Murepavadin Enhances the Killing Efficacy of Ciprofloxacin against Pseudomonas aeruginosa by Inhibiting Drug Efflux
Abstract
:1. Introduction
2. Results
2.1. Combination of Murepavadin with Ciprofloxacin Increases the Killing Efficacy against P. aeruginosa
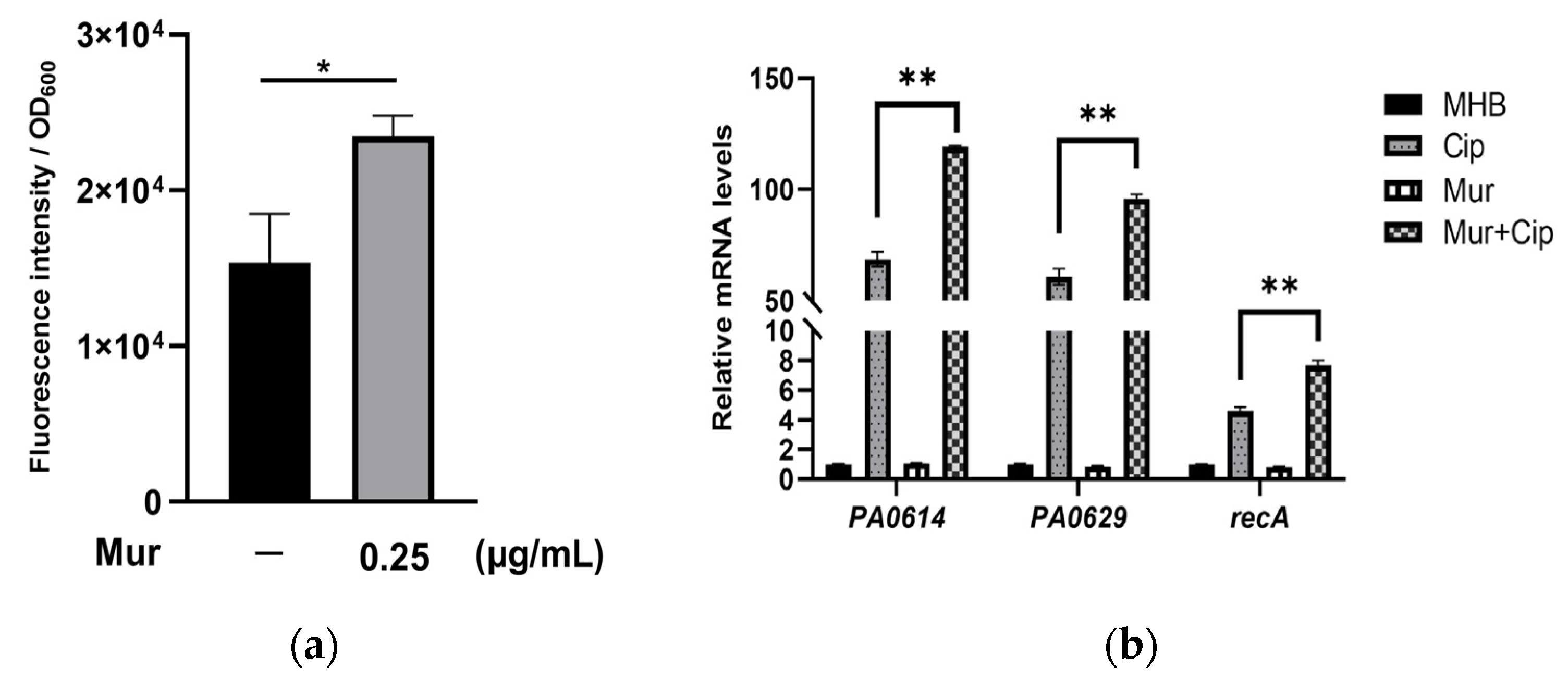
2.2. Murepavadin Enhances Intracellular Accumulation of Ciprofloxacin by Suppressing Drug Efflux
2.3. Murepavadin Enhances Bactericidal Effects of Ciprofloxacin against P. aeruginosa Clinical Isolates
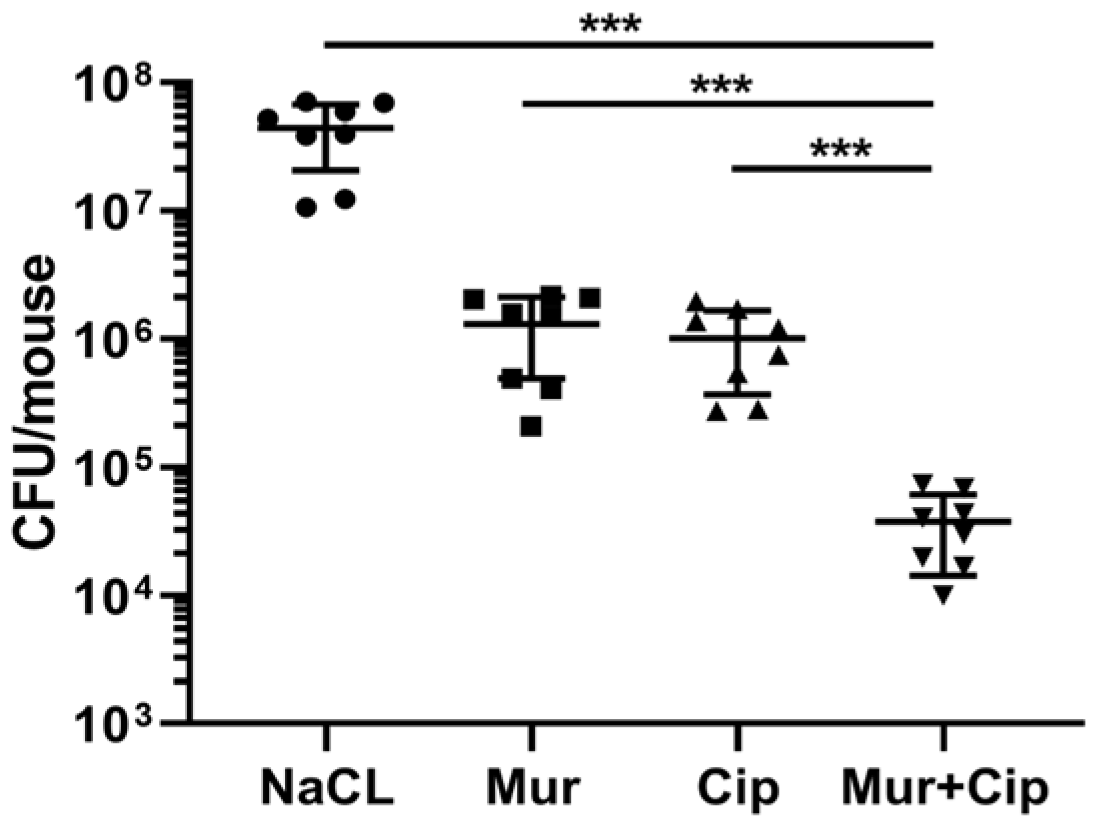
2.4. The Murepavadin–Ciprofloxacin Combination Displays a Synergistic Therapeutic Effect In Vivo
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Growth Conditions
4.2. Minimal Inhibitory Concentration (MIC) Determination
4.3. Time-Dependent Killing Assays
4.4. Intracellular Accumulation of Ciprofloxacin
4.5. Ethidium Bromide Influx Assay
4.6. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR)
4.7. Murine Lung Infection Model
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin-Loeches, I.; Dale, G.E.; Torres, A. Murepavadin: A new antibiotic class in the pipeline. Expert. Rev. Anti Infect. Ther. 2018, 16, 259–268. [Google Scholar] [CrossRef]
- Zheng, M.; Lupoli, T.J. Counteracting antibiotic resistance enzymes and efflux pumps. Curr. Opin. Microbiol. 2023, 75, 102334. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.; Chan, S.Y.; Song, Q.; Li, P.; Huang, W. The Strategies of Pathogen-Oriented Therapy on Circumventing Antimicrobial Resistance. Research 2020, 2020, 2016201. [Google Scholar] [CrossRef]
- Zhu, M.; Tse, M.W.; Weller, J.; Chen, J.; Blainey, P.C. The future of antibiotics begins with discovering new combinations. Ann. N. Y. Acad. Sci. 2021, 1496, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Silby, M.W.; Winstanley, C.; Godfrey, S.A.; Levy, S.B.; Jackson, R.W. Pseudomonas genomes: Diverse and adaptable. FEMS Microbiol. Rev. 2011, 35, 652–680. [Google Scholar] [CrossRef]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, S.; Hayes, D., Jr.; Wozniak, D.J. Cystic Fibrosis and Pseudomonas aeruginosa: The Host-Microbe Interface. Clin. Microbiol. Rev. 2019, 32, e00138-18. [Google Scholar] [CrossRef] [PubMed]
- Sathe, N.; Beech, P.; Croft, L.; Suphioglu, C.; Kapat, A.; Athan, E. Pseudomonas aeruginosa: Infections and novel approaches to treatment “Knowing the enemy” the threat of Pseudomonas aeruginosa and exploring novel approaches to treatment. Infect. Med. 2023, 2, 178–194. [Google Scholar] [CrossRef]
- Moradali, M.F.; Ghods, S.; Rehm, B.H. Pseudomonas aeruginosa Lifestyle: A Paradigm for Adaptation, Survival, and Persistence. Front. Cell Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef]
- Srinivas, N.; Jetter, P.; Ueberbacher, B.J.; Werneburg, M.; Zerbe, K.; Steinmann, J.; Van der Meijden, B.; Bernardini, F.; Lederer, A.; Dias, R.L.; et al. Peptidomimetic antibiotics target outer-membrane biogenesis in Pseudomonas aeruginosa. Science 2010, 327, 1010–1013. [Google Scholar] [CrossRef]
- Andolina, G.; Bencze, L.C.; Zerbe, K.; Muller, M.; Steinmann, J.; Kocherla, H.; Mondal, M.; Sobek, J.; Moehle, K.; Malojcic, G.; et al. A Peptidomimetic Antibiotic Interacts with the Periplasmic Domain of LptD from Pseudomonas aeruginosa. ACS Chem. Biol. 2018, 13, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gros, J.; Cajal, Y.; Marques, A.M.; Rabanal, F. Synthesis of the Antimicrobial Peptide Murepavadin Using Novel Coupling Agents. Biomolecules 2024, 14, 526. [Google Scholar] [CrossRef] [PubMed]
- Amponnawarat, A.; Chompunud Na Ayudhya, C.; Ali, H. Murepavadin, a Small Molecule Host Defense Peptide Mimetic, Activates Mast Cells via MRGPRX2 and MrgprB2. Front. Immunol. 2021, 12, 689410. [Google Scholar] [CrossRef]
- Diez-Aguilar, M.; Hernandez-Garcia, M.; Morosini, M.I.; Fluit, A.; Tunney, M.M.; Huertas, N.; Del Campo, R.; Obrecht, D.; Bernardini, F.; Ekkelenkamp, M.; et al. Murepavadin antimicrobial activity against and resistance development in cystic fibrosis Pseudomonas aeruginosa isolates. J. Antimicrob. Chemother. 2021, 76, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.S.; Henderson, I.R.; Capon, R.J.; Blaskovich, M.A.T. Antibiotics in the clinical pipeline as of December 2022. J. Antibiot. 2023, 76, 431–473. [Google Scholar] [CrossRef]
- Kelly, K.R.; Brooks, B.W. Global Aquatic Hazard Assessment of Ciprofloxacin: Exceedances of Antibiotic Resistance Development and Ecotoxicological Thresholds. Prog. Mol. Biol. Transl. Sci. 2018, 159, 59–77. [Google Scholar]
- Rehman, A.; Patrick, W.M.; Lamont, I.L. Mechanisms of ciprofloxacin resistance in Pseudomonas aeruginosa: New approaches to an old problem. J. Med. Microbiol. 2019, 68, 1–10. [Google Scholar] [CrossRef]
- Gales, A.C.; Jones, R.N.; Turnidge, J.; Rennie, R.; Ramphal, R. Characterization of Pseudomonas aeruginosa isolates: Occurrence rates, antimicrobial susceptibility patterns, and molecular typing in the global SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin. Infect. Dis. 2001, 32 (Suppl. S2), S146–S155. [Google Scholar] [CrossRef] [PubMed]
- Podlesek, Z.; Zgur Bertok, D. The DNA Damage Inducible SOS Response Is a Key Player in the Generation of Bacterial Persister Cells and Population Wide Tolerance. Front. Microbiol. 2020, 11, 1785. [Google Scholar] [CrossRef]
- Long, Y.; Fu, W.; Wang, S.; Deng, X.; Jin, Y.; Bai, F.; Cheng, Z.; Wu, W. Fis Contributes to Resistance of Pseudomonas aeruginosa to Ciprofloxacin by Regulating Pyocin Synthesis. J. Bacteriol. 2020, 202, e00064-20. [Google Scholar] [CrossRef]
- Lim, D.J.; Skinner, D.; McLemore, J.; Rivers, N.; Elder, J.B.; Allen, M.; Koch, C.; West, J.; Zhang, S.; Thompson, H.M.; et al. In-vitro evaluation of a ciprofloxacin and azithromycin sinus stent for Pseudomonas aeruginosa biofilms. Int. Forum Allergy Rhinol. 2020, 10, 121–127. [Google Scholar] [CrossRef] [PubMed]
- De Soyza, A.; Aksamit, T.; Bandel, T.J.; Criollo, M.; Elborn, J.S.; Operschall, E.; Polverino, E.; Roth, K.; Winthrop, K.L.; Wilson, R. RESPIRE 1: A phase III placebo-controlled randomised trial of ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Eur. Respir. J. 2018, 51, 1702052. [Google Scholar] [CrossRef]
- Aksamit, T.; De Soyza, A.; Bandel, T.J.; Criollo, M.; Elborn, J.S.; Operschall, E.; Polverino, E.; Roth, K.; Winthrop, K.L.; Wilson, R. RESPIRE 2: A phase III placebo-controlled randomised trial of ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Eur. Respir. J. 2018, 51, 1702053. [Google Scholar] [CrossRef] [PubMed]
- Haworth, C.S.; Bilton, D.; Chalmers, J.D.; Davis, A.M.; Froehlich, J.; Gonda, I.; Thompson, B.; Wanner, A.; O’Donnell, A.E. Inhaled liposomal ciprofloxacin in patients with non-cystic fibrosis bronchiectasis and chronic lung infection with Pseudomonas aeruginosa (ORBIT-3 and ORBIT-4): Two phase 3, randomised controlled trials. Lancet Respir. Med. 2019, 7, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Coates, A.R.M.; Hu, Y.; Holt, J.; Yeh, P. Antibiotic combination therapy against resistant bacterial infections: Synergy, rejuvenation and resistance reduction. Expert. Rev. Anti Infect. Ther. 2020, 18, 5–15. [Google Scholar] [CrossRef]
- Fischbach, M.A. Combination therapies for combating antimicrobial resistance. Curr. Opin. Microbiol. 2011, 14, 519–523. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Q.; Jin, L.; Wang, R.; Yin, Y.; Sun, S.; Zhang, J.; Wang, H. In vitro Synergistic Activity of Antimicrobial Combinations Against bla (KPC) and bla (NDM)-Producing Enterobacterales With bla (IMP) or mcr Genes. Front. Microbiol. 2020, 11, 533209. [Google Scholar] [CrossRef]
- Wei, X.; Gao, J.; Xu, C.; Pan, X.; Jin, Y.; Bai, F.; Cheng, Z.; Lamont, I.L.; Pletzer, D.; Wu, W. Murepavadin induces envelope stress response and enhances the killing efficacies of beta-lactam antibiotics by impairing the outer membrane integrity of Pseudomonas aeruginosa. Microbiol. Spectr. 2023, 11, e0125723. [Google Scholar] [CrossRef]
- Sader, H.S.; Dale, G.E.; Rhomberg, P.R.; Flamm, R.K. Antimicrobial Activity of Murepavadin Tested against Clinical Isolates of Pseudomonas aeruginosa from the United States, Europe, and China. Antimicrob. Agents Chemother. 2018, 62, e00311–e00318. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, M100, 33rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2023. [Google Scholar]
- Bhattacharyya, T.; Sharma, A.; Akhter, J.; Pathania, R. The small molecule IITR08027 restores the antibacterial activity of fluoroquinolones against multidrug-resistant Acinetobacter baumannii by efflux inhibition. Int. J. Antimicrob. Agents 2017, 50, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.M.; Piddock, L.J. How to Measure Export via Bacterial Multidrug Resistance Efflux Pumps. mBio 2016, 7, e00840-16. [Google Scholar] [CrossRef]
- Pan, X.; Wu, W. Murine Acute Pneumonia Model of Pseudomonas aeruginosa Lung Infection. Bio Protoc. 2020, 10, e3805. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Liu, Y.; Zhou, J.; Long, Y.; Liu, C.; Xia, B.; Shi, J.; Fan, Z.; Liang, Y.; Chen, S.; et al. Combination of Azithromycin and Gentamicin for Efficient Treatment of Pseudomonas aeruginosa Infections. J. Infect. Dis. 2019, 220, 1667–1678. [Google Scholar] [CrossRef]
- Sabnis, A.; Hagart, K.L.; Klockner, A.; Becce, M.; Evans, L.E.; Furniss, R.C.D.; Mavridou, D.A.; Murphy, R.; Stevens, M.M.; Davies, J.C.; et al. Colistin kills bacteria by targeting lipopolysaccharide in the cytoplasmic membrane. Elife 2021, 10, e65836. [Google Scholar] [CrossRef]
- Spiers, D.E.; Candas, V. Relationship of skin surface area to body mass in the immature rat: A reexamination. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1984, 56, 240–243. [Google Scholar] [CrossRef]
- Panthi, V.K.; Fairfull-Smith, K.E.; Islam, N. Ciprofloxacin-Loaded Inhalable Formulations against Lower Respiratory Tract Infections: Challenges, Recent Advances, and Future Perspectives. Pharmaceutics 2024, 16, 648. [Google Scholar] [CrossRef]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D.; Jabbour, J.F.; Kanj, S.S. Current choices of antibiotic treatment for Pseudomonas aeruginosa infections. Curr. Opin. Infect. Dis. 2020, 33, 464–473. [Google Scholar] [CrossRef]
- Gorham, J.; Taccone, F.S.; Hites, M. How to Use Nebulized Antibiotics in Severe Respiratory Infections. Antibiotics 2023, 12, 267. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Schneider-Futschik, E.K. Current and Emerging Inhaled Antibiotics for Chronic Pulmonary Pseudomonas aeruginosa and Staphylococcus aureus Infections in Cystic Fibrosis. Antibiotics 2023, 12, 484. [Google Scholar] [CrossRef] [PubMed]
- Tejada, S.; Ramirez-Estrada, S.; Forero, C.G.; Gallego, M.; Soriano, J.B.; Cardinal-Fernandez, P.A.; Ehrmann, S.; Rello, J. Safety and Efficacy of Devices Delivering Inhaled Antibiotics among Adults with Non-Cystic Fibrosis Bronchiectasis: A Systematic Review and a Network Meta-Analysis. Antibiotics 2022, 11, 275. [Google Scholar] [CrossRef]
- Lorusso, A.B.; Carrara, J.A.; Barroso, C.D.N.; Tuon, F.F.; Faoro, H. Role of Efflux Pumps on Antimicrobial Resistance in Pseudomonas aeruginosa. Int. J. Mol. Sci. 2022, 23, 15779. [Google Scholar] [CrossRef] [PubMed]
- Aeschlimann, J.R. The role of multidrug efflux pumps in the antibiotic resistance of Pseudomonas aeruginosa and other gram-negative bacteria. Insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 2003, 23, 916–924. [Google Scholar] [CrossRef]
- Blair, J.M.; Piddock, L.J. Structure, function and inhibition of RND efflux pumps in Gram-negative bacteria: An update. Curr. Opin. Microbiol. 2009, 12, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Gao, J.; Zhou, D.; Xu, C.; Chen, P.; Chen, S.; Zhang, Y.; Liu, X.; Li, G.; Zhu, G.; et al. Murepavadin promotes the killing efficacies of aminoglycoside antibiotics against Pseudomonas aeruginosa by enhancing membrane potential. Antimicrob. Agents Chemother. 2024, 68, e0153923. [Google Scholar] [CrossRef]
- Mathee, K. Forensic investigation into the origin of Pseudomonas aeruginosa PA14-old but not lost. J. Med. Microbiol. 2018, 67, 1019–1021. [Google Scholar] [CrossRef]
- Grace, A.; Sahu, R.; Owen, D.R.; Dennis, V.A. Pseudomonas aeruginosa reference strains PAO1 and PA14: A genomic, phenotypic, and therapeutic review. Front. Microbiol. 2022, 13, 1023523. [Google Scholar] [CrossRef]
- Rehman, A.; Jeukens, J.; Levesque, R.C.; Lamont, I.L. Gene-Gene Interactions Dictate Ciprofloxacin Resistance in Pseudomonas aeruginosa and Facilitate Prediction of Resistance Phenotype from Genome Sequence Data. Antimicrob. Agents Chemother. 2021, 65, e0269620. [Google Scholar] [CrossRef]
- Haidar, G.; Philips, N.J.; Shields, R.K.; Snyder, D.; Cheng, S.; Potoski, B.A.; Doi, Y.; Hao, B.; Press, E.G.; Cooper, V.S.; et al. Ceftolozane-Tazobactam for the Treatment of Multidrug-Resistant Pseudomonas aeruginosa Infections: Clinical Effectiveness and Evolution of Resistance. Clin. Infect. Dis. 2017, 65, 110–120. [Google Scholar] [CrossRef]
- Torres, A.; Zhong, N.; Pachl, J.; Timsit, J.F.; Kollef, M.; Chen, Z.; Song, J.; Taylor, D.; Laud, P.J.; Stone, G.G.; et al. Ceftazidime-avibactam versus meropenem in nosocomial pneumonia, including ventilator-associated pneumonia (REPROVE): A randomised, double-blind, phase 3 non-inferiority trial. Lancet Infect. Dis. 2018, 18, 285–295. [Google Scholar] [CrossRef]
- Reynolds, D.; Kollef, M. The Epidemiology and Pathogenesis and Treatment of Pseudomonas aeruginosa Infections: An Update. Drugs 2021, 81, 2117–2131. [Google Scholar] [CrossRef] [PubMed]
- Hajji, M.; Jebali, H.; Mrad, A.; Blel, Y.; Brahmi, N.; Kheder, R.; Beji, S.; Fatma, L.B.; Smaoui, W.; Krid, M.; et al. Nephrotoxicity of Ciprofloxacin: Five Cases and a Review of the Literature. Drug Saf. Case Rep. 2018, 5, 17. [Google Scholar] [CrossRef]
- Campbell, R.E.; Chen, C.H.; Edelstein, C.L. Overview of Antibiotic-Induced Nephrotoxicity. Kidney Int. Rep. 2023, 8, 2211–2225. [Google Scholar] [CrossRef] [PubMed]
- Viveiros, M.; Rodrigues, L.; Martins, M.; Couto, I.; Spengler, G.; Martins, A.; Amaral, L. Evaluation of efflux activity of bacteria by a semi-automated fluorometric system. Methods Mol. Biol. 2010, 642, 159–172. [Google Scholar]
- Maurya, A.; Dwivedi, G.R.; Darokar, M.P.; Srivastava, S.K. Antibacterial and synergy of clavine alkaloid lysergol and its derivatives against nalidixic acid-resistant Escherichia coli. Chem. Biol. Drug Des. 2013, 81, 484–490. [Google Scholar] [CrossRef]
- Moo, C.L.; Osman, M.A.; Yang, S.K.; Yap, W.S.; Ismail, S.; Lim, S.H.; Chong, C.M.; Lai, K.S. Antimicrobial activity and mode of action of 1,8-cineol against carbapenemase-producing Klebsiella pneumoniae. Sci. Rep. 2021, 11, 20824. [Google Scholar] [CrossRef] [PubMed]
- Liberati, N.T.; Urbach, J.M.; Miyata, S.; Lee, D.G.; Drenkard, E.; Wu, G.; Villanueva, J.; Wei, T.; Ausubel, F.M. An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants. Proc. Natl Acad. Sci. USA 2006, 103, 2833–2838. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Zhou, D.; Xu, C.; Chen, P.; Chen, S.; Cheng, Z.; Jin, Y.; Jin, S.; Wu, W. Murepavadin Enhances the Killing Efficacy of Ciprofloxacin against Pseudomonas aeruginosa by Inhibiting Drug Efflux. Antibiotics 2024, 13, 810. https://doi.org/10.3390/antibiotics13090810
Wei X, Zhou D, Xu C, Chen P, Chen S, Cheng Z, Jin Y, Jin S, Wu W. Murepavadin Enhances the Killing Efficacy of Ciprofloxacin against Pseudomonas aeruginosa by Inhibiting Drug Efflux. Antibiotics. 2024; 13(9):810. https://doi.org/10.3390/antibiotics13090810
Chicago/Turabian StyleWei, Xiaoya, Dandan Zhou, Congjuan Xu, Ping Chen, Shuiping Chen, Zhihui Cheng, Yongxin Jin, Shouguang Jin, and Weihui Wu. 2024. "Murepavadin Enhances the Killing Efficacy of Ciprofloxacin against Pseudomonas aeruginosa by Inhibiting Drug Efflux" Antibiotics 13, no. 9: 810. https://doi.org/10.3390/antibiotics13090810




