Commercial Silver-Based Dressings: In Vitro and Clinical Studies in Treatment of Chronic and Burn Wounds
Abstract
1. Introduction
2. Methods Section
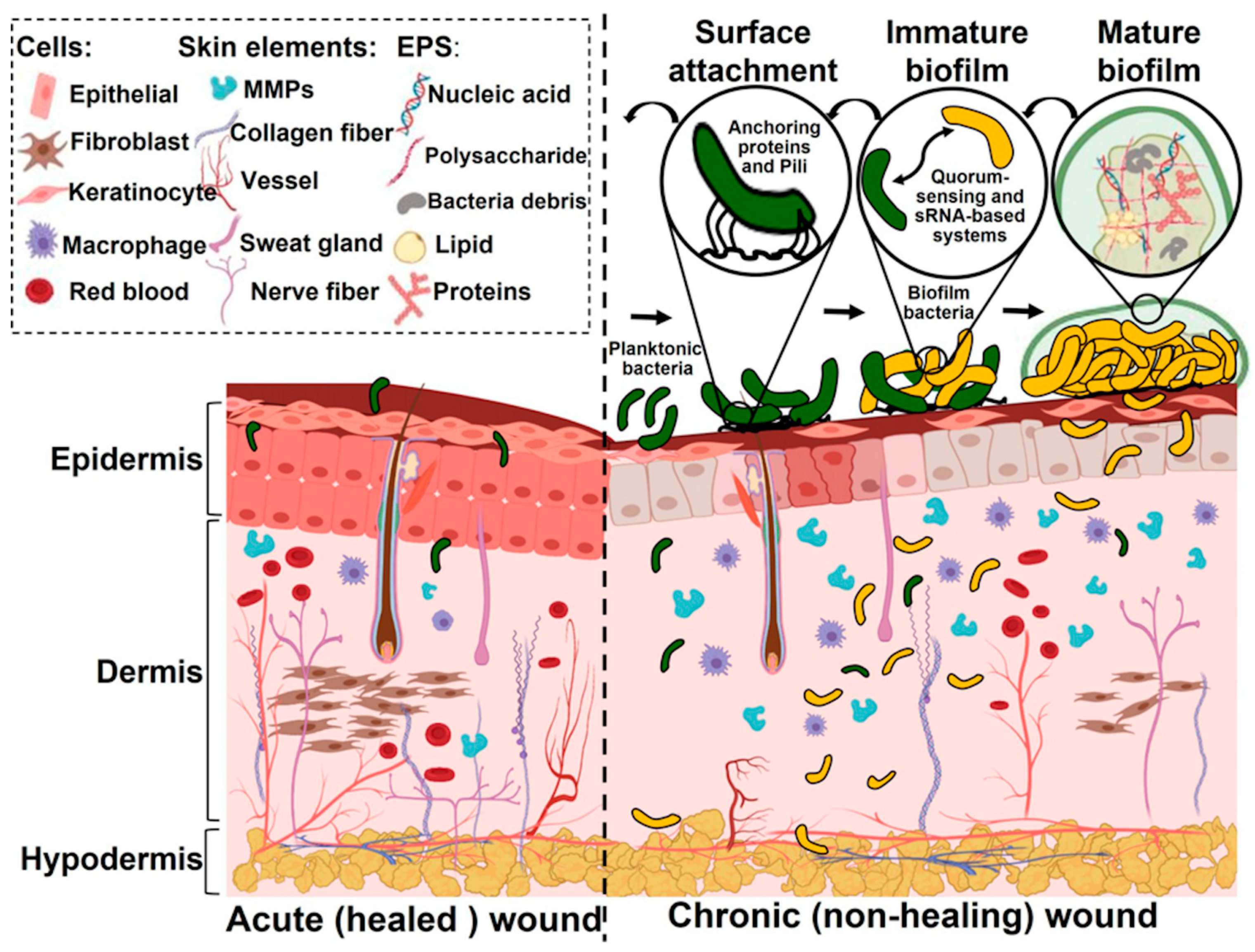
3. Bacterial Infection and Biofilms
4. Wound Dressings
5. Silver-Based Dressings
5.1. Forms of Silver and Additives in Dressings
5.2. Release Characteristics
5.3. Toxicity
5.4. Role of the Dressing Matrix
6. Clinical Studies
- Treatment duration;
- Sample size and diverse demographics;
- Potential biases in the study, including where the funding is coming from;
- Safety profile of the dressing;
- Bacterial load, depth of wound;
- Consideration of both the patient and physician perspective;
- Statistical methods used to analyze results, i.e., are the results of statistical significance;
- Description of the limitations of the study;
- Comparison of what worked and what did not work provides insight;
- Placebo/control effects are not always studied, as in comparing two silver dressings;
- Time to heal for participants who did not heal during the study are often excluded.
7. Concluding Thoughts and Future Scope
Author Contributions
Funding
Conflicts of Interest
References
- Thaarup, I.C.; Iversen, A.K.S.; Lichtenberg, M.; Bjarnsholt, T.; Jakobsen, T.H. Biofilm Survival Strategies in Chronic Wounds. Microorganisms 2022, 10, 775. [Google Scholar] [CrossRef]
- Järbrink, K.; Ni, G.; Sönnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J. Prevalence and Incidence of Chronic Wounds and Related Complications: A Protocol for a Systematic Review. Syst. Rev. 2016, 5, 152. [Google Scholar] [CrossRef] [PubMed]
- Heyer, K.; Herberger, K.; Protz, K.; Glaeske, G.; Augustin, M. Epidemiology of Chronic Wounds in Germany: Analysis of Statutory Health Insurance Data. Wound Repair Regen. 2016, 24, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Paladini, F.; Pollini, M. Antimicrobial Silver Nanoparticles for Wound Healing Application: Progress and Future Trends. Materials 2019, 12, 2540. [Google Scholar] [CrossRef] [PubMed]
- Guest, J.F.; Ayoub, N.; McIlwraith, T.; Uchegbu, I.; Gerrish, A.; Weidlich, D.; Vowden, K.; Vowden, P. Health Economic Burden That Wounds Impose on the National Health Service in the UK. BMJ Open 2015, 5, e009283. [Google Scholar] [CrossRef] [PubMed]
- Asaad, A.; Badr, S. Surgical Site Infections in Developing Countries: Current Burden and Future Challenges. Clin. Microbiol. Open Access 2016, 5, 1000e136. [Google Scholar] [CrossRef]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health J. Int. Soc. Pharmacoecon. Outcomes Res. 2018, 21, 27–32. [Google Scholar] [CrossRef]
- Highmore, C.J.; Melaugh, G.; Morris, R.J.; Parker, J.; Direito, S.O.L.; Romero, M.; Soukarieh, F.; Robertson, S.N.; Bamford, N.C. Translational Challenges and Opportunities in Biofilm Science: A BRIEF for the Future. npj Biofilms Microbiomes 2022, 8, 68. [Google Scholar] [CrossRef]
- Wijesooriya, L.I.; Waidyathilake, D. Antimicrobial Properties of Nonantibiotic Agents for Effective Treatment of Localized Wound Infections: A Minireview. Int. J. Low. Extrem. Wounds 2022, 21, 207–218. [Google Scholar] [CrossRef]
- Gould, L.J.; Alderden, J.; Aslam, R.; Barbul, A.; Bogie, K.M.; El Masry, M.; Graves, L.Y.; White-Chu, E.F.; Ahmed, A.; Boanca, K.; et al. WHS Guidelines for the Treatment of Pressure Ulcers—2023 Update. Wound Repair Regen. 2024, 32, 6–33. [Google Scholar] [CrossRef]
- Nímia, H.H.; Carvalho, V.F.; Isaac, C.; Souza, F.Á.; Gemperli, R.; Paggiaro, A.O. Comparative Study of Silver Sulfadiazine with Other Materials for Healing and Infection Prevention in Burns: A Systematic Review and Meta-Analysis. Burns 2019, 45, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Young, A.; McNaught, C.-E. The Physiology of Wound Healing. Surg. Oxf. 2017, 35, 473–477. [Google Scholar] [CrossRef]
- Wang, P.-H.; Huang, B.-S.; Horng, H.-C.; Yeh, C.-C.; Chen, Y.-J. Wound Healing. J. Chin. Med. Assoc. JCMA 2018, 81, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Govindaraju, P.; Todd, L.; Shetye, S.; Monslow, J.; Puré, E. CD44-Dependent Inflammation, Fibrogenesis, and Collagenolysis Regulates Extracellular Matrix Remodeling and Tensile Strength during Cutaneous Wound Healing. Matrix Biol. 2019, 75–76, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Goh, T.C.; Bajuri, M.Y.; Nadarajah, S.C.; Abdul Rashid, A.H.; Baharuddin, S.; Zamri, K.S. Clinical and Bacteriological Profile of Diabetic Foot Infections in a Tertiary Care. J. Foot Ankle Res. 2020, 13, 36. [Google Scholar] [CrossRef]
- Huang, D.; Wang, J.; Ren, K.; Ji, J. Functionalized Biomaterials to Combat Biofilms. Biomater. Sci. 2020, 8, 4052–4066. [Google Scholar] [CrossRef]
- Guzmán-Soto, I.; McTiernan, C.; Gonzalez-Gomez, M.; Ross, A.; Gupta, K.; Suuronen, E.J.; Mah, T.-F.; Griffith, M.; Alarcon, E.I. Mimicking Biofilm Formation and Development: Recent Progress in in Vitro and in Vivo Biofilm Models. iScience 2021, 24, 102443. [Google Scholar] [CrossRef]
- Bahamondez-Canas, T.F.; Heersema, L.A.; Smyth, H.D.C. Current Status of In Vitro Models and Assays for Susceptibility Testing for Wound Biofilm Infections. Biomedicines 2019, 7, 34. [Google Scholar] [CrossRef]
- Darvishi, S.; Tavakoli, S.; Kharaziha, M.; Girault, H.H.; Kaminski, C.F.; Mela, I. Advances in the Sensing and Treatment of Wound Biofilms. Angew. Chem. Int. Ed. 2022, 61, e202112218. [Google Scholar] [CrossRef]
- Warriner, R.; Burrell, R. Infection and the Chronic Wound: A Focus on Silver. Adv. Skin Wound Care 2005, 18 (Suppl. 1), 2–12. [Google Scholar] [CrossRef]
- Serena, T.E.; Jalodi, O.; Serena, L.; Patel, K.; Mynti, M. Evaluation of the Combination of a Biofilm-Disrupting Agent and Negative Pressure Wound Therapy: A Case Series. J. Wound Care 2021, 30, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, F.; Liu, J.; Yao, H.; Wang, Y. Effect of Silver-Containing Hydrofiber Dressing on Burn Wound Healing: A Meta-Analysis and Systematic Review. J. Cosmet. Dermatol. 2023, 22, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Bourdillon, K.A.; Delury, C.P.; Cullen, B.M. Biofilms and Delayed Healing—An in Vitro Evaluation of Silver- and Iodine-Containing Dressings and Their Effect on Bacterial and Human Cells. Int. Wound J. 2017, 14, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.D.S.; Bernardes, M.J.C.; Gonçalves, R.C.; Vilela, M.S.; Silva, M.V.M.D.; Oliveira, V.D.S.; Rocha, M.R.D.; Vinaud, M.C.; Galdino, H.; Lino, R.D.S. Treatment of Experimentally Induced Partial-Thickness Burns in Rats with Different Silver-Impregnated Dressings. Acta Cir. Bras. 2022, 37, e370801. [Google Scholar] [CrossRef] [PubMed]
- Kostenko, V.; Lyczak, J.; Turner, K.; Martinuzzi, R.J. Impact of Silver-Containing Wound Dressings on Bacterial Biofilm Viability and Susceptibility to Antibiotics during Prolonged Treatment. Antimicrob. Agents Chemother. 2010, 54, 5120–5131. [Google Scholar] [CrossRef]
- May, A.; Kopecki, Z.; Carney, B.; Cowin, A. Antimicrobial Silver Dressings: A Review of Emerging Issues for Modern Wound Care. ANZ J. Surg. 2022, 92, 379–384. [Google Scholar] [CrossRef]
- Metcalf, D.G.; Bowler, P.G. Clinical Impact of an Anti-Biofilm Hydrofiber Dressing in Hard-to-Heal Wounds Previously Managed with Traditional Antimicrobial Products and Systemic Antibiotics. Burns Trauma 2020, 8, tkaa004. [Google Scholar] [CrossRef]
- Doherty, C.; Byrne, C.V.; Baqader, S.; El-Chami, C.; McBain, A.J.; Thomason, H.A. Anti-Biofilm Effects and Healing Promotion by Silver Oxynitrate-Based Dressings. Sci. Rep. 2023, 13, 2014. [Google Scholar] [CrossRef]
- Said, J.; Walker, M.; Parsons, D.; Stapleton, P.; Beezer, A.E.; Gaisford, S. An in Vitro Test of the Efficacy of an Anti-Biofilm Wound Dressing. Int. J. Pharm. 2014, 474, 177–181. [Google Scholar] [CrossRef]
- Nešporová, K.; Pavlík, V.; Šafránková, B.; Vágnerová, H.; Odráška, P.; Žídek, O.; Císařová, N.; Skoroplyas, S.; Kubala, L.; Velebný, V. Effects of Wound Dressings Containing Silver on Skin and Immune Cells. Sci. Rep. 2020, 10, 15216. [Google Scholar] [CrossRef]
- Bowler, P.G.; Parsons, D. Combatting Wound Biofilm and Recalcitrance with a Novel Anti-Biofilm Hydrofiber® Wound Dressing. Wound Med. 2016, 14, 6–11. [Google Scholar] [CrossRef]
- Seth, A.K.; Zhong, A.; Nguyen, K.T.; Hong, S.J.; Leung, K.P.; Galiano, R.D.; Mustoe, T.A. Impact of a Novel, Antimicrobial Dressing on in Vivo, Pseudomonas aeruginosa Wound Biofilm: Quantitative Comparative Analysis Using a Rabbit Ear Model. Wound Repair Regen. 2014, 22, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Regulski, M.; Myntti, M.F.; James, G.A. Anti-Biofilm Efficacy of Commonly Used Wound Care Products in In Vitro Settings. Antibiotics 2023, 12, 536. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.C.; Gil, J.; Solis, M.; Higa, A.; Mills, A.; Simms, C.; Pena, P.V.; Li, J.; Raut, V. Antimicrobial Effectiveness of Wound Matrices Containing Native Extracellular Matrix with Polyhexamethylene Biguanide. Int. Wound J. 2022, 19, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Suleman, L.; Purcell, L.; Thomas, H.; Westgate, S. Use of Internally Validated in Vitro Biofilm Models to Assess Antibiofilm Performance of Silver-Containing Gelling Fibre Dressings. J. Wound Care 2020, 29, 154–161. [Google Scholar] [CrossRef]
- Kim, H.; Makin, I.; Skiba, J.; Ho, A.; Housler, G.; Stojadinovic, A.; Izadjoo, M. Antibacterial Efficacy Testing of a Bioelectric Wound Dressing Against Clinical Wound Pathogens. Open Microbiol. J. 2014, 8, 15–21. [Google Scholar] [CrossRef]
- White, R.; Cowan, T.; Glover, D. Evidence-Based Dressing Selection. J. Wound Care 2011, 20, 4–8. [Google Scholar] [CrossRef]
- Karlsmark, T.; Agerslev, R.H.; Bendz, S.H.; Larsen, J.R.; Roed-Petersen, J.; Andersen, K.E. Clinical Performance of a New Silver Dressing, Contreet Foam, for Chronic Exuding Venous Leg Ulcers. J. Wound Care 2003, 12, 351–354. [Google Scholar] [CrossRef]
- Jørgensen, B.; Price, P.; Andersen, K.E.; Gottrup, F.; Bech-Thomsen, N.; Scanlon, E.; Kirsner, R.; Rheinen, H.; Roed-Petersen, J.; Romanelli, M.; et al. The Silver-Releasing Foam Dressing, Contreet Foam, Promotes Faster Healing of Critically Colonised Venous Leg Ulcers: A Randomised, Controlled Trial. Int. Wound J. 2005, 2, 64–73. [Google Scholar] [CrossRef]
- Meaume, S.; Vallet, D.; Morere, M.N.; Téot, L. Evaluation of a Silver-Releasing Hydroalginate Dressing in Chronic Wounds with Signs of Local Infection. J. Wound Care 2005, 14, 411–419. [Google Scholar] [CrossRef]
- Münter, K.C.; Beele, H.; Russell, L.; Crespi, A.; Gröchenig, E.; Basse, P.; Alikadic, N.; Fraulin, F.; Dahl, C.; Jemma, A.P. Effect of a Sustained Silver-Releasing Dressing on Ulcers with Delayed Healing: The CONTOP Study. J. Wound Care 2006, 15, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Jude, E.B.; Apelqvist, J.; Spraul, M.; Martini, J.; Silver Dressing Study Group. Prospective Randomized Controlled Study of Hydrofiber Dressing Containing Ionic Silver or Calcium Alginate Dressings in Non-Ischaemic Diabetic Foot Ulcers. Diabet. Med. J. Br. Diabet. Assoc. 2007, 24, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Lazareth, I.; Meaume, S.; Sigal-Grinberg, M.L.; Combemale, P.; Guyadec, T.L.; Zagnoli, A.; Perrot, J.-L.; Sauvadet, A.; Bohbot, S. The Role of a Silver Releasing Lipido-Colloid Contact Layer in Venous Leg Ulcers Presenting Inflammatory Signs Suggesting Heavy Bacterial Colonization: Results of a Randomized Controlled Study. Wounds Compend. Clin. Res. Pract. 2008, 20, 158–166. [Google Scholar]
- Gago, M.; Garcia, F.; Gaztelu, V.; Verdu, J.; Lopez, P.; Nolasco, A. A Comparison of Three Silver-Containing Dressings in the Treatment of Infected, Chronic Wounds. Wounds Compend. Clin. Res. Pract. 2008, 20, 273–278. [Google Scholar]
- Michaels, J.A.; Campbell, B.; King, B.; Palfreyman, S.J.; Shackley, P.; Stevenson, M. Randomized Controlled Trial and Cost-Effectiveness Analysis of Silver-Donating Antimicrobial Dressings for Venous Leg Ulcers (VULCAN Trial). Br. J. Surg. 2009, 96, 1147–1156. [Google Scholar] [CrossRef]
- Miller, C.N.; Newall, N.; Kapp, S.E.; Lewin, G.; Karimi, L.; Carville, K.; Gliddon, T.; Santamaria, N.M. A Randomized-Controlled Trial Comparing Cadexomer Iodine and Nanocrystalline Silver on the Healing of Leg Ulcers. Wound Repair Regen. 2010, 18, 359–367. [Google Scholar] [CrossRef]
- Chuangsuwanich, A.; Charnsanti, O.; Lohsiriwat, V.; Kangwanpoom, C.; Thong-In, N. The Efficacy of Silver Mesh Dressing Compared with Silver Sulfadiazine Cream for the Treatment of Pressure Ulcers. J. Med. Assoc. Thail. Chotmaihet Thangphaet 2011, 94, 559–565. [Google Scholar]
- Lazareth, I.; Meaume, S.; Sigal-Grinberg, M.L.; Combemale, P.; Le Guyadec, T.; Zagnoli, A. Efficacy of a Silver Lipidocolloid Dressing on Heavily Colonised Wounds: A Republished RCT. J. Wound Care 2012, 21, 96–102. [Google Scholar] [CrossRef]
- Harding, K.; Gottrup, F.; Jawień, A.; Mikosiński, J.; Twardowska-Saucha, K.; Kaczmarek, S.; Sopata, M.; Shearman, C.; Pieronne, A.; Kommala, D. A Prospective, Multi-Centre, Randomised, Open Label, Parallel, Comparative Study to Evaluate Effects of AQUACEL® Ag and Urgotul® Silver Dressing on Healing of Chronic Venous Leg Ulcers. Int. Wound J. 2012, 9, 285–294. [Google Scholar] [CrossRef]
- Senet, P.; Bause, R.; Jørgensen, B.; Fogh, K. Clinical Efficacy of a Silver-Releasing Foam Dressing in Venous Leg Ulcer Healing: A Randomised Controlled Trial. Int. Wound J. 2014, 11, 649–655. [Google Scholar] [CrossRef]
- Brouillard, C.; Bursztejn, A.-C.; Latarche, C.; Cuny, J.-F.; Truchetet, F.; Goullé, J.-P.; Schmutz, J.-L. Silver Absorption and Toxicity Evaluation of Silver Wound Dressings in 40 Patients with Chronic Wounds. J. Eur. Acad. Dermatol. Venereol. JEADV 2018, 32, 2295–2299. [Google Scholar] [CrossRef] [PubMed]
- Hurd, T.; Woodmansey, E.J.; Watkins, H.M.A. A Retrospective Review of the Use of a Nanocrystalline Silver Dressing in the Management of Open Chronic Wounds in the Community. Int. Wound J. 2021, 18, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Lee, H.-C.; Chen, C.-L.; Kuo, M.-C.; Ramachandran, S.; Chen, R.-F.; Kuo, Y.-R. The Effects of Silver-Releasing Foam Dressings on Diabetic Foot Ulcer Healing. J. Clin. Med. 2021, 10, 1495. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Guo, Y.; Li, B.; Zheng, J.; Tang, Z.; Shu, M. Application Effect of Silver-Containing Dressings in the Repair of Chronic Refractory Wounds. Evid.-Based Complement. Altern. Med. ECAM 2022, 2022, 3616923. [Google Scholar] [CrossRef] [PubMed]
- Dissemond, J.; Aare, K.; Ozer, K.; Gandhi, D.; Ryan, J.L.; DeKoven, M. Aquacel Ag Advantage/Ag+ Extra and Cutimed Sorbact in the Management of Hard-to-Heal Wounds: A Cohort Study. J. Wound Care 2023, 32, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Lafontaine, N.; Jolley, J.; Kyi, M.; King, S.; Iacobaccio, L.; Staunton, E.; Wilson, B.; Seymour, C.; Rogasch, S.; Wraight, P. Prospective Randomised Placebo-Controlled Trial Assessing the Efficacy of Silver Dressings to Enhance Healing of Acute Diabetes-Related Foot Ulcers. Diabetologia 2023, 66, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Tredget, E.E.; Shankowsky, H.A.; Groeneveld, A.; Burrell, R. A Matched-Pair, Randomized Study Evaluating the Efficacy and Safety of Acticoat Silver-Coated Dressing for the Treatment of Burn Wounds. J. Burn Care Rehabil. 1998, 19, 531–537. [Google Scholar] [CrossRef]
- Caruso, D.M.; Foster, K.N.; Hermans, M.H.E.; Rick, C. Aquacel Ag in the Management of Partial-Thickness Burns: Results of a Clinical Trial. J. Burn Care Rehabil. 2004, 25, 89–97. [Google Scholar] [CrossRef]
- Varas, R.P.; O’Keeffe, T.; Namias, N.; Pizano, L.R.; Quintana, O.D.; Herrero Tellachea, M.; Rashid, Q.; Ward, C.G. A Prospective, Randomized Trial of Acticoat versus Silver Sulfadiazine in the Treatment of Partial-Thickness Burns: Which Method Is Less Painful? J. Burn Care Rehabil. 2005, 26, 344–347. [Google Scholar] [CrossRef]
- Caruso, D.M.; Foster, K.N.; Blome-Eberwein, S.A.; Twomey, J.A.; Herndon, D.N.; Luterman, A.; Silverstein, P.; Antimarino, J.R.; Bauer, G.J. Randomized Clinical Study of Hydrofiber Dressing with Silver or Silver Sulfadiazine in the Management of Partial-Thickness Burns. J. Burn Care Res. 2006, 27, 298–309. [Google Scholar] [CrossRef]
- Huang, Y.; Li, X.; Liao, Z.; Zhang, G.; Liu, Q.; Tang, J.; Peng, Y.; Liu, X.; Luo, Q. A Randomized Comparative Trial between Acticoat and SD-Ag in the Treatment of Residual Burn Wounds, Including Safety Analysis. Burns 2007, 33, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Paddock, H.N.; Fabia, R.; Giles, S.; Hayes, J.; Lowell, W.; Besner, G.E. A Silver Impregnated Antimicrobial Dressing Reduces Hospital Length of Stay for Pediatric Patients with Burns. J. Burn Care Res. 2007, 28, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Jester, I.; Bohn, I.; Hannmann, T.; Waag, K.-L.; Loff, S. Comparison of Two Silver Dressings for Wound Management in Pediatric Burns. Wounds Compend. Clin. Res. Pract. 2008, 20, 303–308. [Google Scholar]
- Glat, P.M.; Kubat, W.D.; Hsu, J.F.; Copty, T.; Burkey, B.A.; Davis, W.; Goodwin, I. Randomized Clinical Study of SilvaSorb Gel in Comparison to Silvadene Silver Sulfadiazine Cream in the Management of Partial-Thickness Burns. J. Burn Care Res. 2009, 30, 262–267. [Google Scholar] [CrossRef]
- Muangman, P.; Muangman, S.; Opasanon, S.; Keorochana, K.; Chuntrasakul, C. Benefit of Hydrocolloid SSD Dressing in the Outpatient Management of Partial Thickness Burns. J. Med. Assoc. Thail. Chotmaihet Thangphaet 2009, 92, 1300–1305. [Google Scholar]
- Muangman, P.; Pundee, C.; Opasanon, S. A Prospective, Randomized Trial of Silver Containing Hydrofiber Dressing versus 1% Silver Sulfadiazine for the Treatment of Partial Thickness Burns. Int. Wound J. 2010, 7, 271–276. [Google Scholar] [CrossRef]
- Opasanon, S.; Muangman, P.; Namviriyachote, N. Clinical Effectiveness of Alginate Silver Dressing in Outpatient Management of Partial-Thickness Burns. Int. Wound J. 2010, 7, 467–471. [Google Scholar] [CrossRef]
- Silverstein, P.; Heimbach, D.; Meites, H.; Latenser, B.; Mozingo, D.; Mullins, F.; Garner, W.; Turkowski, J.; Shupp, J.; Glat, P.; et al. An Open, Parallel, Randomized, Comparative, Multicenter Study to Evaluate the Cost-Effectiveness, Performance, Tolerance, and Safety of a Silver-Containing Soft Silicone Foam Dressing (Intervention) vs Silver Sulfadiazine Cream. J. Burn Care Res. 2011, 32, 617–626. [Google Scholar] [CrossRef]
- Mabrouk, A.; Boughdadi, N.; Helal, H.; Zaki, B.; Maher, A. Moist Occlusive Dressing (Aquacel® Ag) versus Moist Open Dressing (MEBO®) in the Management of Partial-Thickness Facial Burns: A Comparative Study in Ain Shams University. Burns 2011, 38, 396–403. [Google Scholar] [CrossRef]
- Duteille, F.; Jeffery, S.L.A. A Phase II Prospective, Non-Comparative Assessment of a New Silver Sodium Carboxymethylcellulose (AQUACEL® Ag BURN) Glove in the Management of Partial Thickness Hand Burns. Burns 2012, 38, 1041–1050. [Google Scholar] [CrossRef]
- Yarboro, D.D. A Comparative Study of the Dressings Silver Sulfadiazine and Aquacel Ag in the Management of Superficial Partial-Thickness Burns. Adv. Skin Wound Care 2013, 26, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Verbelen, J.; Hoeksema, H.; Heyneman, A.; Pirayesh, A.; Monstrey, S. Aquacel® Ag Dressing versus ActicoatTM Dressing in Partial Thickness Burns: A Prospective, Randomized, Controlled Study in 100 Patients. Part 1: Burn Wound Healing. Burns 2014, 40, 416–427. [Google Scholar] [CrossRef]
- Gee Kee, E.L.; Kimble, R.M.; Cuttle, L.; Khan, A.; Stockton, K.A. Randomized Controlled Trial of Three Burns Dressings for Partial Thickness Burns in Children. Burns 2015, 41, 946–955. [Google Scholar] [CrossRef]
- Brown, M.; Dalziel, S.R.; Herd, E.; Johnson, K.; Wong She, R.; Shepherd, M. A Randomized Controlled Study of Silver-Based Burns Dressing in a Pediatric Emergency Department. J. Burn Care Res. 2016, 37, e340–e347. [Google Scholar] [CrossRef] [PubMed]
- Housler, G.J.; Cross, S.; Marcel, V.; Kennedy, D.O.; Husband, M.; Register, A.; Roberts, T.; Grubbs, S.; Dudewicz, D.; Setka, N.; et al. A Prospective Randomized Controlled Two-Arm Clinical Study Evaluating the Efficacy of a Bioelectric Dressing System for Blister Management in US Army Ranger Recruits. J. Spec. Oper. Med. 2017, 17, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Hundeshagen, G.; Collins, V.N.; Wurzer, P.; Sherman, W.; Nunez Lopez, O.; Sheaffer, J.; Herndon, D.N.; Finnerty, C.C.; Branski, L.K. A Prospective, Randomized, Controlled Trial Comparing the Outpatient Treatment of Pediatric and Adult Partial-Thickness Burns with Suprathel or Mepilex Ag. J. Burn Care Res. 2018, 39, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Aurora, A.; Beasy, A.; Rizzo, J.A.; Chung, K.K. The Use of a Silver-Nylon Dressing During Evacuation of Military Burn Casualties. J. Burn Care Res. 2018, 39, 593–597. [Google Scholar] [CrossRef]
- Moreira, S.S.; de Camargo, M.C.; Caetano, R.; Alves, M.R.; Itria, A.; Pereira, T.V.; Lopes, L.C. Efficacy and Costs of Nanocrystalline Silver Dressings versus 1% Silver Sulfadiazine Dressings to Treat Burns in Adults in the Outpatient Setting: A Randomized Clinical Trial. Burns 2022, 48, 568–576. [Google Scholar] [CrossRef]
- Chan, R.K.; Nuutila, K.; Mathew-Steiner, S.S.; Diaz, V.; Anselmo, K.; Batchinsky, M.; Carlsson, A.; Ghosh, N.; Sen, C.K.; Roy, S. A Prospective, Randomized, Controlled Study to Evaluate the Effectiveness of a Fabric-Based Wireless Electroceutical Dressing Compared to Standard-of-Care Treatment Against Acute Trauma and Burn Wound Biofilm Infection. Adv. Wound Care 2024, 13, 1–13. [Google Scholar] [CrossRef]
- Sen, C.K.; Roy, S.; Mathew-Steiner, S.S.; Gordillo, G.M. Biofilm Management in Wound Care. Plast. Reconstr. Surg. 2021, 148, 275e–288e. [Google Scholar] [CrossRef]
- Geesey, G.G.; Richardson, W.T.; Yeomans, H.G.; Irvin, R.T.; Costerton, J.W. Microscopic Examination of Natural Sessile Bacterial Populations from an Alpine Stream. Can. J. Microbiol. 1977, 23, 1733–1736. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Geesey, G.G.; Cheng, K.J. How Bacteria Stick. Sci. Am. 1978, 238, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Cámara, M.; Green, W.; MacPhee, C.E.; Rakowska, P.D.; Raval, R.; Richardson, M.C.; Slater-Jefferies, J.; Steventon, K.; Webb, J.S. Economic Significance of Biofilms: A Multidisciplinary and Cross-Sectoral Challenge. npj Biofilms Microbiomes 2022, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Hill, K.E.; Malic, S.; Thomas, D.W.; Williams, D.W. Antimicrobial Tolerance and the Significance of Persister Cells in Recalcitrant Chronic Wound Biofilms. Wound Repair Regen. 2011, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fux, C.A.; Costerton, J.W.; Stewart, P.S.; Stoodley, P. Survival Strategies of Infectious Biofilms. Trends Microbiol. 2005, 13, 34–40. [Google Scholar] [CrossRef]
- Keren, I.; Kaldalu, N.; Spoering, A.; Wang, Y.; Lewis, K. Persister Cells and Tolerance to Antimicrobials. FEMS Microbiol. Lett. 2004, 230, 13–18. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef]
- Bessa, L.J.; Fazii, P.; Di Giulio, M.; Cellini, L. Bacterial Isolates from Infected Wounds and Their Antibiotic Susceptibility Pattern: Some Remarks about Wound Infection. Int. Wound J. 2015, 12, 47–52. [Google Scholar] [CrossRef]
- Kirketerp-Møller, K.; Jensen, P.Ø.; Fazli, M.; Madsen, K.G.; Pedersen, J.; Moser, C.; Tolker-Nielsen, T.; Høiby, N.; Givskov, M.; Bjarnsholt, T. Distribution, Organization, and Ecology of Bacteria in Chronic Wounds. J. Clin. Microbiol. 2008, 46, 2717–2722. [Google Scholar] [CrossRef]
- Fazli, M.; Bjarnsholt, T.; Kirketerp-Møller, K.; Jørgensen, B.; Andersen, A.S.; Krogfelt, K.A.; Givskov, M.; Tolker-Nielsen, T. Nonrandom Distribution of Pseudomonas aeruginosa and Staphylococcus aureus in Chronic Wounds. J. Clin. Microbiol. 2009, 47, 4084–4089. [Google Scholar] [CrossRef]
- Metcalf, D.G.; Bowler, P.G. Biofilm Delays Wound Healing: A Review of the Evidence. Burns Trauma 2015, 1, 5–12. [Google Scholar] [CrossRef] [PubMed]
- James, G.A.; Swogger, E.; Wolcott, R.; Pulcini, E.d.; Secor, P.; Sestrich, J.; Costerton, J.W.; Stewart, P.S. Biofilms in Chronic Wounds. Wound Repair Regen. 2008, 16, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Posnett, J.; Franks, P.J. The Burden of Chronic Wounds in the UK. Nurs. Times 2008, 104, 44–45. [Google Scholar] [PubMed]
- Harding, K.; Posnett, J.; Vowden, K. A New Methodology for Costing Wound Care. Int. Wound J. 2013, 10, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Wolcott, R.D.; Rhoads, D.D.; Dowd, S.E. Biofilms and Chronic Wound Inflammation. J. Wound Care 2008, 17, 333–341. [Google Scholar] [CrossRef] [PubMed]
- White, R.J.; Cutting, K.F. Wound Biofilms-Are They Visible? J. Wound Care 2012, 21, 140–141. [Google Scholar] [CrossRef]
- Versey, Z.; da Cruz Nizer, W.S.; Russell, E.; Zigic, S.; DeZeeuw, K.G.; Marek, J.E.; Overhage, J.; Cassol, E. Biofilm-Innate Immune Interface: Contribution to Chronic Wound Formation. Front. Immunol. 2021, 12, 648554. [Google Scholar] [CrossRef]
- James, G.A.; Ge Zhao, A.; Usui, M.; Underwood, R.A.; Nguyen, H.; Beyenal, H.; deLancey Pulcini, E.; Agostinho Hunt, A.; Bernstein, H.C.; Fleckman, P.; et al. Microsensor and Transcriptomic Signatures of Oxygen Depletion in Biofilms Associated with Chronic Wounds. Wound Repair Regen. 2016, 24, 373–383. [Google Scholar] [CrossRef]
- Malone, M.; Swanson, T. Biofilm-Based Wound Care: The Importance of Debridement in Biofilm Treatment Strategies. Br. J. Community Nurs. 2017, 22, S20–S25. [Google Scholar] [CrossRef]
- Nusbaum, A.G.; Gil, J.; Rippy, M.K.; Warne, B.; Valdes, J.; Claro, A.; Davis, S.C. Effective Method to Remove Wound Bacteria: Comparison of Various Debridement Modalities in an in Vivo Porcine Model. J. Surg. Res. 2012, 176, 701–707. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, D.; Zhao, F. Updates on Recent Clinical Assessment of Commercial Chronic Wound Care Products. Adv. Healthc. Mater. 2023, 12, e2300556. [Google Scholar] [CrossRef] [PubMed]
- Simões, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent Advances on Antimicrobial Wound Dressing: A Review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.K.; Wang, B.; Shrestha, S. Silver in Health and Medicinal Applications, Amazon Kindle e-book ed.: Seattle, WA, USA. 2021. Available online: https://a.co/d/cGPPwNl (accessed on 3 September 2024).
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef] [PubMed]
- Rybka, M.; Mazurek, Ł.; Konop, M. Beneficial Effect of Wound Dressings Containing Silver and Silver Nanoparticles in Wound Healing-From Experimental Studies to Clinical Practice. Life 2022, 13, 69. [Google Scholar] [CrossRef]
- Bedlovičová, Z.; Strapáč, I.; Baláž, M.; Salayová, A. A Brief Overview on Antioxidant Activity Determination of Silver Nanoparticles. Molecules 2020, 25, 3191. [Google Scholar] [CrossRef]
- Sullivan, T.P.; Eaglstein, W.H.; Davis, S.C.; Mertz, P. The Pig as a Model for Human Wound Healing. Wound Repair Regen. 2001, 9, 66–76. [Google Scholar] [CrossRef]
- Meyer, W.; Görgen, S.; Schlesinger, C. Structural and Histochemical Aspects of Epidermis Development of Fetal Porcine Skin. Am. J. Anat. 1986, 176, 207–219. [Google Scholar] [CrossRef]
- Roy, S.; Biswas, S.; Khanna, S.; Gordillo, G.; Bergdall, V.; Green, J.; Marsh, C.B.; Gould, L.J.; Sen, C.K. Characterization of a Preclinical Model of Chronic Ischemic Wound. Physiol. Genomics 2009, 37, 211–224. [Google Scholar] [CrossRef]
- Patil, P.; Martin, J.R.; Sarett, S.M.; Pollins, A.C.; Cardwell, N.L.; Davidson, J.M.; Guelcher, S.A.; Nanney, L.B.; Duvall, C.L. Porcine Ischemic Wound-Healing Model for Preclinical Testing of Degradable Biomaterials. Tissue Eng. Part C Methods 2017, 23, 754–762. [Google Scholar] [CrossRef]
- Trejo-Hernández, A.; Andrade-Domínguez, A.; Hernández, M.; Encarnación, S. Interspecies Competition Triggers Virulence and Mutability in Candida Albicans—Pseudomonas Aeruginosa Mixed Biofilms. ISME J. 2014, 8, 1974–1988. [Google Scholar] [CrossRef]
- Lansdown, A.B.G.; Williams, A.; Chandler, S.; Benfield, S. Silver Absorption and Antibacterial Efficacy of Silver Dressings. J. Wound Care 2005, 14, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Thuptimdang, P.; Limpiyakorn, T.; McEvoy, J.; Prüß, B.M.; Khan, E. Effect of Silver Nanoparticles on Pseudomonas Putida Biofilms at Different Stages of Maturity. J. Hazard. Mater. 2015, 290, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.Ø.; Bjarnsholt, T.; Phipps, R.; Rasmussen, T.B.; Calum, H.; Christoffersen, L.; Moser, C.; Williams, P.; Pressler, T.; Givskov, M.; et al. Rapid Necrotic Killing of Polymorphonuclear Leukocytes Is Caused by Quorum-Sensing-Controlled Production of Rhamnolipid by Pseudomonas aeruginosa. Microbiol. Read. Engl. 2007, 153, 1329–1338. [Google Scholar] [CrossRef]
- Pearson, J.P.; Pesci, E.C.; Iglewski, B.H. Roles of Pseudomonas Aeruginosa Las and Rhl Quorum-Sensing Systems in Control of Elastase and Rhamnolipid Biosynthesis Genes. J. Bacteriol. 1997, 179, 5756–5767. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.G.; Tran, P.L.; Haley, C.L.; Kruzek, C.; Colmer-Hamood, J.A.; Myntti, M.; Hamood, A.N. Next Science Wound Gel Technology, a Novel Agent That Inhibits Biofilm Development by Gram-Positive and Gram-Negative Wound Pathogens. Antimicrob. Agents Chemother. 2014, 58, 3060–3072. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.L., Jr. Silver Sulfadiazine—A New Topical Therapy for Pseudomonas in Burns: Therapy of Pseudomonas Infection in Burns. Arch. Surg. 1968, 96, 184–188. [Google Scholar] [CrossRef]
- Percival, S.L.; Mayer, D.; Salisbury, A.-M. Efficacy of a Surfactant-Based Wound Dressing on Biofilm Control. Wound Repair Regen. 2017, 25, 767–773. [Google Scholar] [CrossRef]
- Ahmadi, M.; Adibhesami, M. The Effect of Silver Nanoparticles on Wounds Contaminated with Pseudomonas aeruginosa in Mice: An Experimental Study. Iran. J. Pharm. Res. IJPR 2017, 16, 661–669. [Google Scholar]
- Chaudhari, P.R.; Masurkar, S.A.; Shidore, V.B.; Kamble, S.P. Effect of Biosynthesized Silver Nanoparticles on Staphylococcus aureus Biofilm Quenching and Prevention of Biofilm Formation. Nano-Micro Lett. 2012, 4, 34–39. [Google Scholar] [CrossRef]
- Habash, M.B.; Park, A.J.; Vis, E.C.; Harris, R.J.; Khursigara, C.M. Synergy of Silver Nanoparticles and Aztreonam against Pseudomonas aeruginosa PAO1 Biofilms. Antimicrob. Agents Chemother. 2014, 58, 5818–5830. [Google Scholar] [CrossRef]
- Khansa, I.; Schoenbrunner, A.R.; Kraft, C.T.; Janis, J.E. Silver in Wound Care-Friend or Foe?: A Comprehensive Review. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2390. [Google Scholar] [CrossRef] [PubMed]
- Poon, V.K.M.; Burd, A. In Vitro Cytotoxity of Silver: Implication for Clinical Wound Care. Burns 2004, 30, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Maghsoudi, H.; Monshizadeh, S.; Mesgari, M. A Comparative Study of the Burn Wound Healing Properties of Saline-Soaked Dressing and Silver Sulfadiazine in Rats. Indian J. Surg. 2011, 73, 24–27. [Google Scholar] [CrossRef]
- Cho Lee, A.-R.; Leem, H.; Lee, J.; Park, K.C. Reversal of Silver Sulfadiazine-Impaired Wound Healing by Epidermal Growth Factor. Biomaterials 2005, 26, 4670–4676. [Google Scholar] [CrossRef] [PubMed]
- Rosen, J.; Landriscina, A.; Kutner, A.; Adler, B.L.; Krausz, A.E.; Nosanchuk, J.D.; Friedman, A.J. Silver Sulfadiazine Retards Wound Healing in Mice via Alterations in Cytokine Expression. J. Investig. Dermatol. 2015, 135, 1459–1462. [Google Scholar] [CrossRef]
- Qian, L.-W.; Fourcaudot, A.B.; Leung, K.P. Silver Sulfadiazine Retards Wound Healing and Increases Hypertrophic Scarring in a Rabbit Ear Excisional Wound Model. J. Burn Care Res. 2017, 38, e418–e422. [Google Scholar] [CrossRef] [PubMed]
- Burd, A.; Kwok, C.H.; Hung, S.C.; Chan, H.S.; Gu, H.; Lam, W.K.; Huang, L. A Comparative Study of the Cytotoxicity of Silver-Based Dressings in Monolayer Cell, Tissue Explant, and Animal Models. Wound Repair Regen. 2007, 15, 94–104. [Google Scholar] [CrossRef]
- Capella-Monsonís, H.; Tilbury, M.A.; Wall, J.G.; Zeugolis, D.I. Porcine Mesothelium Matrix as a Biomaterial for Wound Healing Applications. Mater. Today Bio 2020, 7, 100057. [Google Scholar] [CrossRef]
- Dissemond, J.; Böttrich, J.G.; Braunwarth, H.; Hilt, J.; Wilken, P.; Münter, K.-C. Evidence for Silver in Wound Care—Meta-Analysis of Clinical Studies from 2000–2015. JDDG J. Dtsch. Dermatol. Ges. 2017, 15, 524–535. [Google Scholar] [CrossRef]
- Vermeulen, H.; van Hattem, J.M.; Storm-Versloot, M.N.; Ubbink, D.T. Topical Silver for Treating Infected Wounds. Cochrane Database Syst. Rev. 2007, CD005486. [Google Scholar] [CrossRef]
- Storm-Versloot, M.N.; Vos, C.G.; Ubbink, D.T.; Vermeulen, H. Topical Silver for Preventing Wound Infection. Cochrane Database Syst. Rev. 2010, CD006478. [Google Scholar] [CrossRef] [PubMed]
- Schaper, N.C.; van Netten, J.J.; Apelqvist, J.; Bus, S.A.; Hinchliffe, R.J.; Lipsky, B.A.; IWGDF Editorial Board. Practical Guidelines on the Prevention and Management of Diabetic Foot Disease (IWGDF 2019 Update). Diabetes Metab. Res. Rev. 2020, 36, e3266. [Google Scholar] [CrossRef] [PubMed]
- Leaper, D. Appropriate Use of Silver Dressings in Wounds: International Consensus Document. Int. Wound J. 2012, 9, 461–464. [Google Scholar] [CrossRef] [PubMed]

| Dressings | Silver Content | Description | Notable Characteristics | Findings Related to Biofilms |
|---|---|---|---|---|
| Aquacel® Ag+ Extra™ (ConvaTec) | 0.17 mg/cm2 | Hydrofiber™ Technology and Ag+ Technology—Two layers of a needle-punched nonwoven fleece of sodium silver CMC (carboxy methyl cellulose) fibers enhanced with EDTA and benzethonium chloride stitched with a high-purity cellulose thread. CMC forms a gel in contact with wound fluid. | Dressing formulated for disruption of mature biofilms [29]. Combinations of metal chelators (binds ions) and surfactants (softens EPS layer) [29]. Negative effect on fibroblast proliferation [23]. EDTA+BT may cause cytotoxicity [28]. | Biofilms were made with a colony-drip flow reactor. Pseudomonas aeruginosa (PA) biofilms (72 h, confirmed by SEM) were exposed to the dressing for 24 h and had 8.8 log10 bacteria as compared to the control with 9.2 log10 bacteria (not significant). With the 24 h biofilm, 9.2 log10 bacteria for the control versus 6.6 log10 bacteria with the dressing were observed [23]. Biofilms were grown in a CDC reactor for 72 h. Dressings were effective against Staphylococcus aureus (SA) and PA biofilms and Candida (CA) yeast biofilms (high levels of extracellular material). Multispecies bacteria (SA, PA, CA) were grown on porous polycarbonate in a CDFR flow reactor for 72 h, and the dressing was found to be effective [30]. With SA and PA biofilms a 4-log10 decrease over 5 days (9 log10 to 4 log10, 1 log10 every day), the addition of bacteria on 5th day did not result in biofilm re-formation [31]. In a porcine ex-vivo model, a 72 h grown biofilm was applied to the skin, and cultured for another 24 h. Biofilm viability was 13% as compared to 77% for the control [28]. In an in vivo murine model, colony biofilm was grown (72 h) on membranes and applied to the full-thickness excisional wound and, after 3 days of observation, no reduction in wound area or epithelization as compared to controls was noted [28]. PA- and PA+SA-infected dermal punch wounds were made in rabbit ears. Test dressing decreased bacterial counts and improved wound healing (p < 0.05), but dressing was not effective against the SA within the wound [32]. |
| Aquacel Ag Extra (ConvaTec) | 1.2% w/w ionic silver | Composed of sodium carboxymethylcellulose (CMC) fibers impregnated with ionic silver, enforced within strengthening fibers. | Ag+ released into broth (TSB) 28.1 ± 1.4 μg/mL in 24 h and 1.4 ± 0.1 μg/mL over 7 days [25]. In cell culture media with 10% FBS, 18.1 μg/mL Ag+ released after 72 h of agitation [30]. 107 ppm Ag released into de-epithelized porcine skin explants in 24 h [30]. Acute cytotoxic response towards HaCaT keratinocytes and primary human dermal fibroblasts [30]. | Extracellular polymeric substrate (EPS) embedded colonies of 45–600 μm for PA (7.6 log10 bacteria) and for MRSA (6.2 log10 bacteria), colony thickness of 54–88 μm were formed. An E. coli biofilm (5.6 log10 bacteria), 10–70 μm in diameter with 5–12 μm thickness, was formed. Upon exposure to the wound dressing, there were 2.8 log10 and 1.5 log10 decrease for PA, 2.9 and 1.6 log10 decrease for MRSA, and 3.5 and 1.8 log10 decrease for E.coli biofilms over 24 h and 7 days, respectively [25]. Biofilms were studied with an in vitro Drip Flow reactor. Dressing impeded new biofilm formation for PA (4.3 log10 decrease) and SA (2.3 log10 decrease). For SA and PA mixed species biofilms grown on hydroxyapatite for 3 days prior to treatment, exposure to dressings for 24 h resulted in 3.4 log10 decrease for SA and 1.3 log10 decrease for PA [33]. Deep reticular dermal wound infected with MRSA for 72 h to form biofilm in a porcine model, debrided, and treated with wound dressing. A 1 log10 decrease in MRSA on day 4, 2 log10 decrease in MRSA on day 8, and day 11 as compared to control were observed. Day 11 observations were 90% reepithelization, with marked angiogenesis and white cell infiltration, and granulation tissue formation approaching 76–100% [34]. |
| Acticoat™ 7 (Smith & Nephew) | 1.70 mg/cm2 | Two rayon/polyester non-woven inner cores laminated between three layers of nanocrystalline silver-coated high-density polyethylene mesh, designed to be the barrier against bacterial invasion. | Ag+ release in broth (TSB) 11.7 ± 0.8 µg/mL in 24 h and 8.0 ± 0.6 µg/mL in 7 days [25]. In cell culture media with 10% FBS, 18.1 μg/mL Ag+ released after 72 h [30]. Ag+ in de-epithelized porcine skin explants was 143 ppm Ag in 24 h. Acute toxic response towards HaCaT keratinocytes and primary human dermal fibroblasts Fibroblast proliferation decreased [23]. | In a Drip Flow reactor, dressing impeded new biofilm formation for both PA and SA. With mature SA and PA mixed species biofilms grown for 3 days, exposure to dressings for 24 h led to a 3.4 log10 decrease for SA and a 1.3 log10 decrease for PA [33]. PA biofilm had 7.6 log10 bacteria, MRSA biofilm had 6.2 log10 bacteria, and E. coli biofilm had log 5.610. For the PA biofilm, there was a 4.2 log10 decrease in bacteria in 2 h, and a 4.5 log10 decrease after 7 days; for MRSA, there was a 4.6 log10 decrease for both 24 h and 7 days. For the E.coli biofilms, there was a 4.8 and 5.0 log10 decrease in bacteria over 24 h and 7 days, respectively [25]. There was significant silver accumulation in the biofilms [25]. Dressing did not destroy biofilms for MRSA and PA [31]. |
| BDWG | No silver | Polyethylene glycol (PEG) gel containing benzalkonium chloride (0.13 wt%), citric acid (3.41%), and sodium citrate (3.57%). Manifested severe cytotoxicity towards fibroblasts, and fibroblast proliferation was compromised [34]. | Using an in vitro Drip Flow reactor, the dressing impeded new biofilm formation for both PA and SA. With SA and PA mixed species biofilms exposure to dressings for 24 h led to a significant decrease in bacteria (5.9 log10 decrease for SA and 6.6 log10 decrease for PA) [33]. A deep reticular porcine dermal wound model infected with MRSA (72 h biofilms) was debrided and then treated with wound dressing. A 2 log10 reduction in MRSA counts was observed after 4 days, and a 3 log10 decrease after 8 days and 11 days. The wound approached 80% reepithelization on day 11, along with marked angiogenesis, and granulation tissue formation approached 76–100% [34]. | |
| Biatain Ag (Coloplast) | 1 mg/cm2 | Hydrophilic polyurethane hydro cellular, silver ions in the form of a complex (formerly Contreet Foam). | ||
| Biatain Alginate Ag (Coloplast) | 0.95 mg/cm2 | An alginate dressing consists of calcium alginate, carboxymethylcellulose (CMC), and an ionic silver complex. | ||
| Contreet Foam (Coloplast) | 1 mg/cm2 | A soft hydrophilic polyurethane foam containing silver. Foam bonded to a semi-permeable polyurethane film. Silver ions are hydroactivated in the presence of fluid or wound exudate. In vitro studies show that silver release is sustained for 7 days, and the release is proportional to the amount of exudate absorbed. | ||
| Exufiber Ag+ (Mölnlycke) | A dressing made with PVA and hydroxypropyl cellulose gel with Ag2SO4 | Biofilms were grown on plates in a CDC reactor for 72 h and exposed to dressings for 24 h. Dressings were effective against SA and PA biofilms separately; for multispecies biofilms, the dressings were not effective [35]. | ||
| Ialugen SSD | 120 µg/cm2 | A dressing impregnated with cream containing Na hyaluronate, SSD, macrogol 4000, and 85% glycerol. | Silver in de-epithelized porcine skin explants: 188 ppm Ag in 24 h [30]. Dressing showed acute toxic response towards keratinocytes and primary human dermal fibroblasts. | |
| Kerracel® Ag (3M) | 0.2 mg/cm2/1.7% (w/w) Ag Oxysalts (Ag7NO11) [28] | A dressing formulated with Ag oxysalts™, a non-woven sterile wound dressing using a mix of 100% carboxymethylcellulose (CMC), cellulose fibers, and silver to create a barrier against bacterial growth for as long as 7 days. | Biofilms were grown in a CDC reactor for 72 h. Dressings were effective against SA and PA biofilms separately and ineffective against Candida yeast biofilms (24 h exposure). With multispecies biofilms on nonporous polycarbonate, the dressing was very effective but not so when the biofilms were grown on porous polycarbonate (better representation of hard-to-heal exudating wound) [35]. A 72 h grown biofilm was placed in porcine ex vivo skin, cultured for 24 h to allow for attachment, and dressing was applied for 24 h. Dressing led to 14% biofilm viability as compared to 75% for control. A 72 h colony biofilm grown on membranes was applied to the full-thickness excisional wound in a murine model. Exposure to dressing for 3 days led to a smaller wound area in PA and SA biofilms, although not statistically significant. Wound area and reepithelization were 34% for PA, control 15%, 31% for SA, and 14% for control. Macrophage reduction within the granulation tissue in SA biofilm-infected wounds was significant [28]. The study used an in vitro Drip Flow reactor. Dressing impeded new biofilm formation for both PA and SA. However, for SA and PA mixed species, exposure to dressings for 24 h led to a 0.8 log10 decrease for SA and a 0.3 log10 decrease for PA [33]. | |
| Maxorb Ag+ Extra (Medline Industries) | A dressing that uses CMC and calcium alginate with AgNaZrPO4 | Biofilms were grown in a CDC reactor for 72 h. Exposure to dressing for 24 h indicated that the dressings were effective against SA and PA biofilms separately and ineffective against Candida yeast biofilms. For a multispecies biofilm grown in a CDFR flow reactor biofilm on porous polycarbonate (better representation of hard-to-heal wound), the dressing was not effective [35]. | ||
| Mepilex Ag (Mölnlycke) | 1.25 mg/cm2 | A dressing composed of absorbent polyurethane foam with a composite of silver and activated carbon. The silver source is silver sulfate which releases silver ions. The outer film is permeable to water vapor and impervious to liquids [24]. There is always a layer with silicone adhesives that stays in contact with the wound. | The study was with partial-thickness burn in rats. The effect of dressing on the inflammatory phase (7 days), proliferative phase (14 days), and remodeling of the wound (30 days) was examined. Necrosis was noted, possibly due to poorer wound hydration due to the absorption of the wound exudate. Observations were as follows: higher inflammatory infiltration of healing PMN cells during the 7 days; on day 14, less hemorrhage, more angiogenesis, and more granulation tissue; and on day 30, more fibroblasts to promote wound closure [24]. | |
| Primatrix Ag (Integra) | 165 µg/cm2 | A dressing containing fetal bovine Type III collagen and silver. | A deep reticular porcine dermal wound was infected with MRSA for 72 h to form a biofilm, debrided, and then treated with wound dressing. There was a 2-log10 decrease reduction in MRSA counts obtained from biofilms on days 4, 8, and 11 as compared to the controls. The wound approached 85% reepithelization on day 11. Marked angiogenesis, along with white cell infiltration, was observed on day 11. Granulation tissue formation approached 76–100% on day 11 [34]. | |
| Procellera™ (Vomaris) | Ag: 0.9 mg/cm2 Zn: 0.3 mg/cm2 | Microcurrent-generating antimicrobial wound dressing consists of a matrix of alternating silver and zinc dots held in position on a polyester substrate with a biocompatible binder. | Antibacterial efficacy against β-lactamase bacteria, multidrug-resistant bacteria, and MRSA. Ineffective with Enterococcus bacteria [36]. | |
| Promogran™ PRISMA (3M) | 1% silver-ORC contains 25% w/w ionically bound Ag. Ag: 20 µg/cm2 | A sterile, freeze-dried composite of 44% oxidized regenerated cellulose (ORC), 55% collagen, and 1% silver-ORC. | Did not inhibit dermal fibroblast growth [23]. | PA biofilms made with colony-drip flow reactor (72 h, confirmed by SEM) upon exposure to dressing for 24 h led to 7.8 log10 bacteria as compared to the control of 9.2 log10, not a significant effect. Results for less mature 24 h biofilm were 9.2 log10 bacteria with control gauze versus 6.5 log10 with dressing. Gentamycin-treated biofilm reduction in BPA (bacterial proteases) was 77% compared to the control, possibly due to the ORC/collagen matrix [23]. Not very effective against MRSA [34]. |
| Polysheet metallic Ag | <25 µg/cm2 | A dressing with polyelectrolyte and polyvinyl alcohol polymeric sheet containing ionic and metallic silver. | Studies were conducted with an in vitro Drip Flow reactor. Dressing impeded new biofilm formation for PA but not for SA. For SA and PA mixed species biofilms, exposure to dressings for 24 h led to a 0.5 log10 decrease for SA and a 4.6 log10 decrease for PA [33]. | |
| PU Foam Ag salt | 0.35–0.4 mg/cm2 | A dressing with metallic silver and starch copolymers on a polyurethane membrane. | The amount of silver released into the broth (TSB): 14.7 ± 0.7 µg/mL Ag release in 24 h and then drops to 2.6 ± 0.1 µg/mL in 7 days (TSB). | Biofilms were grown in a Drip Flow reactor. The dressing was effective for thwarting new biofilm formation for PA but not for SA [33]. |
| Silvercel (3M) | 740 µg/cm2 to 863 µg/cm2, diffuse coating of Ag, (Ag+ or Ag coated fibers [30] 9 wt% silver in dressing [24] | A dressing composed of nonwoven hydroalginate, calcium alginate, guluronic acid (high-G) strength of 32%, sodium carboxymethylcellulose (8%), and nylon fibers (51%) covered with elemental silver (9%). | Silver in the de-epithelized porcine skin explants—111 ppm Ag in 24 h Acute toxic response towards keratinocytes and primary human dermal fibroblasts. | With dressing, the following were observed: a 1.9 log10 decrease in 24 h; after 7 days, a 0.9 log decrease for PA; and 2.6 and 1.6 log10 decrease for MRSA biofilms, and a 3.1 and 1.4 log10 decrease for E.coli biofilms [25]. Did not destroy biofilms for MRSA and PA [31]. In a study involving partial-thickness burns in rats, the dressing exhibited a decrease in necrosis, wound exudate, odor, as well as more granulation tissue helping wound healing. The presence of alginate possibly promoted better wound hydration and autolytic debridement [24]. |
| Silverlon (Silverlon) | 5.46 mg/cm2 | A dressing with silver on nylon cores. | The release of Ag into broth (TSB) was 8.1 ± 0.4 µg/mL in 24 h and 13.9 ± 0.7 µg/mL in 7 days. | With dressing, a 1.2 log10 decrease in PA biofilms was observed over 24 h, and after 7 days, a 2.9 log10 decrease; there were also a 1.6 and 2.3 log10 decrease in MRSA biofilms, and a 1.0 and 3.0 log10 decrease for E.coli biofilms for 24 h and 7 days [25]. |
| Silver sulfadiazine | 1 wt% micronized silver sulfadiazine in gel | A gel with stearyl alcohol, polyethylene glycol hexadecyl ether, liquid petrolatum, propylene glycol, methylparaben, propylparaben, butylhydroxytoluene, and purified water. | Partial-thickness burns were induced in rats, and the wounds were monitored during the inflammatory phase (7 days), proliferative phase (14 days), and remodeling phase (30 days). Dressing increased necrosis, possibly because the gel did not promote the hydration of the wound bed [24]. | |
| Tegaderm Ag mesh (3M) | 8 mL of silver per gram of dressing | Particles of silver sulfate are coated on the surface of cotton fibers. When wound exudate, sterile normal saline, sterile water, or liquid hydrogel comes in contact with the dressing, the silver sulfate dissolves, releasing silver ions in the dressing rapidly and over time. | ||
| UrgoClean Ag (Urgo Medical) | A dressing with lipid-colloid and poly-absorbent fiber with Ag2SO4. | Biofilms were grown in a CDC reactor for 72 h. The dressing was effective against SA and PA biofilms separately but ineffective against the Candida yeast biofilms. For the multispecies biofilms (SA, PA, CA) grown in a CDFR flow reactor on porous polycarbonate (better representation of hard-to-heal wound), the dressing was not effective [35]. | ||
| Urgotul Ag (Urgo Medical) | 3.5% ionic Ag [37] | Non-occlusive, non-adhesive, flexible lipid-colloid dressing comprising a polyester mesh impregnated with hydrocolloid and petroleum jelly particles and silver. |
| Dressings | Clinical Method Summary | Quantitative Results | Year [Ref.] |
|---|---|---|---|
| Contreet Foam | Uncontrolled open study. Treatment of bacteria-infected chronic venous leg ulcers in 25 patients over four weeks. Assessment: healing in terms of wound-bed tissue composition, odor, pain, dressing performance, and effect on the per-ulcer area. | 4 weeks: A mean reduction of 56% in the ulcer area (15.6 to 6.9 cm2) was noted. Week 1 observations were a mean reduction of 25% in granulation tissue from dull to healthy and that wound odor was significantly reduced. Half of the patients showed an increase in ulcer area after the removal of the Ag dressing (after 4 weeks). | 2003 [38] |
| Contreet silver-based foam dressing as compared to a control dressing (Allevyn Hydrocelluar) | A multicenter study (15 centers in 7 countries). Open block-randomized and controlled 4-week study of 129 patients (Contreet: 65, Allevyn: 64) with colonized chronic venous leg ulcers. | A decrease in odor was noted after 1 week of treatment for 83% with the Contreet versus 53% in the control group. Lower maceration was observed after 4 weeks in the Contreet foam (37%) as compared to control (48%). A 45% median reduction in ulcer area was observed as compared to 25% in the control group, suggesting a faster healing process. | 2005 [39] |
| Silvercel (silver-hydroalginate) compared with control Algosteril (calcium-hydroalginate) | A multicenter (13 centers) randomized, two-arm parallel-group study over 4 weeks of 99 patients (51 test group, 48 control group), with venous leg ulcer (71) or pressure ulcer (28); assessment performed over 4 weeks. | Fewer patients developed clinical infection (33%) compared to control (46%, p = 0.223). No patient in the test group required antibiotics as compared to the control (10.5%). Greater wound closure rate for the test group (0.32 ± 0.57 cm2/day) as compared to control (0.16 ± 0.40 cm2/day, p = 0.024). Reduction in wound severity score was greater in the test group (−32 ± 17%) as compared to the control group (−23 ± 25%; p = 0.034). The total modified ASEPSIS (wound scoring method) score over 14 days did not significantly differ between the test (104.2 ± 72.8) and control groups (95.4 ± 62.2; p = 0.791). | 2005 [40] |
| Contreet Foam Outcome Program Comparison with Aquacel Ag, Actisorb, Acticoat Control-local best practice | Randomized controlled trial: A total of 619 patients with ulcers of varying etiologies were treated for four weeks; patients were either treated with the silver foam dressing (326 patients) or with local best practice (293). The objective was to assess the effects on wound area reduction, slough and maceration, exudate level, overall wound progress, exudate handling, ease of use, odor, pain, time spent on dressing changes, and mean wear time of the dressing. | Median ulcer area reduction upon final visit: Ag foam—47.1%, control—31.8% (p = 0.0019). Mean slough on the final visit: Ag foam—7%, control—8.9%. Mean macerated peri-ulcer skin: Ag foam—10.9%, control—16.7% (p = 0.0383). The odor was absent within 1 week. Superior exudate handling as compared to other Ag dressings was noted. | 2006 [41] |
| Aquacel (1.2% ionic silver, AQ) and Algosteril (Calcium Alginate, CA) | A prospective, stratified, randomized, open-labeled, controlled, multicenter study, diabetic patients with non-ischemic Wagner Grade 1 or 2 diabetic foot ulcers (>1 cm2 area). A total of 134 patients’ wound dimensions were measured at 0, 4, 8 weeks, and upon healing. Standardized surgical debridement and callus removal were performed. | AQ-dressed ulcers showed a depth reduction of 0.25 ± 0.49 cm compared to 0.13 ± 0.37 cm in the CA-dressed ulcers (p = 0.04), An 8-week ulcer area reduction of 58.1% (AQ) vs. 60.5 (CA) (p = 0.948) was noted. The AQ group showed a healing speed of 0.29 ± 0.33 cm2 per week, compared to 0.26 ± 0.90 cm2/week for the control (p = 0.993). The 100% healing time was marginally lower for AQ (53 days) as compared to CA (58 days) (p = 0.34). Infected ulcers had a more favorable outcome with AQ vs. CA with systemic antibiotics. | 2007 [42] |
| Urgotul Ag vs. Urgotul | Open-labeled, randomized controlled trial studying venous leg ulcers with heavy bacterial colonization in 102 patients, where 80% of the wounds were not progressing with the previous treatment. | Week 0: Mean ulcer area 20.0 ± 17.8 cm2. Week 4: Mean surface area decreased by 6.5 ± 13.4 cm2 (median: 4.2 cm2) and 1.3 ± 9.0 cm2 (median: 1.1 cm2) in Ag dressing versus control groups, respectively (p = 0.023). Week 4: Bacterial colonization was not clinically observed in 39.2% of Ag dressing versus 16.7% in the control group. | 2008 [43] |
| Group 1: Acticoat; Group 2: Comfeet Ag hydrocolloid/Biatain Ag polyurethane foam; Group 3—Aquacel Ag | Prospective, comparative study on 75 patients, with 25 in each group. Wounds: leg ulcers, pressure ulcers, diabetic foot ulcers, and post-traumatic ulcers. All wounds showed clinical signs of infection. | Resolution of clinical signs of infection: Group 1—2.52 ± 1.29 weeks, Group 2—3.88 ± 0.44 weeks, Group 3—3.80 ± 0.58 weeks No clinical sign of infection: Week 2—60% for Group 1, 4% for Group 2 and 8% for Group 3 Fewer treatments were required in Group 1 to eliminate infection. | 2008 [44] |
| Aquacel® Ag, Acticoat™, Acticoat™ 7, Acticoat™ Absorbent, Contreet® Foam, Urgotul SSD versus non-silver dressings | In a multicenter study, 213 patients with active ulceration of the lower leg were presented for >6 weeks (107 patients had a random assignment to Ag dressings). The focus was on assessing the effectiveness of silver-donating antimicrobial dressings as a category. | No significant difference in the proportion of ulcers healed at 12 weeks: 59.6% for silver and 56.7% for control dressings. The overall median time to healing was 67 days for antimicrobial dressings and 58 days for the control group (p = 0.048). No significant differences were observed between the groups in terms of health-related quality of life. A significantly higher cost was associated with silver dressings. | 2009 [45] |
| Acticoat™ compared with Iodosorb cadexomer iodine | The study used a parallel-group, open-labeled randomized controlled trial (TBSA). Participants had a lower leg ulcer with an ankle brachial pressure index of 0.6 or above, the wound was 15 cm or less in diameter and had evidence of critical colonization. Sample of 281 participants, with 140 for Acticoat and 141 for Iodosorb, in a 12-week study. | Similar overall healing rate for silver dressing (64%) compared to iodine (63%), with a similar daily healing rate. Acticoat and Iodosorb were comparable in terms of the number of wounds healed. Acticoat was associated with a quicker healing rate during the first 2 weeks of treatment, but this was not sustained beyond that time. Silver dressing showed a significantly higher rate of healing for wounds that did not heal in the 12 weeks (larger, older wounds). | 2010 [46] |
| Tegaderm Ag mesh dressing compared to silver sulfadiazine cream | Randomized clinical trial in a single hospital for 8 weeks with 40 patients for treating pressure ulcers; study conducted detailed microbiologic studies of the wounds. | SSD cream application is labor-intensive and expensive. The mean healing rate in the eighth week was lower (25.06%) in the SSD group as compared to the mesh group (36.95%, not statistically significant, p = 0.507). Pressure Ulcer Scale for Healing (PUSH) score, an indicator of ulcer severity, was higher initially as well as in the eighth week in the SSD group compared to the mesh group (p = 0.473). Difficult to conclude anything definitive from the microbiologic studies and needs statistical analysis. | 2011 [47] |
| Urgotul Ag versus Urgotul (without Ag) | This was an open-labeled randomized controlled trial (not double-blind) for 4 weeks (followed for additional 4 weeks). Patients with venous leg ulcers (VLUs) showed at least three out of five clinical signs of bacterial colonization. A total of 99 patients (51 with silver and 48 control) participated in the study. | At week 4, the median wound closure rate was 0.145 cm2/day for Urgotul Silver vs. 0.044 cm2/day for the control group at week 4 (p = 0.009). At week 8, the median decrease in wound size was 5.9 cm2 for the Urgotul Silver group compared to 0.8 cm2 for the control group (p = 0.002). 55% of ulcers showed a >40% decrease in wound area for the Urgotul Silver group compared to 35%% for the control group. At week 4, 39.2% of ulcers showed no clinical signs of colonization as compared to 16.7% in the control group. Local adverse events were comparable in both groups. | 2012 [48] |
| Aquacel Ag dressing compared to Urgotul Ag | Two-arm parallel multi-center open-labeled randomized controlled clinical trial for 8 weeks with 281 patients with chronic venous leg ulcers across 43 centers in multiple countries. | After 8 weeks, there was a relative wound size reduction of 49.65% ± 52.53% in the Aquacel group as compared to 42.81% ± 60.00% in the Urgotul® group (p = 0.3158). At week 8, 39.5% of ulcers in the Aquacel group showed no clinical signs of heavy bacterial colonization, along with 32.5% of ulcers in the Urgotul® Silver group (no significant difference). A total of 15% of subjects in the Aquacel group had healed ulcers, while 15.9% of subjects in the Urgotul® Silver group had healed wounds (p = 0.0899). The inclusion of a placebo/control group would have been useful. | 2012 [49] |
| Biatain Ag vs. Biatain | The study was a double-blinded controlled study with 181 patients (87 control) and conducted across 38 centers in five countries. Patients with venous or predominantly venous leg ulcers were recruited. The 6-week treatment period was followed by a 4-week open study with only Biatain; observations on days 0, 28, 42, and 70. | Biatain Ag demonstrated a greater wound area reduction (42%) compared to Biatain after 6 weeks of treatment (35%) (p = 0.0853). This would be more significant if older, larger ulcers were considered. Healing rate: Biatain Ag showed a Gilman rate of 0.67 mm/week compared to 0.53 mm/week for Biatain (French group: control 0.33 mm/week) (p = 0.0852). Both groups reported similar frequencies of local inflammatory signs after 6 and 10 weeks of treatment. Adverse events (maceration, eczema, pain, and burn) were observed in six events in Biatain Ag versus four in Biatain. A country-wise discrepancy was evident in the study. | 2014 [50] |
| Aquacel Ag | The study was designed to evaluate the systemic absorption of silver in patients (criteria: silver levels > 0.5 µg/mL) with chronic inflammatory wounds and its association with silver toxicity. The study was a longitudinal, observational, multicenter, open-labeled pilot study using 40 elderly (patients mostly female, average age 74.3 years). | Dressing changed every 2 days between the initial day and day 28 of the treatment period. Mean wound surface area reduction was 22.8% (p = 0.041), along with a decrease in the fibrin percentage (beneficial for wound healing) between day 0 and day 28. Half the patients showed increased silver levels. There was no argyria or systemic toxicity. Elimination of silver from the body was slow and could result in cumulative toxicity, especially for elderly patients. The study recommends against long-term silver dressing use. | 2018 [51] |
| Aquacel® Ag+ Extra™ (All patients previously managed with traditional silver (26%), iodine (23%) or poly hexamethylene biguanide (PHMB) (11%) containing products or systemic antibiotics (12%)) | The study recruited 65 patients with wounds ranging in duration from 1 week to 20 years (median duration: 12 months). 47 cases (72%) had stagnant wounds, and 15 cases (23%) had deteriorating wounds, while 3 wounds were not recorded; observations were made for 1–11 weeks. Participants also had clinical signs of infection or critical colonization. | Observations were as follows: 17% of wounds healed, 62% of wounds showed improvement, 14% of wounds remained the same, and 8% of wounds deteriorated. Moderate exudate (52% n = 24) and high exudate (37% n = 34) levels before treatment led to low (31%, n = 20) and moderate (43% n = 28) levels, respectively, after treatment. Biofilms were observed in 49% and slough in 42% of wounds. After applying the dressing, wound bed tissue was 63% granulated. Healthy wound bed tissues increased from 33% to 67% after treatment. Necrotic, slough biofilm reduced from 92% to 40% following treatment. Peri-wound skin health improved in 67% of cases. | 2020 [27] |
| Acticoat™ Flex 7 (nano-Ag) with dressings without nano Ag | Retrospective study: 330 patients and 2242 patients control group in community centers with various types of wounds, including pressure injuries, diabetic foot ulcers, and venous leg ulcers (used Bates–Jensen Wound Assessment Tool). | Sustained silver release over 7 days. The mean time between dressing changes was 3.98 days vs. 1.87 days in control (p < 0.01), reducing nurse visits. The mean healing time for wounds treated with Acticoat 7 was significantly shorter (10.46 weeks) compared to wounds with control dressing (25.49 weeks). Only 0.9% of patients treated with Acticoat 7 dressing developed a systemic infection, compared to 3% in the comparative group. Potential for bias and no control for confounding variables, e.g., concurrent treatments. | 2021 [52] |
| Biatain® Ag Non-Adhesive Foam versus silver sulfadiazine | 60 adult patients diagnosed with type 2 diabetes mellitus, with diabetic foot ulcers (DFU) area of at least 1 cm2 were recruited. Treatment Group: Biatain® Ag Non-Adhesive Foam dressing applied at least every two days (38 patients). Control Group: 1% SSD cream applied once or twice per day (22 patients) A 4-week study, where debridement was performed during weekly visits, if necessary. | Enterococcus faecalis and Staphylococcus aureus were isolated from the wound culture in both groups. The proportion of the wound healed at week 4 in the SSD group was 27.00 ± 4.95%, while Biatain was 76.43 ± 7.41% (p < 0.0001). Silver foam facilitated wound closure faster than SSD in the patient population with HbA1c > 7% (59.94 ± 8.00% vs. 14.21 ± 3.72%, p = 0.027) and in patients with positive microbial isolates in their wound culture (60.87 ± 4.06% vs. 37.50 ± 5.89%, p = 0.020). | 2021 [53] |
| Biatain alginate Ag versus gauze (some with iodoform) | 40 patients in observation and 40 patients in the control group. Debridement and Biatain Alginate Ag were applied to the wounds. Dressing changed every 1 to 3 days. Assessment at 7, 14 days, and 1 month after treatment. The study observed the frequency of dressing changes, granulation tissue growth, wound formation, and healing time. | Pain score (VAS) was significantly different between Bitain and the control group (p < 0.05). Better outcomes in wound scar healing were observed as compared to the control group (p < 0.05). Enhanced granulation tissue growth was significantly higher in observation vs. control. Bacterial load was significantly lower than in the control group. | 2022 [54] |
| Aquacel Ag+ versus Sorbact dressing (Cutimed Sorbact, Essity, retains exudate, no release of any antimicrobials) | Retrospective Patient Chart Audit with 350 patient charts: 200 with Aquacel Ag+ and 150 with Sorbact. Data analyzed separately for Germany and the US (DFU and venous leg ulcers). | Unclear why specific dressings were chosen for specific patients. Germany: Wound percent reduction and wound closure comparable; greater proportion of Sorbact users needed surgery (0 vs. 11%, p = 0.039). US: Wounds were worsening before the use of Aquacel (49% vs. 34%, p = 0.01), regression analysis suggests that it was 3.53 times more likely to have wound healed in Aquacel cohort (p = 0.033). | 2023 [55] |
| Acticoat versus SoC | Prospective, open-labeled, randomized, placebo-controlled trial for acute diabetes-related foot ulcers, with 63 patients with Acticoat and 55 with SoC. The primary endpoint was the proportion of ulcers healed at 12 weeks. | Observation of ulcers healed at 12 weeks: 75% in the control group and 69% in the silver group (p = 0.49). No significant difference in complete ulcer healing (p = 0.53), osteomyelitis, need for amputation or antibiotic treatment between the silver and control groups. | 2023 [56] |
| Dressings | Clinical Method Summary | Quantitative Results | Year [Ref.] |
|---|---|---|---|
| Acticoat vs. 0.5% silver nitrate | Randomized 30 burn patients with symmetric wounds. | The frequency of burn wound sepsis (>105 organisms per gram of tissue) was less in the Acticoat-treated wounds than in those treated with silver nitrate (5 vs. 16), as well as the observations of secondary bacteremia (1 vs. 5). Dressing removal was less painful with the Acticoat than with silver nitrate. | 1998 [57] |
| Aquacel Ag | Phase II multicenter, open-labeled, noncomparative trial, where 24 patients with fresh superficial, mid-dermal, or mixed partial-thickness burns covering 5% to 20% of total body surface area (TBSA) were studied; trial lasted for 158 days. | Up to 77% of patients achieved over 95% re-epithelialization within 14 ± 3 days. The mean time for complete healing was 11.6 days.Significant reduction in pain between the baseline and post-burn days three and five. Positive reviews of conformability and ease of use were noted. | 2004 [58] |
| Acticoat vs. SSD | Prospective Randomized Trial of adults with partial-thickness burns, with 14 patients, with a focus on pain management during dressing change. | Mean pain scores for wounds treated with Acticoat were significantly lower (3.2) as compared to those treated with SSD (7.9) (p < 0.0001). | 2005 [59] |
| Aquacel Ag versus SSD | A comparative cost-effectiveness study comparing Aquacel Ag and SSD for superficial mid-dermal or mixed partial-thickness burns covering 5% to 40% TBSA (total body surface area). The 21-day study involved 84 patients, with 42 patients randomly assigned to each of the two treatment groups (mean age of 26.8 years, and 69.5% were men). | Aquacel® Ag dressing had 73.8% of patients achieving full re-epithelialization, compared to 60.0% achieving full re-epithelialization in the silver sulfadiazine group (not significant, p = 0.222). Silver sulfadiazine was found to have significantly greater flexibility and ease of movement. Adverse events were comparable between the two dressings, though Aquacel was associated with lower pain Total cost with Aquacel was found to be less than SSD. | 2006 [60] |
| Acticoat versus SSD | Multi-center randomized experimental design with blinding and positive parallel control. Work was performed at four burn centers across the country, with 98 patients with 166 residual wounds, comprising 79 men and 19 women, aged 18–63 years, with an average burn size of 54.17% TBSA. (5 g of SSD–Ag per 80 cm2); 20 days of medication. | Healing time for wounds treated with Acticoat was 12.42 ± 5.40 days, 3.35 days less than the control group (p < 0.01). At 15 days post-treatment, the healing percentage for the Acticoat group was 97.37%, higher than the control group but not significantly different. At the 6th day post-treatment, the bacterial clearance rate for the Acticoat group was 16.67% and, on the 12th day, it was 26.67%, both significantly higher than the control group, though no differences at the end of the study. | 2007 [61] |
| Aquacel Ag and SSD | 39 pediatric patients with partial-thickness burns treated with Aquacel Ag, 40 with SSD; the objective was to compare the hospital length of stay. | Patients treated with Aquacel Ag had a significantly shorter mean hospital stay (3.8 days) compared to those treated with SSD (5.9 days) (p = 0.001). Aquacel Ag adhered to the burn, reducing pain. | 2007 [62] |
| Urgotul SSD vs. Contreet Ag | A retrospective cohort study was performed with 2 groups of 20 burns until wounds healed or grafted. | Pain was “absent or slight” in 61 (92%) dressing changes with Urgotul SSD and in 60 (85%) of the dressing changes with Contreet Ag. The dressing application was comparable. Contreet Ag had a greater ability to absorb exudate than Urgotul SSD. | 2008 [63] |
| Silvasorb gel vs. Silvadene SSD | In a prospective, randomized study of 24 patients aged 2 months to 18 years, TBSA burns ranging from 1% to 40% were observed for 21 days or until full re-epithelialization. | SilvaSorb Gel was associated with significantly less pain compared to Silvadene, respectively (p = 0.004). No significant differences in the number of dressing changes (p = 0.383), re-epithelialization (p = 0.449), and rate of infection between the two dressings. | 2009 [64] |
| Urgotul SSD (petroleum jelly with SSD) versus Silvadene SSD | 68 patients with partial-thickness burn wounds less than 15%; monitored percentage of wound infection, total cost of wound dressing, pain medication, level of pain, and time of wound healing. | Time of wound closure was significantly shorter in the Urgotul SSD-treated group (10 ± 4 days bin Urgotul SSD- versus 12 ± 6 in 1% silver sulfadiazine-treated group) between both groups (p < 0.05). Average pain scores and pain medication in Urgotul SSD-treated group were significantly lower than the silver sulfadiazine-treated group (3 ± 1 versus 6 ± 2), p < 0.05. | 2009 [65] |
| Aquacel Ag vs. 1% SSD | A prospective, randomized trial, 70 patients were equally divided, all with partial-thickness burns. | Time-to-wound closure was significantly shorter in the Aquacel® Ag-treated group compared to the silver sulfadiazine-treated group (10 ± 3 days vs. 13.7 ± 4.3 days, p < 0.02). Number of hospital visits for dressing changes was significantly lower in the Aquacel® Ag-treated group (3.5 ± 1 visits) compared to the silver sulfadiazine-treated group (13.7 ± 4 visits, p < 0.001). Average pain scores during dressing changes were significantly lower in the Aquacel® Ag group than in the silver sulfadiazine group on days 1, 3, and 7. The scores were 4.1 ± 2.1, 2.1 ± 1.8, and 0.9 ± 1.4 for the Aquacel® Ag group versus 6.1 ± 2.3, 5.2 ± 2.1, and 3.3 ± 1.9 for the silver sulfadiazine group, respectively (p < 0.02). Total cost of treatment was significantly lower for the Aquacel® Ag group (52 ± 29 US dollars) compared to the silver sulfadiazine group (93 ± 36 US dollars, p < 0.01). | 2010 [66] |
| Askina Calgitrol Ag (silver alginate/polyurethane foam) vs. SSD | 65 patients with partial-thickness burn wounds, less than 24 h post-burn, with TBSA less than 15%; in the Askina Calgitrol Ag® group (30), dressings were changed every 5 days, in the SSD group (35), dressings were changed daily. | Time to healing was significantly shorter in the Askina Calgitrol Ag® group (7 ± 3.51 days) compared to the 1% Ag SD group (14 ± 4.18 days) (p < 0.02). Askina Calgitrol Ag® group had significantly lower pain scores compared to the 1% SSD group (2.23 ± 1.87 vs. 6.08 ± 2.33) (p < 0.02). Nursing time was significantly reduced in the Askina Calgitrol Ag® group (p < 0.02). | 2010 [67] |
| Mepilex Ag vs. SSD | Open, parallel, randomized, comparative, multicenter study with patients, 5 years and older, with partial-thickness thermal burns (2.5–20% TBSA); a total of 101 patients. | Mean healing rates were 71.7% for the Mepilex Ag group and 60.8% for the SSD group. Mean time to discharge from inpatient hospital care was shorter for the Mepilex Ag group (5.62 days) compared to the SSD group (8.31 days) (p = 0.034), and no significant difference in average healing time was observed. Less pain upon application and during wear in the acute stages of wound healing with Mepilex Ag (statistically significant). More cost effective than SSD (data from subsamples of patients). | 2011 [68] |
| Aquacel Ag vs. moist open burn ointment (MEBO) | 40 patients with partial-thickness facial burns were equally divided between silver dressing and control. | Aquacel® Ag group had a mean time of 10.5 days for reepithelization, compared to 12.4 days for the MEBO® group (p < 0.05). Aquacel® Ag group had softer, better-quality scars, though with some hyperpigmentation. Higher patient comfort was observed with Aquacel® Ag. | 2011 [69] |
| Aquacel Ag Burn Glove | Phase II non-comparative assessment of the management of partial thickness hand burns using a glove. 23 patients (mean age 41.2 years, male participants 74%) participated. The duration of treatment was 21 days. | A mean decrease in hand burn area from 29.4% at the baseline to 8.6% at the final evaluation, with 70% of hand burns fully re-epithelialized over 15.6 days. The mean pain score was 1.15 at rest and 2.29 during movement (0–10 range). Glove was well tolerated by patients. | 2012 [70] |
| Aquacel Ag vs. SSD | Randomized trial of superficial partial-thickness burns, with 24 subjects, 18 men and 6 women, aged between 19 and 53 years. | The number of treatments required for 100% re-epithelialization was higher for the SSD group (10.27 ± 7.46) compared to the Aquacel Ag group (4.10 ± 1.38) (p = 0.02). SSD group reported a mean pain score of 4.70 ± 2.22, while the Aquacel Ag group reported a score of 2.92 ± 1.12. (p = 0.03). | 2013 [71] |
| Aquacel® Ag and Acticoat | A prospective, randomized, controlled study of 100 patients with partial-thickness burns. | No significant differences between the dressings in terms of wound healing and bacterial colonization (p = 0.226–0.941), Aquacel® Ag had advantages regarding nurse experience (p < 0.001 to 0.125). Patients experienced similar baseline pain with both dressings. Reduced frequency of dressing changes in Aquacel group should be beneficial for the patient and nurse. | 2014 [72] |
| Mepilex™ Ag vs. Acticoat™ and Acticoat™ + Mepitel™ Ag | Children aged 0–15 years with acute partial-thickness burns (superficial partial to deep partial thickness) and TBSA of ≤10%, a total of 103 participants. | Median days to 95% re-epithelialization were 9.50 days for Acticoat™, 10.00 days for Acticoat™ + Mepitel™, and 7.00 days for Mepilex AgTM (statistically significant) Mepilex Ag™ silicone dressings decreased the FLACC score (nurse’s observation of pain) by 37%, as compared to Acticoat™ (p = 0.002). Silicone-based dressings are useful for pediatric population since it reduces pain and wound trauma. | 2015 [73] |
| Acticoat™ vs. Aquacel Ag | A single-blind, randomized controlled study in a Pediatric Emergency Department, included 89 children with superficial or mid-dermal burns (<10% TBSA), who were randomized to receive either the Acticoat™ (n = 45) or Aquacel® Ag (n = 44) dressings. | No significant difference between the groups in terms of percentage epithelialization by day 10, with Acticoat™ showing 93 ± 14% and Aquacel® Ag showing 94 ± 17% (p = 0.89). No significant difference in infection and escalation of care. Aquacel® Ag dressings (59) required significantly fewer dressing changes compared to Acticoat (102) (p = 0.03) | 2016 [74] |
| Procellera™ + Standard of Care (SoC) versus SoC (moleskin and Tegaderm) | A prospective randomized controlled two-arm Clinical Study for blister management. The study involved 80 Ranger recruits as participants in a 14-day study. | No significant difference in wound healing rates between the SoC group and the SoC + Procellera group (p = 0.528). No significant difference in pain management between the SoC and SoC + Procellera groups. | 2017 [75] |
| Mepilex A vs. Suprathel (DL-polylactic acid membrane) | A prospective randomized controlled trial comparing the outpatient treatment of pediatric and adult partial-thickness burns. 29 adults and 33 pediatric patients (almost equally split between two dressings). TBSA: 1–29% in Meiplex Ag and 1–20% in Suprathel group. | The median time to complete reepithelialization was 12 days for both groups (p = 0.75). Suprathel reported better overall scar quality, and Mepilex Ag increased stiffness of burned skin at 1 month post-burn. Patients experienced less pain with Suprathel (only for first 5 days, p = 0.03). | 2018 [76] |
| Silverlon vs. SSD or mafenide acetate (considered topical antimicrobials) | A 10-year retrospective analysis on a total of 987 combat burn casualties, with 184 patients in Group 1 (Silverlon) and 803 in Group 2 (topical antimicrobial); 49% of the cohort had third-degree burns. | The incidence of wound infection was 5.4% in Group 1 and 9.5% in Group 2 (p = 0.08), the overall mortality rate did not differ significantly between the groups (8% in Group 1). The incidence of bacteremia was 4.3% in Group 1 and 5.5% in Group 2, showing no significant difference (p = 1.0). Topical antimicrobials application was painful. | 2018 [77] |
| Acticoat Flex 3 vs. 1% SSD | A randomized, single-center, single-blind trial involving 100 adults aged 18–65 with second-degree burns. | Reepithelization: Acticoat: 48% (24/50 patients), SSD: 52% (26/50 patients) (p = 0.56). Number of dressing changes: Acticoat fewer than SSD (p < 0.001) | 2022 [78] |
| Procellera™ versus SoC (Standard of Care) | A single-center prospective, randomized controlled clinical trial with 38 patients with dermal burn/traumatic wounds. Procellera dressing compared with SOC: silver nylon, SSD ointment, bacitracin, xeroform, 5% sulfamylon solution, and Manuka honey, observations at 7-day. | In 52% of the Procellera-treated wounds, little to no biofilm could be detected by scanning electron microscopy compared to only 24% of SoC-treated wounds; Procellera lowered the increase in biofilm versus SoC (p < 0.05). | 2024 [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shrestha, S.; Wang, B.; Dutta, P.K. Commercial Silver-Based Dressings: In Vitro and Clinical Studies in Treatment of Chronic and Burn Wounds. Antibiotics 2024, 13, 910. https://doi.org/10.3390/antibiotics13090910
Shrestha S, Wang B, Dutta PK. Commercial Silver-Based Dressings: In Vitro and Clinical Studies in Treatment of Chronic and Burn Wounds. Antibiotics. 2024; 13(9):910. https://doi.org/10.3390/antibiotics13090910
Chicago/Turabian StyleShrestha, Sweta, Bo Wang, and Prabir K. Dutta. 2024. "Commercial Silver-Based Dressings: In Vitro and Clinical Studies in Treatment of Chronic and Burn Wounds" Antibiotics 13, no. 9: 910. https://doi.org/10.3390/antibiotics13090910
APA StyleShrestha, S., Wang, B., & Dutta, P. K. (2024). Commercial Silver-Based Dressings: In Vitro and Clinical Studies in Treatment of Chronic and Burn Wounds. Antibiotics, 13(9), 910. https://doi.org/10.3390/antibiotics13090910








