Efficacy and Safety of Oral Neomycin for the Decolonization of Carbapenem-Resistant Enterobacterales: An Open-Label Randomized Controlled Trial
Abstract
1. Introduction
2. Results
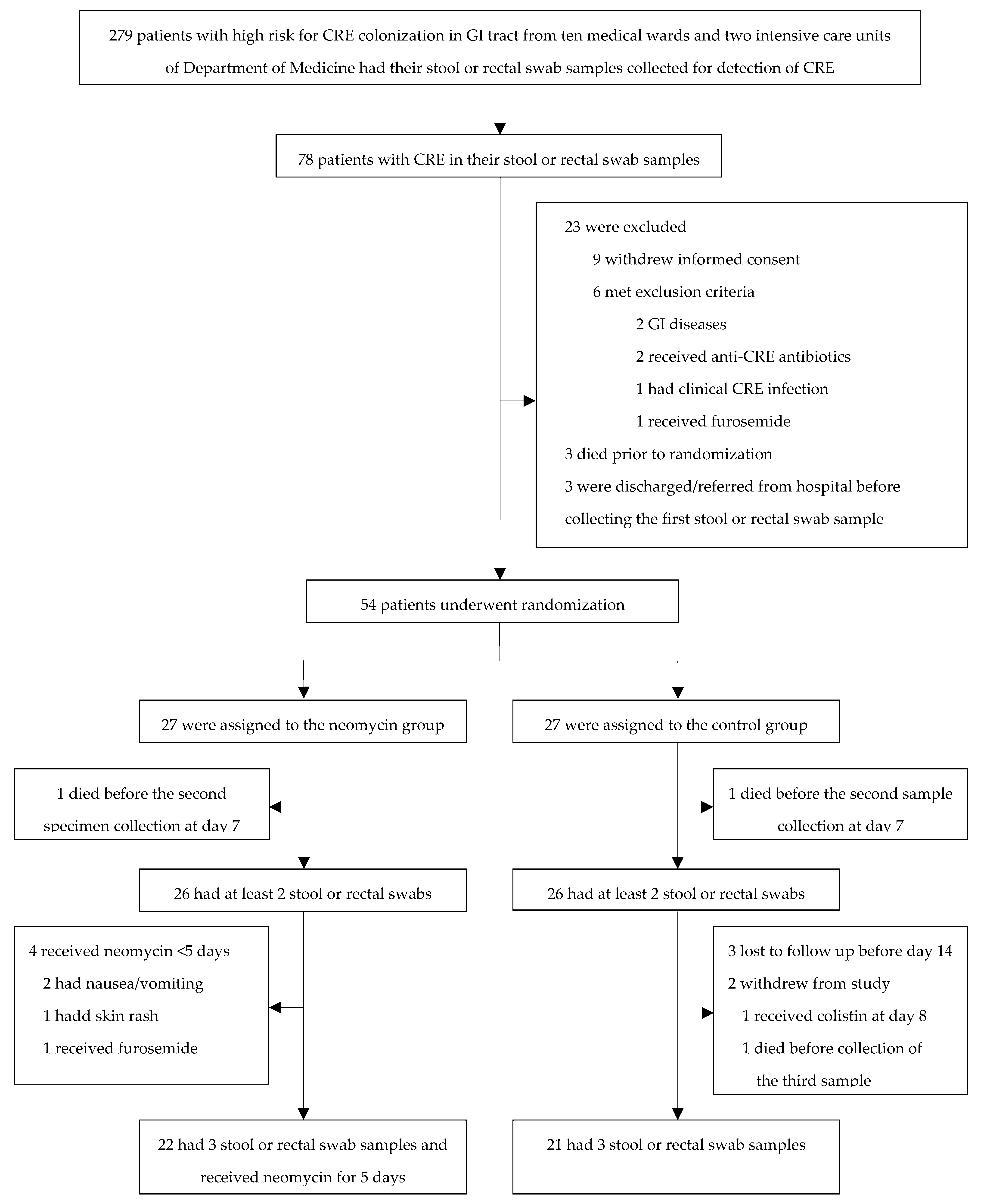
2.1. Inclusion of Participants
2.2. Baseline Characteristics of Participants
2.3. Efficacy of Neomycin for the Decolonization of CRE at Day 7 ± 2 and Day 14 ± 2
2.4. Species of CRE Isolates
2.5. Safety and Tolerability
2.6. Susceptibility Rates of CRE Isolates
3. Discussion
4. Materials and Methods
4.1. Study Design and Setting
4.2. Participants
4.3. Detection of CRE
4.4. Intervention
4.5. Follow-Up of Participants
4.6. Study Outcomes
4.7. Sample Size Estimation and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. WHO Publishes List of Bacteria for Which New Antibiotics are Urgently Needed 2017. 27 February 2017. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 13 January 2023).
- Manning, M.L.; Septimus, E.J.; Ashley, E.S.D.; Cosgrove, S.E.; Fakih, M.G.; Schweon, S.J.; Myers, F.E.; Moody, J.A. Antimicrobial stewardship and infection prevention-leveraging the synergy: A position paper update. Am. J. Infect. Control 2018, 46, 364–368. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Division (AMR). Global Antimicrobial Resistance and Use Surveillance system (GLASS) Report: 2022. 9 December 2022. Available online: https://www.who.int/publications/i/item/9789240062702 (accessed on 10 January 2023).
- National Antimicrobial Resistance Surveillance, Thailand (NARST). Antimicrobial Resistance 2000–2022; National Institute of Health, Department of Medical Sciences: Bangkok, Thailand, 2023. [Google Scholar]
- So-Ngern, A.; Osaithai, N.; Meesing, A.; Chumpangern, W. Mortality rate and factors associated with mortality of carbapenem-resistant Enterobacteriaceae infection. Drug Target Insights 2023, 17, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Tangsawad, W.; Kositamongkol, C.; Chongtrakool, P.; Phisalprapa, P.; Jitmuang, A. The burden of carbapenem-resistant Enterobacterales infection in a large Thai tertiary care hospital. Front. Pharmacol. 2022, 13, 972900. [Google Scholar] [CrossRef] [PubMed]
- Nasomsong, W.; Nulsopapon, P.; Changpradub, D.; Pongchaidecha, M.; Pungcharoenkijkul, S.; Juntanawiwat, P.; Simsiriporn, W.; Santimaleeworagun, W. The Potential Use of Ceftazidime-Avibactam against Carbapenem Resistant Klebsiella pneumoniae Clinical Isolates Harboring Different Carbapenemase Types in a Thai University Hospital. Drug Des. Devel. Ther. 2021, 15, 3095–3104. [Google Scholar] [CrossRef] [PubMed]
- Nulsopapon, P.; Pongchaidecha, M.; Nasomsong, W.; Polwichai, P.; Suphankong, S.; Sirichote, P.; Chaisomboonpan, S.; Santimaleeworagun, W. Antimicrobial Activity Profiles and Potential Antimicrobial Regimens against Carbapenem-Resistant Enterobacterales Isolated from Multi-Centers in Western Thailand. Antibiotics 2022, 11, 355. [Google Scholar] [CrossRef] [PubMed]
- National Drug System Development Committee. National Essential Medicines List of Thailand. 14 August 2022. Available online: https://ndi.fda.moph.go.th/ndi_news_detail/index/295 (accessed on 18 February 2024).
- Zhou, R.; Fang, X.; Zhang, J.; Zheng, X.; Shangguan, S.; Chen, S.; Shen, Y.; Liu, Z.; Li, J.; Zhang, R.; et al. Impact of carbapenem resistance on mortality in patients infected with Enterobacteriaceae: A systematic review and meta-analysis. BMJ Open 2021, 11, e054971. [Google Scholar] [CrossRef] [PubMed]
- Wangchinda, W.; Laohasakprasit, K.; Lerdlamyong, K.; Thamlikitkul, V. Epidemiology of Carbapenem-Resistant Enterobacterales Infection and Colonization in Hospitalized Patients at a University Hospital in Thailand. Infect. Drug Resist. 2022, 15, 2199–2210. [Google Scholar] [CrossRef] [PubMed]
- Hassoun-Kheir, N.; Hussien, K.; Karram, M.; Saffuri, M.; Badaan, S.; Peleg, S.; Aboelhega, W.; Warman, S.; Alon, T.; Pollak, D.; et al. Clinical significance and burden of carbapenem-resistant Enterobacterales (CRE) colonization acquisition in hospitalized patients. Antimicrob. Resist. Infect. Control 2023, 12, 129. [Google Scholar] [CrossRef]
- Pérez-Galera, S.; Bravo-Ferrer, J.M.; Paniagua, M.; Kostyanev, T.; De Kraker, M.E.A.; Feifel, J.; Sojo-Dorado, J.; Schotsman, J.; Cantón, R.; Daikos, G.L.; et al. Risk factors for infections caused by carbapenem-resistant Enterobacterales: An international matched case-control-control study (EURECA). EClinicalMedicine 2023, 57, 101871. [Google Scholar] [CrossRef]
- McConville, T.H.; Sullivan, S.B.; Gomez-Simmonds, A.; Whittier, S.; Uhlemann, A.C. Carbapenem-resistant Enterobacteriaceae colonization (CRE) and subsequent risk of infection and 90-day mortality in critically ill patients, an observational study. PLoS ONE 2017, 12, e0186195. [Google Scholar] [CrossRef]
- Tacconelli, E.; Mazzaferri, F.; De Smet, A.M.; Bragantini, D.; Eggimann, P.; Huttner, B.D.; Kuijper, E.J.; Lucet, J.-C.; Mutters, N.T.; Sanguinetti, M.; et al. ESCMID-EUCIC clinical guidelines on decolonization of multidrug-resistant Gram-negative bacteria carriers. Clin. Microbiol. Infect. 2019, 25, 807–817. [Google Scholar] [CrossRef]
- Saidel-Odes, L.; Polachek, H.; Peled, N.; Riesenberg, K.; Schlaeffer, F.; Trabelsi, Y.; Eskira, S.; Yousef, B.; Smolykov, R.; Codish, S.; et al. A Randomized, Double-Blind, Placebo-Controlled Trial of Selective Digestive Decontamination Using Oral Gentamicin and Oral Polymyxin E for Eradication of Carbapenem-Resistant Klebsiella pneumoniae Carriage. Infect. Control Hosp. Epidemiol. 2012, 33, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Oren, I.; Sprecher, H.; Finkelstein, R.; Hadad, S.; Neuberger, A.; Hussein, K.; Raz-Pasteur, A.; Lavi, N.; Saad, E.; Henig, I.; et al. Eradication of carbapenem-resistant Enterobacteriaceae gastrointestinal colonization with nonabsorbable oral antibiotic treatment: A prospective controlled trial. Am. J. Infect. Control 2013, 41, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Stoma, I.; Karpov, I.; Iskrov, I.; Krivenko, S.; Uss, A.; Vlasenkova, S.; Lendina, I.; Cherniak, V.; Suvorov, D. Decolonization of Intestinal Carriage of MDR/XDR Gram-Negative Bacteria with Oral Colistin in Patients with Hematological Malignancies: Results of a Randomized Controlled Trial. Mediterr. J. Hematol. Infect. Dis. 2018, 10, e2018030. [Google Scholar] [CrossRef] [PubMed]
- Fariñas, M.C.; González-Rico, C.; Fernández-Martínez, M.; Fortún, J.; Escudero-Sanchez, R.; Moreno, A.; Bodro, M.; Muñoz, P.; Valerio, M.; Montejo, M.; et al. Oral decontamination with colistin plus neomycin in solid organ transplant recipients colonized by multidrug-resistant Enterobacterales: A multicentre, randomized, controlled, open-label, parallel-group clinical trial. Clin. Microbiol. Infect. 2021, 27, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Lübbert, C.; Faucheux, S.; Becker-Rux, D.; Laudi, S.; Dürrbeck, A.; Busch, T.; Gastmeier, P.; Eckmanns, T.; Rodloff, A.C.; Kaisers, U.X. Rapid emergence of secondary resistance to gentamicin and colistin following selective digestive decontamination in patients with KPC-2-producing Klebsiella pneumoniae: A single-centre experience. Int. J. Antimicrob. Agents 2013, 42, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Huttner, B.D.; De Lastours, V.; Wassenberg, M.; Maharshak, N.; Mauris, A.; Galperine, T.; Zanichelli, V.; Kapel, N.; Bellanger, A.; Olearo, F.; et al. A 5-day course of oral antibiotics followed by faecal transplantation to eradicate carriage of multidrug-resistant Enterobacteriaceae: A randomized clinical trial. Clin. Microbiol. Infect. 2019, 25, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Carrara, E.; Retamar, P.; Tängdén, T.; Bitterman, R.; Bonomo, R.A.; De Waele, J.; Daikos, G.L.; Akova, M.; Harbarth, S.; et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin. Microbiol. Infect. 2022, 28, 521–547. [Google Scholar] [CrossRef] [PubMed]
- Righi, E.; Mutters, N.T.; Guirao, X.; Del Toro, M.D.; Eckmann, C.; Friedrich, A.W.; Giannella, M.; Kluytmans, J.; Presterl, E.; Christaki, E.; et al. ESCMID/EUCIC clinical practice guidelines on perioperative antibiotic prophylaxis in patients colonized by multidrug-resistant Gram-negative bacteria before surgery. Clin. Microbiol. Infect. 2023, 29, 463–479. [Google Scholar] [CrossRef]
- Macareño-Castro, J.; Solano-Salazar, A.; Dong, L.T.; Mohiuddin, M.; Espinoza, J.L. Fecal microbiota transplantation for Carbapenem-Resistant Enterobacteriaceae: A systematic review. J. Infect. 2022, 84, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Veirup, N.; Kyriakopoulos, C. Neomycin Treasure Island (FL); StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560603/ (accessed on 5 February 2024).
- Hu, Y.; Liu, L.; Zhang, X.; Feng, Y.; Zong, Z. In Vitro Activity of Neomycin, Streptomycin, Paromomycin and Apramycin against Carbapenem-Resistant Enterobacteriaceae Clinical Strains. Front. Microbiol. 2017, 8, 2275. [Google Scholar] [CrossRef] [PubMed]
- Yungyuen, T.; Chatsuwan, T.; Plongla, R.; Kanthawong, S.; Yordpratum, U.; Voravuthikunchai, S.P.; Chusri, S.; Saeloh, D.; Samosornsuk, W.; Suwantarat, N.; et al. Nationwide Surveillance and Molecular Characterization of Critically Drug-Resistant Gram-Negative Bacteria: Results of the Research University Network Thailand Study. Antimicrob. Agents Chemother. 2021, 65, e0067521. [Google Scholar] [CrossRef] [PubMed]
- Tiengrim, S.; Thamlikitkul, V. In Vitro Activity of Neomycin, Gentamicin and Amikacin against Carbapenem-Resistant Enterobacterales (CRE) isolated from Patients at Siriraj Hospital, Thailand. Infect. Chemother. 2024, 56 (Suppl. S1), S131. [Google Scholar]
- Doi, Y.; Wachino, J.; Arakawa, Y. Aminoglycoside Resistance: The Emergence of Acquired 16S Ribosomal RNA Methyltransferases. Infect. Dis. Clin. N. Am. 2016, 30, 523–537. [Google Scholar] [CrossRef]
- Ma, J.; Song, X.; Li, M.; Yu, Z.; Cheng, W.; Yu, Z.; Zhang, W.; Zhang, Y.; Shen, A.; Sun, H.; et al. Global spread of carbapenem-resistant Enterobacteriaceae: Epidemiological features, resistance mechanisms, detection and therapy. Microbiol. Res. 2023, 266, 127249. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Suscetibility Testing, 33rd ed.; CLSI Supplement M100 [Electronic]; Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2023; Available online: https://clsi.org/standards/products/microbiology/documents/m100/ (accessed on 12 December 2023).
| Characteristics | Neomycin Group (n = 26) | Control Group (n = 26) | p |
|---|---|---|---|
| Male | 8 (30.8%) | 15 (57.7%) | 0.05 |
| Mean age (SD), years | 63.6 (20.9) | 71.7 (14.1) | 0.11 |
| Alive at discharge from hospital | 22 (84.6%) | 17 (65.4%) | 0.11 |
| Status before/on admission | |||
| Underlying disease | 22 (84.6%) | 26 (100.0%) | 0.11 |
| Heart disease | 4 (15.4%) | 10 (38.5%) | 0.06 |
| Pulmonary disease | 2 (7.7%) | 7 (26.9%) | 0.14 |
| Hypertension | 15 (57.7%) | 18 (69.2%) | 0.39 |
| Diabetes mellitus | 9 (34.6%) | 10 (38.5%) | 0.77 |
| Renal disease | 3 (11.5%) | 7 (26.9%) | 0.16 |
| Cerebrovascular disease | 11 (42.3%) | 6 (23.1%) | 0.14 |
| Gastrointestinal and liver disease | 2 (7.7%) | 2 (7.7%) | 1.00 |
| Hematologic malignancy | 2 (7.7%) | 1 (3.8%) | 1.00 |
| Solid malignancy | 2 (7.7%) | 4 (15.4%) | 0.67 |
| Autoimmune disease | 3 (11.5%) | 1 (3.8%) | 0.61 |
| Median Karnofsky performance status scale (IQR) | 50.0 (40.0–80.0) | 50.0 (40.0–60.0) | 0.32 |
| Transfer from long-term care facility | 2 (7.7%) | 4 (15.4%) | 0.67 |
| Previous hospitalization within 3 months | 15 (57.7%) | 13 (50.0%) | 0.58 |
| Urinary catheter | 1 (3.8%) | 4 (15.4%) | 0.35 |
| Nasogastric tube | 3 (11.5%) | 6 (23.1%) | 0.47 |
| Tracheostomy tube | 5 (19.2%) | 5 (19.2%) | 1.00 |
| Previous major surgery within 3 months | 2 (7.7%) | 1 (3.8%) | 0.55 |
| Previous antibiotic use within 3 months | 15 (57.7%) | 15 (57.7%) | 1.00 |
| Previous immunosuppressive agent use within 3 months | 4 (15.4%) | 4 (15.4%) | 1.00 |
| Previous CRE infection | 0 (0.0%) | 1 (3.8%) | 1.00 |
| Previous CRE colonization | 2 (7.7%) | 4 (15.4%) | 0.67 |
| Status during admission | |||
| Indwelling central intravascular line | 5 (19.2%) | 3 (11.5%) | 0.70 |
| Mechanical ventilator | 13 (50.0%) | 14 (53.8%) | 0.78 |
| Indwelling urinary catheter | 21 (80.0%) | 21 (80.0%) | 1.00 |
| Major surgery | 5 (19.2%) | 0 (0.0%) | 0.05 |
| Chemotherapy | 3 (11.5%) | 0 (0.0%) | 0.24 |
| Diagnosis | |||
| Heart disease | 2 (7.7%) | 4 (15.4%) | 0.67 |
| Pulmonary disease | 2 (7.7%) | 6 (23.1%) | 0.25 |
| Renal disease | 2 (7.7%) | 3 (11.5%) | 1.00 |
| Cerebrovascular disease | 5 (19.2%) | 1 (3.8%) | 0.19 |
| Hematologic malignancy | 2 (7.7%) | 1 (3.8%) | 1.00 |
| Solid malignancy | 4 (15.4%) | 1 (3.8%) | 0.35 |
| Autoimmune disease | 3 (11.5%) | 0 (0.0%) | 0.24 |
| Previous or current documented or presumed infection during this admission up to the end of the study | 21 (80.8%) | 26 (100.0%) | 0.05 |
| Had received or receiving antibiotics during this admission up to the end of the study | 26 (100.0%) | 26 (100.0%) | 1.00 |
| Species of CRE isolates | 28 CRE isolates | 28 CRE isolates | |
| E. coli | 8 (28.6%) | 10 (35.7%) | 0.78 |
| Non-E. coli | 20 (71.4%) | 18 (61.5%) | 0.78 |
| Susceptibility of neomycin against 28 CRE isolates | 26 (92.9%) | 26 (92.9%) | 1.00 |
| Susceptibility of gentamicin against 28 CRE isolates | 14 (50.0%) | 13 (46.4%) | 0.80 |
| Susceptibility of amikacin against 28 CRE isolates | 13 (46.4%) | 15 (53.6%) | 0.80 |
| MIC50 of neomycin against 28 CRE isolates | 2 mg/L | 2 mg/L | |
| MIC90 of neomycin against 28 CRE isolates | 8 mg/L | 8 mg/L | |
| MIC range of neomycin against 28 CRE isolates | 1–32 mg/L | 1– > 128 mg/L |
| Persistence of CRE Fecal Carriage | Neomycin Group (n = 26) | Control Group (n = 26) | p |
|---|---|---|---|
| Day 1 | 26 (100.0%) | 26 (100.0%) | |
| Day 7 ± 2 | 12 (46.2%) | 21 (80.8%) | 0.01 |
| Day 14 ± 2 | 19 (73.1%) | 16 (61.5%) | 0.56 |
| 21 (80.8%) | 0.74 |
| Susceptibility of CRE to | Neomycin Group | Control Group | p |
|---|---|---|---|
| neomycin on day 1 | 26/28 (92.9%) | 26/28 (92.9%) | 1.00 |
| neomycin on day 7 ± 2 | 12/14 (85.7%) | 20/22 (90.9%) | 0.64 |
| neomycin on day 14 ± 2 | 18/22 (81.8%) | 15/17 (88.2%) | 0.68 |
| p | 0.50 | 0.87 | |
| gentamicin on day 1 | 14/28 (50.0%) | 13/28 (46.4%) | 1.00 |
| gentamicin on day 7 ± 2 | 7/14 (50.0%) | 10/22 (45.4%) | 0.94 |
| gentamicin on day 14 ± 2 | 12/22 (54.4%) | 9/17 (52.9%) | 0.82 |
| p | 0.94 | 0.95 | |
| amikacin on day 1 | 13/28 (46.4%) | 15/28 (53.6%) | 0.79 |
| amikacin on day 7 ± 2 | 6/14 (42.9%) | 9/22 (40.9%) | 0.82 |
| amikacin on day 14 ± 2 | 12/22 (54.5%) | 9/17 (52.9%) | 0.82 |
| p | 0.76 | 0.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tancharoen, L.; Srisomnuek, A.; Tiengrim, S.; Thamthaweechok, N.; Tangkorskul, T.; Thamlikitkul, V. Efficacy and Safety of Oral Neomycin for the Decolonization of Carbapenem-Resistant Enterobacterales: An Open-Label Randomized Controlled Trial. Antibiotics 2024, 13, 781. https://doi.org/10.3390/antibiotics13080781
Tancharoen L, Srisomnuek A, Tiengrim S, Thamthaweechok N, Tangkorskul T, Thamlikitkul V. Efficacy and Safety of Oral Neomycin for the Decolonization of Carbapenem-Resistant Enterobacterales: An Open-Label Randomized Controlled Trial. Antibiotics. 2024; 13(8):781. https://doi.org/10.3390/antibiotics13080781
Chicago/Turabian StyleTancharoen, Lalita, Ananya Srisomnuek, Surapee Tiengrim, Narisara Thamthaweechok, Teerawit Tangkorskul, and Visanu Thamlikitkul. 2024. "Efficacy and Safety of Oral Neomycin for the Decolonization of Carbapenem-Resistant Enterobacterales: An Open-Label Randomized Controlled Trial" Antibiotics 13, no. 8: 781. https://doi.org/10.3390/antibiotics13080781
APA StyleTancharoen, L., Srisomnuek, A., Tiengrim, S., Thamthaweechok, N., Tangkorskul, T., & Thamlikitkul, V. (2024). Efficacy and Safety of Oral Neomycin for the Decolonization of Carbapenem-Resistant Enterobacterales: An Open-Label Randomized Controlled Trial. Antibiotics, 13(8), 781. https://doi.org/10.3390/antibiotics13080781





