urine | INT B | 87 | M | |
| 212710 | 14 November 2023 | ΝDM | Rectal surveillance swab | SURGB | 80 | M | |
| 212971 | 21 November 2023 | ΝDM | Urine | NS | 56 | M | |
| 214397 | 22 November 2023 | VIM | BAL | SURGB | 78 | M | |
| 215548 | 24 November 2023 | ΝDM | BAL | ICU A | 61 | M | |
| 214929 | 25 November 2023 | VIM and NDM | Wound | CARD | 70 | F | |
| 214898 | 28 November 2023 | VIM | Blood | INT B | 87 | F | |
| 219774 | 29 November 2023 | ΝDM | Urine | INT B | 81 | F | |
| December 2023 | 229861 | 6 December 2023 | VIM | BAL | SURGB | 79 | M |
| 231056 | 7 December 2023 | ΝDM | Urine | CARD | 92 | M | |
| 231677 | 9 December 2023 | ΝDM | Urine | INT A | 81 | F | |
| 232043 | 9 December 2023 | ΝDM | Wound | CARDSURG | 57 | F | |
| 232474 | 10 December 2023 | ΝDM | Blood | ICU A | 43 | M | |
| 236598 | 15 December 2023 | VIM | Urine | INT B | 86 | M | |
| 238830 | 19 December 2023 | ΝDM | Urine | NEPHR | 80 | F | |
| 240501 | 21 December 2023 | VIM | Blood | BH | 13 | M | |
| 242396 | 25 December 2023 | VIM | CVC | ICU A | 67 | F | |
| 242607 | 25 December 2023 | ΝDM | Urine | INT A | 73 | F | |
| 243772 | 27 December 2023 | ΝDM | Rectal surveillance swab | INT A | 86 | F | |
| 245847 | 31 December 2023 | ΝDM | Urine | INT B | 85 | F | |
| January 2024 | 830 | 2 January 2024 | VIM | Urine | EMERG | 93 | F |
| 71 | 2 January 2024 | ΝDM | Urine | INT B | 62 | F | |
| 590 | 2 January 2024 | ΝDM | Rectal surveillance swab | INT B | 98 | M | |
| 954 | 2 January 2024 | ΝDM | Urine | NEPHR | 80 | F | |
| 8683 | 15 January 2024 | ΝDM | Blood | INT B | 82 | M | |
| 8653 | 15 January 2024 | ΝDM | Sputum | INT B | 67 | F | |
| 11691 | 18 January 2024 | ΝDM | Blood | INT A | 93 | F | |
| 12093 | 19 January 2024 | ΝDM | Blood | INT A | 88 | M | |
| 13707 | 22 January 2024 | ΝDM | Groin surveillance swab | ICU A | 52 | M | |
| 15141 | 23 January 2024 | VIM | Rectal surveillance swab | INT B | 89 | F | |
| 15194 | 23 January 2024 | VIM | Rectal surveillance swab | INT B | 71 | M | |
| 15253 | 23 January 2024 | ΝDM | Groin surveillance swab | INT B | 72 | F | |
| 15186 | 23 January 2024 | VIM | Urine | INT A | 78 | F | |
| 14463 | 23 January 2024 | VIM | Urine | INT A | 87 | F | |
| 15506 | 24 January 2024 | VIM | BAL | SURGB | 45 | M | |
| 16012 | 24 January 2024 | VIM | Rectal surveillance swab | CARD | 54 | F | |
| 20811 | 31 January 2024 | ΝDM | Groin surveillance swab | INT B | 85 | M | |
| 20810 | 31 January 2024 | ΝDM | Groin surveillance swab | INT B | 80 | F | |
| February 2024 | 21862 | 1 February 2024 | VIM | Cerebrospinal fluid | ICU B | 54 | M |
| 23947 | 4 February 2024 | NDM | Bronchial secretions | ICU B | 87 | M | |
| 24299 | 5 February 2024 | NDM | Urine | INT A | 89 | F | |
| 23926 | 5 February 2024 | VIM | BAL | ICU B | 60 | M | |
| 26299 | 8 February 2024 | VIM | Urine | INT B | 74 | M | |
| 26661 | 8 February 2024 | NDM | Groin surveillance swab | INT B | 74 | M | |
| 30608 | 14 February 2024 | NDM | Groin surveillance swab | INT A | 78 | M | |
| 30606 | 14 February 2024 | NDM | Rectal surveillance swab | INT A | 80 | F | |
| 31647 | 15 February 2024 | NDM | Rectal surveillance swab | ICU B | 72 | M | |
| 32632 | 16 February 2024 | VIM | Rectal surveillance swab | INT B | 95 | F | |
| 32639 | 16 February 2024 | NDM | Groin surveillance swab | INT B | 84 | M | |
| 32636 | 16 February 2024 | NDM | Groin surveillance swab | INT B | 87 | M | |
| 33016 | 17 February 2024 | NDM | Blood | INT B | 80 | F | |
| 35019 | 20 February 2024 | NDM | Rectal surveillance swab | INT A | 40 | M | |
| 38019 | 24 February 2024 | NDM | Urine | INT B | 67 | M | |
| 39050 | 26 February 2024 | VIM | Rectal surveillance swab | ICU B | 77 | M | |
| 38901 | 26 February 2024 | NDM | Rectal surveillance swab | INT A | 79 | M | |
| 39189 | 26 February 2024 | NDM | Groin surveillance swab | INT B | 86 | F | |
| 39177 | 26 February 2024 | NDM | Groin surveillance swab | INT B | 78 | F | |
| 41326 | 28 February 2024 | VIM | Rectal surveillance swab | INT A | 76 | M | |
| 41336 | 28 February 2024 | NDM | Groin surveillance swab | INT B | 86 | M | |
| 41257 | 28 February 2024 | NDM | Groin surveillance swab | INT A | 75 | F | |
| 40486 | 28 February 2024 | VIM | Sputum | INT B | 63 | M |
| Isolate ID | Hybrispot Result | NG-Test CARBA 5 Result |
|---|---|---|
| 11102 | blaVIM, sul-1, mut gyrp-T831 | VIM |
| 91845 | blaNDM, sul-1, sul-2, aac(6′)-Ib, mut gyrp-T831 | NDM |
| 127808 | blaVIM, sul-1, aac(6′)-Ib, mut gyrp-T831 | VIM |
| 129750 | blaNDM, sul-1, sul-2, aac(6′)-Ib, mut gyrp-T831 | NDM |
| 138644 | blaNDM, sul-1, sul-2, aac(6′)-Ib, mut gyrp-T831 | NDM |
| 165541 | blaNDM, sul-1, sul-2, aac(6′)-Ib, mut gyrp-T831 | NDM |
| 171353 | blaVIM, sul-1, sul-2, mut gyrp-T831 | VIM |
| 214929 | blaNDM, blaVIM, sul-1 | NDM, VIM |
| Isolate | Antimicrobial Resistance Genes | Virulence Genes |
|---|---|---|
| 91845 | aac(3)-Id aac(6′)-Ib-cr aac(6′)-Ib3 aac(6′)-Il aadA11 aph(3″)-Ib aph(3′)-IIb aph(6)-Id blaNDM-1 blaOXA-10 blaOXA-488 blaPAO catB7 crpP dfrB5 floR fosA msr(E) qacE qnrVC1 rmtF sul1 sul2 | xcpP exoU flgC pchB mbtH-like lasI pilG pscS pscF hcp1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Protonotariou, E.; Meletis, G.; Vlachodimou, N.; Malousi, A.; Tychala, A.; Katsanou, C.; Daviti, A.; Mantzana, P.; Skoura, L. Rapid Reversal of Carbapenemase-Producing Pseudomonas aeruginosa Epidemiology from blaVIM- to blaNDM-harbouring Isolates in a Greek Tertiary Care Hospital. Antibiotics 2024, 13, 762. https://doi.org/10.3390/antibiotics13080762
Protonotariou E, Meletis G, Vlachodimou N, Malousi A, Tychala A, Katsanou C, Daviti A, Mantzana P, Skoura L. Rapid Reversal of Carbapenemase-Producing Pseudomonas aeruginosa Epidemiology from blaVIM- to blaNDM-harbouring Isolates in a Greek Tertiary Care Hospital. Antibiotics. 2024; 13(8):762. https://doi.org/10.3390/antibiotics13080762
Chicago/Turabian StyleProtonotariou, Efthymia, Georgios Meletis, Nikoletta Vlachodimou, Andigoni Malousi, Areti Tychala, Charikleia Katsanou, Aikaterini Daviti, Paraskevi Mantzana, and Lemonia Skoura. 2024. "Rapid Reversal of Carbapenemase-Producing Pseudomonas aeruginosa Epidemiology from blaVIM- to blaNDM-harbouring Isolates in a Greek Tertiary Care Hospital" Antibiotics 13, no. 8: 762. https://doi.org/10.3390/antibiotics13080762





