Three in One with Dual-Functional Hydrogel of Lactoferrin/NZ2114/LMSH Promoting Staphylococcus aureus-Infected Wound Healing
Abstract
:1. Introduction
2. Results and Discussions
2.1. Preparation and Characterization of Lf/NZ2114/LMSH
2.1.1. Preparation Scheme of Lf/NZ2114/LMSH
2.1.2. Formulas and Good Antimicrobial Activity of Lf/NZ2114/LMSH
2.1.3. FIR Analysis of Lf/NZ2114/LMSH (Three in One)
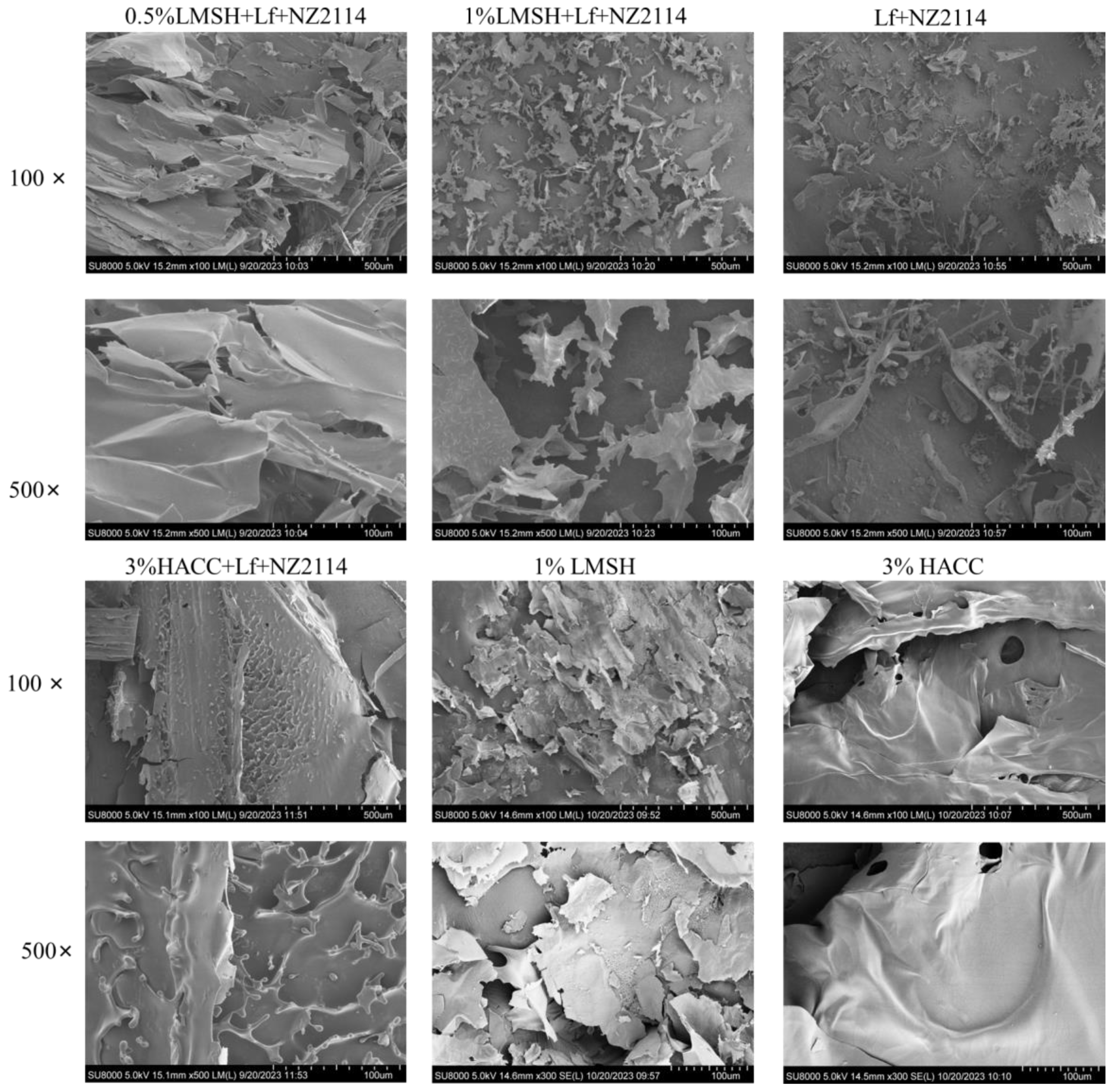
2.1.4. Lamellar Porous Mesh Structure of Lf/NZ2114/LMSH (Three in One)
2.2. Better Shear-Thinning Characteristics of Lf/NZ2114/LMSH
2.3. Good Antimicrobial and Biocompatible Properties
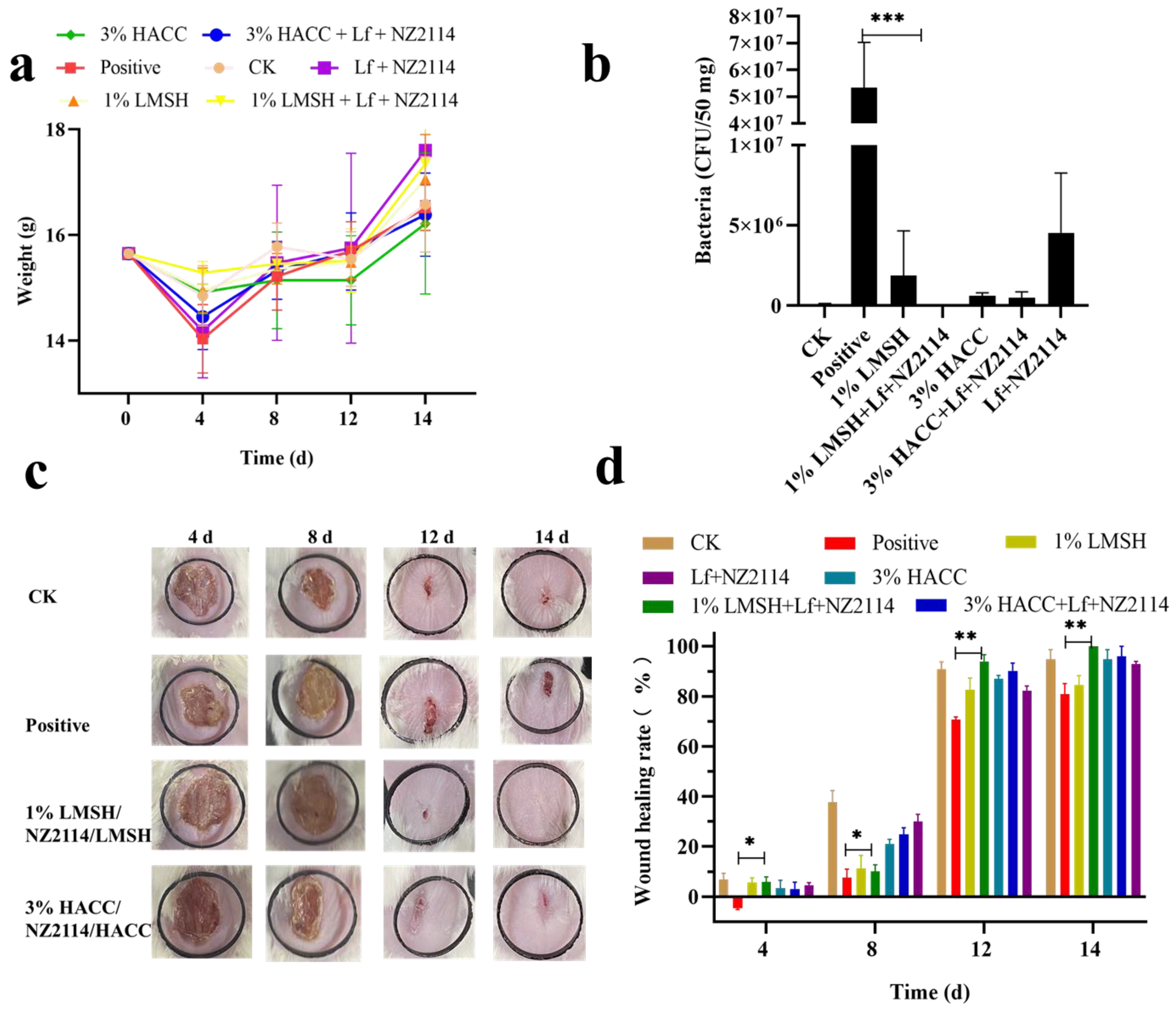
2.4. Antimicrobial and Pro-Healing Effects In Vivo
2.4.1. Wound Infection Model and Treatment
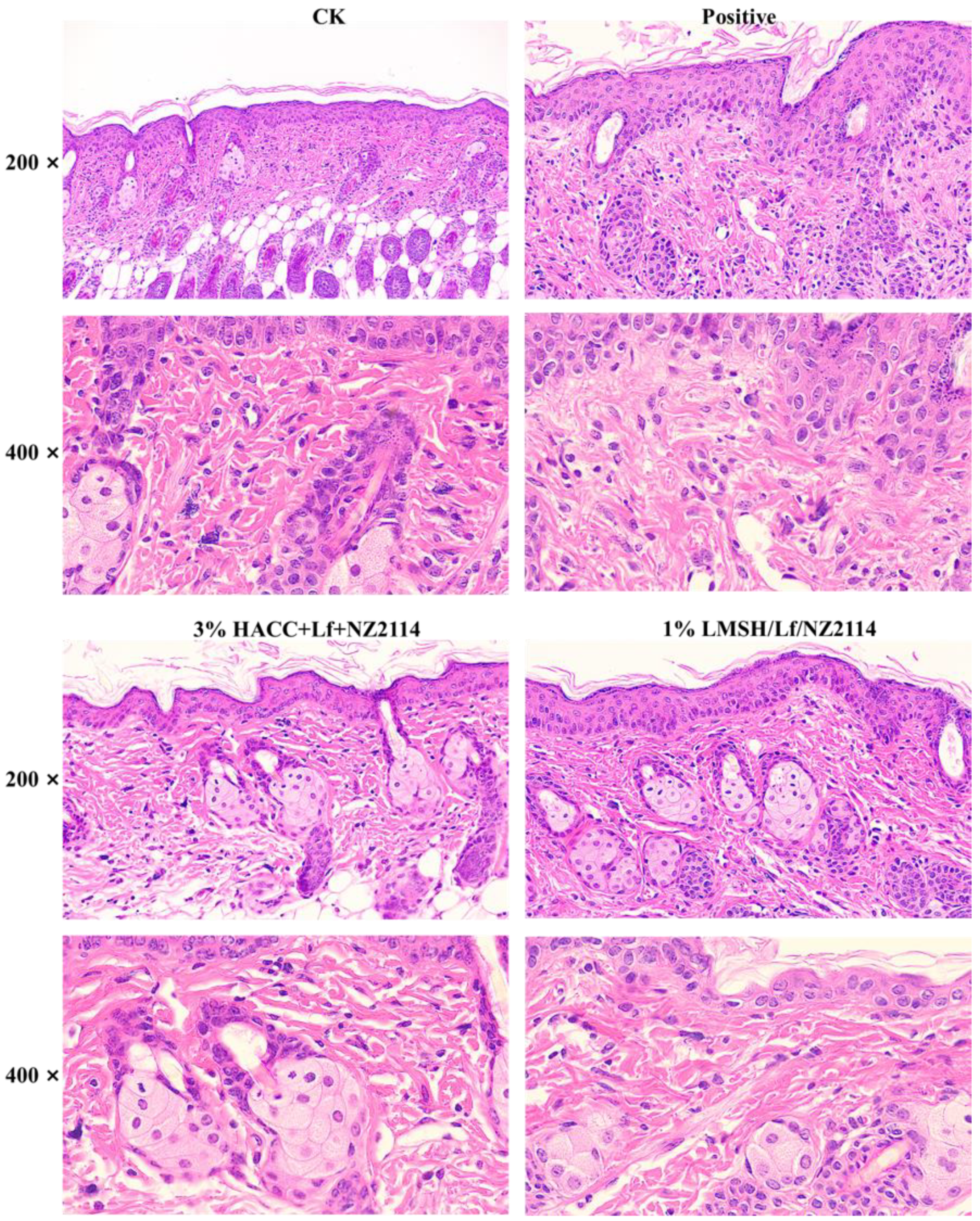
2.4.2. HE Stain of Skin
2.5. The Pro-Healing Mechanism of Lf/NZ2114/LMSH
2.5.1. The qPCR Analysis for Factors IL-6, CD31, EGFR, and VEGF of Skin
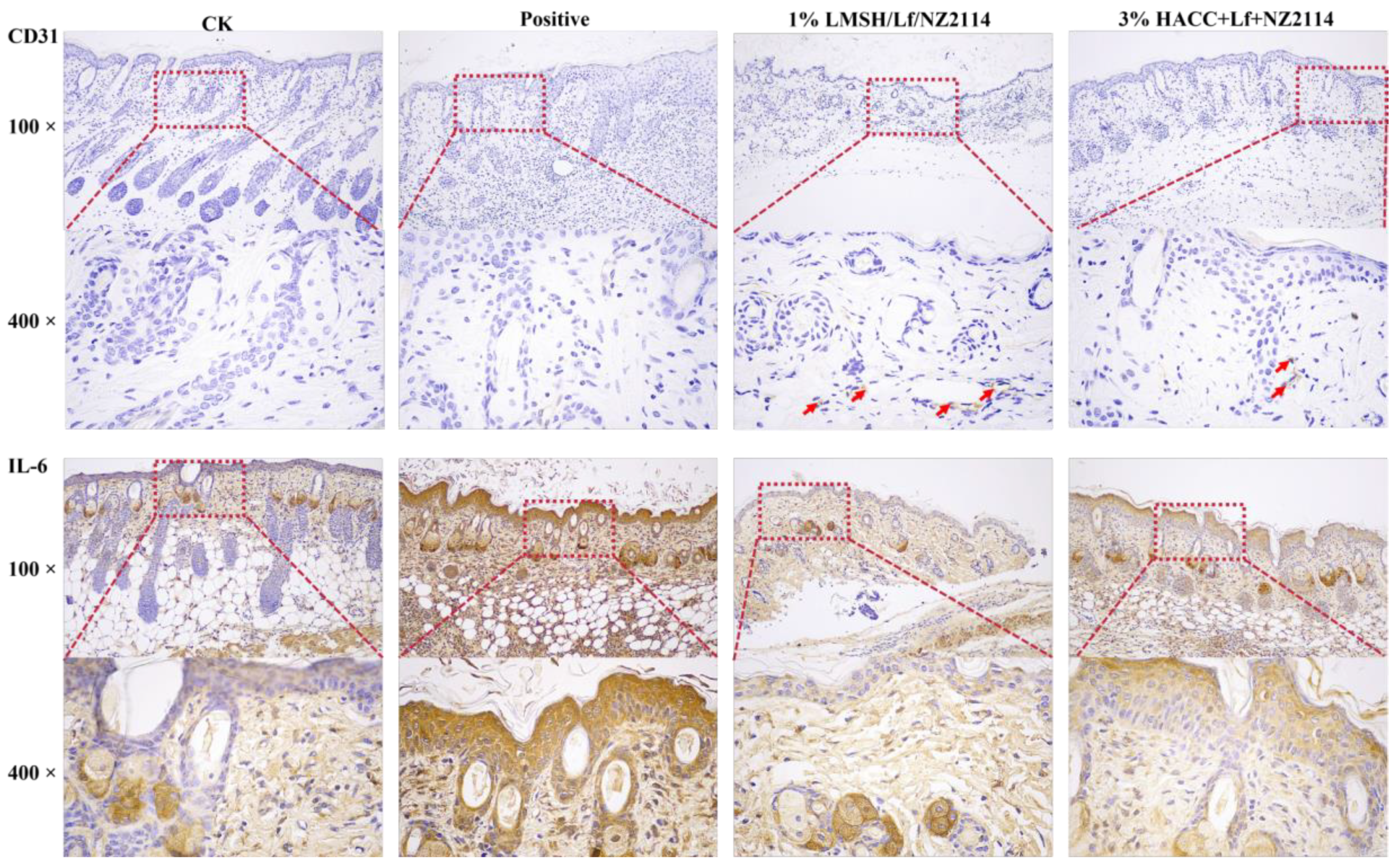
2.5.2. IHC Assay
3. Material and Method
3.1. Materials and Animals
3.2. Method
3.2.1. Preparation of Injectable Hydrogel Lf/NZ2114/LMSH
3.2.2. Antimicrobial Assay, FT–IR, and SEM of Lf/NZ2114/LMSH
3.2.3. Rheological Analysis
3.2.4. Antimicrobial Properties
3.2.5. Biocompatibility
3.2.6. Animal Experiment of Wound Infection
3.2.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Serra, R.; Grande, R.; Butrico, L.; Rossi, A.; Settimio, U.F.; Caroleo, B.; Amato, B.; Gallelli, L.; De Franciscis, S. Chronic wound infections: The role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev. Anti-Infect. Ther. 2015, 13, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Santra, S.; Das, A.; Dixith, S.; Sinha, M.; Ghatak, S.; Ghosh, N.; Banerjee, P.; Khanna, S.; Mathew-Steiner, S. Staphylococcus aureus biofilm infection compromises wound healing by causing deficiencies in granulation tissue collagen. Ann. Surg. 2020, 271, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, N.; Teng, D.; Mao, R.; Hao, Y.; Ma, X.; Wei, L.; Wang, J. Antibacterial peptide nz2114-loaded hydrogel accelerates Staphylococcus aureus-infected wound healing. Appl. Microbiol. Biotechnol. 2022, 106, 3639–3656. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Nunan, R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br. J. Dermatol. 2015, 173, 370–378. [Google Scholar] [CrossRef]
- Huang, C.; Leavitt, T.; Bayer, L.R.; Orgill, D.P. Effect of negative pressure wound therapy on wound healing. Curr. Probl. Surg. 2014, 51, 301–331. [Google Scholar] [CrossRef]
- Nandi, A.; Mondal, S.; Das, S. A review on recent advances in polymer and peptide hydrogels. Soft Matter 2020, 16, 1404–1454. [Google Scholar]
- Krychowiak, M.; Grinholc, M.; Banasiuk, R.; Krauze-Baranowska, M.; Głód, D.; Kawiak, A.; Królicka, A. Combination of silver nanoparticles and drosera binata extract as a possible alternative for antibiotic treatment of burn wound infections caused by resistant Staphylococcus aureus. PLoS ONE 2014, 9, e115727. [Google Scholar] [CrossRef]
- Kaul, S.; Sagar, P.; Gupta, R.; Garg, P.; Priyadarshi, N.; Singhal, N.K. Mechanobactericidal, gold nanostar hydrogel-based bandage for bacteria-infected skin wound healing. ACS Appl. Mater. Interfaces 2022, 14, 44084–44097. [Google Scholar] [CrossRef]
- Chaby, G.; Senet, P.; Vaneau, M.; Martel, P.; Guillaume, J.C.; Meaume, S.; Téot, L.; Debure, C.; Dompmartin, A.; Bachelet, H. Dressings for acute and chronic wounds: A systematic review. Arch. Dermatol. 2007, 143, 1297–1304. [Google Scholar] [CrossRef]
- Kannon, G.; Garrett, A. Moist wound healing with occlusive dressings. Dermatol. Surg. 1995, 21, 175–181. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, R.; Zheng, B.; Guo, W.; Wang, H. Highly stretchable; adhesive; biocompatible, and antibacterial hydrogel dressings for wound healing. Adv. Sci. 2021, 8, 2003627. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Li, D.; Dai, K.; Wang, Y.; Song, P.; Li, H.; Tang, P.; Zhang, Z.; Li, Z.; Zhou, Y. Recent progress of collagen, chitosan, alginate and other hydrogels in skin repair and wound dressing applications. Int. J. Biol. Macromol. 2022, 208, 400–408. [Google Scholar] [CrossRef]
- Mathew, A.; Uthaman, S.; Cho, K.; Cho, C.; Park, I. Injectable hydrogels for delivering biotherapeutic molecules. Int. J. Biol. Macromol. Struct. Funct. Interact. 2018, 110, 17–29. [Google Scholar] [CrossRef]
- Zhao, G.; Sun, Z.; Wang, J.; Xu, Y.; Li, L.; Ge, Y. Electrochemical properties of a highly biocompatible chitosan polymer actuator based on a different nanocarbon/ionic liquid electrode. Polym. Compos. 2017, 269, 118333. [Google Scholar] [CrossRef]
- Seo, J.; Shin, S.; Lee, M.; Cha, J.; Bae, H. Injectable hydrogel derived from chitosan with tunable mechanical properties via hybrid-crosslinking system. Carbohydr. Polym. 2021, 251, 117036. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Xin, Z.; Yongping, L.; Tianlong, Z.; Ma, P.X.; Baolin, G. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 2018, 183, 185–199. [Google Scholar]
- Yuan, H.; Li, W.; Song, C.; Huang, R. An injectable supramolecular nanofiber-reinforced chitosan hydrogel with antibacterial and anti-inflammatory properties as potential carriers for drug delivery. Int. J. Biol. Macromol. 2022, 205, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.; Sheu, M.; Chiang, W.; Chiu, Y.; Tu, C.; Wang, W.; Wu, M.; Wang, Y.; Lu, M.; Ho, H.O. In situ chemically crosslinked injectable hydrogels for the subcutaneous delivery of trastuzumab to treat breast cancer. Acta Biomater. 2019, 86, 280–290. [Google Scholar] [CrossRef]
- Cui, Z.; Milani, A.H.; Greensmith, P.J.; Yan, J.; Adlam, D.J.; Hoyland, J.A.; Kinloch, I.A.; Freemont, A.; Saunders, B.R. A study of physical and covalent hydrogels containing pH-responsive microgel particles and graphene oxide. Langmuir 2014, 30, 13384–13393. [Google Scholar] [CrossRef]
- Torres, J.; Meng, F.; Bhattacharya, S.; Buno, K.; Ahmadzadegan, A.; Madduri, S.; Babiak, P.; Vlachos, P.; Solorio, L.; Yeo, Y.; et al. Interpenetrating networks of collagen and hyaluronic acid that serve as in vitro tissue models for assessing macromolecular transport. Biomacromolecules 2023, 24, 4718–4730. [Google Scholar] [CrossRef]
- Li, Z.; Fan, Z.; Xu, Y.; Lo, W.; Wang, X. pH-sensitive and thermosensitive hydrogels as stem-cell carriers for cardiac therapy. ACS Appl. Mater. Interfaces 2016, 8, 10752–10760. [Google Scholar] [CrossRef] [PubMed]
- Nakahata, M.; Takashima, Y.; Harada, A. Redox-responsive macroscopic gel assembly based on discrete dual interactions. Angew. Chem. Int. Ed. Engl. 2013, 53, 3617–3621. [Google Scholar] [CrossRef] [PubMed]
- Bei, W.; Yuan, Z.; Xin, L.; Ya, M.; Sen, W.; Li, A.; Jie, F. The preparation of lactoferrin/magnesium silicate lithium injectable hydrogel and application in promoting wound healing. Int. J. Biol. Macromol. 2022, 220, 1501–1511. [Google Scholar]
- Zhu, Y.; Hao, W.; Wang, X.; Ouyang, J.; Deng, X.; Yu, H.; Wang, Y. Antimicrobial peptides; conventional antibiotics, and their synergistic utility for the treatment of drug-resistant infections. Med. Res. Rev. 2022, 42, 1377–1422. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 2000, 8, 402–410. [Google Scholar] [CrossRef]
- Zhang, Y.; Teng, D.; Mao, R.; Wang, X.; Xi, D.; Hu, X.; Wang, J. High expression of a plectasin-derived peptide NZ2114 in Pichia pastoris and its pharmacodynamics, postantibiotic and synergy against Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2014, 98, 681–694. [Google Scholar] [CrossRef]
- Antoshin, A.; Shpichka, A.; Huang, G.; Chen, K.; Lu, P.; Svistunov, A.; Lychagin, A.; Lipina, M.M.; Sinelnikov, M.Y.; Reshetov, I.V. Lactoferrin as a regenerative agent: The old-new panacea? Pharmacol. Res. Off. J. Ital. Pharmacol. Soc. 2021, 167, 105564. [Google Scholar] [CrossRef]
- Puddu, P.; Grazia, M.; Filippo, C.; Piera, B.; Sandra, V. Role of endogenous interferon and lps in the immunomodulatory effects of bovine lactoferrin in murine peritoneal macrophages. J. Leukoc. Biol. 2007, 82, 347–353. [Google Scholar] [CrossRef]
- Baveye, S.; Elass, E.; Mazurier, J.L.; Spik, G.; Legrand, D. Lactoferrin: A multifunctional glycoprotein involved in the modulation of the inflammatory process. Clin. Chem. Lab. Med. 1999, 37, 281–286. [Google Scholar] [CrossRef]
- Moreno-Expósito, L.; Illescas-Montes, R.; Melguizo-Rodríguez, L.; Ramos-Torrecillas, C. Multifunctional capacity and therapeutic potential of lactoferrin. Life Sci. 2018, 195, 61–64. [Google Scholar] [CrossRef]
- Kell, D.; Heyden, E.; Pretorius, E. The biology of lactoferrin, an iron-binding protein that can help defend against viruses and bacteria. Front. Immunol. 2020, 11, 1221. [Google Scholar] [CrossRef] [PubMed]
- Elhabal, S.; Ghaffar, S.; Hager, R.; Elzohairy, N.; Khalifa, M.; Mohie, P.; Gad, R.; Omar, N.; Elkomy, M.; Khasawneh, M. Development of thermosensitive hydrogel of amphotericin-b and lactoferrin combination-loaded plga-peg-pei nanoparticles for potential eradication of ocular fungal infections: In-vitro, ex-vivo and in-vivo studies. Int. J. Pharm. 2023, 5, 100174. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Huang, X.; Du, X.; Mo, L.; Ma, C.; Wang, H. Facile synthesis of pH-responsive sodium alginate/carboxymethyl chitosan hydrogel beads promoted by hydrogen bond. Carbohydr. Polym. 2022, 278, 118993. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, K.; Hao, Y.; Liu, M.; Wu, W. Osteoblast/bone-tissue responses to porous surface of polyetheretherketone–nanoporous lithium-doped magnesium silicate blends’ integration with polyetheretherketone. Int. J. Nanomed. 2019, 14, 4975–4989. [Google Scholar] [CrossRef]
- Andersen, F. Final report on the safety assessment of aluminum silicate, calcium silicate, magnesium aluminum silicate, magnesium silicate, magnesium trisilicate, sodium magnesium silicate, zirconium silicate, attapulgite, bentonite, fuller’s earth, hectorite, kaolin, lithium magnesium silicate, lithium magnesium sodium silicate, montmorillonite, pyrophyllite, and zeolite. Int. J. Toxicol. 2003, 22 (Suppl. 1), 37–102. [Google Scholar]
- Cao, L.; Weng, W.; Chen, X.; Zhang, J.; Su, J. Promotion of in vivo degradability, vascularization and osteogenesis of calcium sulfate-based bone cements containing nanoporous lithium doping magnesium silicate. Int. J. Nanomed. 2017, 12, 1341–1352. [Google Scholar] [CrossRef]
- Wang, W.; Cui, J.; Chou, S.; Wen, Y.; Sun, H. Electrospun nanosilicates-based organic/inorganic nanofibers for potential bone tissue engineering. Colloids Surf. B Biointerfaces 2018, 172, 90–97. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Ma, B.; Shao, J.; Liu, H.; Ge, S. Gingipain-responsive thermosensitive hydrogel loaded with sdf-1 facilitatesin situperiodontal tissue regeneration. Acs Appl. Mater. Interfaces 2021, 13, 36880–36893. [Google Scholar] [CrossRef]
- Ramachandran, S.; Chen, S.; Etzler, F. Rheological characterization of hydroxypropylcellulose gels. Drug Dev. Ind. Pharm. 1999, 25, 153–161. [Google Scholar] [CrossRef]
- Wiegand, I.; Kai, H.; Hancock, R. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, N.; Teng, D.; Mao, R.; Hao, Y.; Wang, J. Expression and characterization of the new antimicrobial peptide ap138l-arg26 anti Staphylococcus aureus. Applied microbiology and biotechnology. Appl. Microbiol. Biotechnol. 2024, 108, 111. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Song, L.; Wang, X.; Xu, Y.; Liu, Z.; Zhao, D.; Wang, S.; Fan, X.; Wang, Z.; Gao, C. A bovine lactoferricin-lactoferrampin-encoding Lactobacillus reuteri co21 regulates the intestinal mucosal immunity and enhances the protection of piglets against enterotoxigenic Escherichia coli k88 challenge. Gut Microbes 2021, 13, 1956281. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Zhang, Q.; Mao, R.; Hao, Y.; Ma, X.; Teng, D.; Fan, H.; Wang, J. Effect of NZ2114 against Streptococcus dysgalactiae biofilms and its application in murine mastitis model. Front. Microbiol. 2022, 13, 1010148. [Google Scholar] [CrossRef]
- Yang, C.; Xue, R.; Zhang, Q.; Yang, S.; Liu, P.; Chen, L.; Wang, K.; Zhang, X.; Wei, Y. Nanoclay cross-linked semi-ipn silk sericin/poly(nipam/lmsh) nanocomposite hydrogel: An outstanding antibacterial wound dressing. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 81, 303–313. [Google Scholar] [CrossRef]
- Yuan, N.; Xu, L.; Xu, B.; Zhao, J.; Rong, J. Chitosan derivative-based self-healable hydrogels with enhanced mechanical properties by high-density dynamic ionic interactions. Carbohydr. Polym. 2018, 193, 259–267. [Google Scholar] [CrossRef]
- Sherje, A.; Dravyakar, B.; Kadam, D.; Jadhav, M. Carbohydrate polymers scientific & technological aspects of industrially important polysaccharides. Carbohydr. Polym. 2017, 173, 37–49. [Google Scholar]
- Yang, Y.; Zhang, C.; Bian, X.; Ren, L.; Ma, C.; Xu, Y.; Su, D.; Ai, L.; Song, M.; Zhang, N. Characterization of structural and functional properties of soy protein isolate and sodium alginate interpenetrating polymer network hydrogels. J. Sci. Food. Agric. 2023, 103, 6566–6573. [Google Scholar] [CrossRef]
- Ying, Y.; Huang, Z.; Tu, Y.; Wu, Q.; Li, Z.; Zhang, Y.; Yu, H.; Zeng, A.; Huang, H.; Ye, J. A shear-thinning, ROS-scavenging hydrogel combined with dental pulp stem cells promotes spinal cord repair by inhibiting ferroptosis. Bioact. Mater. 2023, 22, 24–290. [Google Scholar] [CrossRef] [PubMed]
- Tak, N.; Rashid, S.; Kour, P.; Nazir, N.; Zargar, M.; Dar, A. Bergenia stracheyi extract-based hybrid hydrogels of biocompatible polymers with good adhesive, stretching, swelling, self-healing, antibacterial, and antioxidant properties. Int. J. Biol. Macromol. 2023, 234, 123718. [Google Scholar] [CrossRef]
- Dominique, L. Overview of lactoferrin as a natural immune modulator. J. Pediatr. 2016, 173, S10–S15. [Google Scholar]
- Xiong, Y.; Hady, W.; Deslandes, A.; Rey, A.; Fraiss, L.; Kristensen, H.; Yeaman, M.; Bayer, A. Efficacy of NZ2114, a novel plectasin-derived cationic antimicrobial peptide antibiotic, in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2011, 55, 5325–5330. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.; Li, X.; Zhou, Y.; Song, Q.; Zhang, R. Glycosylation-enhanced biocompatibility of the supramolecular hydrogel of an anti-inflammatory drug for topical suppression of inflammation. Acta Biomater. 2018, 73, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Robson, C. Wound infection. A failure of wound healing caused by an imbalance of bacteria. J. Urol. 1998, 159, 1414–1415. [Google Scholar] [CrossRef]
- Veith, A.; Henderson, K.; Spencer, A.; Sligar, A.; Baker, A. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv. Drug Deliv. Rev. 2019, 146, 97–125. [Google Scholar] [CrossRef] [PubMed]
- Toda, M.; Suzuki, T.; Hosono, K.; Kurihara, Y.; Kurihara, H.; Hayashi, I.; Kitasato, H.; Hoka, S.; Majima, M. Roles of calcitonin gene-related peptide in facilitation of wound healing and angiogenesis. Biomed. Pharmacother. 2008, 62, 352–359. [Google Scholar] [CrossRef]
- Ahmed, S.; Saeid, A. The role of phytochemicals in the inflammatory phase of wound healing. Int. J. Mol. Ences 2017, 18, 1068. [Google Scholar]
- Glass, G.; Murphy, G.; Esmaeili, A.; Lai, L.; Nanchahal, J. Systematic review of molecular mechanism of action of negative-pressure wound therapy. Br. J. Surg. 2014, 101, 1627–1636. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Ma, X.; Teng, D.; Mao, R.; Yang, N.; Hao, Y.; Wang, J. Three in One with Dual-Functional Hydrogel of Lactoferrin/NZ2114/LMSH Promoting Staphylococcus aureus-Infected Wound Healing. Antibiotics 2024, 13, 889. https://doi.org/10.3390/antibiotics13090889
Zhang K, Ma X, Teng D, Mao R, Yang N, Hao Y, Wang J. Three in One with Dual-Functional Hydrogel of Lactoferrin/NZ2114/LMSH Promoting Staphylococcus aureus-Infected Wound Healing. Antibiotics. 2024; 13(9):889. https://doi.org/10.3390/antibiotics13090889
Chicago/Turabian StyleZhang, Kun, Xuanxuan Ma, Da Teng, Ruoyu Mao, Na Yang, Ya Hao, and Jianhua Wang. 2024. "Three in One with Dual-Functional Hydrogel of Lactoferrin/NZ2114/LMSH Promoting Staphylococcus aureus-Infected Wound Healing" Antibiotics 13, no. 9: 889. https://doi.org/10.3390/antibiotics13090889








