EDBD—3,6-Epidioxy-1,10-Bisaboladiene—An Endoperoxide Sesquiterpene Obtained from Drimys brasiliensis (Winteraceae) Exhibited Potent Preclinical Efficacy against Schistosoma mansoni Infection
Abstract
1. Introduction
2. Results
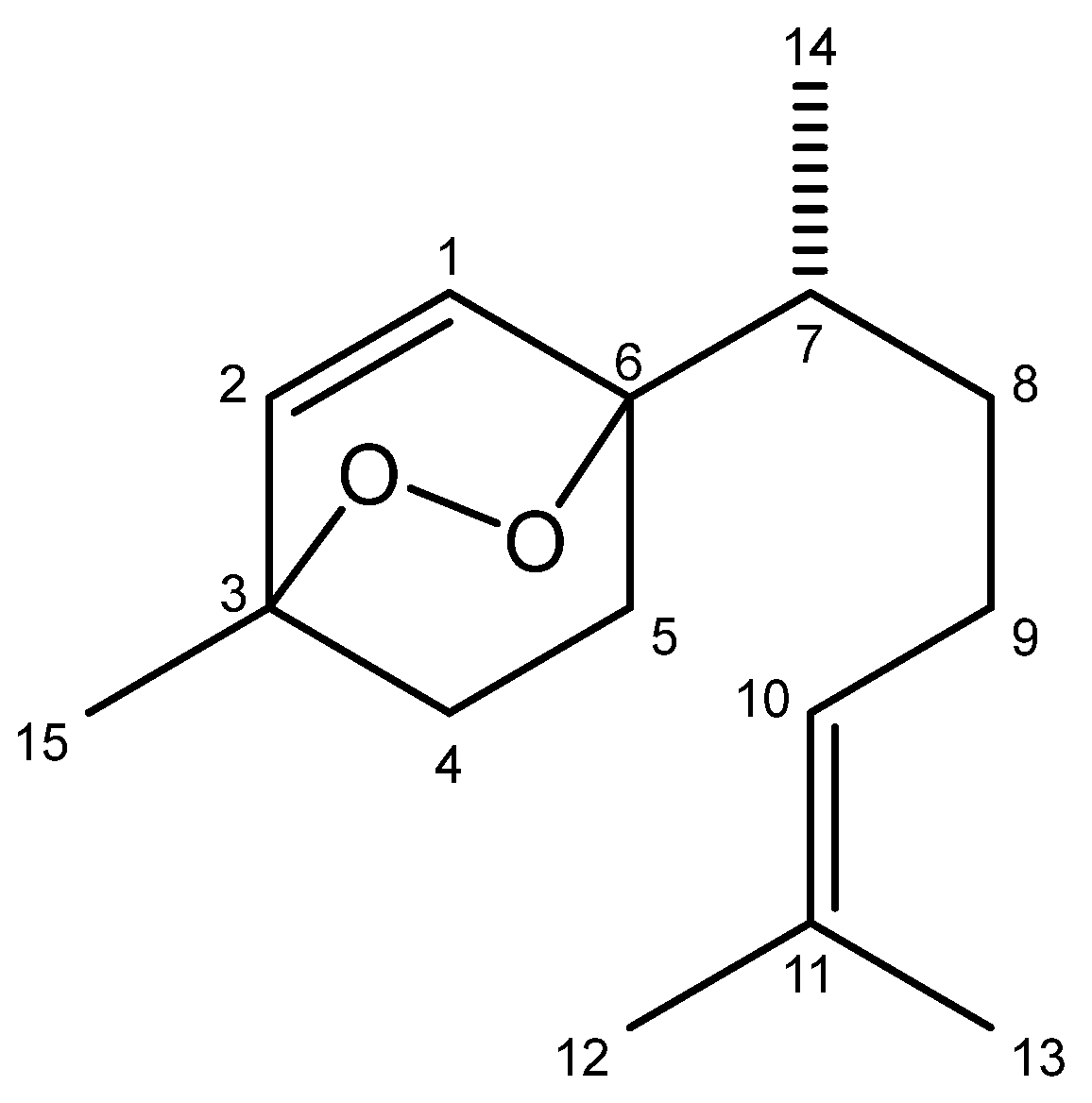
2.1. Chemical Characterization of EDBD
2.2. EDBD Exhibited Antiparasitic Effects on S. mansoni without Toxicity to Mammalian Cells
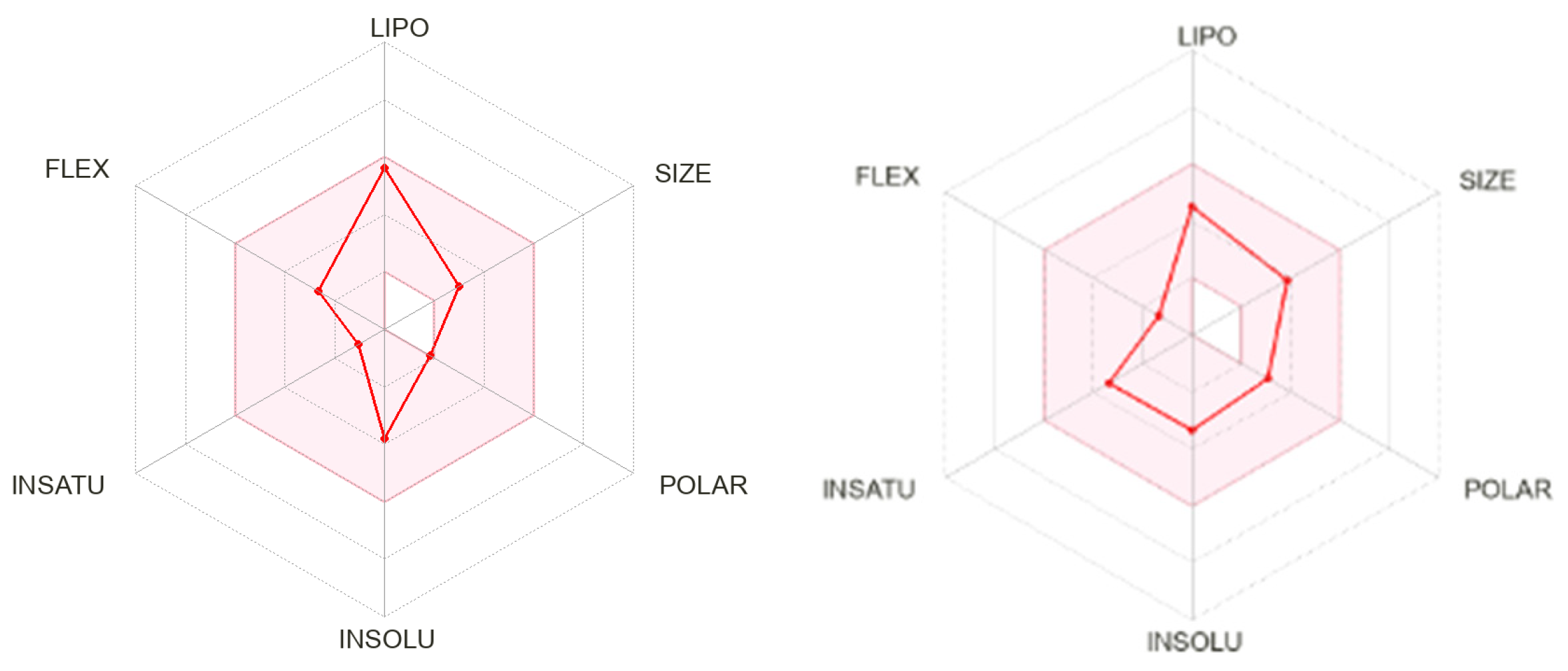
2.3. In Silico Evaluation
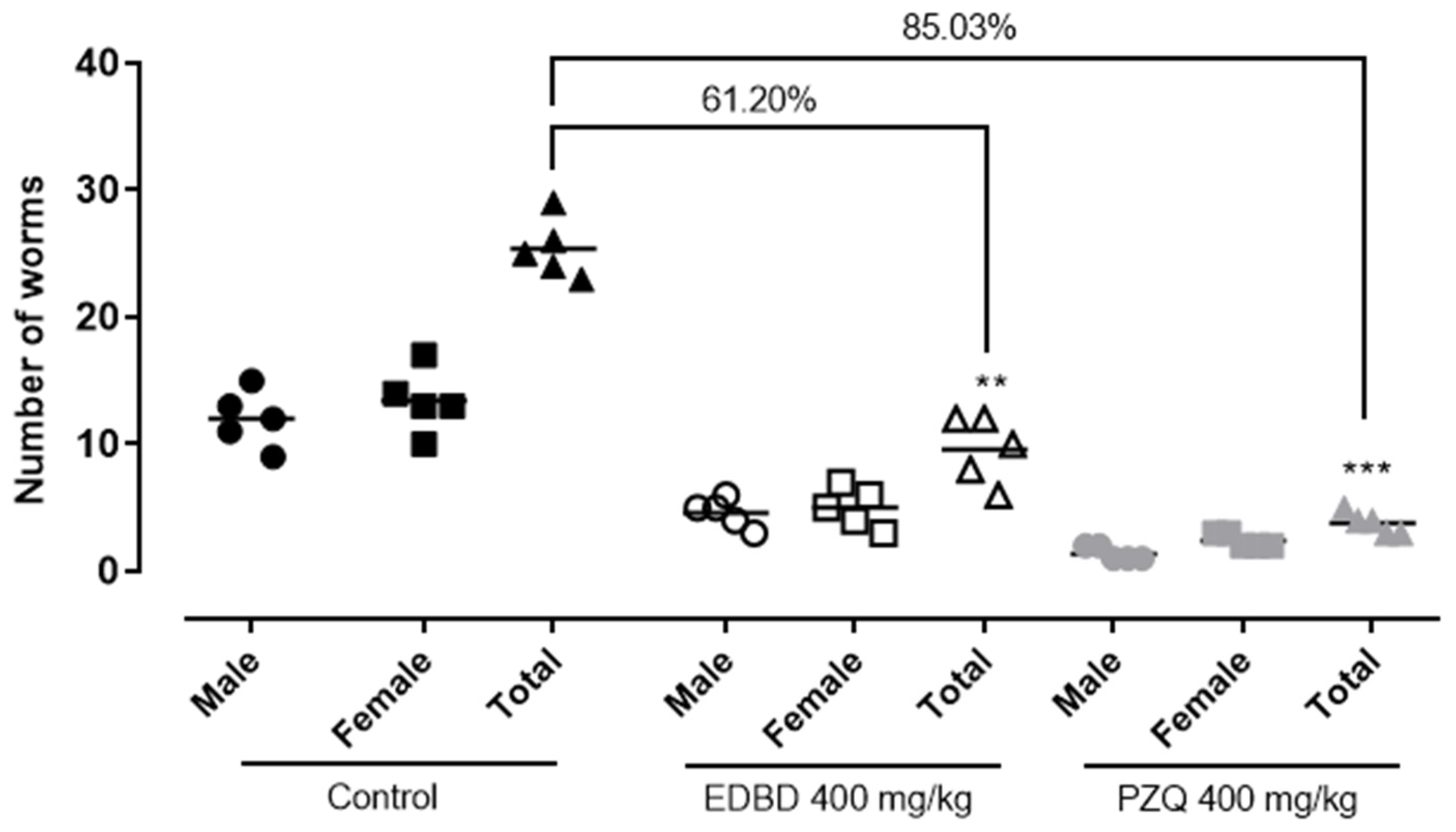
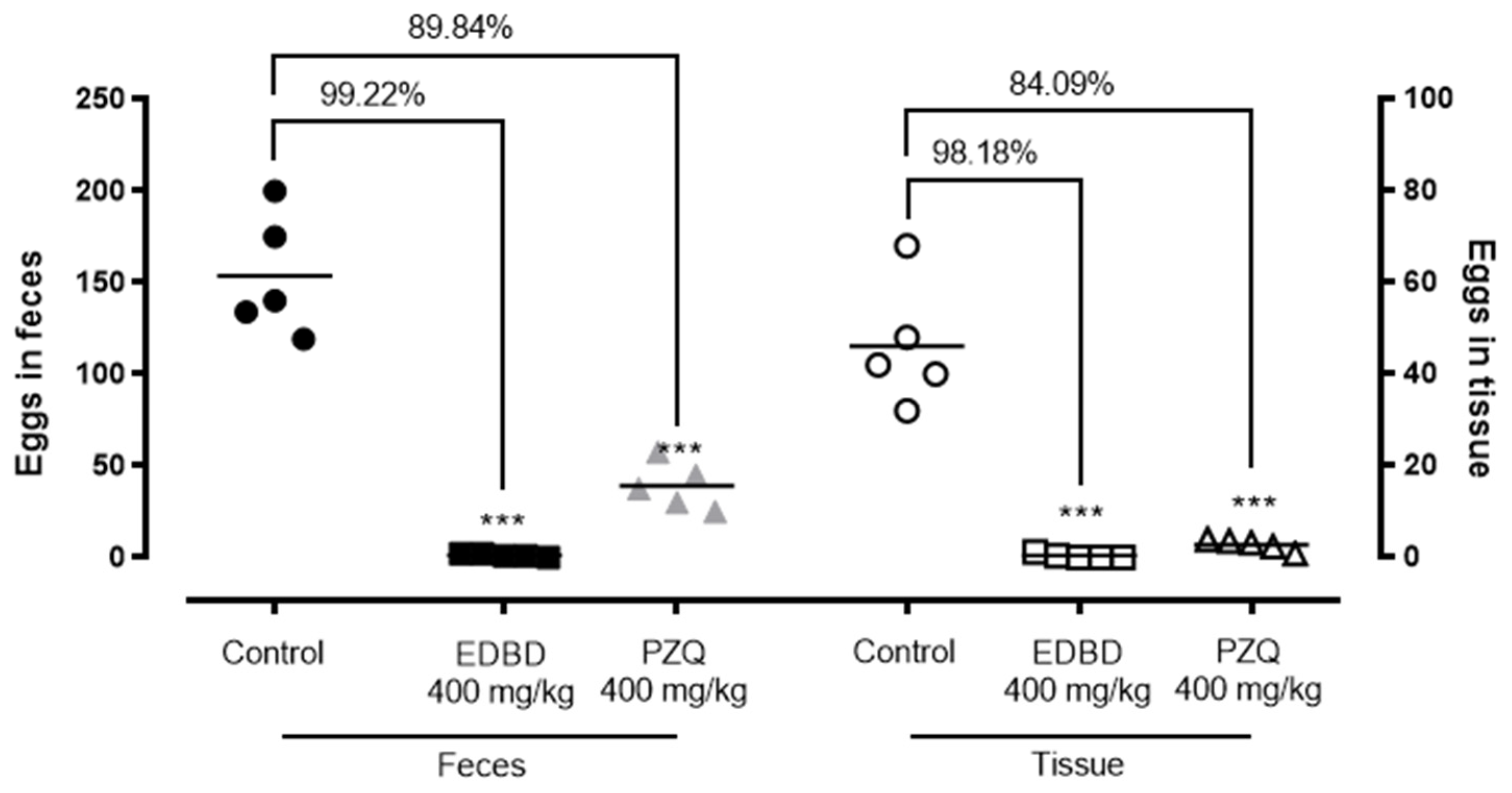
2.4. In Vivo Evaluation of EDBD—Reduction in Adult Worm Parasite Burden and a Decrease in Egg Production
3. Discussion
4. Materials and Methods
4.1. General Procedures
4.2. Plant Material
4.3. Isolation of EDBD (3,6-Epidioxy-Bisabola-1,10-Diene)
4.4. In Silico Analysis
4.5. Animals, Worms and Cells
4.6. In Vitro Antischistosomal Assay
4.7. Toxicity Profile in Mammalian Cells
4.8. In Vivo Therapeutic Efficacy in an Animal Model of Schistosomiasis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Schistosomiasis; World Health Organization: Geneva, Switzerland, 2023; Available online: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (accessed on 16 April 2024).
- Anissuzaman; Tsuji, N. Schistosomiasis and hookworm infection in humans: Disease burden, pathology and anthelmintic vaccines. Parasitol. Int. 2020, 75, 102051. [Google Scholar] [CrossRef] [PubMed]
- McManus, D.P.; Dunne, D.W.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X.-N. Schistosomiasis. Nat. Rev. Dis. Primers 2018, 4, 13–31. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Wang, W.; Wang, L.; Liang, Y.S. Susceptibility or resistance of praziquantel in human schistosomiasis: A review. Parasitol. Res. 2012, 111, 1871–1877. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, N.; Afshar, R.; Baragaña, B.; Bustinduy, A.L.; Caffrey, C.R.; Collins, J.J.; Fusco, D.; Garba, A.; Gardner, M.; Gomes, M.; et al. Perspective on Schistosomiasis Drug Discovery: Highlights from a Schistosomiasis Drug Discovery Workshop at Wellcome Collection, London, September 2022. ACS Infect. Dis. 2023, 9, 1046–1055. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Malheiros, A.; Filho, V.D.; Schmitt, C.B.; Yunes, R.A.; Escalante, A.; Svetaz, L.; Zacchino, S.; Monache, F.R. Antifungal Activity of Drimane Sesquiterpenes from Drimys brasiliensis Using Bioassay-guided Fractionation. J. Pharm. Pharm. Sci. 2005, 8, 335–339. [Google Scholar] [PubMed]
- Claudino, V.; Silva, K.; Filho, V.; Yunes, R.; Monache, F.D.; Giménez, A.; Salamanca, E.; Yapu-Gutierrez, D.; Malheiros, A. Drimanes from Drimys brasiliensis with Leishmanicidal and Antimalarial Activity. Mem. Inst. Oswaldo Cruz 2013, 108, 140–144. [Google Scholar] [CrossRef]
- Ferreira, B.A.; Filho, A.F.N.; Deconte, S.R.; Tomiosso, T.C.; Thevenard, F.; Andrade, S.P.; Lago, J.H.G.; Araújo, F.D.A. Sesquiterpene Polygodial from Drimys brasiliensis (Winteraceae) Down-Regulates Implant-Induced Inflammation and Fibrogenesis in Mice. J. Nat. Prod. 2020, 83, 3698–3705. [Google Scholar] [CrossRef]
- Filho, V.C.; Schlemper, V.; Santos, A.R.S.; Pinheiro, T.R.; Yunes, R.A.; Mendes, G.L.; Calixto, G.L.M.; Monache, F.D. Isolation and Identification of Active Compounds from Drimys winteri Barks. J. Ethnopharmacol. 1998, 62, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Freitas, J.; De Lírio, E.J.; Alves-Araújo, A. Flora of Espírito Santo: Winteraceae. Rodriguesia 2022, 73, e200372021. [Google Scholar] [CrossRef]
- Corrêa, D.S.; Tempone, A.G.; Reimão, J.Q.; Taniwaki, N.N.; Romoff, P.; Fávero, O.A.; Sartorelli, P.; Mecchi, M.C.; Lago, J.H.G. Anti-leishmanial and Anti-trypanosomal Potential of Polygodial Isolated from Stem Barks of Drimys brasiliensis Miers (Winteraceae). Parasitol. Res. 2011, 109, 231–236. [Google Scholar] [CrossRef]
- Simonsen, H.T.; Adsersen, A.; Bremner, P.; Heinrich, M.; Smitt, U.W.; Jaroszewski, J.W. Antifungal Constituents of Melicope borbonica. Phytother. Res. 2004, 18, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Mugumbate, G.; Overington, J.P. The Relationship Between Target-class and the Physicochemical Properties of Anti-bacterial Drugs. Bioorg. Med. Chem. 2015, 23, 5218–5224. [Google Scholar] [CrossRef]
- Lago, E.M.; Xavier, R.P.; Teixeira, T.R.; Silva, L.M.; da Silva Filho, A.A.; de Moraes, J. Antischistosomal agents: State of art and perspectives. Future Med. Chem. 2018, 10, 89–120. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; De Sarkar, S.; Gille, L.; Chatterjee, M. Ascaridole Exerts the Leishmanicidal Activity by Inhibiting Parasite Glycolysis: Anti-leishmanial Activity of Ascaridole. Phytomedicine 2022, 103, 154221. [Google Scholar] [CrossRef] [PubMed]
- Brito, J.R.; Wilairatana, P.; Roquini, D.B.; Parra, B.C.; Gonçalves, M.M.; Souza, D.C.S.; Ferreira, E.A.; Salvadori, M.C.; Teixeira, F.S.; Lago, J.H.G.; et al. Neolignans Isolated from Saururus cernuus L. (Saururaceae) Exhibit Efficacy Against Schistosoma mansoni. Sci. Rep. 2022, 12, 19320. [Google Scholar] [CrossRef]
- Mona, M.H.; Omran, N.E.; Mansoor, M.A.; El-Fakharany, Z.M. Antischistosomal Effect of Holothurin Extracted from Some Egyptian Sea Cucumbers. Pharm. Biol. 2012, 50, 1144–1150. [Google Scholar] [CrossRef]
- Kanyanta, M.; Lengwe, C.; Mambwe, D.; Francisco, K.R.; Liu, L.J.; Uli Sun, Y.; Amarasinghe, D.K.; Caffrey, C.R.; Mubanga Cheuka, P. Activity of N-phenylbenzamide Analogs Against the Neglected Disease Pathogen, Schistosoma mansoni. Bioorg. Med. Chem. Lett. 2023, 82, 129164. [Google Scholar] [CrossRef]
- Rocha, B.; de Morais, L.A.; Viana, M.C.; Carneiro, G. Promising Strategies for Improving Oral Bioavailability of Poor Water-Soluble Drugs. Expert Opin. Drug Discov. 2023, 18, 615–627. [Google Scholar] [CrossRef]
- Mengarda, A.C.; Silva, M.P.; Cirino, M.E.; Morais, T.R.; Conserva, G.A.A.; Lago, J.H.G.; de Moraes, J. Licarin A, a Neolignan Isolated from Nectandra oppositifolia Nees & Mart. (Lauraceae), Exhibited Moderate Preclinical Efficacy Against Schistosoma mansoni Infection. Phytoter. Res. 2021, 35, 4627–5329. [Google Scholar]
- Silva, M.P.; Oliveira, R.M.; Mengarda, A.C.; Roquini, D.B.; Allegretti, S.M.; Salvadori, M.C.; Teixeira, F.S.; de Sousa, D.P.; Pinto, P.L.S.; da Silva Filho, A.A.; et al. Antiparasitic Activity of Nerolidol in a Mouse Model of Schistosomiasis. Int. J. Antimicrob. Agents 2017, 50, 467–472. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.S.; Leal, C.M.; Cajas, R.A.; Gazolla, M.C.; Silva, L.M.; Carvalho, L.S.A.; Lemes, B.L.; de Moura, R.O.; de Almeida, J.; de Moraes, J.; et al. Antiparasitic Properties of 4-nerolidylcatechol from Pothomorphe umbellata (L.) Miq. (Piperaceae) in vitro and in mice Models with Either Prepatent or Patent Schistosoma mansoni Infections. J. Ethnopharmacol. 2023, 313, 116607. [Google Scholar] [CrossRef] [PubMed]
- Hunt, P.R. The C. elegans Model in Toxicity Testing. J. Appl. Toxicol. 2017, 37, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Ryan, K.T.; Wheeler, N.J.; Kamara, I.K.; Johnson, H.; Humphries, J.E.; Zamanian, M.; Chan, J.D. Phenotypic Profiling of Macrocyclic Lactones on Parasitic Schistosoma Flatworms. Antimicrob. Agents Chemother. 2023, 67, e0123022. [Google Scholar] [CrossRef] [PubMed]
- Jatsa, H.B.; Ngo-Sock, E.T.; Tchuente, L.A.T.; Kamtchouing, P. Evaluation of the In Vivo Activity of Different Concentrations of Clerodendrum umbellatum Poir Against Schistosoma mansoni Infection in Mice. Afr. J. Tradit. Complement. Altern. Med. 2009, 6, 216–221. [Google Scholar] [CrossRef]
- Kittur, N.; Castleman, J.D.; Campbell, C.H.; King, C.H.; Colley, D.G. Comparison of Schistosoma mansoni Prevalence and Intensity of Infection, as Determined by the Circulating Cathodic Antigen Urine Assay or by the Kato-Katz Fecal Assay: A Systematic Review. Am. J. Trop. Med. Hyg. 2016, 94, 605–610. [Google Scholar] [CrossRef] [PubMed]
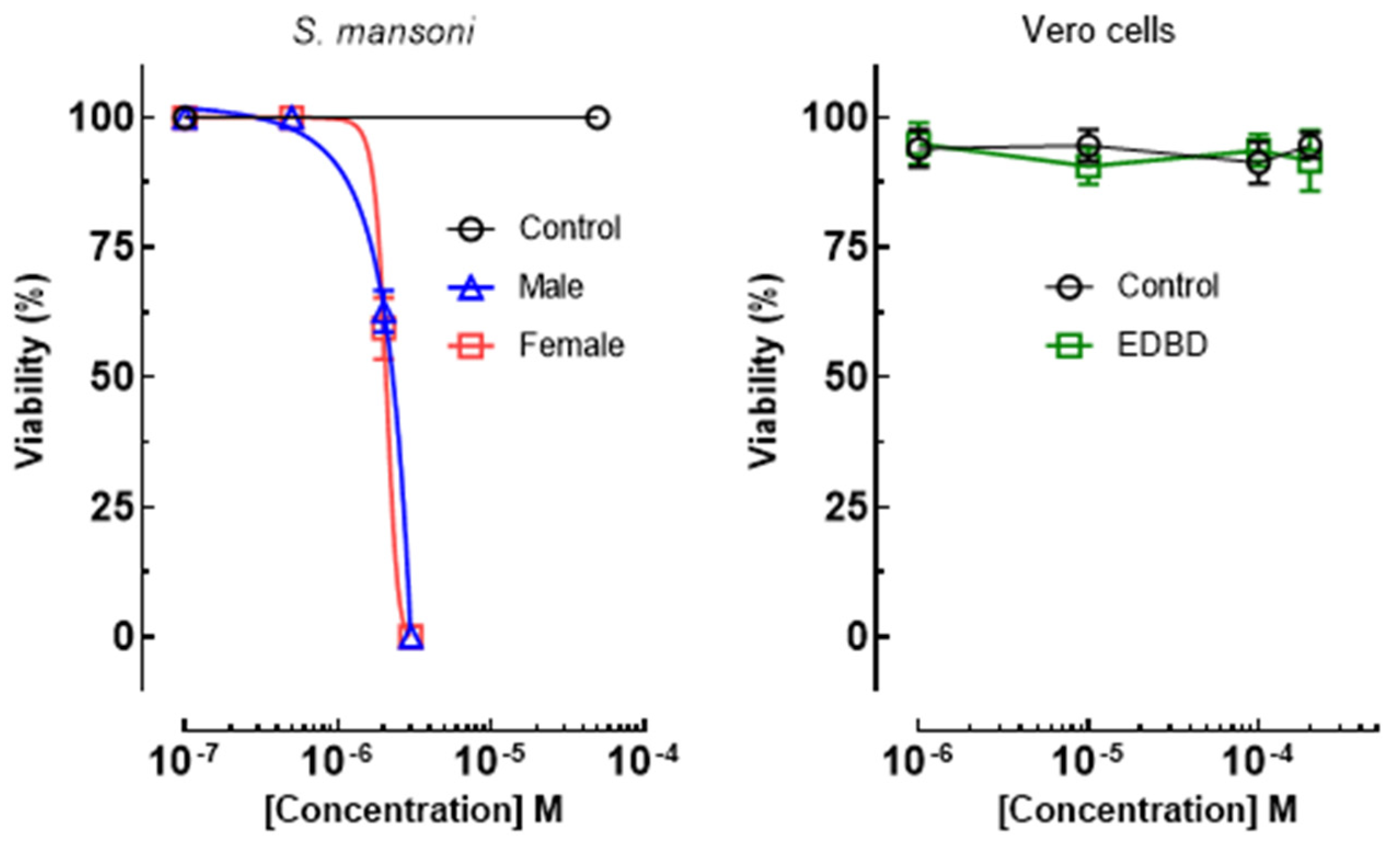
| Compound | S. mansoni Male EC50/μM | S. mansoni Female EC50/μM | Vero Cell CC50/μM | SI a (Male Worms) | SI a (Female Worms) |
|---|---|---|---|---|---|
| EDBD | 4.1 ± 1.2 | 4.1 ± 1.2 | ˃200 | ˃48 | ˃48 |
| PZQ | 1.1 ± 0.7 | 1.3 ± 0.8 | ˃200 | ˃181 | ˃153 |
| Parameters | EDBD | PZQ |
|---|---|---|
| MW | 236.35 Da | 312.41 Da |
| TPSA | 18.46 Å | 40.62 Å |
| Log Po/w | 3.79 | 3.00 |
| Log S | −3.21 | −3.52 |
| Gastroinstestinal absorption | High | High |
| BBB | Yes | Yes |
| Inhibitor CYP1A2 | No | No |
| Inhibitor CYP2C19 | Yes | Yes |
| Inhibitor CYP2C9 | Yes | No |
| Inhibitor CYP2D6 | No | Yes |
| Inhibitor CYP3A4 | No | Yes |
| Lipinksi | Yes | Yes |
| Ghose | Yes | Yes |
| Veber | Yes | Yes |
| Egan | Yes | Yes |
| Muegge | Yes | Yes |
| Bioavaliability Score | 0.55 | 0.55 |
| Leadlikeness | Yes | Yes |
| PAINS | 0 alert | 0 alert |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umehara, E.; Teixeira, T.R.; Cajás, R.A.; Amaro, M.C.; de Moraes, J.; Lago, J.H.G. EDBD—3,6-Epidioxy-1,10-Bisaboladiene—An Endoperoxide Sesquiterpene Obtained from Drimys brasiliensis (Winteraceae) Exhibited Potent Preclinical Efficacy against Schistosoma mansoni Infection. Antibiotics 2024, 13, 779. https://doi.org/10.3390/antibiotics13080779
Umehara E, Teixeira TR, Cajás RA, Amaro MC, de Moraes J, Lago JHG. EDBD—3,6-Epidioxy-1,10-Bisaboladiene—An Endoperoxide Sesquiterpene Obtained from Drimys brasiliensis (Winteraceae) Exhibited Potent Preclinical Efficacy against Schistosoma mansoni Infection. Antibiotics. 2024; 13(8):779. https://doi.org/10.3390/antibiotics13080779
Chicago/Turabian StyleUmehara, Eric, Thainá R. Teixeira, Rayssa A. Cajás, Monique C. Amaro, Josué de Moraes, and João Henrique G. Lago. 2024. "EDBD—3,6-Epidioxy-1,10-Bisaboladiene—An Endoperoxide Sesquiterpene Obtained from Drimys brasiliensis (Winteraceae) Exhibited Potent Preclinical Efficacy against Schistosoma mansoni Infection" Antibiotics 13, no. 8: 779. https://doi.org/10.3390/antibiotics13080779
APA StyleUmehara, E., Teixeira, T. R., Cajás, R. A., Amaro, M. C., de Moraes, J., & Lago, J. H. G. (2024). EDBD—3,6-Epidioxy-1,10-Bisaboladiene—An Endoperoxide Sesquiterpene Obtained from Drimys brasiliensis (Winteraceae) Exhibited Potent Preclinical Efficacy against Schistosoma mansoni Infection. Antibiotics, 13(8), 779. https://doi.org/10.3390/antibiotics13080779







