Novel Antimicrobial Agents Based on Zinc-Doped Hydroxyapatite Loaded with Tetracycline
Abstract
1. Introduction
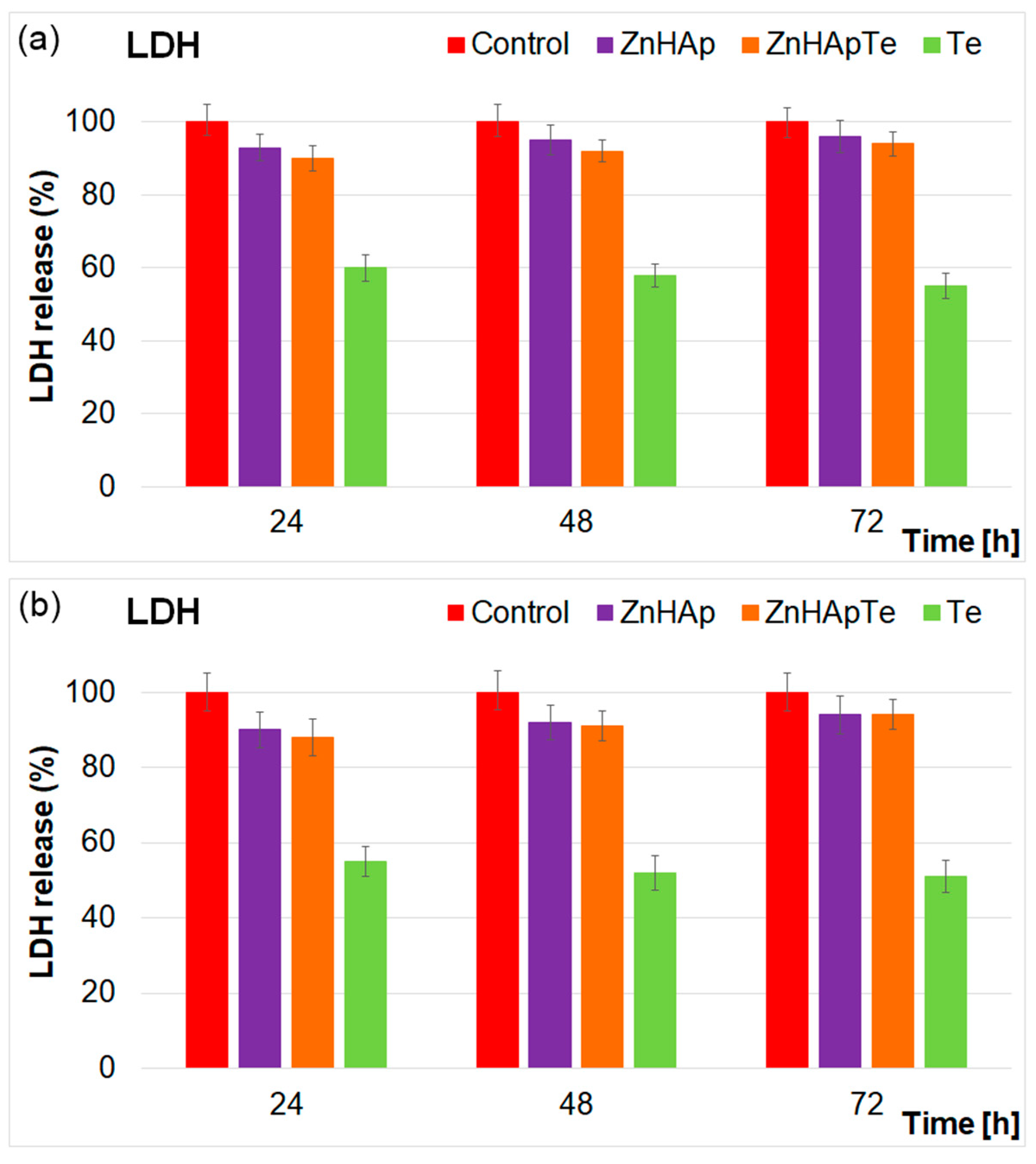
2. Results and Discussions
3. Materials and Methods
3.1. Materials
3.2. Development of Zinc-Doped Hydroxyapatite Enriched with Tetracycline
3.3. X-ray Diffraction (XRD)
3.4. Fourier Transform Infrared Spectroscopy (FTIR)
3.5. X-ray Photoelectron Spectroscopy (XPS)
3.6. Transmission Electron Microscopy (TEM)
3.7. Scanning Electron Microscopy (SEM)
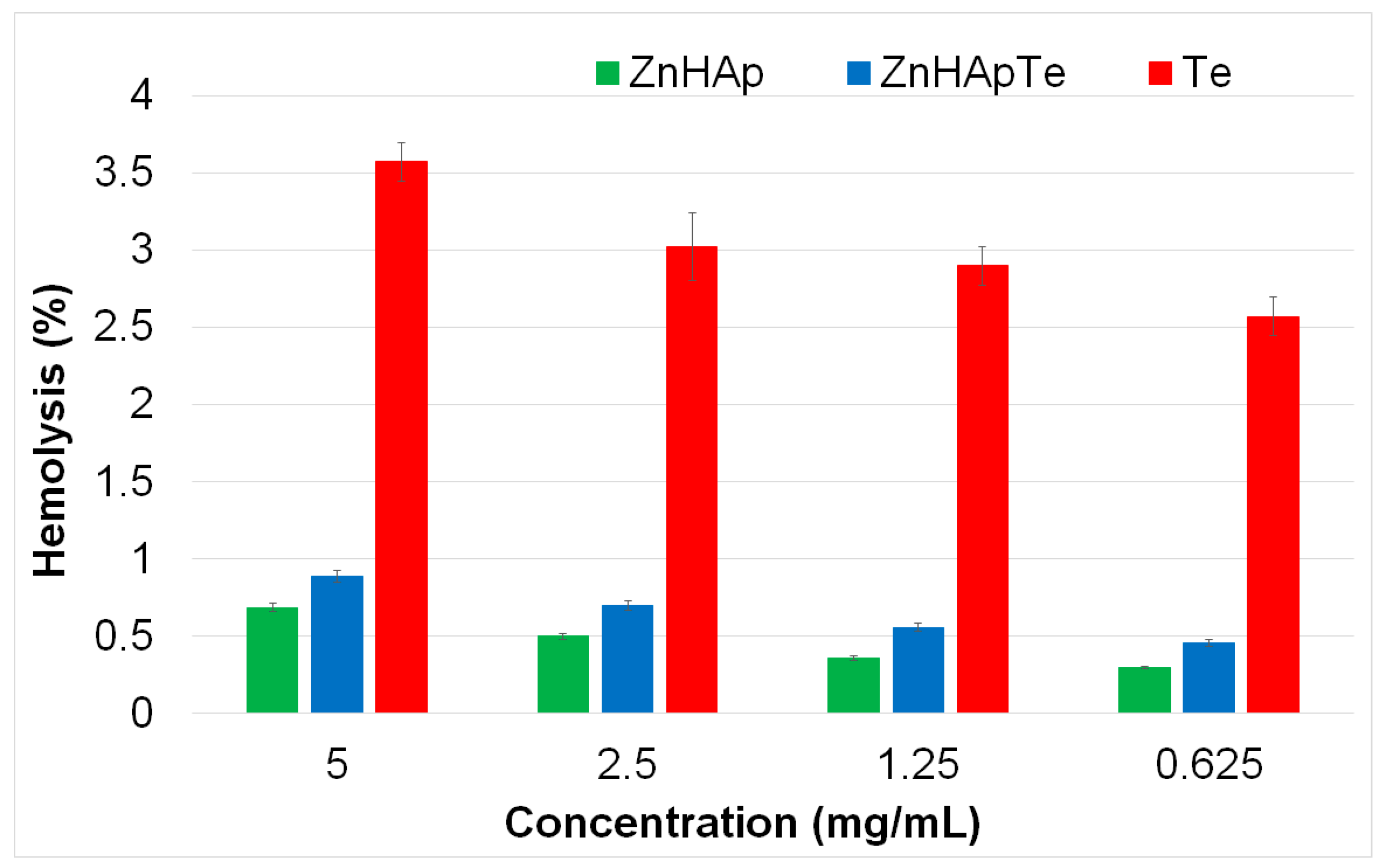
3.8. Hemolysis Assay
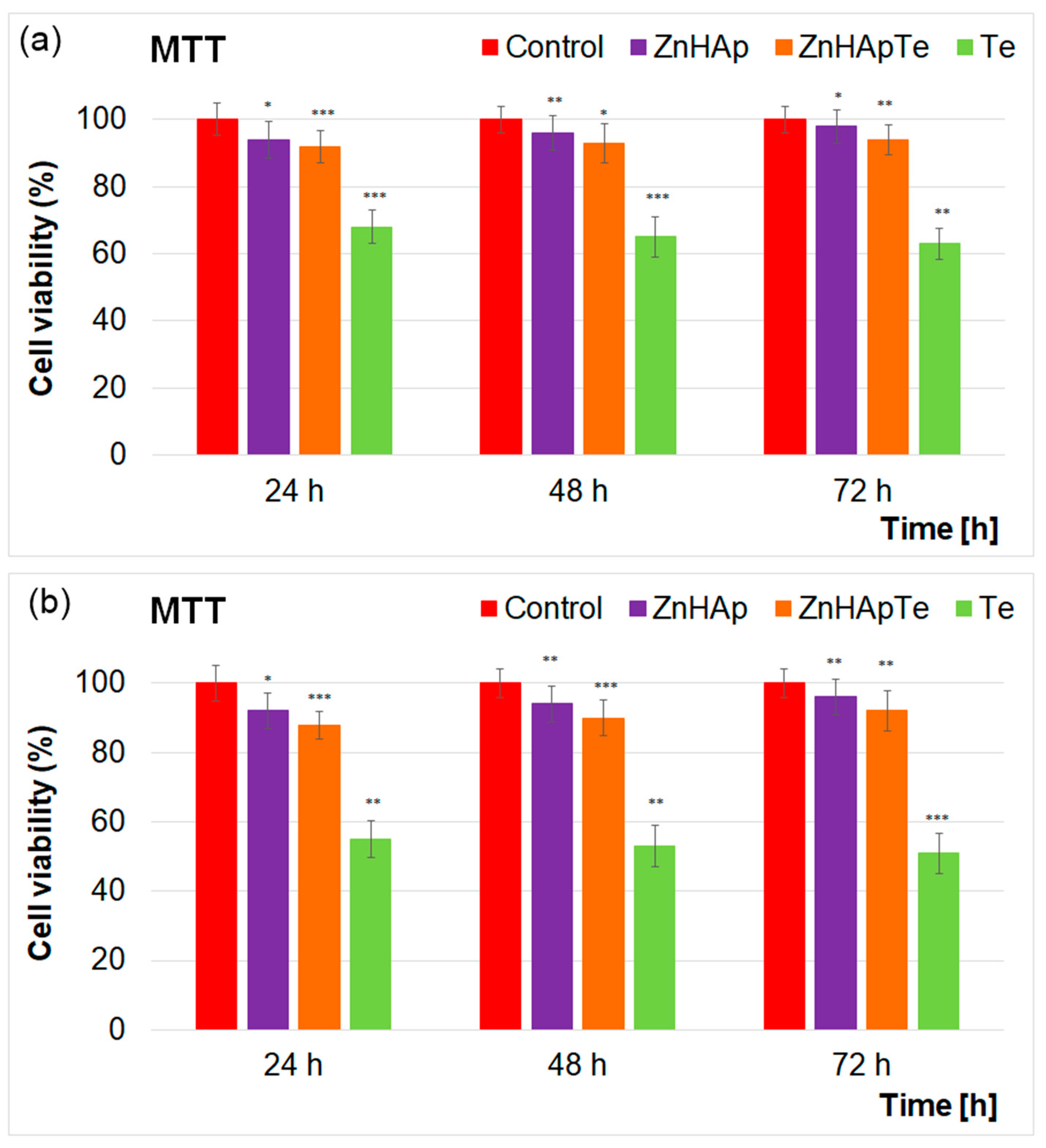
3.9. MTT Assay
3.10. Lactate Dehydrogenase (LDH) Release Measurement
3.11. In Vitro Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mecu, R.; Cîrţînă, D.; Nănescu, V. Hydroxyapatite-antibiotic applications in bone therapy. JRISS 2019, 1, 121–124. [Google Scholar] [CrossRef]
- Maleki-Ghaleh, H.; Siadati, M.H.; Fallah, A.; Koc, B.; Kavanlouei, M.; Khademi-Azandehi, P.; Moradpur-Tari, E.; Omidi, Y.; Barar, J.; Beygi-Khosrowshahi, Y.; et al. Antibacterial and Cellular Behaviors of Novel Zinc-Doped Hydroxyapatite/Graphene Nanocomposite for Bone Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 9564. [Google Scholar] [CrossRef] [PubMed]
- Ofudje, E.A.; Adeogun, A.I.; Idowu, M.A.; Kareem, S.O. Synthesis and characterization of Zn-Doped hydroxyapatite: Scaffold application, antibacterial and bioactivity studies. Heliyon 2019, 5, e01716. [Google Scholar] [CrossRef] [PubMed]
- Ait Said, H.; Noukrati, H.; Oudadesse, H.; Ben Youcef, H.; Lefeuvre, B.; Hakkou, R.; Lahcini, M.; Barroug, A. Formulation and characterization of hydroxyapatite-based composite with enhanced compressive strength and controlled antibiotic release. J. Biomed. Mater. Res. A 2021, 109, 1942–1954. [Google Scholar] [CrossRef]
- Jiang, Y.; Yuan, Z.; Huang, J. Substituted hydroxyapatite: A recent development. Mater. Technol. 2020, 35, 785–796. [Google Scholar] [CrossRef]
- Cinici, B.; Yaba, S.; Kurt, M.; Yalcin, H.C.; Duta, L.; Gunduz, O. Fabrication Strategies for Bioceramic Scaffolds in Bone Tissue Engineering with Generative Design Applications. Biomimetics 2024, 9, 409. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Mondal, S.; Bharathiraja, S.; Manivasagan, P.; Moorthy, M.S.; Oh, J. Optimized Zn-doped hydroxyapatite/doxorubicin bioceramics system for efficient drug delivery and tissue engineering application. Ceram. Int. 2018, 44, 6062–6071. [Google Scholar] [CrossRef]
- Lin, K.; Zhou, Y.; Zhou, Y.; Qu, H.; Chen, F.; Zhu, Y.; Chang, J. Biomimetic hydroxyapatite porous microspheres with co-substituted essential trace elements:Surfactant-free hydrothermal synthesis, enhanced degradation and drug release. J. Mater. Chem. 2011, 21, 16558–16565. [Google Scholar] [CrossRef]
- Sebastiammal, S.; Fathima, A.S.L.; Alarifi, S.; Mahboob, S.; Henry, J.; Kavipriya, M.R.; Govindarajan, M.; Nicoletti, M.; Vaseeharan, B. Synthesis and physicochemical characteristics of Ag-doped hydroxyapatite nanoparticles, and their potential biomedical applications. Environ. Res. 2022, 210, 112979. [Google Scholar] [CrossRef]
- Phatai, P.; Futalan, C.M.; Utara, S.; Khemthong, P.; Kamonwannasit, S. Structural characterization of cerium-doped hydroxyapatite nanoparticles synthesized by an ultrasonic-assisted sol-gel technique. Results Phys. 2018, 10, 956–963. [Google Scholar] [CrossRef]
- Chen, P.; Wang, F.; Qiao, Y.; Zhang, Z. Luminescence of samarium doped hydroxyapatite containing strontium: Effects of doping concentration. J. Rare Earths 2022, 40, 398–405. [Google Scholar] [CrossRef]
- Ishikawa, K.; Miyamoto, Y.; Yuasa, T.; Ito, A.; Nagayama, M.; Suzuki, K. Fabrication of Zn containing apatite cement and its initial evaluation using human osteoblastic cells. Biomaterials 2002, 23, 423–428. [Google Scholar] [CrossRef]
- Wang, X.; Ito, A.; Sogo, Y.; Li, X.; Oyane, A. Zinc-containing apatite layers on external fixation rods promoting cell activity. Acta Biomater. 2010, 6, 962–968. [Google Scholar] [CrossRef]
- Begam, H.; Mandal, S.; Chanda, A.; Mukherjee, J.; Nandi, S.K. Effect of zinc doping on biological properties of biphasic calcium phosphate ceramics in orthopaedic animal model. T. Indian. Ceram. Soc. 2014, 73, 284–292. [Google Scholar] [CrossRef]
- Pasquet, J.; Chevalier, Y.; Pelletier, J.; Couval, E.; Bouvier, D.; Bolzinger, M.A. The contribution of zinc ions to the antimicrobial activity of zinc oxide. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 263–274. [Google Scholar] [CrossRef]
- Lallo da Silva, B.; Abuçafy, M.P.; Berbel Manaia, E.; Oshiro Junior, J.A.; Chiari-Andréo, B.G.; Pietro, R.C.R.; Chiavacci, L.A. Relationship between structure and antimicrobial activity of zinc oxide nanoparticles: An overview. Int. J. Nanomed. 2019, 14, 9395–9410. [Google Scholar] [CrossRef] [PubMed]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Motelica-Heino, M.; Guegan, R.; Buton, N. Evaluation of Antibacterial Activity of Zinc-Doped Hydroxyapatite Colloids and Dispersion Stability Using Ultrasounds. Nanomaterials 2019, 9, 515. [Google Scholar] [CrossRef] [PubMed]
- Klein, N.C.; Cunha, B.A. Tetracyclines. Med. Clin. North. Am. 1995, 79, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Rusu, L.C.; Nedelcu, I.A.; Albu, G.; Sonmez, M.; Voicu, M.; Radulescu, G.M.; Ficai, D.; Ficai, A.; Negrutiu, M.L.; Sinescu, C. Tetracycline loaded collagen/hydroxyapatite composite materials for biomedical applications. J. Nanomater. 2015, 2015, 361969. [Google Scholar] [CrossRef]
- Rivadeneira, J.; Luz, G.M.; Audisio, M.C.; Mano, J.F.; Gorustovich, A.A. Novel antibacterial bioactive glass nanocomposite functionalized with tetracycline hydrochloride. Biomed. Glas. 2015, 1, 128–135. [Google Scholar] [CrossRef]
- Soriano-Souza, C.; Valiense, H.; Mavropoulos, E.; Martinez-Zelaya, V.; Costa, A.M.; Alves, A.T.; Longuinho, M.; Resende, R.; Mourão, C.; Granjeiro, J.; et al. Doxycycline containing hydroxyapatite ceramic microspheres as a bone-targeting drug delivery system. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Predoi, D.; Iconaru, S.-L.; Predoi, M.-V.; Buton, N. Development of Novel Tetracycline and Ciprofloxacin Loaded Silver Doped Hydroxyapatite Suspensions for Biomedical Applications. Antibiotics 2023, 12, 74. [Google Scholar] [CrossRef]
- Madhumathi, K.; Kumar, T.S. Regenerative potential and anti-bacterial activity of tetracycline loaded apatitic nanocarriers for the treatment of periodontitis. Biomed. Mater. 2014, 9, 035002. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, S.; Varadhan, S.R.; Karunanidhi, N. Qualitative analysis on the infrared bands of tetracycline and ampicillin. Proc.-Indian. Natl. Sci. Acad. Part A Phys. Sci. 1996, 62, 309–316. [Google Scholar]
- de Sousa, F.B.; Oliveira, M.F.; Lula, I.S.; Sansiviero, M.T.C.; Cortés, M.E.; Sinisterra, R.D. Study of inclusion compound in solution involving tetracycline and β-cyclodextrin by FTIR-ATR. Vib. Spectrosc. 2008, 46, 57–62. [Google Scholar] [CrossRef]
- Hou, H.; Dai, Z.; Liu, X.; Yao, Y.; Liao, Q.; Yu, C.; Li, D. Reutilization of the expired tetracycline for lithium ion battery anode. Sci. Total Environ. 2018, 630, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Popa, C.L.; Groza, A.; Chapon, P.; Ciobanu, C.S.; Ghita, R.V.; Trusca, R.; Ganciu, M.; Predoi, D. Physicochemical analysis of the polydimethylsiloxane interlayer influence on a hydroxyapatite doped with silver coating. J. Nanomater. 2015, 2015, 250617. [Google Scholar] [CrossRef]
- Predoi, D.; Groza, A.; Iconaru, S.L.; Predoi, G.; Barbuceanu, F.; Guegan, R.; Motelica-Heino, M.S.; Cimpeanu, C. Properties of Basil and Lavender Essential Oils Adsorbed on the Surface of Hydroxyapatite. Materials 2018, 11, 652. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Patil, S.; Shettigar, H.; Bairwa, K.; Jana, S. Spectroscopic Characterization of Chloramphenicol and Tetracycline: An Impact of Biofield Treatment. Pharm. Anal. Acta 2015, 6, 395. [Google Scholar] [CrossRef]
- Tian, M.; Hu, X.; Qu, L.; Zhu, S.; Sun, Y.; Han, G. Versatile and ductile cotton fabric achieved via layer-by-layer self-assembly by consecutive adsorption of graphene doped PEDOT: PSS and chitosan. Carbon. 2016, 96, 166–1174. [Google Scholar] [CrossRef]
- Zhang, N.; Xue, H.; Hu, R. The activity and stability of CeO2@CaO catalysts for the production of biodiesel. RSC Adv. 2018, 8, 32922–32929. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Zhang, H.; Wang, S.; Liu, L.; Tan, X.; Liu, S. Atomic Level Design of CoOH+−Hydroxyapatite@C Catalysts for Superfast Degradation of Organics via Peroxymonosulfate Activation. Chem. Commun. 2018, 54, 4919–4922. [Google Scholar] [CrossRef]
- Hegde, M.S.; Ayyoob, M. O2- and O1- types of oxygen species on Ni and barium-dosed Ni and Cu surfaces. Surf. Sci. 1986, 173, L635–L640. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Vijayakrishnan, V.; Kulkarni, G.U.; Rajumon, M.K. A comparative study of the interaction of oxygen with clusters and single-crystal surfaces of nickel. Appl. Surf. Sci. 1995, 84, 285–289. [Google Scholar] [CrossRef]
- Kulkarni, G.U.; Rao, C.N.R.; Roberts, M.W. Coadsorption of Dioxygen and Water on the Ni(110) Surface: Role of O1--Type Species in the Dissociation of Wate. Langmuir 1995, 11, 2572–2575. [Google Scholar] [CrossRef]
- Guo, J.; Yu, H.; Dong, F.; Zhu, B.; Huang, W.; Zhang, S. High efficiency and stability of Au-Cu/hydroxyapatite catalyst for the oxidation of carbon monoxide. RSC Adv. 2017, 7, 45420–45431. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Physical Electronics Inc.: Chanhassen, MN, USA, 1995. [Google Scholar]
- Bee, S.-L.; Bustami, Y.; Ul-Hamid, A.; Lim, K.; Abdul Hamid, Z.A. Synthesis of Silver Nanoparticle-Decorated Hydroxyapatite Nanocomposite with Combined Bioactivity and Antibacterial Properties. J. Mater. Sci. Mater. Med. 2021, 32, 106. [Google Scholar] [CrossRef]
- Gomes, G.C.; Borghi, F.F.; Ospina, R.O.; L’opez, E.O.; Borges, F.O.; Mello, A. Nd:YAG (532 nm) pulsed laser deposition produces crystalline hydroxyapatite thin coatings at room temperature. Surf. Coat. Technol. 2017, 329, 174–183. [Google Scholar] [CrossRef]
- Sinulingga, K.; Sirait, M.; Siregar, N.; Abdullah, H. Synthesis and characterizations of natural limestone-derived nano-hydroxyapatite (HAp): A comparison study of different metals doped Haps on antibacterial activity. RSC Adv. 2021, 11, 15896–15904. [Google Scholar] [CrossRef] [PubMed]
- Lou, L.; Nelson, A.E.; Heo, G.; Major, P.W. Surface chemical composition of human maxillary first premolar as assessed by X-ray photoelectron spectroscopy (XPS). Appl. Surf. Sci. 2008, 254, 6706–6709. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Zhang, L.; Zuo, Y. Composition of calcium deficient Na-containing carbonate hydroxyapatite modified with Cu (II) and Zn (II) ions. Appl. Surf. Sci. 2008, 254, 2844–2850. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Thian, E.S.; Konishi, T.; Kawanobe, Y.; Lim, P.N.; Choong, C.; Ho, B.; Aizawa, M. Zinc-substituted hydroxyapatite: A biomaterial with enhanced bioactivity and antibacterial properties. J. Mater. Sci. Mater. Med. 2013, 24, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Predoi, D.; Iconaru, S.L.; Deniaud, A.; Chevallet, M.; Michaud-Soret, I.; Buton, N.; Prodan, A.M. Textural, Structural and Biological Evaluation of Hydroxyapatite Doped with Zinc at Low Concentrations. Materials 2017, 10, 229. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Benko, A.; Nocun, M.; Przekora, A. Novel chitosan/agarose/hydroxyapatite nanocomposite scaffold for bone tissue engineering applications: Comprehensive evaluation of biocompatibility and osteoinductivity with the use of osteoblasts and mesenchymal stem cells. Int. J. Nanomed. 2019, 14, 6615–6630. [Google Scholar] [CrossRef]
- Radovanović, Ž.; Veljović, D.; Jokić, B.; Dimitrijević, S.; Bogdanović, G.; Kojić, V.; Petrović, R.; Janaćković, D. Biocompatibility and antimicrobial activity of zinc(II)-doped hydroxyapatite, synthesized by a hydrothermal method. J. Serb. Chem. Soc. 2012, 77, 1787–1798. [Google Scholar] [CrossRef]
- Badea, M.A.; Balas, M.; Popa, M.; Borcan, T.; Bunea, A.-C.; Predoi, D.; Dinischiotu, A. Biological Response of Human Gingival Fibroblasts to Zinc-Doped Hydroxyapatite Designed for Dental Applications—An In vitro Study. Materials 2023, 16, 4145. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Xin, R.; Ge, X.; Leng, Y. Characterization and structural analysis of zinc-substituted hydroxyapatites. Acta Biomater. 2009, 5, 3141–3149. [Google Scholar] [CrossRef]
- Tank, K.P.; Chudasama, K.S.; Thaker, V.S.; Joshi, M.J. Pure and zinc doped nano-hydroxyapatite: Synthesis, characterization, antimicrobial and hemolytic studies. J. Cryst. Growth 2014, 401, 474–479. [Google Scholar] [CrossRef]
- Dayaghi, E.; Bakhsheshi-Rad, H.R.; Hamzah, E.; Akhavan-Farid, A.; Ismail, A.F.; Aziz, M.; Abdolahi, E. Magnesium-zinc scaffold loaded with tetracycline for tissue engineering application: In vitro cell biology and antibacterial activity assessment. Mater. Sci. Eng. C 2019, 102, 53–65. [Google Scholar] [CrossRef]
- Shao, W.; Liu, H.; Wang, S.; Wu, J.; Huang, M.; Min, H.; Liu, X. Controlled release and antibacterial activity of tetracyclinehydrochloride-loaded bacterial cellulose composite membranes. Carbohydr. Polym. 2016, 145, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.S.; Santos, K.D.; Fernandes, M.H. Cell-induced response by tetracyclines on human bone marrow colonized hydroxyapatite and Bonelike. Acta Biomater. 2008, 4, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Higgins, P.; Draper, M.; Nelson, M. Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry. Former. Curr. Med. Chem. 2011, 10, 132–152. [Google Scholar] [CrossRef]
- Kładna, A.; Michalska, T.; Berczyński, P.; Kruk, I.; Aboul-Enein, H.Y. Evaluation of the antioxidant activity of tetracycline antibiotics in vitro. Luminescence 2012, 27, 249–255. [Google Scholar] [CrossRef]
- Sano, T.; Ozaki, K.; Kodama, Y.; Matsuura, T.; Narama, I. Antimicrobial Agent, Tetracycline, Enhanced Upper Alimentarytract Candida Albicans Infection and Its Related Mucosal Proliferation in Alloxan-Induced Diabetic Rats. Toxicol. Pathol. 2012, 40, 1014–1019. [Google Scholar] [CrossRef]
- Heman-Ackah, S.M. Comparison of Tetracycline Action on Staphylococcus Aureus and Escherichia coli by Microbial Kinetics. Antimicrob. Agents Chemother. 1976, 10, 223–228. [Google Scholar] [CrossRef]
- Campbell, P.J.; Heseltine, W.W. An Apparent Growth Stimulant for Candida albicans Released from Tetracycline-Treated Bacterial Flora. J. Hyg. 1960, 58, 95–97. [Google Scholar] [CrossRef]
- Li, B.; Webster, T.J. Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopaedic infections. J. Orthop. Res. 2018, 36, 22–32. [Google Scholar] [CrossRef]
- Hemeg, H.A. Nanomaterials for alternative antibacterial therapy. Int. J. Nanomed. 2017, 12, 8211–8225. [Google Scholar] [CrossRef]
- Song, W.; Zhang, J.; Guo, J.; Zhang, J.; Ding, F.; Li, L.; Sun, Z. Role of the dissolved zinc ion and reactive oxygen species in cytotoxicity of ZnO nanoparticles. Toxicol. Lett. 2010, 199, 389–397. [Google Scholar] [CrossRef]
- Li, Y.; Liao, C.; Tjong, S.C. Recent Advances in Zinc Oxide Nanostructures with Antimicrobial Activities. Int. J. Mol. Sci. 2020, 21, 8836. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Tang, X.; Zhang, B.; He, L.; Shi, Y. Antimicrobial activity and synergistic antibacterial mechanism of a combination of zinc and rare-earth scandium against Escherichia coli. Mater. Technol. 2019, 35, 797–806. [Google Scholar] [CrossRef]
- Stafford, S.L.; Bokil, N.J.; Achard, M.E.; Kapetanovic, R.; Schembri, M.A.; McEwan, A.G.; Sweet, M.J. Metal ions in macrophage antimicrobial pathways: Emerging roles for zinc and copper. Biosci. Rep. 2013, 33, e00049. [Google Scholar] [CrossRef]
- Riduan, S.N.; Zhang, Y. Recent Advances of Zinc-based Antimicrobial Materials. Chem. Asian J. 2021, 16, 2588. [Google Scholar] [CrossRef]
- Mendes, C.R.; Dilarri, G.; Forsan, C.F.; Sapata, V.D.M.R.; Lopes, P.R.M.; de Moraes, P.B.; Montagnolli, R.N.; Ferreira, H.; Bidoia, E.D. Antibacterial action and target mechanisms of zinc oxide nanoparticles against bacterial pathogens. Sci. Rep. 2022, 12, 2658. [Google Scholar] [CrossRef]
- Caron, A.J.; Ali, I.J.; Delgado, M.J.; Johnson, D.; Reeks, J.M.; Strzhemechny, Y.M.; McGillivray, S.M. Zinc oxide nanoparticles mediate bacterial toxicity in Mueller-Hinton Broth via Zn2+. Front. Microbiol. 2024, 15, 1394078. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, F.; Starosta, A.L.; Arenz, S.; Sohmen, D.; Dönhöfer, A.; Wilson, D.N. Tetracycline antibiotics and resistance mechanisms. Biol. Chem. 2014, 395, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Schnappinger, D.; Hillen, W. Tetracyclines: Antibiotic action, uptake, and resistance mechanisms. Arch. Microbiol. 1996, 165, 359–369. [Google Scholar] [CrossRef]
- Speer, B.S.; Shoemaker, N.B.; Salyers, A.A. Bacterial resistance to tetracycline: Mechanisms, transfer, and clinical significance. Clin. Microbiol. Rev. 1992, 5, 387–399. [Google Scholar] [CrossRef]
- Marily, C. Roberts, Tetracycline resistance determinants: Mechanisms of action, regulation of expression, genetic mobility, and distribution. FEMS Microbiol. Rev. 1996, 19, 1–24. [Google Scholar] [CrossRef]
- Connell, S.R.; Tracz, D.M.; Nierhaus, K.H.; Taylor, D.E. Ribosomal protection proteins and their mechanism of tetracycline resistance. Antimicrob. Agents Chemother. 2003, 47, 3675–3681. [Google Scholar] [CrossRef]
- Grossman, T.H. Tetracycline Antibiotics and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025387. [Google Scholar] [CrossRef] [PubMed]
- Sheykhsaran, E.; Baghi, H.B.; Soroush, M.H.; Ghotaslou, R. An overview of tetracyclines and related resistance mechanisms. Rev. Med. Microbiol. 2019, 30, 69–75. [Google Scholar] [CrossRef]
- Iconaru, S.L.; Motelica-Heino, M.; Predoi, D. Study on europium-doped hydroxyapatite nanoparticles by Fourier transform infrared spectroscopy and their antimicrobial properties. J. Spectro. 2013, 2013, 284285. [Google Scholar] [CrossRef]
- Iconaru, S.L.; Predoi, M.V.; Chapon, P.; Gaiaschi, S.; Rokosz, K.; Raaen, S.; Motelica-Heino, M.; Predoi, D. Investigation of Spin Coating Cerium-Doped Hydroxyapatite Thin Films with Antifungal Properties. Coatings 2021, 11, 464. [Google Scholar] [CrossRef]
- Casa Software Ltd. CasaXPS: Processing Software for XPS, AES, SIMS and More. 2009. Available online: www.casaxps.com (accessed on 1 May 2024).
- Das, D.; Nath, B.C.; Phukon, P.; Dolui, S.K. Synthesis of ZnO nanoparticles and evaluation of antioxidant and cytotoxic activity. Colloids Surf. B Biointerfaces 2013, 111, 556–560. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Rokosz, K.; Raaen, S.; Negrila, C.C.; Buton, N.; Ghegoiu, L.; Badea, M.L. Physico-Chemical and Biological Features of Fluorine-Substituted Hydroxyapatite Suspensions. Materials 2024, 17, 3404. [Google Scholar] [CrossRef]
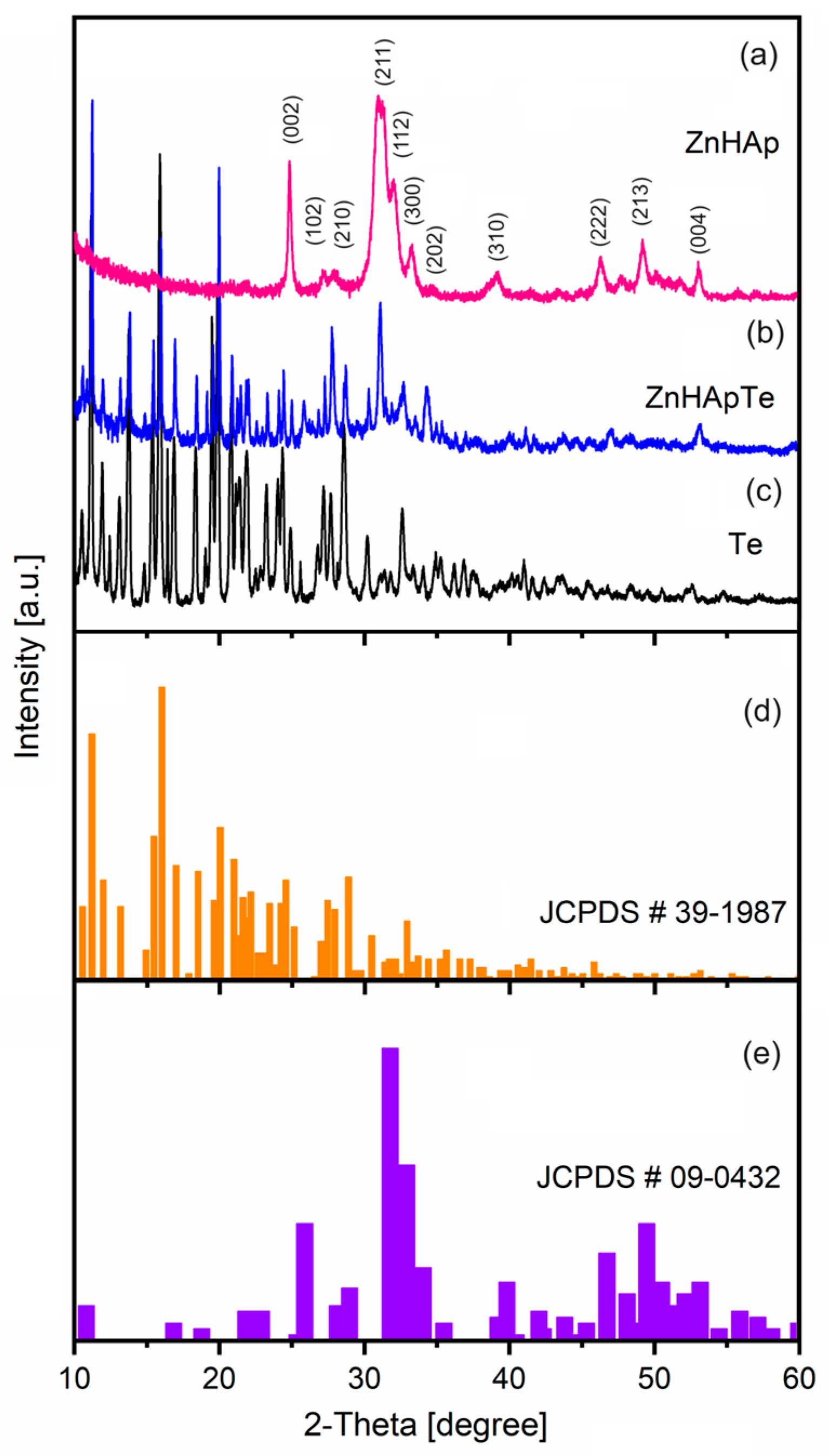
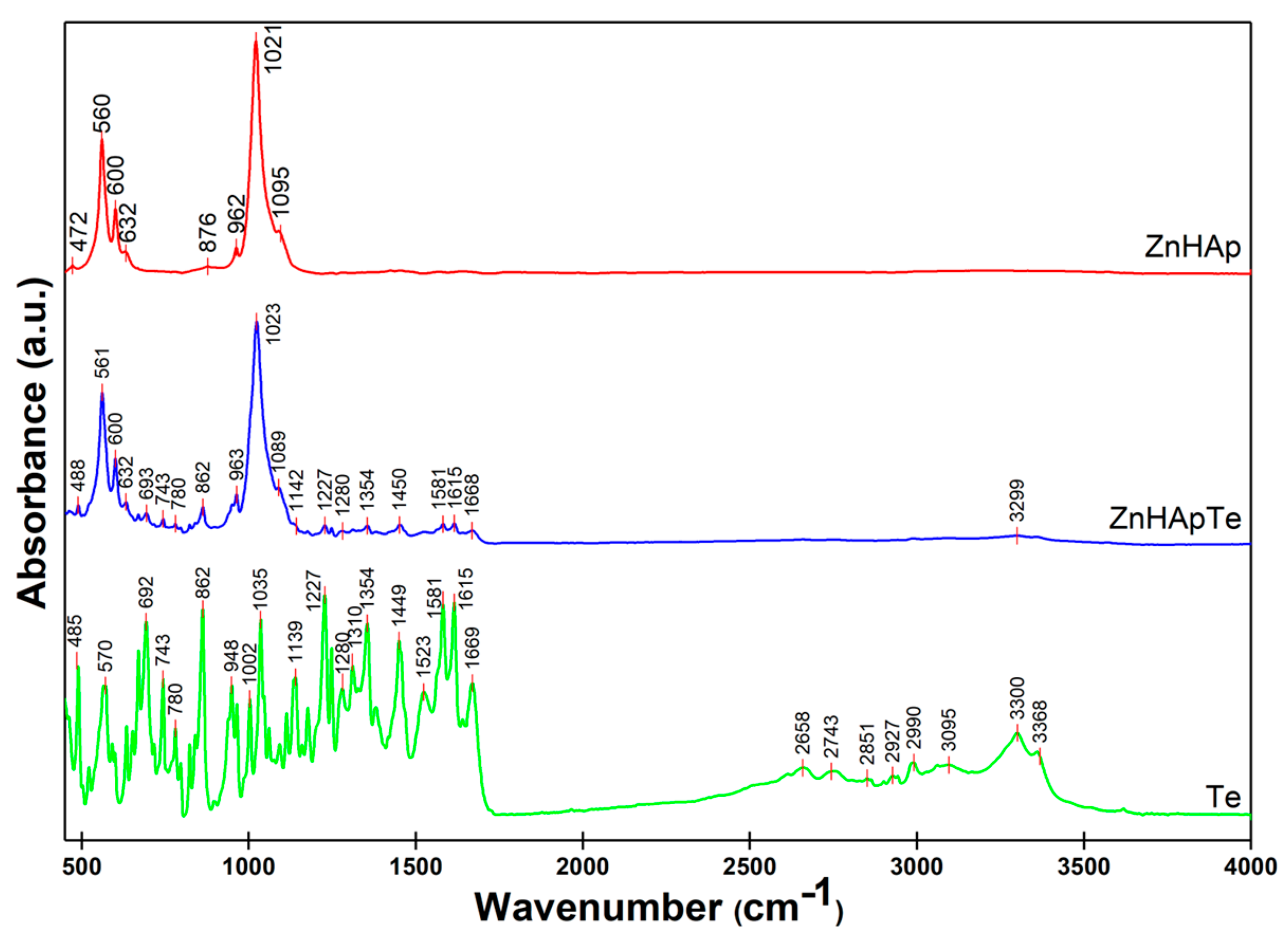
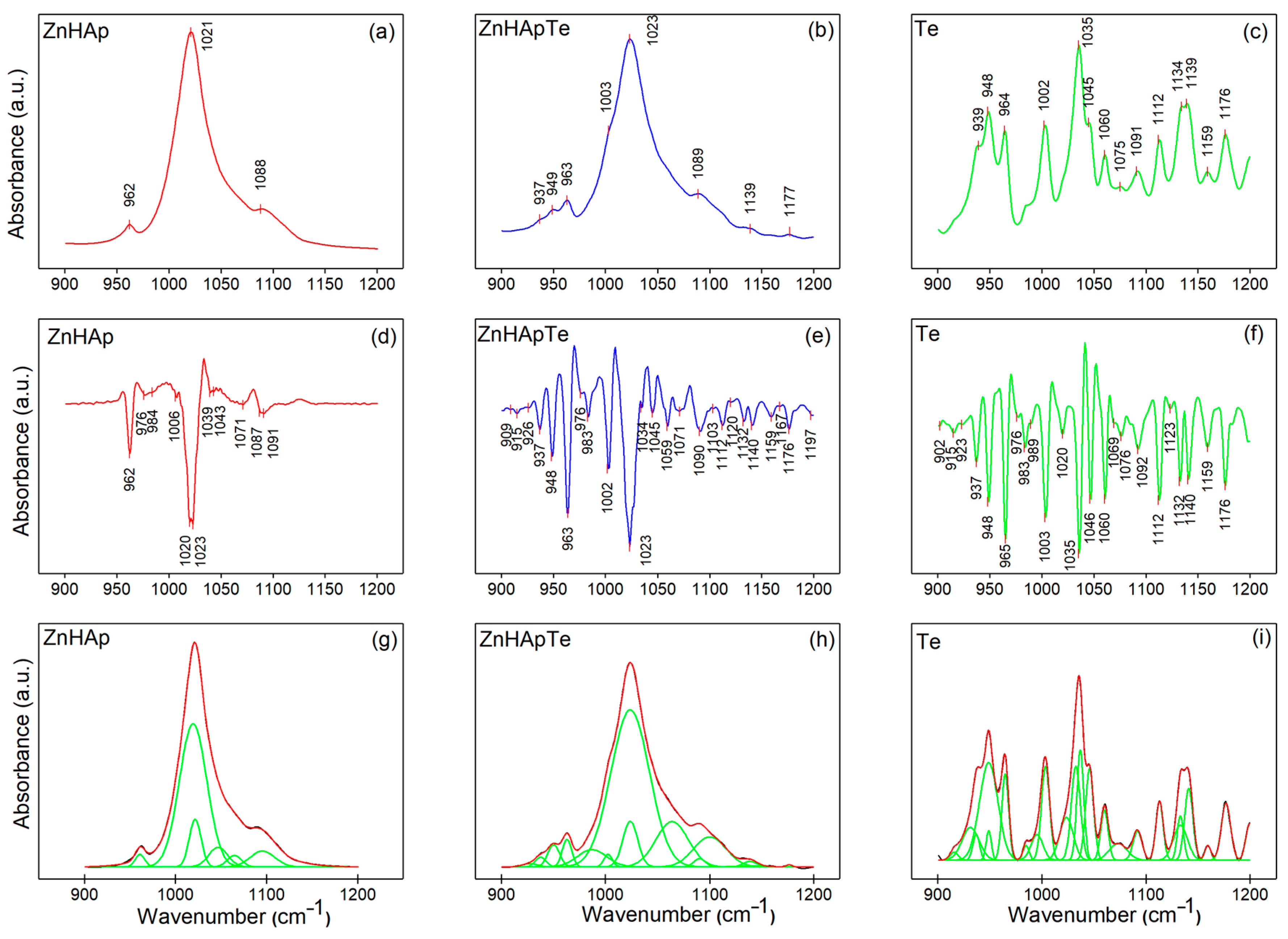
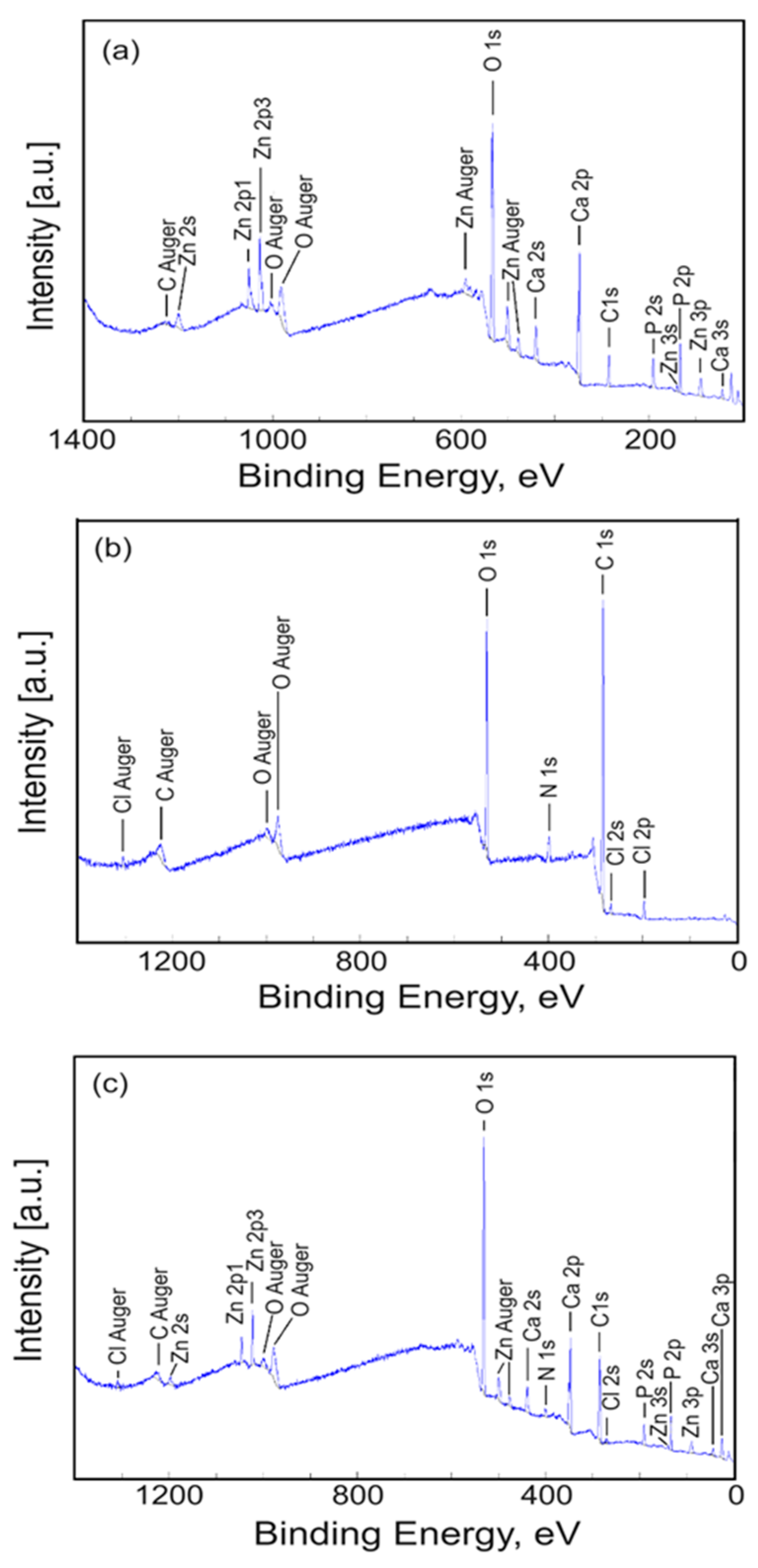


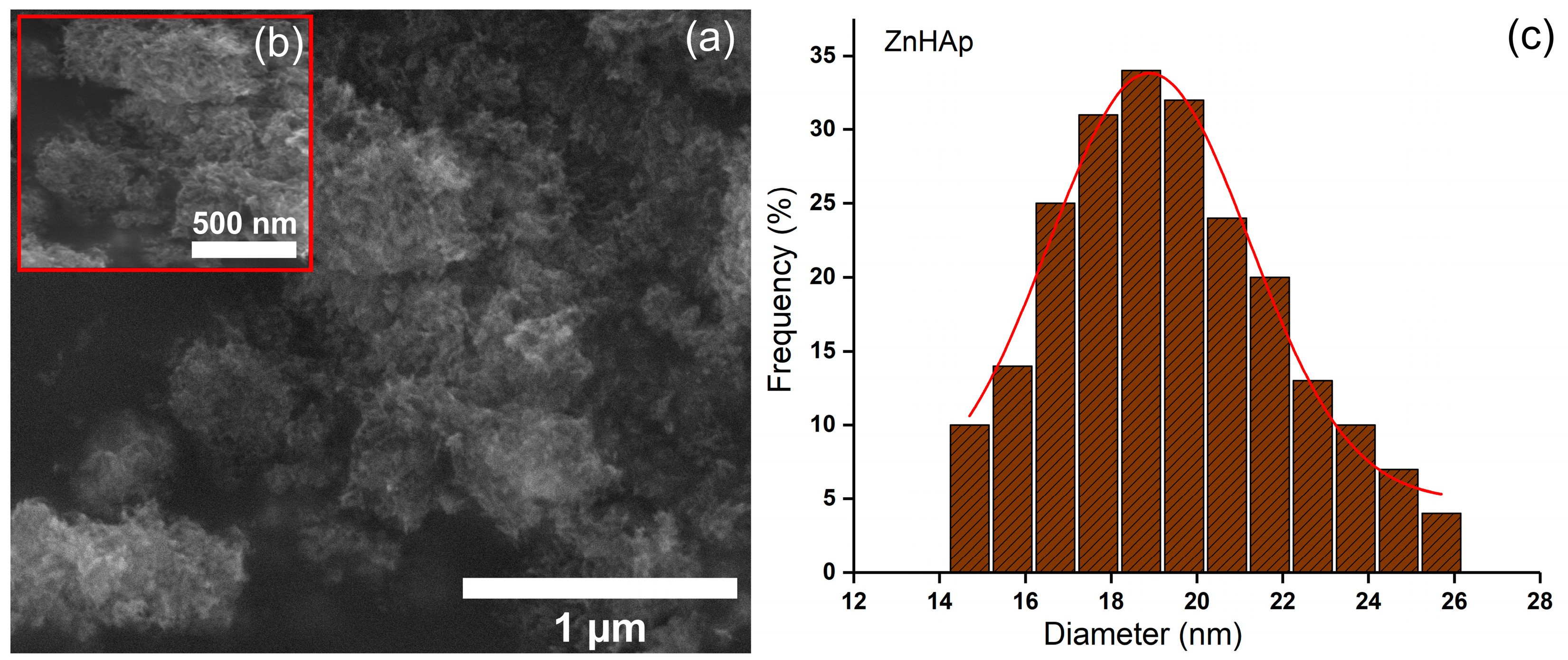

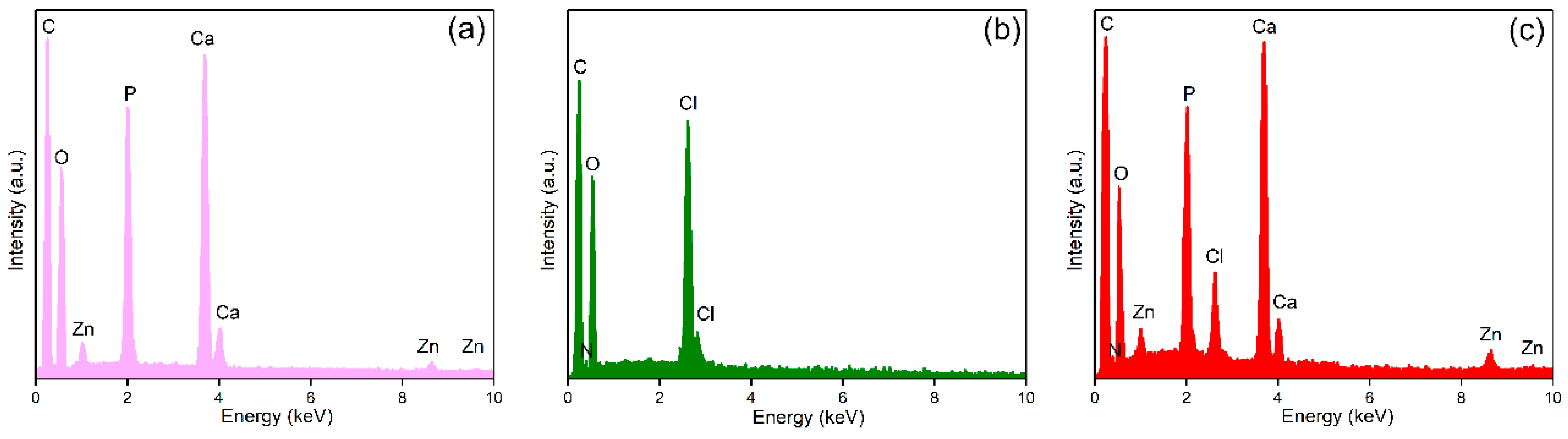

| Sample | Ca | P | O | Zn | N | Cl |
|---|---|---|---|---|---|---|
| ZnHAp | 16.9 | 10.68 | 71.49 | 0.93 | - | - |
| ZnHApTe | 20.18 | 12.65 | 51.6 | 0.92 | 0.95 | 13.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iconaru, S.L.; Predoi, D.; Ciobanu, C.S.; Negrila, C.C.; Trusca, R.; Raaen, S.; Rokosz, K.; Ghegoiu, L.; Badea, M.L.; Cimpeanu, C. Novel Antimicrobial Agents Based on Zinc-Doped Hydroxyapatite Loaded with Tetracycline. Antibiotics 2024, 13, 803. https://doi.org/10.3390/antibiotics13090803
Iconaru SL, Predoi D, Ciobanu CS, Negrila CC, Trusca R, Raaen S, Rokosz K, Ghegoiu L, Badea ML, Cimpeanu C. Novel Antimicrobial Agents Based on Zinc-Doped Hydroxyapatite Loaded with Tetracycline. Antibiotics. 2024; 13(9):803. https://doi.org/10.3390/antibiotics13090803
Chicago/Turabian StyleIconaru, Simona Liliana, Daniela Predoi, Carmen Steluta Ciobanu, Catalin Constantin Negrila, Roxana Trusca, Steinar Raaen, Krzysztof Rokosz, Liliana Ghegoiu, Monica Luminita Badea, and Carmen Cimpeanu. 2024. "Novel Antimicrobial Agents Based on Zinc-Doped Hydroxyapatite Loaded with Tetracycline" Antibiotics 13, no. 9: 803. https://doi.org/10.3390/antibiotics13090803
APA StyleIconaru, S. L., Predoi, D., Ciobanu, C. S., Negrila, C. C., Trusca, R., Raaen, S., Rokosz, K., Ghegoiu, L., Badea, M. L., & Cimpeanu, C. (2024). Novel Antimicrobial Agents Based on Zinc-Doped Hydroxyapatite Loaded with Tetracycline. Antibiotics, 13(9), 803. https://doi.org/10.3390/antibiotics13090803










