Abstract
Antimicrobial resistance poses a major threat to human health worldwide and the implementation of antimicrobial stewardship programs (ASPs), including antimicrobial de-escalation (ADE), is a multifaceted tool for minimizing unnecessary or inappropriate antibiotic exposure. This was a prospective observational study of 142 non-Intensive Care Unit (ICU) patients with microbiologically documented infection who were initially administered empirical antimicrobial therapy and admitted to the medical wards of 6 tertiary-care hospitals in Greece from January 2017 to December 2018. Patients were divided into two groups, the ADE and non-ADE group, based on whether ADE was applied or not, respectively. Exploratory end-points were ADE feasibility, safety and efficacy. ADE was applied in 76 patients at a median time of 4 days (IQR: 3, 5). An increased likelihood of ADE was observed in patients with urinary tract (OR: 10.04, 95% CI: 2.91, 34.57; p < 0.001), skin and soft tissue (OR: 16.28, 95% CI: 1.68, 158.08; p = 0.016) and bloodstream infections (OR: 2.52, 95% CI: 1, 6.36; p = 0.05). Factors significantly associated with higher rates of ADE were clarithromycin administration, diagnosis of urinary tract infection (UTI), isolation of E. coli, age and symptoms type on admission. Mortality was lower in the ADE group (18.4% vs. 30.3% p < 0.1) and ADE was not significantly associated with the probability of death (p = 0.432). ADE was associated with favorable clinical outcomes and can be performed even in settings with high prevalence of multi-drug resistant (MDR) pathogens without compromising safety.
1. Introduction
The World Health Organization (WHO) has declared antimicrobial resistance (AMR) as one of the top ten public health threats affecting humanity worldwide [1]. The global burden associated with AMR was an estimated 4.95 million deaths in 2019, of which 1.27 million were directly attributable to drug resistance [2]. In 2015, recognizing the urgent need to combat AMR, the World Health Assembly endorsed a global action plan, with one of the five strategic objectives outlined being the optimization of the use of antimicrobial medicines in human and animal health, thereby recommending the implementation of ASP programs [3]. Antimicrobial stewardship (AMS), which encompasses the coordinated interventions designed to improve and measure the appropriate use of antimicrobial agents in terms of selection, dose, route, and duration of administration, is now recognized as an essential tool for minimizing inappropriate or unnecessary antibiotic use and reducing both AMR rates and associated healthcare costs [4,5,6]. ADE is a key component of ASP and refers to the discontinuation of one or more components of combination empirical therapy and/or the change from a broad-spectrum to a narrower spectrum antimicrobial [7,8]. ADE feasibility and efficacy are strongly correlated with various factors, such the relevance of diagnosis, early and adequate collection of microbiological samples, pathogen isolation, appropriateness of empirical treatment, lower baseline severity, or clinical resolution at the time of culture positivity [8,9].
Early administration of empirical antimicrobial therapy in patients with suspected infection is crucial and results in favorable outcomes [10]. Notably, the impact is even greater in patients with confirmed or suspected sepsis, and the Surviving Sepsis Campaign recommends the early administration of empirical broad-spectrum antimicrobial regimens, ideally within the first hour of patient clinical assessment [11,12,13]. However, this practice can lead to antimicrobial overconsumption, thus gradually contributing to AMR emergence and occurrence of antibiotic-related adverse events.
To the best of our knowledge, the vast majority of studies assessing ADE value were conducted in ICU settings [14,15,16,17,18,19,20,21,22], and little is, so far, known about ADE safety and efficacy in patients hospitalized in common wards. The aim of the present study was to evaluate the feasibility, safety, and efficacy of ADE in patients with microbiologically documented infection admitted to Greek medical wards with recognized prevalence of MDR pathogens.
2. Results
A total of 142 patients (male: n = 75, 52.8%) fulfilled the inclusion criteria and were analyzed. Mean age was 73.7 years (SD: 15.5) and median Charlson’s Comorbidity Index (CCI) was 5 (IQR: 5, 7). One in three patients (n = 46, 32.4%) reported an antimicrobials receipt in the last trimester for a median of two courses (IQR: 1, 6). On admission, the majority of the study population presented with sepsis (n = 80, 56.3%), and 18 patients were in septic shock (12.7%); their median Sequential Organ Failure Assessment (SOFA) and quick Sequential Organ Failure Assessment (qSOFA) scores were 3 (IQR: 1, 4) and 1 (IQR: 0, 1), respectively. The urinary tract was the most common site of infection (n = 95, 69.9%), followed by the lung (n = 19, 13.4%) and intra-abdominal sources (n = 8, 5.6%). About half of the infections (n = 66, 46.5%) were bacteraemic, and the incidence of primary bacteremia was 6.3% (n = 9). E. coli (n = 72, 50.7%), K. pneumoniae (n = 19, 13.4%), and P. aeurginosa (n = 11, 7.8%) were the predominant pathogens identified, and the most frequent sources of isolation were the urinary tract (n = 86, 60.6%) and the bloodstream (n = 71, 52.2%). Detailed patients’ characteristics classified using ADE status are depicted in Table 1.

Table 1.
Study population characteristics classified by de-escalation status.
The median time from hospital admission to the antimicrobial susceptibility testing report was 3 days (IQR: 3, 4), and ADE was applied in 76 patients (53.5%) at a median time of 4 days (IQR: 3, 5). The rest of the cohort was managed with either the initial (n = 29, 20.4%) or escalated (n = 37, 26.1%) antimicrobial therapy. The reported reasons for which ADE was not performed were as follows: (i) non-permissive antimicrobial susceptibility testing (n = 11, 16.7%), (ii) patient’s non-clinical improvement (n = 9, 13.6%), (iii) occurrence of a new infection (n = 4, 6.1%), (iv) type of isolated pathogen (n = 2, 3%), and (v) physician’s decision (n = 40, 60.6%).
In the multivariable logistic regression analysis (Table 2), upon adjustment for several confounders (e.g., sex, residence type, symptoms, SOFA score on admission, and source of infection), UTIs (OR: 10.04, 95% CI: 2.91, 34.57; p < 0.001), skin and soft tissue infections (SSTIs) (OR: 16.28, 95% CI: 1.68, 158.08; p = 0.016), and bloodstream infections (BSIs) (OR: 2.52, 95% CI: 1, 6.36; p = 0.05) were associated with an increased likelihood of ADE. Moreover, ADE was more likely to be applied in female patients (OR: 2.65, 95% CI: 1.09, 6.43; p = 0.031) and those that reported symptoms other than fever and disorder of consciousness on admission (OR: 3.44, 95% CI: 1.37, 8.67; p = 0.009).

Table 2.
Multivariable logistic regression analysis of factors associated with ADE.
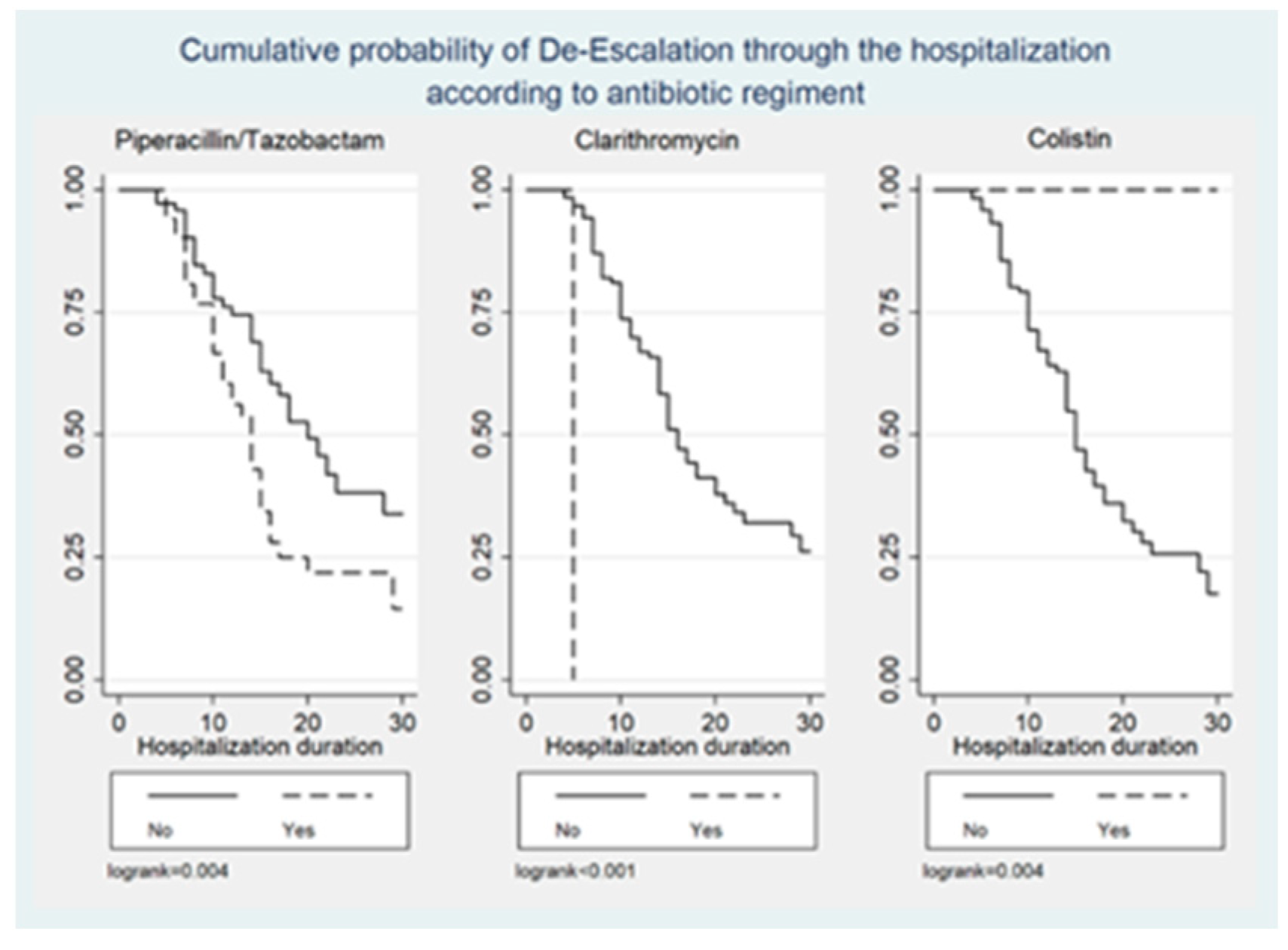
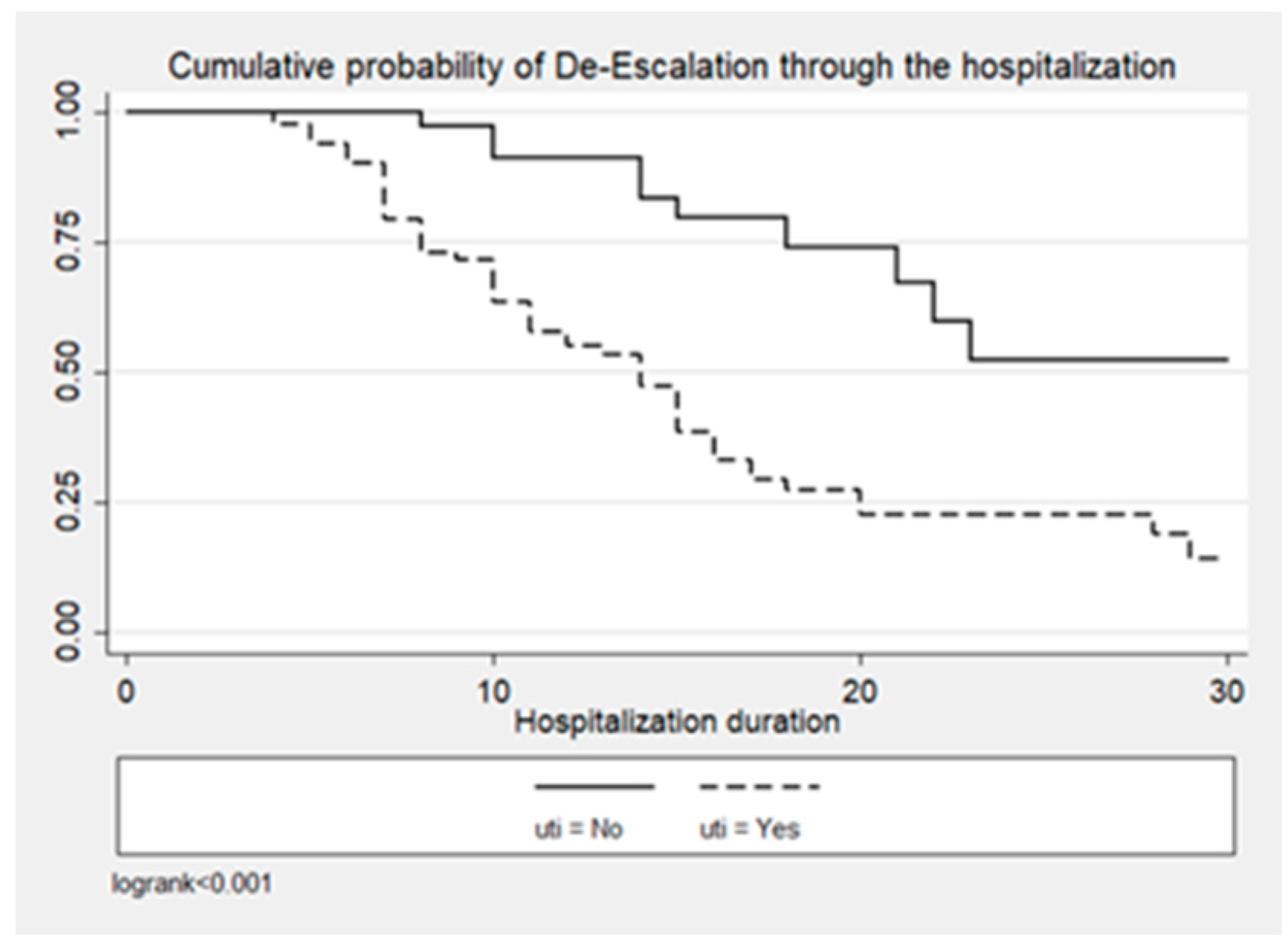
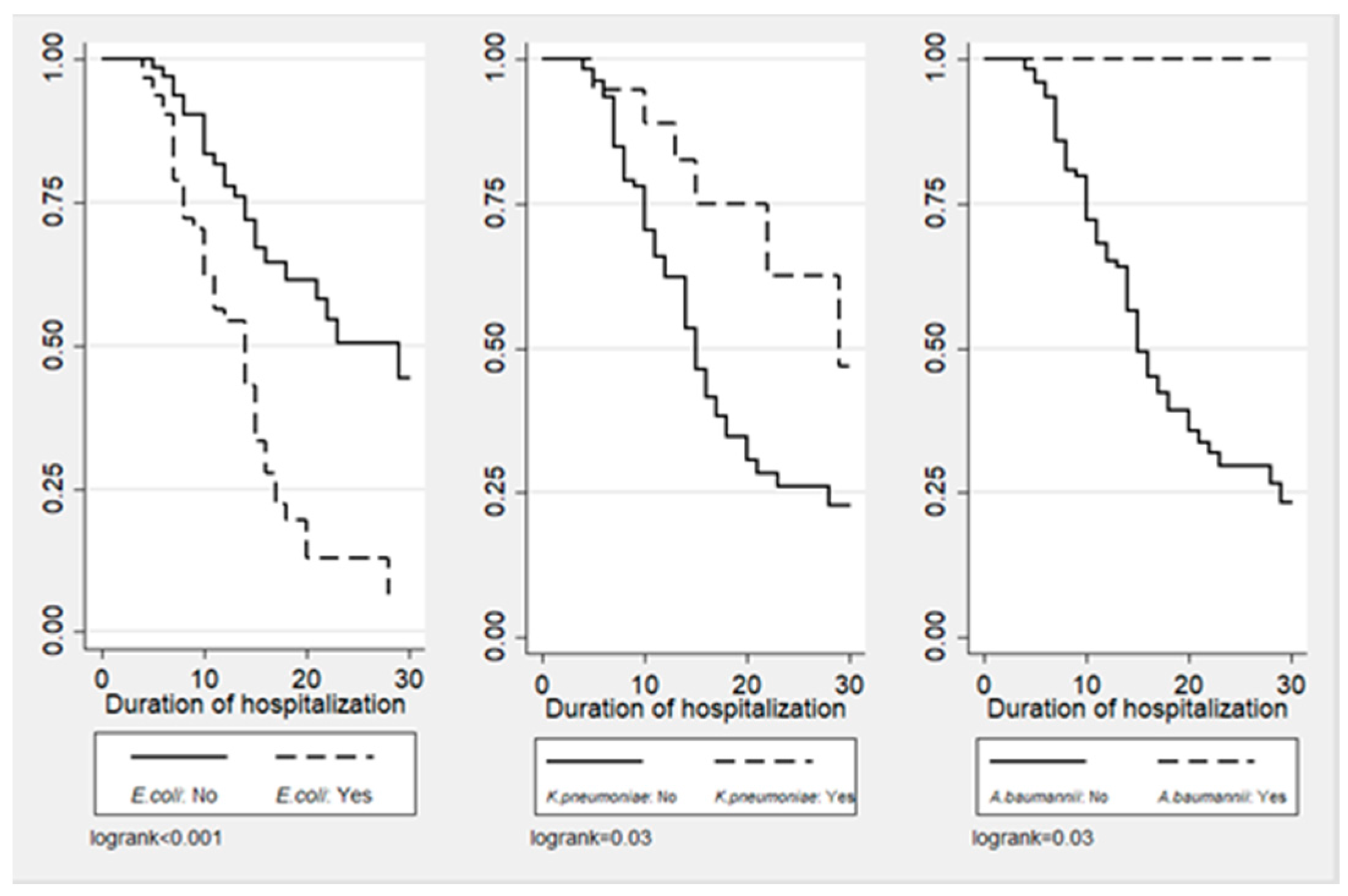
Figure 1, Figure 2 and Figure 3 illustrate the cumulative probability of ADE by initial antimicrobial regimens administered, UTI as a source of infection, and isolated pathogens, respectively. UTI as a source had a higher cumulative probability of ADE compared to all other sources grouped together. Regimens containing clarithromycin had the highest, whereas regimens containing colistin had the least cumulative probability of ADE. Similarly, recovery of E. coli was more likely to be associated with ADE compared to K. pneumoniae and A. baumannii.
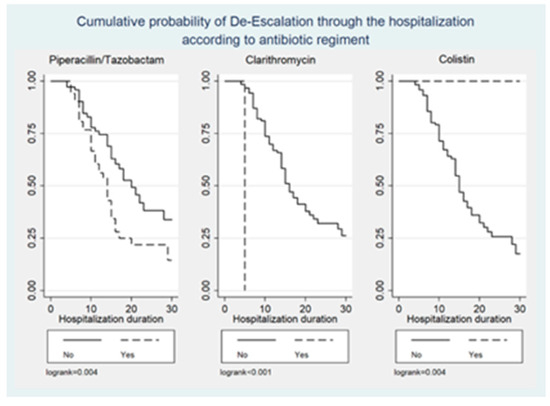
Figure 1.
Cumulative probability of ADE by initial antimicrobial regimens administered.
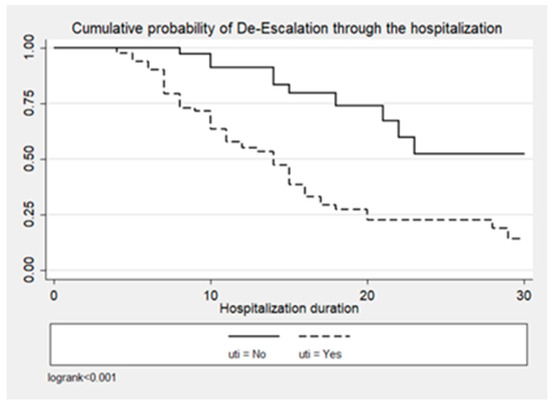
Figure 2.
Cumulative probability of ADE by UTI as source of infection.
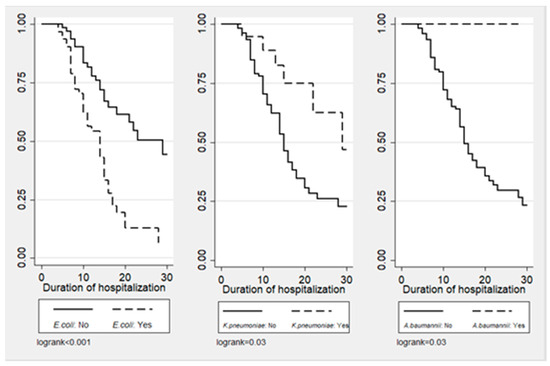
Figure 3.
Cumulative probability of ADE by type of isolated pathogen.
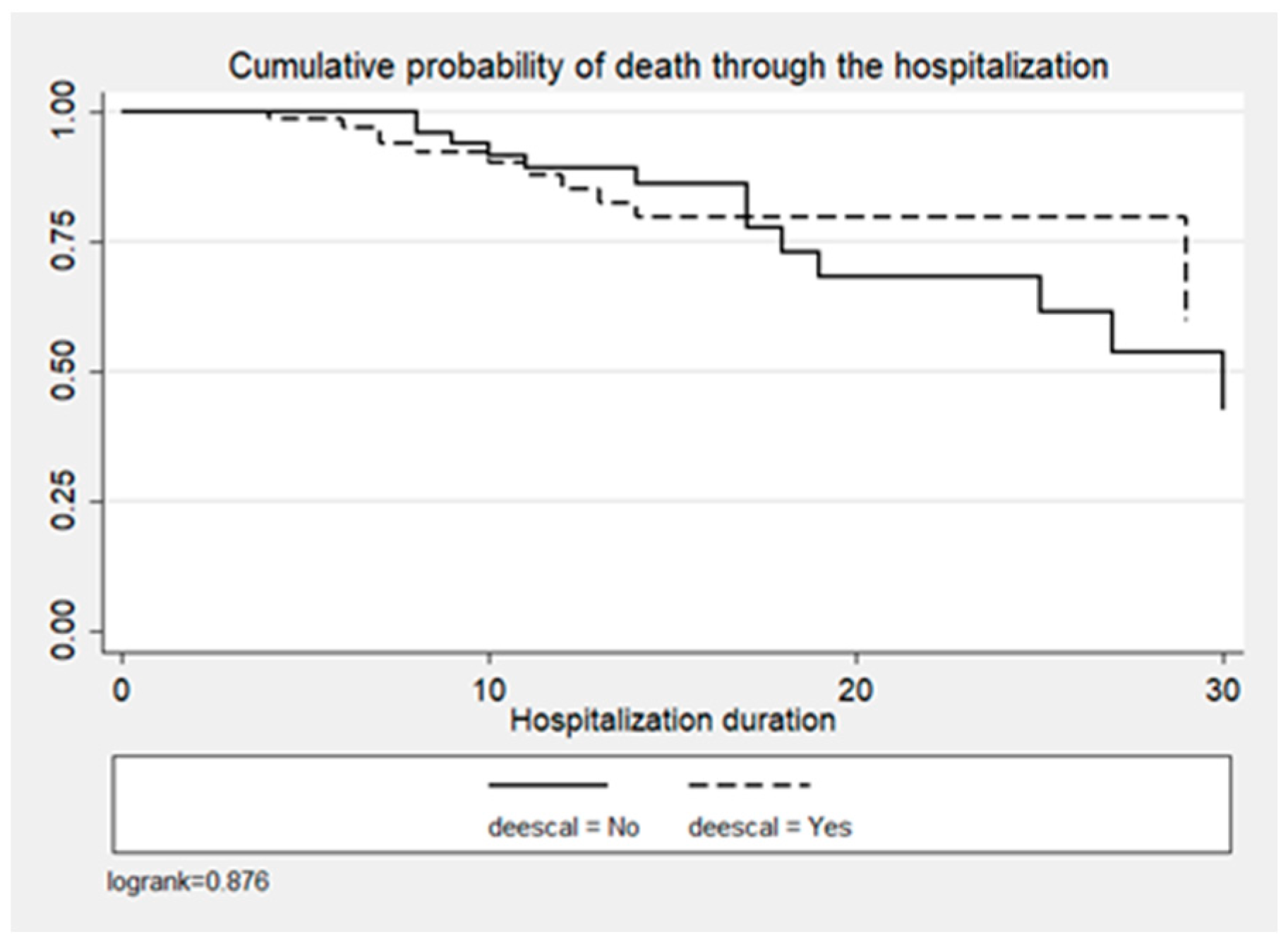
In the entire cohort, crude mortality was 23.9% (n = 34), 18.4% (n = 14) for the ADE group vs. 30.3% (n = 20) for the non-ADE group (p < 0.1) (Table 1). ADE was not significantly associated with the probability of death (p = 0.432), as shown in Figure 4 and Table 3.
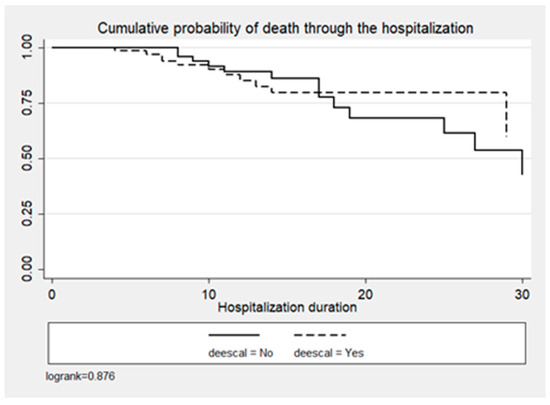
Figure 4.
Cumulative probability of death by time since admission and DE decision.

Table 3.
Multivariable Cox proportional hazards model for the probability of death.
Mean length of hospital stay (LOS) was 12.5 (IQR: 8, 16) days and was significantly lower in the ADE group (11, IQR: 7, 15 vs. 14 IQR: 8, 19; p = 0.047). Antimicrobial therapy lasted for a median of 12.5 days (IQR: 10, 15) [ADE group: 12 days (IQR: 10, 14) vs. non-ADE group: 13 days (IQR: 9, 15); p = 0.9]. At the 30-day follow up, patients were antibiotic-free for a median of 4 days (IQR: 0, 14), and no difference was reported between the ADE and non-ADE groups (Table 1). Stress doses of hydrocortisone were administered in ten patients (median duration: 3.5 days, IQR: 3, 3.5) (Table 1). Vasopressor therapy was started on the third day of hospitalization in five (3.52%), on the ADE day in four (2.82%), on the third day upon ADE initiation in two (1.41%), and on day 28 in three (2.11%) patients, and the median duration of the therapy was 3.5 days (IQR: 2, 4.5) (Table 1). The need for both hydrocortisone and vasopressors administration was shown to be independent of ADE. Superinfection was observed in nine patients, of whom two were diagnosed with CDI, and XDR bacteria colonization was reported in two others. Acute kidney injury (AKI) emerged in 6.3% (n = 9) and multi-organ failure in 4.2% (n = 6), regardless of ADE status.
3. Discussion
The present study evaluated the feasibility, safety, and efficacy of ADE in patients with microbiologically documented infection admitted to medical wards with recognized prevalence of MDR pathogens. ADE was applied in the majority of the study population at a median of 4 days, which corresponds to a time frame that allows for the reporting of most diagnostic results and the assessment of a patient’s clinical response to empiric therapy [11,23]. The diagnosis of UTI, SSTI, and BSI was found to be predictive of ADE, thus suggesting that an ADE approach is appropriate and can be generally performed, as shown in previously published research [24,25,26,27,28,29,30]. Within the factors strongly associated with the probability of ADE, clarithromycin administration in patients with bacteremia or pneumonia conferred the highest ADE rate. This finding should not be surprising, since the addition of clarithromycin to the treatment regimen is well acknowledged to lead to better survival rates in patients with severe community-acquired pneumonia (CAP), decrease sepsis recurrence, and shorten time to recovery [31,32,33,34]. Most of the participating hospitals are members of a large sepsis study group, with a series of publications verifying the beneficial-immunomodulatory role of clarithromycin as adjunctive treatment in patients with CAP and nosocomial pneumonia, as well as sepsis caused by Gram-negative pathogens [31,32,33,34,35,36,37]. Furthermore, mortality rate, as well as median LOS, was lower in the ADE group, and the probability of death was proved to be independent of ADE, demonstrating that safety was not compromised. Lastly, the profile of the patient most eligible for ADE was delineated, mainly referring to a patient with clinical stability, sepsis from a UTI source and mostly caused by E. coli, or sepsis with symptoms and signs excluding an unknown origin. Profiling the eligible patient for ADE is an important tool for the local AMS team.
Our findings are in-line with previously published data reporting the favorable effect of ADE on mortality. A total of two randomized control trials (RCT) and twelve cohort studies evaluating ADE in critical care settings were included in the meta-analysis performed by Tabah et al., and the pooled estimated mortality was shown to favor ADE (relative risk [RR] 0.68, 95% CI: 0.52–0.88) [8]. The ADE effect on 30-day all-cause mortality in patients with BSI or pneumonia was addressed by Paul et al., and 19 studies (RCT: n = 3; observational studies: n = 16) conducted in non-ICU and ICU settings were analyzed. In approximately 4000 patients, mortality did not differ between the ADE group and the group in which the empiric antimicrobial therapy was maintained (OR: 0.83, 95% CI: 0.59–1.16) [27]. Ohji et al. sought to evaluate ADE effectiveness and safety for various types of infection by measuring the all-cause mortality. In the subset of patients experiencing community- or ICU-acquired pneumonia, as in the previous study, the 30-day mortality rate was significantly lower in the ADE group compared to the control group (OR: 0.50, 95% CI: 0.29–0.87; p = 0.01 and OR: 0.34, 95% CI: 0.17–0.68; p = 0.002, respectively), whereas it did not differ among those diagnosed with BSI, UTI, and ventilator-associated pneumonia or among those presenting with septic shock. Of note, the authors commented on the absence of high-quality evidence [29]. A trend towards lower mortality rate (RR: 0.74, 95% CI: 0.54–1.03; p = 0.005) was reported in patients with severe sepsis or septic shock in whom ADE was applied in the meta-analysis designed by Guo et al. [38]. The DIANA study, a prospective cohort study conducted in 152 ICUs across 28 countries, attempted to assess the feasibility of ADE in 1495 critically ill patients, as well as the ADE impact on clinical cure at day 7 following the empirical therapy initiation. No difference in the 28-day mortality rate was found between the ADE and no-ADE group, but there was a 30% increased likelihood of clinical cure at day 7 in de-escalated patients (RR: 1.32, 95% CI: 1.14–1.64) [14]. A recent publication of our group in the ICU setting showed that albeit ADE was feasible in a small percentage of septic ICU patients due to the antibiogram of the infecting pathogen, when applied, it was associated with lower all-cause 28-day mortality (13.3% vs. 36.7%, OR 0.27, 95% CI 0.11–0.66, p = 0.006) with a subsequent collateral decrease in the SOFA score. ICU and hospital mortality were also lower. Furthermore, a Cox multivariable regression analysis revealed that ADE was a significant factor for 28-day survival (HR 0.31, 95% CI 0.14–0.70, p = 0.005) [15]. Lastly, in several other studies conducted in the emergency room department, ICU setting, or medical ward, ADE did not confer increased mortality rates [19,25,28,39,40,41].
Although ADE does not seem to have a measurable impact on LOS in the literature, in our study, it resulted in significantly shorter LOS. In the meta-analysis performed by Ambaras Khan et al. that involved more than 2000 patients diagnosed with pneumonia and admitted to an ICU, despite the low quality of evidence, a statistically significant LOS reduction was observed in the ADE group (mean reduction: 5.96 days; 95% CI −8.39, −3.52) [16]. In a retrospective analysis of 240 patients who received piperacillin/tazobactam and vancomycin with the most commonly documented indications being sepsis and pneumonia, median LOS was 4 days shorter in ADE patients (6 vs. 10 days, p = 0.0003) [28]. Similarly, in a prospective analysis of carbapenem prescriptions and ADE performance, ADE reduced the median hospital stay by five days (p = 0.030) [16]. On the contrary, in several other studies and meta-analyses, including a non-blinded RCT, no changes in LOS were observed based on ADE status [14,20,27,40].
As a cornerstone of AMS strategies, ADE appears to be a safe policy for the prudent use of antimicrobials in patients with microbiologically documented infection based on the aforementioned. Nevertheless, robust data are lacking regarding the safety of ADE in patients with suspected infection and negative cultures. In the study of Moehring, R. et al., an antibiotic opt-out protocol was implemented as a randomized controlled trial, to decrease unnecessary antimicrobial use in patients with suspected sepsis. The study was conducted in ten Unites States (US) hospitals from September 2018 through May 2020 on non-ICU patients on broad spectrum antibiotics with negative blood cultures. The authors used an exhaustive 23-item safety check-list to ensure the safety of patients when proceeding with the opt-out randomization. Despite that, only antibiotics were stopped in only 59 out of 383 patients in the intervention arm, whereas in 299 patients, the treating physicians responded with the opt-out strategy. The treating physician’s belief that stopping antibiotics was unsafe (31%) was the second most common reason after treatment of local infection (76%) for antibiotic continuation. The length of therapy was similar in both the intervention and the control group, albeit the exposure of patients in the rank-3 agent group (extended spectrum) was statistically lower in the intervention group [42]. These data show that even under optimal conditions, antibiotic de-escalation has to overcome hurdles based on the physicians’ behaviors and established beliefs. In our opinion, in real practice, creating a similar safety check-list based on clinical and laboratory parameters which are locally available is an important tool in the hands of the AMS team. De-escalating antibiotics in patients with negative blood cultures, particularly in settings where molecular diagnostics are already available, may be feasible and safe as long as the clinical parameters are stable or ameliorating and regularly used biomarkers are in the same direction. However, the building of trust between the members of the team and the treating physicians remains the milestone for the success of these programs.
Regarding the rest of the exploratory end-points, the median duration of antimicrobial therapy was almost the same between the two groups. In the literature, antibiotic treatment duration varied and was reportedly decreased [17,19], similar [18,20], or longer in patients undergoing ADE [14]. Antimicrobial therapy duration in cases of ADE implementation is of major importance and should be carefully assessed [43]. Longer courses of antimicrobial therapy may result in increased incidence of MDR pathogens and adverse events, such as superinfections and CDI. On the other hand, shorter antimicrobial courses can be safely utilized in hospitalized patients with common infections (e.g., UTIs, intra-abdominal, or respiratory tract infections) to achieve clinical and microbiological resolution without affecting mortality or recurrence rates [44].
The rate of superinfections remained low in our cohort (n = 9, 6.3%) and was slightly higher in patients undergoing ADE (n = 5 (6.6%) vs. n = 4 (6.1%); p = 0.3). An increased incidence of superinfection in the ADE group (n = 16 (27%) vs. n = 6 (11%); p = 0.003) was also recorded by Leone et al. [40], whereas no difference in superinfection rates between ADE and empirical treatment continuation groups was found in other observational studies [21,22]. The increased occurrence of superinfections, observed both in our study and the RCT conducted by Leone et al., should be interpreted with caution, and the small number of patients experiencing a new infection should be taken into account [40].
There is a paucity of data regarding the effect of ADE on rates of C. difficile infection (CDI). It is only evaluated in a retrospective cohort study of 808 patients diagnosed with Enterobacteriales BSI and managed with antipseudomonal β-lactam (APBL) therapy for more or less than 48 h. The empirical use of APBL therapy > 48 h was found to be an independent risk factor for CDI [HR: 3.56, 95% CI: 1.48–9.92; p = 0.004], thus suggesting that early de-escalation of APBL using clinical risk assessment tools or rapid diagnostic testing may reduce the incidence of CDI [45].
Lastly, XDR pathogen colonization was rare in both patient groups, although it should be noted that patients were followed until hospital discharge, a time frame that may not be sufficient to assess the emergence, as well as the outcome, of MDR/XDR pathogens. To date, there is a lack of clinical data regarding the impact of ADE and AMR emergence. The few studies exploring AMR as an outcome reported no or a very limited effect of ADE on the individual or local prevalence of MDR/XDR bacteria [46]. In the DIANA study, a trend towards reduced MDR acquisition in the ADE group was noted (7.5% vs. 11.9; p = 0.06) [14]. Similarly, the acquisition rate of extended spectrum beta-lactamase (ESBL)-producing Enterobacterales in 182 patients with ventilator-associated pneumonia was lower in the ADE-group (1.4% vs. 8.2%; p = 0.07) [20]. In a prospective study on the evaluation of ASP-guided carbapenem ADE, a significantly lower incidence of carbapenem-resistant A. baumannii was revealed in the ADE-group (2% vs. 7.3%; p = 0.042) [47]. De Bus et al. sought to evaluate determinants of ADE and assessed whether the latter is associated with AMR emergence; in the analysis of 478 anti-pseudomonal beta-lactam prescriptions in tertiary ICUs, the risk of resistance emergence at day 14 was higher in the ADE group (30.6% vs. 23.5%; p = 0.22), but this may be partially attributed to the longer duration of treatment, which probably acted as a confounding factor [19]. In the analysis of Gonzalez et al., an increase in MDR bacteria colonization was detected in the ADE group (15.3% vs. 10.7; p = 0.1) of ICU patients treated for sepsis [48].
The findings of our study should be interpreted in light of certain limitations, such as the small number of patients analyzed, its observational character that automatically induces inclusion bias due to lack of adjustment for clinical stability, and the absence of a matched-comparison group to optimize the conclusions drawn. However, the fact that our data are in line with the previous publication regarding our group in the ICU setting supports the safety of DE in settings with a high prevalence of multidrug resistance despite compromised feasibility owing to the possibly MDR-infecting pathogens [15]. The study was conducted before the launch of new antibiotics (beta-lactams with beta lactamase inhibitors) in Greece and before the COVID-19 pandemic. As resistance issues seem to be on the rise during the pandemic years, the conclusions of our study can be used as a guide to local AMS teams in order to rekindle the battle against MDR pathogens not only in ICUs, but also in general wards [49,50].
4. Materials and Methods
4.1. Study Setting
This was a prospective observational cohort study conducted in the medical wards of 6 tertiary-care hospitals in Greece over a 2-year period (January 2017–December 2018). The participating centers [Attikon University General Hospital (Athens), General Hospital of Athens “G. Gennimatas” (Athens), Konstantopouleio General Hospital (Athens), Thriasio General Hospital of Elefsina (Attica), Laikon General Hospital (Athens), and “Papageorgiou” General Hospital of Thessaloniki (Thessaloniki)] are listed among the largest hospitals in Athens (Capital) and Thessaloniki (second most populated) metropolitan areas. The decision of de-escalation was at the discretion of the treating physicians. Local Antimicrobial Stewardship teams overlooked the antibiotic decisions, as per standard procedures.
4.2. Study Population and Data Collection
Adult patients aged ≥ 18 years with microbiologically documented infection, either at the time of hospital admission or acquired during their hospital stay, who were administered empirical antimicrobial therapy were analyzed. Patients in whom infection was suspected, but was not microbiologically confirmed, or in whom there was a lack of data regarding variables of interest, were excluded.
Data were prospectively extracted from routine care patient charts, including the following: demographic characteristics, CCI, residence type, receipt of antimicrobials in the past trimester, admitting diagnosis, illness severity assessed by SOFA and qSOFA scores, presence of sepsis or septic shock on admission and at preselected time points during hospitalization, vasopressors and/or hydrocortisone administration on admission and during hospitalization, development of AKI during or after the septic episode, and LOS. Other variables recorded were as follows: site of the microbiologically confirmed infection, identified pathogen, specimens of pathogen isolation, antimicrobial susceptibility testing report, empirical antimicrobial therapy administered, time interval from empirical antibiotic therapy initiation to applied ADE, duration of antimicrobial therapy, new episodes of infection (including CDI), and colonization with XDR pathogens.
Each participating institute was responsible for the data entry of its patients. Three of the authors (V.R., G.P., and H.G.) were responsible for the evaluation of the quality of entered data, data interpretation in cases of uncertainty, and communication with physicians of participating centers for additional information.
4.3. Definitions
Sepsis was defined as a life-threatening organ dysfunction caused by a dysregulated host response to an infection. It was presumed in patients with suspected or confirmed infection and SOFA score ≥ 2 or a SOFA score change ≥ 2 [51]. Septic shock was defined as a subset of sepsis in which profound circulatory, cellular, and metabolic abnormalities are associated with a greater risk of mortality than with sepsis alone. It was presumed in patients presenting with (i) persisting hypotension and requiring vasopressors to maintain mean arterial pressure (MAP) > 65 mmHg and/or (ii) blood lactate > 2 mmol/L despite adequate fluid resuscitation [51].
The site of infection was assessed according to criteria proposed by the US Center for Disease Control and Prevention [52].
Antibiotic-resistant pathogens were classified as MDR if they were non-susceptible to at least one agent in three or more antimicrobial categories, as extensively drug-resistant (XDR) if they were non-susceptible to at least one agent in all but two or fewer antimicrobial categories, and as pandrug-resistant (PDR) if they were non-susceptible to all agents in the available antimicrobial categories [53].
ADE was defined as the discontinuation of one or more components of combination empirical therapy and/or the change from a broad-spectrum to a narrower spectrum antimicrobial [7,8]. “Antimicrobial escalation” was defined as the addition of or switch to an antibiotic with a broader spectrum of activity. “No change” in antibiotic regimens administered was defined as the maintenance of the initial empirical antimicrobial therapy. For the purposes of the study, the patients were divided into two groups: the ADE group, in which DE was applied, and the non-ADE group, in which either the empirical antimicrobial therapy was maintained or escalation was performed.
Primary bacteremia was defined as the bacteremia for which no source of infection was documented. Bacteremia was defined as secondary when laboratory examination revealed infection by the same microorganism at a distant site in the same time or up to three days earlier.
AKI was defined by increased serum creatinine levels and reduced urinary output lasting a maximum of 7 days [54].
Superinfection was defined as an infection following a previous one, especially when caused by microorganisms that were resistant or have become resistant to the antibiotics used against the first infection [55].
4.4. Outcomes
We sought to assess the safety and efficacy of ADE in medical wards with recognized prevalence of MDR pathogens. Therefore, this study’s primary exploratory endpoints were: (i) the rate and feasibility of ADE in patients with microbiologically documented infection, (ii) the factors associated with ADE decision, and (iii) mortality in ADE vs. non-ADE groups. Secondary endpoints included duration of antimicrobial therapy, antibiotic-free days, need for vasopressors or/and hydrocortisone administration, incidence of new infections, including C. difficile and fungal infections, XDR pathogen colonization, development of AKI after the septic episode, and LOS.
4.5. Statistical Analysis
Baseline characteristics were summarized using descriptive statistics, including mean and standard deviation (SD) for normally distributed variables, medians and interquartile ranges (IQRs) for non-normally distributed variables, and absolute (N) and relative (%) frequencies for categorical ones. T-test and Mann–Whitney tests were used to compare continuous variables, while chi-square and Fisher’s exact tests were used for the categorical ones.
Survival analysis techniques, including multivariable Cox proportional hazards models, log-rank tests, and Kaplan–Meier curves, were conducted to evaluate the association of de-escalation to survival within 30 days upon hospital admission. Cumulative probability of recovery is presented as median (95% CI) time.
Factors possibly associated with DE were evaluated via multiple logistic regression models. Moreover, DE as decision was also evaluated as an outcome using the Kaplan–Meier estimator, and possible associations of other factors with DE decision were analyzed through Cox proportional hazard models.
STATA/MP13 (Stata Corp., College Station, TX, USA) was used for the analyses performed, and statistical significance was set at the 0.05 level.
4.6. Ethical Issues
This study was supported by the Hellenic Society of Chemotherapy. The protocol was approved by the institutional review boards of the participating hospitals and was conducted in accordance with the Helsinki Declaration of Human Rights. In compliance with the local regulations, the use of an informed consent form was waived because of the non-interventional character of the study and the use of anonymous clinical data in the analysis.
5. Conclusions
In the present study, patients with microbiologically documented infection admitted to Greek medical wards with recognized prevalence of MDR bacteria were prone to ADE, and when ADE was eventually applied, it was associated with favorable outcomes. Although no causality can be demonstrated, careful clinical selection of patients can result in a reduction in unnecessary antibiotic consumption without compromising safety. Our observations strengthen the importance of striving the process of de-escalation as part of ASP, even in areas with prevalent MDR. Exploitation of locally produced data can lead to the recognition of profiles of eligible patients and safely guide the efforts of the relevant antimicrobial stewardship teams.
Antibiotic overuse, irrespective of antibiotic class, is integrally linked to AMR, and the implementation of ASP and ADE is crucial. Well-designed targeted studies are needed to understand the benefits and risks of ADE in patients admitted to medical settings. An efficient ASP should incorporate a wide range of measures, including (i) optimization of empiric therapy with infection-site-specific guidelines, (ii) up-to-date local resistance data and assessments of the individual risks of resistant pathogens, (iii) review at the second or third empirical antimicrobial therapy with relevant microbiology results and clinical progress, and (iv) early cessation of antibiotics in unproven infection. Involvement of an infection specialist or pharmacist is beneficial, and rapid diagnostics may play a pivotal role [45].
Author Contributions
Conceptualization, G.P. and H.G.; methodology, G.P. and H.G.; software, V.R.; validation, V.R., G.P. and H.G.; formal analysis, E.P.; investigation, V.R., G.P., A.M. (Anastasia Mousouli), A.K., S.P., A.M. (Aikaterini Masgala), S.S., V.A., C.G., N.A., K.A., C.T., A.P., M.S., G.L.D. and H.G.; data curation, V.R., G.P. and H.G.; writing—original draft preparation, V.R.; writing—review and editing, G.P., A.M. (Anastasia Mousouli), A.K., S.P., E.P., A.M. (Aikaterini Masgala), S.S., V.A., C.G., N.A., K.A., C.T., A.P., M.S., G.L.D. and H.G.; visualization, V.R.; supervision, G.P. and H.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
We state that the present study, a prospective, non-interventional, multicenter, observational study, was conducted after IRB approval. Ethic Committee Name for centers located in Attica Region: Institutional Review Board, Attikon University Hospital, Athens, Greece. Approval Code: 1821A/22.09.2016. Approval Date: 25 October 2016. Ethic Committee Name for centers located in Thessaloniki Region: Institutional Review Board, Papageorgiou General Hospital, Aristotle University of Thessaloniki. Approval Code:35/18.01.2017. Approval Date: 13 February 2017.
Informed Consent Statement
Not applicable.
Data Availability Statement
Raw data is not available due to patient confidentiality, but can be provided upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organization. Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 6 June 2024).
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Action Plan on Antimicrobial Resistance. Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 6 June 2024).
- Barlam, T.F.; Cosgrove, S.E.; Abbo, L.M.; MacDougall, C.; Schuetz, A.N.; Septimus, E.J.; Srinivasan, A.; Dellit, T.H.; Falck-Ytter, Y.T.; Fishman, N.O.; et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin. Infect. Dis. 2016, 62, e51–e77. [Google Scholar] [CrossRef]
- Society for Healthcare Epidemiology of America; Infectious Diseases Society of America; Pediatric Infectious Diseases Society. Policy statement on amtimicrobial stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Diseases Society (PIDS). Infect. Control Hosp. Epidemiol. 2012, 33, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Nathwani, D.; Varghese, D.; Stephens, J.; Ansari, W.; Martin, S.; Charbonneau, C. Value of hospital antimicrobial stewardship programs [ASPs]: A systematic review. Antimicrob. Resist. Infect. Control 2019, 8, 35. [Google Scholar] [CrossRef]
- De Waele, J.J.; Schouten, J.; Beovic, B.; Tabah, A.; Leone, M. Antimicrobial de-escalation as part of antimicrobial stewardship in intensive care: No simple answers to simple questions-a viewpoint of experts. Intensive Care Med. 2020, 46, 236–244. [Google Scholar] [CrossRef]
- Tabah, A.; Cotta, M.O.; Garnacho-Montero, J.; Schouten, J.; Roberts, J.A.; Lipman, J.; Tacey, M.; Timsit, J.F.; Leone, M.; Zahar, J.R.; et al. A Systematic Review of the Definitions, Determinants, and Clinical Outcomes of Antimicrobial De-escalation in the Intensive Care Unit. Clin. Infect. Dis. 2016, 62, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, C.; Pastene, B.; Cassir, N.; Martin-Loeches, I.; Leone, M. Efficacy and safety of antimicrobial de-escalation as a clinical strategy. Expert Rev. Anti. Infect. Ther. 2019, 17, 79–88. [Google Scholar] [CrossRef]
- Ferrer, R.; Martin-Loeches, I.; Phillips, G.; Osborn, T.M.; Townsend, S.; Dellinger, R.P.; Artigas, A.; Schorr, C.; Levy, M.M. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: Results from a guideline-based performance improvement program. Crit. Care Med. 2014, 42, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, e1063–e1143. [Google Scholar] [CrossRef]
- Kim, R.Y.; Ng, A.M.; Persaud, A.K.; Furmanek, S.P.; Kothari, Y.N.; Price, J.D.; Wiemken, T.L.; Saad, M.A.; Guardiola, J.J.; Cavallazzi, R.S. Antibiotic Timing and Outcomes in Sepsis. Am. J. Med. Sci. 2018, 355, 524–529. [Google Scholar] [CrossRef]
- Liu, V.X.; Fielding-Singh, V.; Greene, J.D.; Baker, J.M.; Iwashyna, T.J.; Bhattacharya, J.; Escobar, G.J. The Timing of Early Antibiotics and Hospital Mortality in Sepsis. Am. J. Respir. Crit. Care Med. 2017, 196, 856–863. [Google Scholar] [CrossRef] [PubMed]
- De Bus, L.; Depuydt, P.; Steen, J.; Dhaese, S.; De Smet, K.; Tabah, A.; Akova, M.; Cotta, M.O.; De Pascale, G.; Dimopoulos, G.; et al. Antimicrobial de-escalation in the critically ill patient and assessment of clinical cure: The DIANA study. Intensive Care Med. 2020, 46, 1404–1417. [Google Scholar] [CrossRef] [PubMed]
- Routsi, C.; Gkoufa, A.; Arvaniti, K.; Kokkoris, S.; Tourtoglou, A.; Theodorou, V.; Vemvetsou, A.; Kassianidis, G.; Amerikanou, A.; Paramythiotou, E.; et al. De-escalation of antimicrobial therapy in ICU settings with high prevalence of multidrug-resistant bacteria: A multicentre prospective observational cohort study in patients with sepsis or septic shock. J. Antimicrob. Chemother. 2020, 75, 3665–3674. [Google Scholar] [CrossRef] [PubMed]
- Ambaras Khan, R.; Aziz, Z. Antibiotic de-escalation in patients with pneumonia in the intensive care unit: A systematic review and meta-analysis. Int. J. Clin. Pract. 2018, 72, e13245. [Google Scholar] [CrossRef]
- Li, H.; Yang, C.H.; Huang, L.O.; Cui, Y.H.; Xu, D.; Wu, C.R.; Tang, J.G. Antibiotics De-Escalation in the Treatment of Ventilator-Associated Pneumonia in Trauma Patients: A Retrospective Study on Propensity Score Matching Method. Chin. Med. J. 2018, 131, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Trupka, T.; Fisher, K.; Micek, S.T.; Juang, P.; Kollef, M.H. Enhanced antimicrobial de-escalation for pneumonia in mechanically ventilated patients: A cross-over study. Crit. Care 2017, 21, 180. [Google Scholar] [CrossRef]
- De Bus, L.; Denys, W.; Catteeuw, J.; Gadeyne, B.; Vermeulen, K.; Boelens, J.; Claeys, G.; De Waele, J.J.; Decruyenaere, J.; Depuydt, P.O. Impact of de-escalation of beta-lactam antibiotics on the emergence of antibiotic resistance in ICU patients: A retrospective observational study. Intensive Care Med. 2016, 42, 1029–1039. [Google Scholar] [CrossRef]
- Weiss, E.; Zahar, J.R.; Garrouste-Orgeas, M.; Ruckly, S.; Essaied, W.; Schwebel, C.; Timsit, J.F.; OUTCOMEREA Study Group. De-escalation of pivotal beta-lactam in ventilator-associated pneumonia does not impact outcome and marginally affects MDR acquisition. Intensive Care Med. 2016, 42, 2098–2100. [Google Scholar] [CrossRef] [PubMed]
- Joffe, A.R.; Muscedere, J.; Marshall, J.C.; Su, Y.; Heyland, D.K.; Canadian Critical Care Trials Group. The safety of targeted antibiotic therapy for ventilator-associated pneumonia: A multicenter observational study. J. Crit. Care 2008, 23, 82–90. [Google Scholar] [CrossRef]
- Alvarez-Lerma, F.; Alvarez, B.; Luque, P.; Ruiz, F.; Dominguez-Roldan, J.M.; Quintana, E.; Sanz-Rodriguez, C.; ADANN Study Group. Empiric broad-spectrum antibiotic therapy of nosocomial pneumonia in the intensive care unit: A prospective observational study. Crit. Care 2006, 10, R78. [Google Scholar] [CrossRef]
- Infectious Diseases Society of America (IDSA). Combating antimicrobial resistance: Policy recommendations to save lives. Clin. Infect. Dis. 2011, 52, S397–S428. [Google Scholar] [CrossRef]
- Alshareef, H.; Alfahad, W.; Albaadani, A.; Alyazid, H.; Talib, R.B. Impact of antibiotic de-escalation on hospitalized patients with urinary tract infections: A retrospective cohort single center study. J. Infect. Public Health 2020, 13, 985–990. [Google Scholar] [CrossRef]
- Sadyrbaeva-Dolgova, S.; Aznarte-Padial, P.; Jimenez-Morales, A.; Expósito-Ruiz, M.; Calleja-Hernández, M.Á.; Hidalgo-Tenorio, C. Pharmacist recommendations for carbapenem de-escalation in urinary tract infection within an antimicrobial stewardship program. J. Infect. Public Health 2020, 13, 558–563. [Google Scholar] [CrossRef]
- Lee, C.C.; Wang, J.L.; Lee, C.H.; Hung, Y.P.; Hong, M.Y.; Tang, H.J.; Ko, W.C. Clinical benefits of antimicrobial de-escalation in adults with community-onset monomicrobial Escherichia coli, Klebsiella species and Proteus mirabilis bacteremia. Int. J. Antimicrob. Agents 2017, 50, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Dickstein, Y.; Raz-Pasteur, A. Antibiotic de-escalation for bloodstream infections and pneumonia: Systematic review and meta-analysis. Clin. Microbiol. Infect. 2016, 22, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Ohl, C.; Johnson, J.; Williamson, J.; Beardsley, J.; Luther, V. Frequency of empiric antibiotic de-escalation in an acute care hospital with an established Antimicrobial Stewardship Program. BMC Infect. Dis. 2016, 16, 751. [Google Scholar] [CrossRef]
- Ohji, G.; Doi, A.; Yamamoto, S.; Iwata, K. Is de-escalation of antimicrobials effective? A systematic review and meta-analysis. Int. J. Infect. Dis. 2016, 49, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Khasawneh, F.A.; Karim, A.; Mahmood, T.; Ahmed, S.; Jaffri, S.F.; Tate, M.E.; Mehmood, M. Antibiotic de-escalation in bacteremic urinary tract infections: Potential opportunities and effect on outcome. Infection 2014, 42, 829–834. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Siampanos, A.; Bolanou, A.; Doulou, S.; Kakavoulis, N.; Tsiakos, K.; Katopodis, S.; Schinas, G.; Skorda, L.; Alexiou, Z.; et al. Clarithromycin for early anti-inflammatory responses in community-acquired pneumonia in Greece (ACCESS): A randomised, double-blind, placebo-controlled trial. Lancet Respir. Med. 2024, 12, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Kyriazopoulou, E.; Sinapidis, D.; Halvatzis, S.; Velissaris, D.; Alexiou, N.; Kosmas, V.; Adami, M.E.; Kyprianou, M.; Kyprianou, A.; Stefos, A.; et al. Survival benefit associated with clarithromycin in severe community-acquired pneumonia: A matched comparator study. Int. J. Antimicrob. Agents 2020, 55, 105836. [Google Scholar] [CrossRef]
- Karakike, E.; Scicluna, B.P.; Roumpoutsou, M.; Mitrou, I.; Karampela, N.; Karageorgos, A.; Psaroulis, K.; Massa, E.; Pitsoulis, A.; Chaloulis, P.; et al. Effect of intravenous clarithromycin in patients with sepsis, respiratory and multiple organ dysfunction syndrome: A randomized clinical trial. Crit. Care 2022, 26, 183. [Google Scholar] [CrossRef] [PubMed]
- Giamarellos-Bourboulis, E.J.; Mylona, V.; Antonopoulou, A.; Tsangaris, I.; Koutelidakis, I.; Marioli, A.; Raftogiannis, M.; Kopterides, P.; Lymberopoulou, K.; Mouktaroudi, M.; et al. Effect of clarithromycin in patients with suspected Gram-negative sepsis: Results of a randomized controlled trial. J. Antimicrob. Chemother. 2014, 69, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Tsaganos, T.; Raftogiannis, M.; Pratikaki, M.; Christodoulou, S.; Kotanidou, A.; Papadomichelakis, E.; Armaganidis, A.; Routsi, C.; Giamarellos-Bourboulis, E.J. Clarithromycin Leads to Long-Term Survival and Cost Benefit in Ventilator-Associated Pneumonia and Sepsis. Antimicrob. Agents Chemother. 2016, 60, 3640–3646. [Google Scholar] [CrossRef] [PubMed]
- Spyridaki, A.; Raftogiannis, M.; Antonopoulou, A.; Tsaganos, T.; Routsi, C.; Baziaka, F.; Karagianni, V.; Mouktaroudi, M.; Koutoukas, P.; Pelekanou, A.; et al. Effect of clarithromycin in inflammatory markers of patients with ventilator-associated pneumonia and sepsis caused by Gram-negative bacteria: Results from a randomized clinical study. Antimicrob. Agents Chemother. 2012, 56, 3819–3825. [Google Scholar] [CrossRef] [PubMed]
- Giamarellos-Bourboulis, E.J.; Pechère, J.C.; Routsi, C.; Plachouras, D.; Kollias, S.; Raftogiannis, M.; Zervakis, D.; Baziaka, F.; Koronaios, A.; Antonopoulou, A.; et al. Effect of clarithromycin in patients with sepsis and ventilator-associated pneumonia. Clin. Infect. Dis. 2008, 46, 1157–1164. [Google Scholar] [CrossRef]
- Guo, Y.; Gao, W.; Yang, H.; Ma, C.; Sui, S. De-escalation of empiric antibiotics in patients with severe sepsis or septic shock: A meta-analysis. Heart Lung 2016, 45, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Corcione, S.; Mornese Pinna, S.; Lupia, T.; Trentalange, A.; Germanò, E.; Cavallo, R.; Lupia, E.; De Rosa, F.G. Antibiotic De-escalation Experience in the Setting of Emergency Department: A Retrospective, Observational Study. J. Clin. Med. 2021, 10, 3285. [Google Scholar] [CrossRef]
- Leone, M.; Bechis, C.; Baumstarck, K.; Lefrant, J.Y.; Albanèse, J.; Jaber, S.; Lepape, A.; Constantin, J.M.; Papazian, L.; Bruder, N.; et al. De-escalation versus continuation of empirical antimicrobial treatment in severe sepsis: A multicenter non-blinded randomized noninferiority trial. Intensive Care Med. 2014, 40, 1399–1408. [Google Scholar] [CrossRef]
- Yamana, H.; Matsui, H.; Tagami, T.; Hirashima, J.; Fushimi, K.; Yasunaga, H. De-escalation versus continuation of empirical antimicrobial therapy in community-acquired pneumonia. J. Infect. 2016, 73, 314–325. [Google Scholar] [CrossRef]
- Moehring, R.W.; Yarrington, M.E.; Warren, B.G.; Lokhnygina, Y.; Atkinson, E.; Bankston, A.; Collucio, J.; David, M.Z.; Davis, A.E.; Davis, J.; et al. Evaluation of an Opt-Out Protocol for Antibiotic De-Escalation in Patients with Suspected Sepsis: A Multicenter, Randomized, Controlled Trial. Clin. Infect Dis. 2023, 76, 433–442. [Google Scholar] [CrossRef]
- Tabah, A.; Bassetti, M.; Kollef, M.H.; Zahar, J.R.; Paiva, J.A.; Timsit, J.F.; Roberts, J.A.; Schouten, J.; Giamarellou, H.; Rello, J.; et al. Antimicrobial de-escalation in critically ill patients: A position statement from a task force of the European Society of Intensive Care Medicine (ESICM) and European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Critically Ill Patients Study Group (ESGCIP). Intensive Care Med. 2020, 46, 245–265. [Google Scholar] [PubMed]
- Royer, S.; DeMerle, K.M.; Dickson, R.P.; Prescott, H.C. Shorter Versus Longer Courses of Antibiotics for Infection in Hospitalized Patients: A Systematic Review and Meta-Analysis. J. Hosp. Med. 2018, 13, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Seddon, M.M.; Bookstaver, P.B.; Justo, J.A.; Kohn, J.; Rac, H.; Haggard, E.; Mediwala, K.N.; Dash, S.; Al-Hasan, M.N. Role of Early De-escalation of Antimicrobial Therapy on Risk of Clostridioides difficile Infection Following Enterobacteriaceae Bloodstream Infections. Clin. Infect. Dis. 2019, 69, 414–420. [Google Scholar] [CrossRef]
- Umpleby, H.; Dushianthan, A.; Catton, T.; Saeed, K. Antimicrobial stewardship programmes focused on de-escalation: A narrative review of efficacy and risks. J. Emerg. Crit. Care Med. 2022, 6, 23. [Google Scholar] [CrossRef]
- Lew, K.Y.; Ng, T.M.; Tan, M.; Tan, S.H.; Lew, E.L.; Ling, L.M.; Ang, B.; Lye, D.; Teng, C.B. Safety and clinical outcomes of carbapenem de-escalation as part of an antimicrobial stewardship programme in an ESBL-endemic setting. J. Antimicrob. Chemother. 2015, 70, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.; Cravoisy, A.; Barraud, D.; Conrad, M.; Nace, L.; Lemarié, J.; Bollaert, P.E.; Gibot, S. Factors influencing the implementation of antibiotic de-escalation and impact of this strategy in critically ill patients. Crit. Care 2013, 17, R140. [Google Scholar] [CrossRef] [PubMed]
- Polemis, M.; Mandilara, G.; Pappa, O.; Argyropoulou, A.; Perivolioti, E.; Koudoumnakis, N.; Pournaras, S.; Vasilakopoulou, A.; Vourli, S.; Katsifa, H.; et al. COVID-19 and Antimicrobial Resistance: Data from the Greek Electronic System for the Surveillance of Antimicrobial Resistance-WHONET-Greece (January 2018–March 2021). Life 2021, 11, 996. [Google Scholar] [CrossRef] [PubMed]
- Palaiopanos, K.; Krystallaki, D.; Mellou, K.; Kotoulas, P.; Kavakioti, C.A.; Vorre, S.; Vertsioti, G.; Gkova, M.; Maragkos, A.; Tryfinopoulou, K.; et al. Healthcare-associated infections and antimicrobial use in acute care hospitals in Greece, 2022; results of the third point prevalence survey. Antimicrob. Resist. Infect. Control 2024, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control 2008, 36, 309–332. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Romagnani, P.; Ashuntantang, G.; Ronco, C.; Zarbock, A.; Anders, H.J. Acute kidney injury. Nat. Rev. Dis. Primers 2021, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services. Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States. 2013. Available online: https://www.cdc.gov/antimicrobial-resistance/media/pdfs/ar-threats-2013-508.pdf?CDC_AAref_Val=https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf (accessed on 6 May 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).