Genomic Analysis of Enterobacter Species Isolated from Patients in United States Hospitals
Abstract
1. Introduction
2. Materials and Methods
3. Results
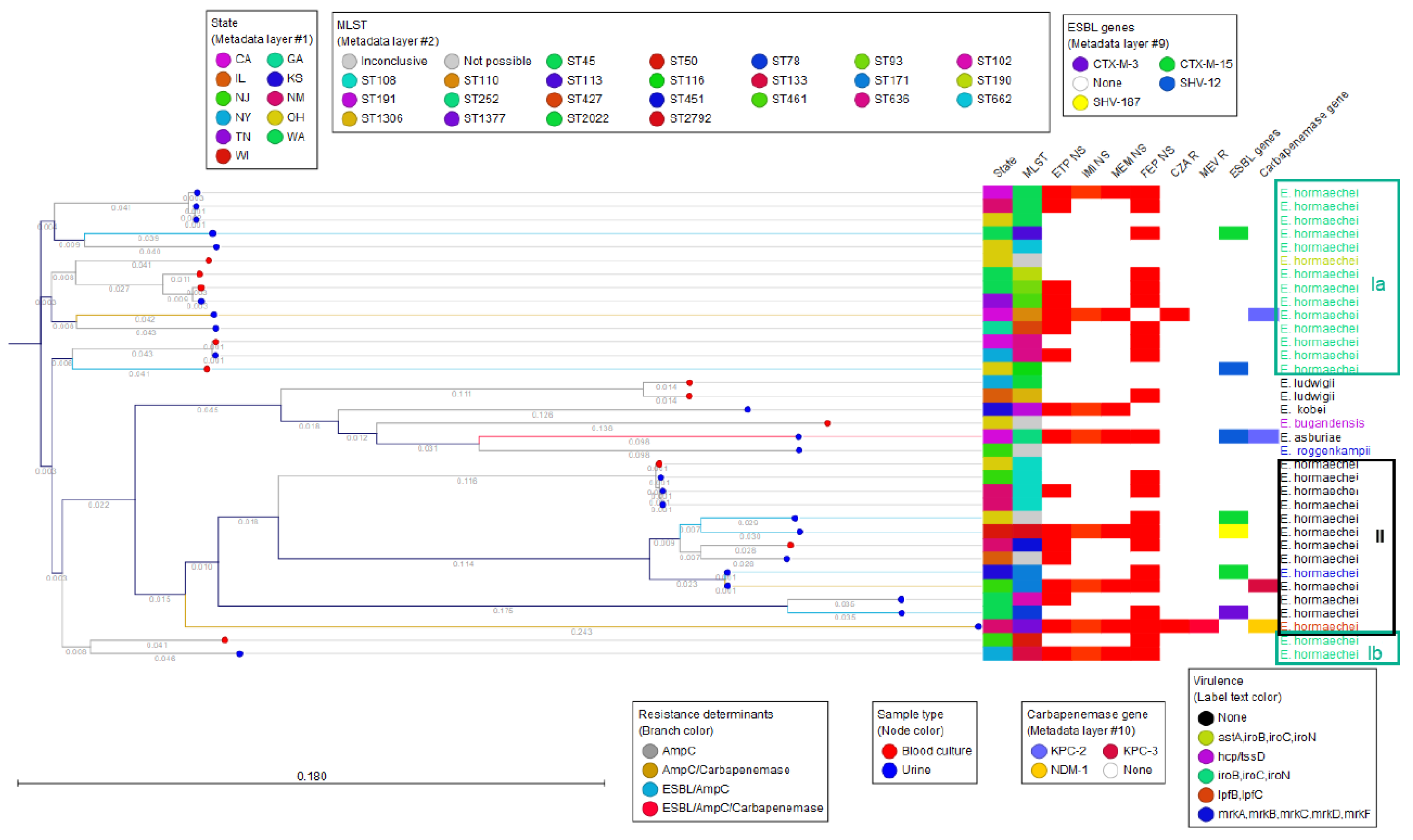
3.1. Enterobacter Species Identification and MLST Typing
3.2. Phenotypic Antimicrobial Susceptibility Testing Results for Beta-Lactams
3.3. Phenotypic and Genotypic Results for Aminoglycosides and Fluoroquinolones
3.4. AmpC Diversity
3.5. ESBLs and Other Beta Lactamases
3.6. Carbapenem Resistance Phenotypes and Genotypes
3.7. Virulence Genes
3.8. Pathogen Detection of E. hormaechei
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davin-Regli, A.; Lavigne, J.-P.; Pagès, J.-M. Enterobacter spp.: Update on Taxonomy, Clinical Aspects, and Emerging Antimicrobial Resistance. Clin. Microbiol. Rev. 2019, 32, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Jadimurthy, R.; Mayegowda, S.B.; Nayak, S.; Mohan, C.D.; Rangappa, K.S. Escaping mechanisms of ESKAPE pathogens from antibiotics and their targeting by natural compounds. Biotechnol. Rep. 2022, 34, e00728. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Fang, R.; Zhang, Y.; Chen, L.; Huang, N.; Yu, K.; Zhou, C.; Cao, J.; Zhou, T. Characterization of resistance mechanisms of Enterobacter cloacae Complex co-resistant to carbapenem and colistin. BMC Microbiol. 2021, 21, 208. [Google Scholar] [CrossRef] [PubMed]
- Sutton, G.G.; Brinkac, L.M.; Clarke, T.H.; Fouts, D.E. Enterobacter hormaechei subsp. hoffmannii subsp. nov., Enterobacter hormaechei subsp. xiangfangensis comb. nov., Enterobacter roggenkampii sp. nov., and Enterobacter muelleri is a later heterotypic synonym of Enterobacter asburiae based on computational analysis of sequenced Enterobacter genomes. F1000Research 2018, 7, 521. [Google Scholar] [CrossRef]
- Iversen, C.; Lehner, A.; Mullane, N.; Bidlas, E.; Cleenwerck, I.; Marugg, J.; Fanning, S.; Stephan, R.; Joosten, H. The taxonomy of Enterobacter sakazakii: Proposal of a new genus Cronobacter gen. nov. and descriptions of Cronobacter sakazakii comb. nov. Cronobacter sakazakii subsp. sakazakii, comb. nov., Cronobacter sakazakii subsp. malonaticus subsp. nov., Cronobacter turicensis sp. nov., Cronobacter muytjensii sp. nov., Cronobacter dublinensis sp. nov. and Cronobacter genomospecies 1. BMC Evol. Biol. 2007, 7, 64. [Google Scholar] [CrossRef]
- Horinouchi, N.; Shiota, S.; Takakura, T.; Yoshida, A.; Kikuchi, K.; Nishizono, A.; Miyazaki, E. Bacteremia caused by Enterobacter asburiae misidentified biochemically as Cronobacter sakazakii and accurately identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry: A case report. J. Med. Case Rep. 2022, 16, 19. [Google Scholar] [CrossRef]
- Yigit, H.; Anderson, G.J.; Biddle, J.W.; Steward, C.D.; Rasheed, J.K.; Valera, L.L.; McGowan, J.E.; Tenover, F.C. Carbapenem resistance in a clinical isolate of Enterobacter aerogenes is associated with decreased expression of OmpF and OmpC porin analogs. Antimicrob. Agents Chemother. 2002, 46, 3817–3822. [Google Scholar] [CrossRef]
- Annavajhala, M.K.; Gomez-Simmonds, A.; Uhlemann, A.-C. Multidrug-Resistant Enterobacter cloacae Complex Emerging as a Global, Diversifying Threat. Front. Microbiol. 2019, 10, 44. [Google Scholar] [CrossRef]
- Pasteran, F.; Mendez, T.; Rapoport, M.; Guerriero, L.; Corso, A. Controlling false-positive results obtained with the Hodge and Masuda assays for detection of class a carbapenemase in species of enterobacteriaceae by incorporating boronic Acid. J. Clin. Microbiol. 2010, 48, 1323–1332. [Google Scholar] [CrossRef]
- Intra, J.; Carcione, D.; Sala, R.M.; Siracusa, C.; Brambilla, P.; Leoni, V. Antimicrobial Resistance Patterns of Enterobacter cloacae and Klebsiella aerogenes Strains Isolated from Clinical Specimens: A Twenty-Year Surveillance Study. Antibiotics 2023, 12, 775. [Google Scholar] [CrossRef]
- Tickler, I.A.; Kawa, D.; Obradovich, A.E.; Fang, F.C.; Tenover, F.C. The Healthcare Associated Infections Consortium. Characterization of Carbapenemase- and ESBL-Producing Gram-Negative Bacilli Isolated from Patients with Urinary Tract and Bloodstream Infections. Antibiotics. 2023, 12, 1386. [Google Scholar] [CrossRef] [PubMed]
- Ciufo, S.; Kannan, S.; Sharma, S.; Badretdin, A.; Clark, K.; Turner, S.; Brover, S.; Schoch, C.L.; Kimchi, A.; DiCuccio, M. Using average nucleotide identity to improve taxonomic assignments in prokaryotic genomes at the NCBI. Int. J. Syst. Evol. Microbiol. 2018, 68, 2386–2392. [Google Scholar] [CrossRef] [PubMed]
- CLSI Standard M02; Performance Standards for Antimicrobial Disk Susceptibility Tests. 13th ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018.
- CLSI Supplement M100; Performance Standards for Antimicrobial Susceptibility Testing. 34th ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2024.
- The European Committee on Antimicrobial Susceptibility Testing. EUCAST: Clinical Breakpoints and Dosing of Antibiotics 2024. Available online: https://www.eucast.org/clinical_breakpoints/ (accessed on 8 April 2024).
- Kaas, R.S.; Leekitcharoenphon, P.; Aarestrup, F.M.; Lund, O. Solving the problem of comparing whole bacterial genomes across different sequencing platforms. PLoS ONE 2014, 9, e104984. [Google Scholar] [CrossRef] [PubMed]
- Minarini, L.A.R.; Darini, A.L.C. Mutations in the quinolone resistance-determining regions of gyrA and parC in Enterobacteriaceae isolates from Brazil. Braz. J. Microbiol. 2012, 43, 1309–1314. [Google Scholar] [CrossRef]
- Sarangi, J.; Matsuo, N.; Nonogaki, R.; Hayashi, M.; Kawamura, K.; Suzuki, M.; Jin, W.; Tamai, K.; Ogawa, M.; Wachino, J.-I.; et al. Molecular Epidemiology of Enterobacter cloacae Complex Isolates with Reduced Carbapenem Susceptibility Recovered by Blood Culture. Jpn. J. Infect. Dis. 2022, 75, 41–48. [Google Scholar] [CrossRef]
- Flury, B.B.; Ellington, M.J.; Hopkins, K.L.; Turton, J.F.; Doumith, M.; Loy, R.; Staves, P.; Hinic, V.; Frei, R.; Woodford, N. Association of Novel Nonsynonymous Single Nucleotide Polymorphisms in AMPD with Cephalosporin Resistance and Phylogenetic Variations in ampC, ampR, ompF, and ompC in Enterobacter cloacae Isolates That Are Highly Resistant to Carbapenems. Antimicrob. Agents Chemother. 2016, 60, 2383–2390. [Google Scholar] [CrossRef]
- Anderson, A.J.G.; Morrell, B.; Campos, G.L.; Valvano, M.A. Distribution and diversity of type VI secretion system clusters in Enterobacter bugandensis and Enterobacter cloacae. Microb. Genom. 2023, 9, 001148. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yang, X.; Shen, X. Confirmed and potential roles of bacterial T6SSs in the intestinal ecosystem. Front. Microbiol. 2019, 10, 1484. [Google Scholar] [CrossRef]
- Khonsari, M.S.; Behzadi, P.; Foroohi, F. The prevalence of type 3 fimbriae in Uropathogenic Escherichia coli isolated from clinical urine samples. Meta Gene 2021, 28, 100881. [Google Scholar] [CrossRef]
- Morhart, P.; Gerlach, R.G.; Kunz, C.; Held, J.; Valenza, G.; Wölfle, J.; Reutter, H.; Hanslik, G.J.; Fahlbusch, F.B. Application of Next-Generation Sequencing to Enterobacter hormaechei Subspecies Analysis during a Neonatal Intensive Care Unit Outbreak. Children 2023, 10, 1696. [Google Scholar] [CrossRef]
- Candela, A.; Guerrero-López, A.; Mateos, M.; Gómez-Asenjo, A.; Arroyo, M.J.; Hernandez-García, M.; del Campo, R.; Cercenado, E.; Cuénod, A.; Méndez, G.; et al. Automatic Discrimination of Species within the Enterobacter cloacae Complex Using Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry and Supervised Algorithms. J. Clin. Microbiol. 2023, 61, e0104922. [Google Scholar] [CrossRef] [PubMed]
- Chavda, K.D.; Chen, L.; Fouts, D.E.; Sutton, G.; Brinkac, L.; Jenkins, S.G.; Bonomo, R.A.; Adams, M.D.; Kreiswirth, B.N. Comprehensive Genome Analysis of Carbapenemase-Producing Enterobacter spp.: New Insights into Phylogeny, Population Structure, and Resistance Mechanisms. MBio 2016, 7, e02093-16. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, H.; Stindl, S.; Ludwig, W.; Stumpf, A.; Mehlen, A.; Monget, D.; Pierard, D.; Ziesing, S.; Heesemann, J.; Roggenkamp, A.; et al. Enterobacter hormaechei subsp. oharae subsp. nov., E. hormaechei subsp. hormaechei comb. nov., and E. hormaechei subsp. steigerwaltii subsp. nov., three new subspecies of clinical importance. J. Clin. Microbiol. 2005, 43, 3297–3303. [Google Scholar] [CrossRef] [PubMed]
- Papanicolaou, G.A.; Medeiros, A.A.; Jacoby, G.A. Novel plasmid-mediated beta-lactamase (MIR-1) conferring resistance to oxyimino- and alpha-methoxy beta-lactams in clinical isolates of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 1990, 34, 2200–2209. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Hu, Y.; Zong, Z. Reexamining the Association of AmpC Variants with Enterobacter Species in the Context of Updated Taxonomy. Antimicrob. Agents Chemother. 2021, 65, e0159621. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Zhu, M.; Li, Y.; Huang, D.; Wang, L.; Yan, C.; Zhang, L.; Dong, F.; Lu, J.; Lin, X.; et al. Whole-Genome Sequencing-Based Species Classification, Multilocus Sequence Typing, and Antimicrobial Resistance Mechanism Analysis of the Enterobacter cloacae Complex in Southern China. Microbiol. Spectr. 2022, 10, e0216022. [Google Scholar] [CrossRef]
- Fouad, A.; Simner, P.J.; Nicolau, D.P.; Asempa, T.E. Comparison of BD Phoenix and disk diffusion to broth microdilution for determining cefepime susceptibility among carbapenem-resistant Enterobacterales. J. Clin. Microbiol. 2024, 62, e0152023. [Google Scholar] [CrossRef]
- Thomson, K.S. Extended-spectrum-beta-lactamase, AmpC, and Carbapenemase issues. J. Clin. Microbiol. 2010, 48, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Xie, X.; Yu, H.; Jia, W.; Shan, B.; Huang, B.; Qu, F.; Niu, S.; Lv, J.; Gao, Q.; et al. Epidemiological characteristics and molecular features of carbapenem-resistant Enterobacter strains in China: A multicenter genomic study. Emerg. Microbes Infect. 2023, 12, 2148562. [Google Scholar] [CrossRef] [PubMed]
- Manandhar, S.; Nguyen, Q.; Nguyen, T.N.T.; Pham, D.T.; Rabaa, M.A.; Dongol, S.; Basnyat, B.; Dixit, S.M.; Baker, S.; Karkey, A. Genomic epidemiology, antimicrobial resistance and virulence factors of Enterobacter cloacae complex causing potential community-onset bloodstream infections in a tertiary care hospital of Nepal. JAC Antimicrob. Resist. 2022, 4, dlac050. [Google Scholar] [CrossRef]


| Isolate | Isolated from | Organism by K-mer Spectra | Identification by ANI (GenBank) | Identification by MALDI-TOF * |
|---|---|---|---|---|
| 17193 | Blood culture | Enterobacter hormaechei subsp. steigerwaltii | Enterobacter hormaechei | Enterobacter hormaechei * |
| 17317 | Blood culture | Enterobacter hormaechei subsp. steigerwaltii | Enterobacter hormaechei | Enterobacter hormaechei * |
| 17461 | Blood culture | Enterobacter ludwigii | Enterobacter ludwigii | Enterobacter ludwigii * |
| 17536 | Blood culture | Enterobacter hormaechei subsp. steigerwaltii | Enterobacter hormaechei | Enterobacter hormaechei ** |
| 17571 | Blood culture | Enterobacter hormaechei subsp. steigerwaltii | Enterobacter hormaechei | Enterobacter hormaechei ** |
| 17597 | Blood culture | Enterobacter hormaechei subsp. steigerwaltii | Enterobacter hormaechei | Enterobacter hormaechei * |
| 17616 | Blood culture | Enterobacter hormaechei subsp. steigerwaltii | Enterobacter hormaechei | Enterobacter hormaechei ** |
| 17618 | Blood culture | Enterobacter hormaechei subsp. steigerwaltii | Enterobacter hormaechei | Enterobacter hormaechei * |
| 17623 | Blood culture | Enterobacter hormaechei subsp. steigerwaltii | Enterobacter hormaechei | Enterobacter hormaechei * |
| 17627 | Blood culture | Enterobacter bugandensis | Enterobacter bugandensis | Enterobacter bugandensis |
| 17825 | Blood culture | Enterobacter ludwigii | Enterobacter ludwigii | Enterobacter ludwigii * |
| 17180 | Urine | Enterobacter hormaechei | Enterobacter hormaechei | Enterobacter hormaechei * |
| 17181 | Urine | Enterobacter asburiae | Enterobacter asburiae | Enterobacter asburiae * |
| 17208 | Urine | Enterobacter hormaechei subsp. steigerwaltii | Enterobacter hormaechei | Enterobacter hormaechei * |
| 17246 | Urine | Enterobacter kobei | Enterobacter kobei | Enterobacter kobei * |
| 17307 | Urine | Enterobacter hormaechei subsp. steigerwaltii | Enterobacter hormaechei | Enterobacter hormaechei * |
| 17308 | Urine | Enterobacter hormaechei subsp. steigerwaltii | Enterobacter hormaechei | Enterobacter hormaechei * |
| 17310 | Urine | Enterobacter roggenkampii | Enterobacter roggenkampii | Enterobacter roggenkampii |
| 17328 | Urine | Enterobacter hormaechei subsp. steigerwaltii | Enterobacter hormaechei | Enterobacter hormaechei * |
| 17437 | Urine | Enterobacter hormaechei subsp. steigerwaltii | Enterobacter hormaechei | Enterobacter hormaechei * |
| 17440 | Urine | Enterobacter hormaechei subsp. steigerwaltii | Enterobacter hormaechei | Enterobacter hormaechei * |
| 17525 | Urine | Enterobacter hormaechei subsp. steigerwaltii | Enterobacter hormaechei | Enterobacter hormaechei * |
| 17526 | Urine | Enterobacter hormaechei subsp. steigerwaltii | Enterobacter hormaechei | Enterobacter hormaechei * |
| 17529 | Urine | Enterobacter hormaechei subsp. steigerwaltii | Enterobacter hormaechei | Enterobacter hormaechei * |
| 17534 | Urine | Enterobacter hormaechei subsp. steigerwaltii | Enterobacter hormaechei | Enterobacter hormaechei * |
| 17549 | Urine | Enterobacter hormaechei subsp. steigerwaltii | Enterobacter hormaechei | Enterobacter hormaechei * |
| 17550 | Urine | Enterobacter hormaechei subsp. steigerwaltii | Enterobacter hormaechei | Enterobacter hormaechei * |
| 17559 | Urine | Enterobacter hormaechei subsp. steigerwaltii | Enterobacter hormaechei | Enterobacter hormaechei * |
| 17600 | Urine | Enterobacter hormaechei subsp. steigerwaltii | Enterobacter hormaechei | Enterobacter hormaechei * |
| 17603 | Urine | Enterobacter hormaechei subsp. steigerwaltii | Enterobacter hormaechei | Enterobacter hormaechei * |
| 17609 | Urine | Enterobacter hormaechei subsp. steigerwaltii | Enterobacter hormaechei | Enterobacter hormaechei * |
| 17714 | Urine | Enterobacter hormaechei subsp. steigerwaltii | Enterobacter hormaechei | Enterobacter hormaechei * |
| 17738 | Urine | Enterobacter hormaechei subsp. steigerwaltii | Enterobacter hormaechei | Enterobacter hormaechei * |
| 17816 | Urine | Enterobacter hormaechei subsp. steigerwaltii | Enterobacter hormaechei | Enterobacter hormaechei |
| 17844 | Urine | Enterobacter hormaechei subsp. steigerwaltii | Enterobacter hormaechei | Enterobacter hormaechei |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tenover, F.C.; Tickler, I.A. Genomic Analysis of Enterobacter Species Isolated from Patients in United States Hospitals. Antibiotics 2024, 13, 865. https://doi.org/10.3390/antibiotics13090865
Tenover FC, Tickler IA. Genomic Analysis of Enterobacter Species Isolated from Patients in United States Hospitals. Antibiotics. 2024; 13(9):865. https://doi.org/10.3390/antibiotics13090865
Chicago/Turabian StyleTenover, Fred C., and Isabella A. Tickler. 2024. "Genomic Analysis of Enterobacter Species Isolated from Patients in United States Hospitals" Antibiotics 13, no. 9: 865. https://doi.org/10.3390/antibiotics13090865
APA StyleTenover, F. C., & Tickler, I. A. (2024). Genomic Analysis of Enterobacter Species Isolated from Patients in United States Hospitals. Antibiotics, 13(9), 865. https://doi.org/10.3390/antibiotics13090865






