An Eco-Friendly Method to Synthesize Potent Antimicrobial Tricyclic Flavonoids
Abstract
1. Introduction
2. Results and Discussion
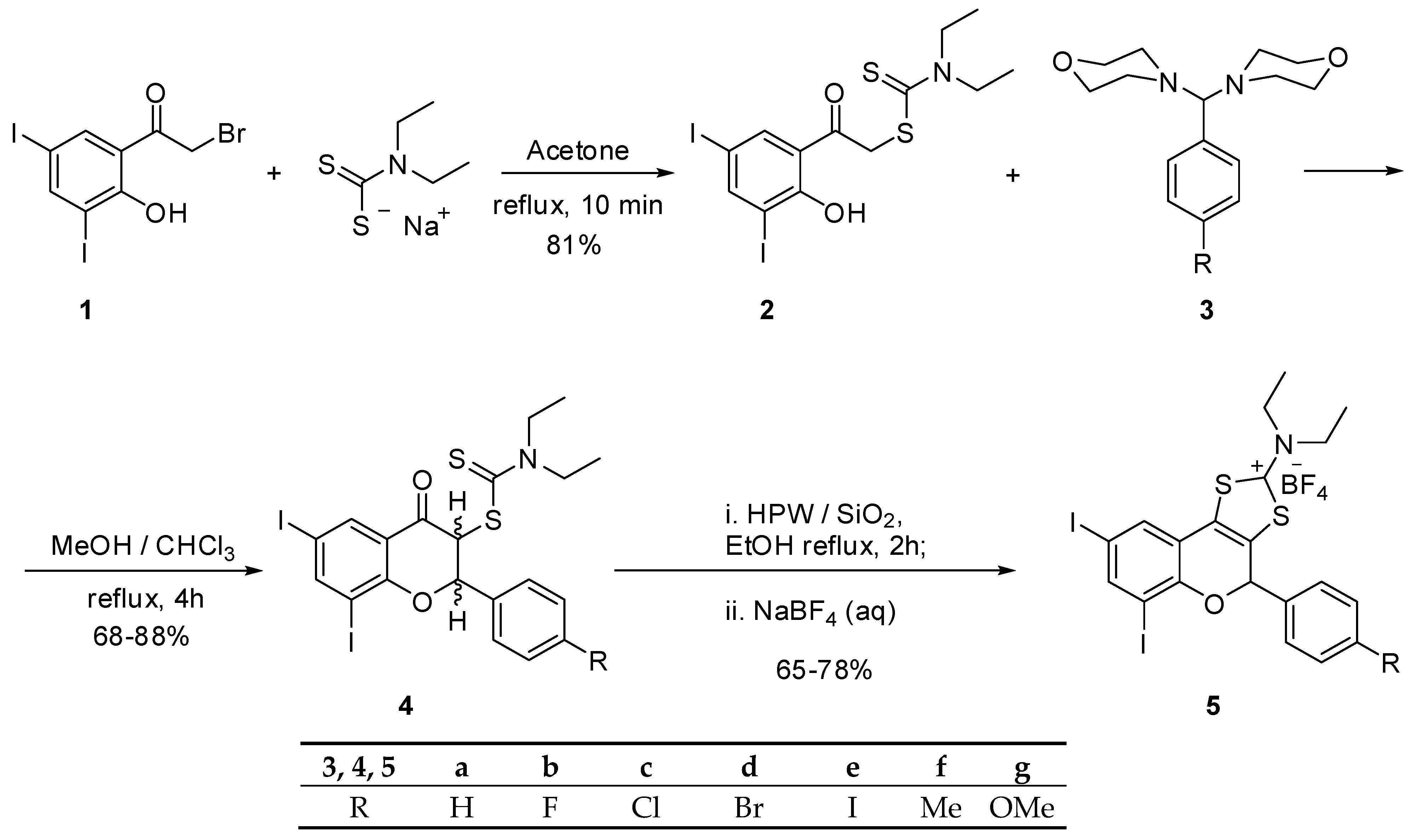
2.1. The Synthesis of Tricyclic Flavonoids
2.2. Flavonoids 5a–g Exhibit Potent Antimicrobial Activity
3. Materials and Methods
3.1. Chemistry
3.1.1. General Procedure for 6,8-diiodo-2-(4-iodophenyl)-4-oxochroman-3-yl N,N-diethyldithiocarbamate (4e)
3.1.2. General Procedure for 2-N,N-Diethylamino-6,8-diiodo-4-(4-iodophenyl)-4H-1,3-dithiol[4,5-c]chromen-2-ylium Tetrafluoroborate (5e)
3.2. Microbial Strains and Culture Conditions
3.3. Antibacterial Susceptibility Testing: Determination of the Minimum Inhibitory Concentration and the Minimum Bactericidal/Fungicidal Concentration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef] [PubMed]
- The World Bank. Drug-Resistant Infections: A Threat to Our Economic Future; World Bank: Washington, DC, USA, 2017. [Google Scholar]
- Zhu, Y.; Huang, W.E.; Yang, Q. Clinical perspective of antimicrobial resistance in bacteria. Infect. Drug Resist. 2022, 15, 735–746. [Google Scholar] [CrossRef]
- Chinemerem Nwobodo, D.; Ugwu, M.C.; Oliseloke Anie, C.; Al-Ouqaili, M.T.S.; Chinedu Ikem, J.; Victor Chigozie, U.; Saki, M. Antibiotic resistance: The challenges and some emerging strategies for tackling a global menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Andayani, N.; Mahdani, W.; Nisyra, M.; Agustin, H. Distribution and antibacterial susceptibility pattern of isolated bacteria from endotracheal aspirates among ventilator-assisted pneumonia patients in Indonesia. Narra J. 2023, 3, e149. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. 2023 Antibacterial Agents in Clinical and Preclinical Development: An Overview and Analysis; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, J.; Xie, Y. Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: An overview. Int. J. Pharm. 2019, 570, 118642. [Google Scholar] [CrossRef]
- Babii, C.; Mihalache, G.; Bahrin, L.G.; Neagu, A.N.; Gostin, I.; Mihai, C.T.; Sarbu, L.G.; Birsa, L.M.; Stefan, M. A novel synthetic flavonoid with potent antibacterial properties: In Vitro activity and proposed mode of action. PLoS ONE 2018, 13, e0194898. [Google Scholar] [CrossRef]
- Mutlu, H.; Barner, L. Getting the terms right: Green, sustainable, or circular chemistry? Macromol. Chem. Phys. 2022, 223, 2200111. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Arends, I.W.C.E.; Hanefeld, U. Green Chemistry and Catalysis; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar] [CrossRef]
- Cernansky, R. Chemistry: Green refill. Nature 2015, 519, 379–380. [Google Scholar] [CrossRef] [PubMed]
- Poliakoff, M.; Licence, P. Green chemistry. Nature 2007, 450, 810–812. [Google Scholar] [CrossRef]
- Babii, C.; Savu, M.; Motrescu, I.; Birsa, L.M.; Sarbu, L.G.; Stefan, M. The antibacterial synthetic flavonoid BrCl-Flav exhibits important anti-Candida activity by damaging cell membrane integrity. Pharmaceuticals 2021, 14, 1130. [Google Scholar] [CrossRef] [PubMed]
- Bahrin, L.G.; Apostu, M.O.; Birsa, L.M.; Stefan, M. The antibacterial properties of sulfur containing flavonoids. Bioorg. Med. Chem. Lett. 2014, 24, 2315–2318. [Google Scholar] [CrossRef]
- Birsa, M.L.; Sarbu, L.G. An improved synthetic method for sensitive iodine containing tricyclic flavonoids. Molecules 2022, 27, 8430. [Google Scholar] [CrossRef]
- Yang, S.; Sun, Y.; Yang, H.; Wan, J. Catalytic wet air oxidation of phenol, nitrobenzene and aniline over the multi-walled carbon nanotubes (MWCNTs) as catalysts. Front. Environ. Sci. Eng. 2015, 9, 436–443. [Google Scholar] [CrossRef]
- Rafiee, E.; Shahbazi, F. One-pot synthesis of dihydropyrimidones using silica-supported heteropoly acid as an efficient and reusable catalyst: Improved protocol conditions for the Biginelli reaction. J. Mol. Catal. A Chem. 2006, 250, 57–61. [Google Scholar] [CrossRef]
- Parghi, K.D.; Satam, J.R.; Jayaram, R.V. Silica supported heteropolyacid catalyzed dehydration of aldoximes to nitriles and alcohols to alkenes. Green Chem. Lett. Rev. 2011, 4, 143–149. [Google Scholar] [CrossRef]
- Sandulache, A.; Cascaval, A.; Toniutti, N.; Giumanini, A.G. New flavones by a novel synthetic route. Tetrahedron 1997, 53, 9813–9822. [Google Scholar] [CrossRef]
- Rukayadi, Y.; Lee, K.; Han, S.; Yong, D.; Hwang, J.K. In vitro activities of panduratin A against clinical Staphylococcus strains. Antimicrob. Agents Chemother. 2009, 53, 4529–4532. [Google Scholar] [CrossRef]
- Thebti, A.; Meddeb, A.; Ben Salem, I.; Bakary, C.; Ayari, S.; Rezgui, F.; Essafi-Benkhadir, K.; Boudabous, A.; Ouzari, H.-I. Antimicrobial activities and mode of flavonoid actions. Antibiotics 2023, 12, 225. [Google Scholar] [CrossRef] [PubMed]
- Narwal, S.; Kumar, S.; Verma, P.K. Synthesis and biological activity of new chalcone scaffolds as prospective antimicrobial agents. Res. Chem. Intermed. 2021, 47, 1625–1641. [Google Scholar] [CrossRef]
- Long, G.-Q.; Li, X.-M.; Wang, D.-D.; Bao, T.-R.-G.; Yang, Y.-C.; Zheng, Y.-Y.; Liu, X.-L.; Sun, X.-D.; Wang, A.-H.; Jia, J.-M. Prenylated flavonoids and derivatives isolated from the root barks of Sophora flavescens as potent antibacterial agents against Staphylococcus aureus. Ind. Crops Prod. 2022, 189, 115834. [Google Scholar] [CrossRef]
- Xu, M.; Wu, P.; Shen, F.; Ji, J.; Rakesh, K.P. Chalcone derivatives and their antibacterial activities: Current development. Bioorg. Chem. 2019, 91, 103133. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-H.; Zheng, C.-J.; Sun, L.-P.; Piao, H.-R. Synthesis of new chalcone derivatives containing a rhodanine-3-acetic acid moiety with potential anti-bacterial activity. Eur. J. Med. Chem. 2010, 45, 5739–5743. [Google Scholar] [CrossRef] [PubMed]
- Bandgar, B.P.; Gawande, S.S. Synthesis and biological screening of a combinatorial library of β-chlorovinyl chalcones as anticancer, anti-inflammatory and antimicrobial agents. Bioorg. Med. Chem. 2010, 18, 2060–2065. [Google Scholar] [CrossRef]
- Edziri, H.; Mastouri, M.; Mahjoub, M.A.; Mighri, Z.; Mahjoub, A.; Verschaeve, L. Antibacterial, antifungal and cytotoxic activities of two flavonoids from Retama raetam flowers. Molecules 2012, 17, 7284–7293. [Google Scholar] [CrossRef]
- Jin, Y.-S. Recent advances in natural antifungal flavonoids and their derivatives. Bioorg. Med. Chem. Lett. 2019, 29, 126589. [Google Scholar] [CrossRef]
- Andrade, J.T.; Santos, F.R.S.; Lima, W.G.; Sousa, C.D.F.; Oliveira, L.S.F.M.; Ribeiro, R.I.M.A.; Gomes, A.J.P.S.; Araújo, M.G.F.; Villar, J.A.F.P.; Ferreira, J.M.S. Design, synthesis, biological activity and structure-activity relationship studies of chalcone derivatives as potential anti-Candida agents. J. Antibiot. 2018, 71, 702–712. [Google Scholar] [CrossRef]
- Ahmad, A.; Wani, M.Y.; Patel, M.; Sobral, A.; Duse, A.G.; Aqlan, F.M.; Al-Bogami, A.S. Synergistic antifungal effect of cyclized chalcone derivatives and fluconazole against Candida albicans. Medchemcomm 2017, 8, 2195–2207. [Google Scholar] [CrossRef]
- Kucerova-Chlupacova, M.; Vyskovska-Tyllova, V.; Richterova-Finkova, L.; Kunes, J.; Buchta, V.; Vejsova, M.; Paterova, P.; Semelkova, L.; Jandourek, O.; Opletalova, V. Novel halogenated pyrazine-based chalcones as potential antimicrobial drugs. Molecules 2016, 21, 1421. [Google Scholar] [CrossRef] [PubMed]
- Kant, R.; Kumar, D.; Agarwal, D.; Gupta, R.D.; Tilak, R.; Awasthi, S.K.; Agarwal, A. Synthesis of newer 1,2,3-triazole linked chalcone and flavone hybrid compounds and evaluation of their antimicrobial and cytotoxic activities. Eur. J. Med. Chem. 2016, 113, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, T.; Zhao, W.; Hu, Z.-Q. Mechanism of action and potential for use of tea catechin as an antiinfective agent. Anti-Infect. Agents Med. Chem. (Former. Curr. Med. Chem. Anti-Infect. Agents) 2007, 6, 57–62. [Google Scholar] [CrossRef]
| Microbial Strains | 5a | 5b | 5c | 5d | 5e | 5f | 5g | DMSO (%) | Control |
|---|---|---|---|---|---|---|---|---|---|
| Staphylococcus aureus ATCC 25923 | 0.97 | 0.97 | 0.48 | 0.97 | 0.48 | 0.97 | 0.97 | 24.87 | 1.95 a/7.81 chl |
| Bacillus subtilis ATCC 6633 | 0.24 | 0.12 | 0.12 | 0.48 | 0.24 | 0.9 | 0.48 | 24.87 | 0.12 a |
| Enterococcus faecium medbio2-2012 | 7.81 | 3.9 | 3.9 | 3.9 | 3.9 | 3.9 | 3.9 | 12.43 | 15.62 chl |
| Escherichia coli ATCC 25922 | 7.81 | 15.62 | 31.25 | 7.81 | 62.50 | 15.62 | 31.25 | 12.43 | 62.50 a/7.81 k |
| Pseudomonas aeruginosa PAO1 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 12.43 | >250 a |
| Acinetobacter pittii Cl2 | 7.81 | 7.81 | 7.81 | 62.50 | 62.50 | 15.62 | 31.25 | 6.21 | >250 a/0.37 cip |
| Candida albicans ATCC 10231 | 15.62 | 7.81 | 7.81 | 7.81 | 7.81 | 15.62 | 15.62 | 6.21 | >500 f |
| Candida krusei Prx | 7.81 | 7.81 | 7.81 | 3.90 | 15.62 | 7.81 | 7.81 | 6.21 | 62.5 f |
| Microbial Strains | 5a | 5b | 5c | 5d | 5e | 5f | 5g | Control |
|---|---|---|---|---|---|---|---|---|
| Staphylococcus aureus ATCC 25923 | 3.90 | 7.81 | 3.90 | 1.97 | 3.90 | 3.90 | 3.90 | 7.8 a |
| Bacillus subtilis ATCC 6633 | 0.9 | 0.9 | 1.9 | 0.9 | 0.48 | 3.9 | 1.95 | 3.9 a |
| Enterococcus faecium medbio2-2012 | 62.5 | 15.62 | 31.25 | 62.5 | 15.62 | 62.5 | 31.25 | >250 chl |
| Escherichia coli ATCC 25922 | 31.25 | 31.25 | 62.50 | 31.25 | 125 | 62.50 | 31.25 | 125 a |
| Pseudomonas aeruginosa PAO1 | 250 | 250 | 250 | 125 | 125 | 125 | 125 | >250 a |
| Acinetobacter pittii Cl2 | 31.25 | 15.62 | 31.25 | 125 | 125 | 62.50 | 31.25 | >250 a |
| Candida albicans ATCC 10231 | 15.62 | 15.62 | 7.81 | 7.81 | 15.62 | 15.62 | 15.62 | >500 f |
| Candida krusei Prx | 15.62 | 15.62 | 7.81 | 7.81 | 31.25 | 7.81 | 15.62 | 62.5 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantea, L.-E.; Moldovan, C.-V.; Savu, M.; Sarbu, L.G.; Stefan, M.; Birsa, M.L. An Eco-Friendly Method to Synthesize Potent Antimicrobial Tricyclic Flavonoids. Antibiotics 2024, 13, 798. https://doi.org/10.3390/antibiotics13090798
Mantea L-E, Moldovan C-V, Savu M, Sarbu LG, Stefan M, Birsa ML. An Eco-Friendly Method to Synthesize Potent Antimicrobial Tricyclic Flavonoids. Antibiotics. 2024; 13(9):798. https://doi.org/10.3390/antibiotics13090798
Chicago/Turabian StyleMantea, Loredana-Elena, Cristina-Veronica Moldovan, Mihaela Savu, Laura Gabriela Sarbu, Marius Stefan, and Mihail Lucian Birsa. 2024. "An Eco-Friendly Method to Synthesize Potent Antimicrobial Tricyclic Flavonoids" Antibiotics 13, no. 9: 798. https://doi.org/10.3390/antibiotics13090798
APA StyleMantea, L.-E., Moldovan, C.-V., Savu, M., Sarbu, L. G., Stefan, M., & Birsa, M. L. (2024). An Eco-Friendly Method to Synthesize Potent Antimicrobial Tricyclic Flavonoids. Antibiotics, 13(9), 798. https://doi.org/10.3390/antibiotics13090798







