Antimicrobial Efficacy of Five Wound Irrigation Solutions in the Biofilm Microenvironment In Vitro and Ex Vivo
Abstract
1. Introduction
2. Results
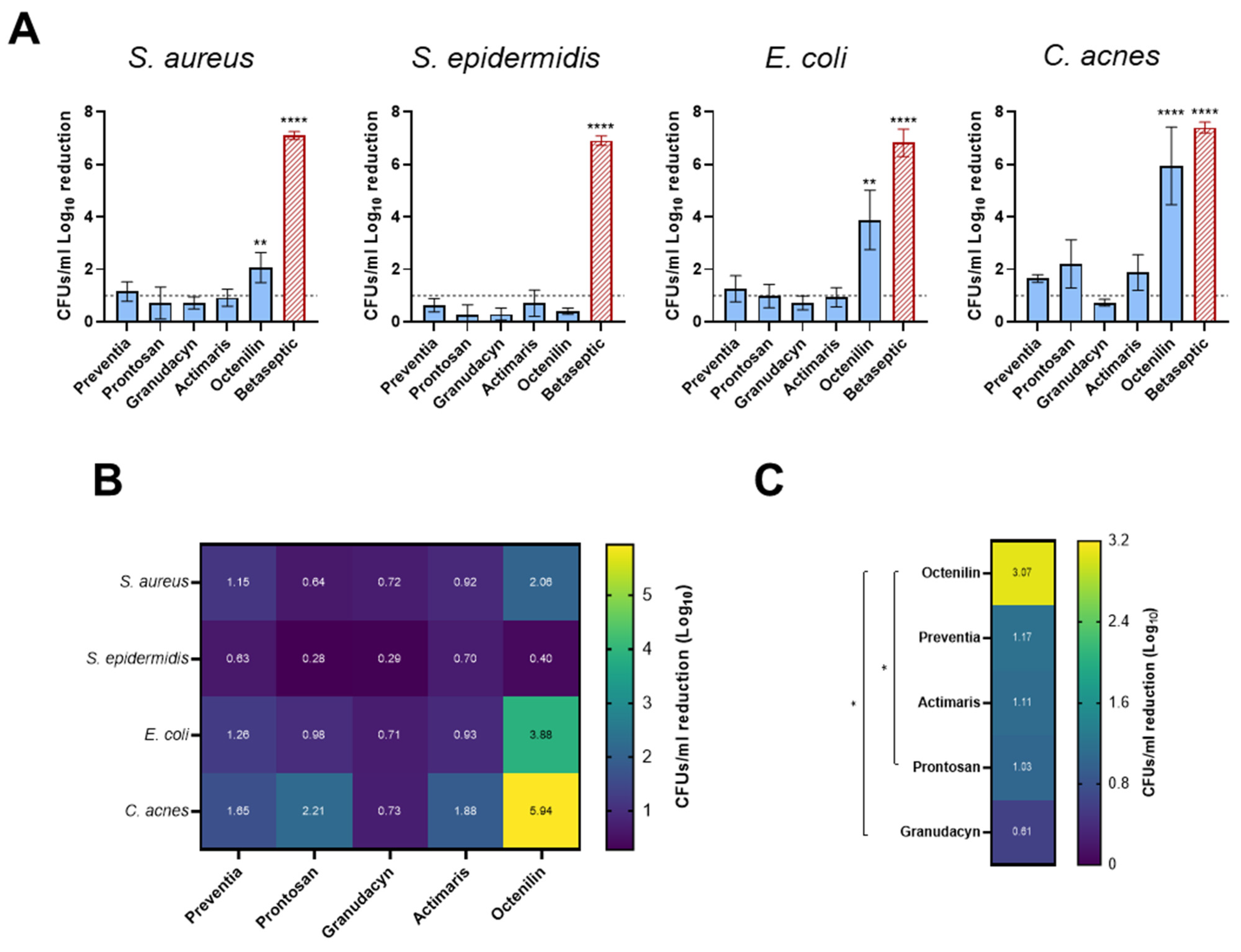
2.1. Biofilms on Titanium Alloy (TAV) Discs In Vitro Assay
2.2. Staphylococcal Abscess Communities (SACs) in Collagen Tissue In Vitro Assay
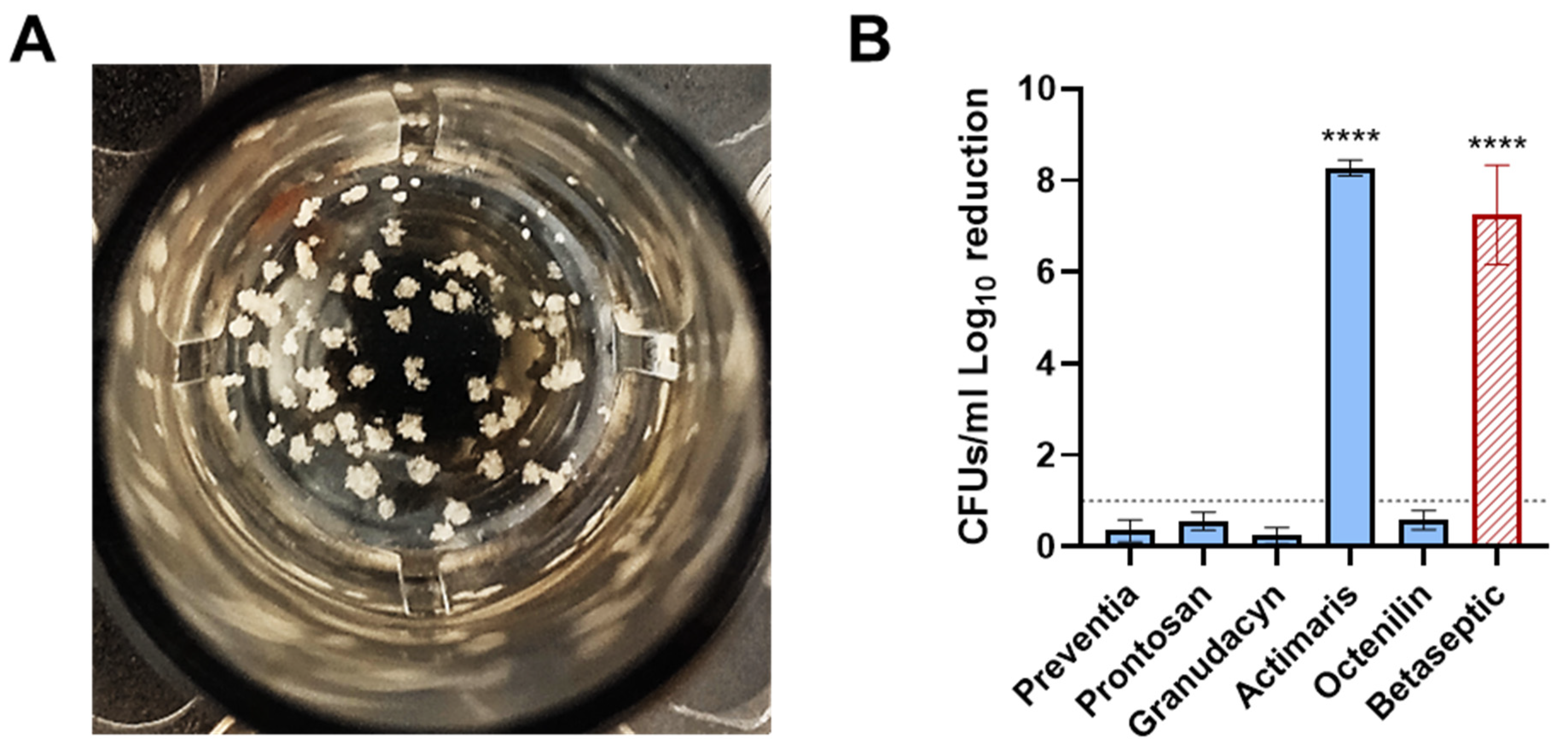
2.3. PJI Sonication Solutions Ex Vivo Assay
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Cultivation
4.2. Biofilm Assays
4.3. Treatment with Irrigation Solutions
4.4. S. aureus Abscess Communities (SACs)
4.5. Sonication Fluids Treatment
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beck, M.; Bolliger, L.; Brand, C.; Christen, B. SIRIS Report 2023. In Annual Report of the Swiss National Joint Registry, Hip and Knee, 2012–2022; SIRIS: Bern, Switzerland, 2024; Available online: https://www.siris-implant.ch/en/Downloads&category=16 (accessed on 20 November 2024).
- Del Pozo, J.L.; Patel, R. Clinical practice. Infection associated with prosthetic joints. N. Engl. J. Med. 2009, 361, 787–794. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Lau, E.; Watson, H.; Schmier, J.K.; Parvizi, J. Economic burden of periprosthetic joint infection in the United States. J. Arthroplast. 2012, 27, 61–65.e61. [Google Scholar] [CrossRef] [PubMed]
- Patel, R. Periprosthetic Joint Infection. N. Engl. J. Med. 2023, 388, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Barberan, J.; Aguilar, L.; Carroquino, G.; Gimenez, M.J.; Sanchez, B.; Martinez, D.; Prieto, J. Conservative treatment of staphylococcal prosthetic joint infections in elderly patients. Am. J. Med. 2006, 119, 993.e7–993.e10. [Google Scholar] [CrossRef] [PubMed]
- Giulieri, S.G.; Graber, P.; Ochsner, P.E.; Zimmerli, W. Management of infection associated with total hip arthroplasty according to a treatment algorithm. Infection 2004, 32, 222–228. [Google Scholar] [CrossRef]
- Laffer, R.R.; Graber, P.; Ochsner, P.E.; Zimmerli, W. Outcome of prosthetic knee-associated infection: Evaluation of 40 consecutive episodes at a single centre. Clin. Microbiol. Infect. 2006, 12, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.R.; Chonko, D.J.; Pigott, M.; Sullivan, A.C.; Moore, K.; Stoodley, P. Mapping bacterial biofilm on explanted orthopedic hardware: An analysis of 14 consecutive cases. APMIS 2023, 131, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Staats, A.; Li, D.; Sullivan, A.C.; Stoodley, P. Biofilm formation in periprosthetic joint infections. Ann. Jt. 2021, 6, 43. [Google Scholar] [CrossRef]
- Rzycki, M.; Drabik, D.; Szostak-Paluch, K.; Hanus-Lorenz, B.; Kraszewski, S. Unraveling the mechanism of octenidine and chlorhexidine on membranes: Does electrostatics matter? Biophys. J. 2021, 120, 3392–3408. [Google Scholar] [CrossRef]
- Salisbury, A.-M.; Mullin, M.; Chen, R.; Percival, S.L. Antibiofilm Efficacy of Polihexanide, Octenidine and Sodium Hypochlorite/Hypochlorous Acid Based Wound Irrigation Solutions against Staphylococcus aureus, Pseudomonas aeruginosa and a Multispecies Biofilm. In Advances in Microbiology, Infectious Diseases and Public Health: Volume 16; Donelli, G., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 53–67. [Google Scholar] [CrossRef]
- Wille, J.; Coenye, T. Biofilm dispersion: The key to biofilm eradication or opening Pandora’s box? Biofilm 2020, 2, 100027. [Google Scholar] [CrossRef] [PubMed]
- Personnic, N.; Doublet, P.; Jarraud, S. Intracellular persister: A stealth agent recalcitrant to antibiotics. Front. Cell Infect. Microbiol. 2023, 13, 1141868. [Google Scholar] [CrossRef] [PubMed]
- Izakovicova, P.; Borens, O.; Trampuz, A. Periprosthetic joint infection: Current concepts and outlook. EFORT Open Rev. 2019, 4, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Renz, N.; Trampuz, A.; Ojeda-Thies, C. Twenty common errors in the diagnosis and treatment of periprosthetic joint infection. Int. Orthop. 2020, 44, 3–14. [Google Scholar] [CrossRef]
- Haddad, F.S.; Sukeik, M.; Alazzawi, S. Is single-stage revision according to a strict protocol effective in treatment of chronic knee arthroplasty infections? Clin. Orthop. Relat. Res. 2015, 473, 8–14. [Google Scholar] [CrossRef]
- Volpin, A.; Sukeik, M.; Alazzawi, S.; Haddad, F.S. Aggressive Early Debridement in Treatment of Acute Periprosthetic Joint Infections After Hip and Knee Replacements. Open Orthop. J. 2016, 10, 669–678. [Google Scholar] [CrossRef]
- Zimmerli, W.; Lew, P.D.; Waldvogel, F.A. Pathogenesis of foreign body infection. Evidence for a local granulocyte defect. J. Clin. Investig. 1984, 73, 1191–1200. [Google Scholar] [CrossRef]
- Anderson, J.T.; Barnes, A.J.; Stambough, J.B. The Role of Antiseptic Irrigation Solutions and Topical Antibiotics in Total Joint Arthroplasty. J. Surg. Orthop. Adv. 2021, 30, 226–230. [Google Scholar]
- Calkins, T.E.; Culvern, C.; Nam, D.; Gerlinger, T.L.; Levine, B.R.; Sporer, S.M.; Della Valle, C.J. Dilute Betadine Lavage Reduces the Risk of Acute Postoperative Periprosthetic Joint Infection in Aseptic Revision Total Knee and Hip Arthroplasty: A Randomized Controlled Trial. J. Arthroplast. 2020, 35, 538–543.e531. [Google Scholar] [CrossRef] [PubMed]
- Blom, A.; Cho, J.; Fleischman, A.; Goswami, K.; Ketonis, C.; Kunutsor, S.K.; Makar, G.; Meeker, D.G.; Morgan-Jones, R.; Ortega-Pena, S.; et al. General Assembly, Prevention, Antiseptic Irrigation Solution: Proceedings of International Consensus on Orthopedic Infections. J. Arthroplast. 2019, 34, S131–S138. [Google Scholar] [CrossRef] [PubMed]
- Bonez, P.C.; Dos Santos Alves, C.F.; Dalmolin, T.V.; Agertt, V.A.; Mizdal, C.R.; Flores Vda, C.; Marques, J.B.; Santos, R.C.; Anraku de Campos, M.M. Chlorhexidine activity against bacterial biofilms. Am. J. Infect. Control 2013, 41, e119–e122. [Google Scholar] [CrossRef] [PubMed]
- Gaur, G.; Predtechenskaya, M.; Voyich, J.M.; James, G.; Stewart, P.S.; Borgogna, T.R. Assessing the Effects of Surgical Irrigation Solutions on Human Neutrophil Interactions with Nascent Staphylococcus aureus Biofilms. Microorganisms 2024, 12, 1951. [Google Scholar] [CrossRef] [PubMed]
- Kadkhoda, Z.; Amarlu, Z.; Eshraghi, S.; Samiei, N. Antimicrobial effect of chlorhexidine on Aggregatibacter actinomycetemcomitans biofilms associated with peri-implantitis. J. Dent. Res. Dent. Clin. Dent. Prospect. 2016, 10, 176–180. [Google Scholar] [CrossRef]
- Lollobrigida, M.; Filardo, S.; Sessa, R.; Di Pietro, M.; Bozzuto, G.; Molinari, A.; Lamazza, L.; Vozza, I.; De Biase, A. Antibacterial Activity and Impact of Different Antiseptics on Biofilm-Contaminated Implant Surfaces. Appl. Sci. 2019, 9, 5467. [Google Scholar] [CrossRef]
- Ready, D.; Theodoridis, G.; Green, I.; Ciric, L.; Pratten, J.; Tay, W.; McDonald, A. In vitro evaluation of the antibiofilm properties of chlorhexidine and delmopinol on dental implant surfaces. Int. J. Antimicrob. Agents 2015, 45, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Ibarburu, B.; Diaz-Navarro, M.; Ibarra, G.; Rivera, A.; Hafian, R.; Irigoyen, A.; Carrillo, R.; Perez-Cano, R.; Munoz, P.; Garcia-Ruano, A.; et al. Efficacy of Povidone Iodine Against Microbial Biofilms in Breast Implants With Different Textures: Results From an in vitro Study. Front. Microbiol. 2022, 13, 868347. [Google Scholar] [CrossRef]
- Semeshchenko, D.; Veiga, M.F.; Visus, M.; Farinati, A.; Huespe, I.; HIBA Hip Surgery Unit; Buttaro, M.A.; Slullitel, P.A. Povidone-iodine and Silver-nitrate are Equally Effective in Eradicating Staphylococcal Biofilm Grown on a Titanium Surface: An in vitro Analysis. J. Hosp. Infect. 2024, 155, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Huang, X.; Lv, W.; Zhou, J. The Toxicity and Antibacterial Effects of Povidone-Iodine Irrigation in Fracture Surgery. Orthop. Surg. 2022, 14, 2286–2297. [Google Scholar] [CrossRef] [PubMed]
- Esin, S.; Kaya, E.; Maisetta, G.; Romanelli, M.; Batoni, G. The antibacterial and antibiofilm activity of Granudacyn in vitro in a 3D collagen wound infection model. J. Wound Care 2022, 31, 908–922. [Google Scholar] [CrossRef]
- Hubner, N.O.; Siebert, J.; Kramer, A. Octenidine dihydrochloride, a modern antiseptic for skin, mucous membranes and wounds. Skin. Pharmacol. Physiol. 2010, 23, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Koburger, T.; Hubner, N.O.; Braun, M.; Siebert, J.; Kramer, A. Standardized comparison of antiseptic efficacy of triclosan, PVP-iodine, octenidine dihydrochloride, polyhexanide and chlorhexidine digluconate. J. Antimicrob. Chemother. 2010, 65, 1712–1719. [Google Scholar] [CrossRef] [PubMed]
- Severing, A.L.; Rembe, J.D.; Koester, V.; Stuermer, E.K. Safety and efficacy profiles of different commercial sodium hypochlorite/hypochlorous acid solutions (NaClO/HClO): Antimicrobial efficacy, cytotoxic impact and physicochemical parameters in vitro. J. Antimicrob. Chemother. 2019, 74, 365–372. [Google Scholar] [CrossRef]
- Ochsner, P.E.; Hailemariam, S. Histology of osteosynthesis associated bone infection. Injury 2006, 37 (Suppl. S2), S49–S58. [Google Scholar] [CrossRef] [PubMed]
- Hofstee, M.I.; Riool, M.; Terjajevs, I.; Thompson, K.; Stoddart, M.J.; Richards, R.G.; Zaat, S.A.J.; Moriarty, T.F. Three-Dimensional In Vitro Staphylococcus aureus Abscess Communities Display Antibiotic Tolerance and Protection from Neutrophil Clearance. Infect. Immun. 2020, 88, e00293-20. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.G.; DeDent, A.C.; Schneewind, O.; Missiakas, D. A play in four acts: Staphylococcus aureus abscess formation. Trends Microbiol. 2011, 19, 225–232. [Google Scholar] [CrossRef]
- Masters, E.A.; Trombetta, R.P.; de Mesy Bentley, K.L.; Boyce, B.F.; Gill, A.L.; Gill, S.R.; Nishitani, K.; Ishikawa, M.; Morita, Y.; Ito, H.; et al. Evolving concepts in bone infection: Redefining “biofilm”, “acute vs. chronic osteomyelitis”, “the immune proteome” and “local antibiotic therapy”. Bone Res. 2019, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Pankey, G.A.; Sabath, L.D. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin. Infect. Dis. 2004, 38, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Amalaradjou, M.A.; Venkitanarayanan, K. Antibiofilm Effect of Octenidine Hydrochloride on Staphylococcus aureus, MRSA and VRSA. Pathogens 2014, 3, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Rivera, E.S.; Weiss, A.; Migas, L.G.; Freiberg, J.A.; Djambazova, K.V.; Neumann, E.K.; Van de Plas, R.; Spraggins, J.M.; Skaar, E.P.; Caprioli, R.M. Imaging mass spectrometry reveals complex lipid distributions across Staphylococcus aureus biofilm layers. J. Mass. Spectrom. Adv. Clin. Lab. 2022, 26, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.H.; Monk, I.R.; Goncalves da Silva, A.; Seemann, T.; Chua, K.Y.L.; Kearns, A.; Hill, R.; Woodford, N.; Bartels, M.D.; Strommenger, B.; et al. Global spread of three multidrug-resistant lineages of Staphylococcus epidermidis. Nat. Microbiol. 2018, 3, 1175–1185. [Google Scholar] [CrossRef]
- Wang, B.; Zhan, Q.; Xiao, Y.; Xu, Y.; Zhao, H.; Rao, L.; Wang, X.; Zhang, J.; Shen, L.; Zhou, Y.; et al. Mupirocin enhances the biofilm formation of Staphylococcus epidermidis in an atlE-dependent manner. Int. J. Antimicrob. Agents 2023, 62, 106904. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.; Mayfield, C.K.; Leong, J.; Deckey, D.G.; Zega, A.; Glasser, J.; Daniels, A.H.; Eberson, C.; Green, A.; Born, C. Early adherence and biofilm formation of Cutibacterium acnes (formerly Propionibacterium acnes) on spinal implant materials. Spine J. 2020, 20, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Gannesen, A.V.; Zdorovenko, E.L.; Botchkova, E.A.; Hardouin, J.; Massier, S.; Kopitsyn, D.S.; Gorbachevskii, M.V.; Kadykova, A.A.; Shashkov, A.S.; Zhurina, M.V.; et al. Composition of the Biofilm Matrix of Cutibacterium acnes Acneic Strain RT5. Front. Microbiol. 2019, 10, 1284. [Google Scholar] [CrossRef]
- McCrate, O.A.; Zhou, X.; Reichhardt, C.; Cegelski, L. Sum of the parts: Composition and architecture of the bacterial extracellular matrix. J. Mol. Biol. 2013, 425, 4286–4294. [Google Scholar] [CrossRef] [PubMed]
- DeFrancesco, A.S.; Masloboeva, N.; Syed, A.K.; DeLoughery, A.; Bradshaw, N.; Li, G.W.; Gilmore, M.S.; Walker, S.; Losick, R. Genome-wide screen for genes involved in eDNA release during biofilm formation by Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 2017, 114, E5969–E5978. [Google Scholar] [CrossRef]
- Dengler, V.; Foulston, L.; DeFrancesco, A.S.; Losick, R. An Electrostatic Net Model for the Role of Extracellular DNA in Biofilm Formation by Staphylococcus aureus. J. Bacteriol. 2015, 197, 3779–3787. [Google Scholar] [CrossRef] [PubMed]
- Foulston, L.; Elsholz, A.K.; DeFrancesco, A.S.; Losick, R. The extracellular matrix of Staphylococcus aureus biofilms comprises cytoplasmic proteins that associate with the cell surface in response to decreasing pH. mBio 2014, 5, 10–128. [Google Scholar] [CrossRef]
- Melo, L.D.R.; Pinto, G.; Oliveira, F.; Vilas-Boas, D.; Almeida, C.; Sillankorva, S.; Cerca, N.; Azeredo, J. The Protective Effect of Staphylococcus epidermidis Biofilm Matrix against Phage Predation. Viruses 2020, 12, 1076. [Google Scholar] [CrossRef] [PubMed]
- Broekhuizen, C.A.; de Boer, L.; Schipper, K.; Jones, C.D.; Quadir, S.; Feldman, R.G.; Dankert, J.; Vandenbroucke-Grauls, C.M.; Weening, J.J.; Zaat, S.A. Peri-implant tissue is an important niche for Staphylococcus epidermidis in experimental biomaterial-associated infection in mice. Infect. Immun. 2007, 75, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Broekhuizen, C.A.; de Boer, L.; Schipper, K.; Jones, C.D.; Quadir, S.; Vandenbroucke-Grauls, C.M.; Zaat, S.A. Staphylococcus epidermidis is cleared from biomaterial implants but persists in peri-implant tissue in mice despite rifampicin/vancomycin treatment. J. Biomed. Mater. Res. A 2008, 85, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Riool, M.; de Boer, L.; Jaspers, V.; van der Loos, C.M.; van Wamel, W.J.B.; Wu, G.; Kwakman, P.H.S.; Zaat, S.A.J. Staphylococcus epidermidis originating from titanium implants infects surrounding tissue and immune cells. Acta Biomater. 2014, 10, 5202–5212. [Google Scholar] [CrossRef]
- Hatlen, T.J.; Miller, L.G. Staphylococcal Skin and Soft Tissue Infections. Infect. Dis. Clin. 2021, 35, 81–105. [Google Scholar] [CrossRef] [PubMed]
- Hofstee, M.I.; Heider, A.; Hackel, S.; Constant, C.; Riool, M.; Richards, R.G.; Moriarty, T.F.; Zaat, S.A.J. In Vitro 3D Staphylococcus aureus Abscess Communities Induce Bone Marrow Cells to Expand into Myeloid-Derived Suppressor Cells. Pathogens 2021, 10, 1446. [Google Scholar] [CrossRef]
- Seiser, S.; Cerbu, D.; Gallhofer, A.; Matiasek, J.; Elbe-Burger, A. Comparative assessment of commercially available wound gels in ex vivo human skin reveals major differences in immune response-modulatory effects. Sci. Rep. 2022, 12, 17481. [Google Scholar] [CrossRef] [PubMed]
- Nekoofar, M.H.; Namazikhah, M.S.; Sheykhrezae, M.S.; Mohammadi, M.M.; Kazemi, A.; Aseeley, Z.; Dummer, P.M. pH of pus collected from periapical abscesses. Int. Endod. J. 2009, 42, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.; Sauermann, R.; Joukhadar, C. Principles of antibiotic penetration into abscess fluid. Pharmacology 2006, 78, 1–10. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Y.; Breslawec, A.P.; Liang, T.; Deng, Z.; Kuperman, L.L.; Yu, Q. Strategy to combat biofilms: A focus on biofilm dispersal enzymes. NPJ Biofilms Microbiomes 2023, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Chua, S.L.; Liu, Y.; Yam, J.K.; Chen, Y.; Vejborg, R.M.; Tan, B.G.; Kjelleberg, S.; Tolker-Nielsen, T.; Givskov, M.; Yang, L. Dispersed cells represent a distinct stage in the transition from bacterial biofilm to planktonic lifestyles. Nat. Commun. 2014, 5, 4462. [Google Scholar] [CrossRef] [PubMed]
- Chua, S.L.; Tan, S.Y.; Rybtke, M.T.; Chen, Y.; Rice, S.A.; Kjelleberg, S.; Tolker-Nielsen, T.; Yang, L.; Givskov, M. Bis-(3′-5′)-cyclic dimeric GMP regulates antimicrobial peptide resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2013, 57, 2066–2075. [Google Scholar] [CrossRef]
- Yan, J.; Bassler, B.L. Surviving as a Community: Antibiotic Tolerance and Persistence in Bacterial Biofilms. Cell Host Microbe 2019, 26, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Tunney, M.M.; Patrick, S.; Gorman, S.P.; Nixon, J.R.; Anderson, N.; Davis, R.I.; Hanna, D.; Ramage, G. Improved detection of infection in hip replacements. A currently underestimated problem. J. Bone Jt. Surg. Br. 1998, 80, 568–572. [Google Scholar] [CrossRef]
- Weinstein, E.J.; Stephens-Shields, A.J.; Newcomb, C.W.; Silibovsky, R.; Nelson, C.L.; O’Donnell, J.A.; Glaser, L.J.; Hsieh, E.; Hanberg, J.S.; Tate, J.P.; et al. Incidence, Microbiological Studies, and Factors Associated With Prosthetic Joint Infection After Total Knee Arthroplasty. JAMA Netw. Open 2023, 6, e2340457. [Google Scholar] [CrossRef]
- Prinz, J.; Wink, M.; Neuhaus, S.; Grob, M.C.; Walt, H.; Bosshard, P.P.; Achermann, Y. Effective Biofilm Eradication on Orthopedic Implants with Methylene Blue Based Antimicrobial Photodynamic Therapy In Vitro. Antibiotics 2023, 12, 118. [Google Scholar] [CrossRef] [PubMed]
| Irrigation Solutions | Company | Active Ingredients | Solvent | Indication by the Company (Package Insert) |
|---|---|---|---|---|
| Preventia® | Hartmann (Heidenheim an der Brenz, Germany) | Polyhexanide (0.1%), poloxamer | H2O |
|
| Prontosan® | B. Braun (Sempach, Switzerland) | Polyhexanide (0.1%), betaine (0.1%) | H2O |
|
| Granudacyn® | Mölnlycke (Schlieren, Switzerland) | Sodium chloride, hypochlorous acid (<0.005%), sodium hypochlorite (<0.005%) | H2O |
|
| ActiMaris® forte | ActiMaris (Appenzell, Switzerland) | Oxychlorit NaOCl (0.2%), sea salt (3%) | H2O |
|
| Octenilin® | Schülke & Mayr (Frauenfeld, Switzerland) | Ethylhexylglycerol, octenidine HCl | H2O |
|
| Growth: Yes, 1; No, 0 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Pathogen | Concentration (CFUs/mL) | Ringer | Preventia | Prontosan | Granudacyn | Actimaris | Octenilin | Betaseptic |
| S. aureus | 1250 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| S. aureus | 2500 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| S. aureus | 2000 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| S. epidermidis | 1250 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| S. aureus | 1250 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| E. cloacae | 1250 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total eradication | 0% | 100% | 100% | 66% | 100% | 100% | 100% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honegger, A.L.; Schweizer, T.A.; Achermann, Y.; Bosshard, P.P. Antimicrobial Efficacy of Five Wound Irrigation Solutions in the Biofilm Microenvironment In Vitro and Ex Vivo. Antibiotics 2025, 14, 25. https://doi.org/10.3390/antibiotics14010025
Honegger AL, Schweizer TA, Achermann Y, Bosshard PP. Antimicrobial Efficacy of Five Wound Irrigation Solutions in the Biofilm Microenvironment In Vitro and Ex Vivo. Antibiotics. 2025; 14(1):25. https://doi.org/10.3390/antibiotics14010025
Chicago/Turabian StyleHonegger, Anja L., Tiziano A. Schweizer, Yvonne Achermann, and Philipp P. Bosshard. 2025. "Antimicrobial Efficacy of Five Wound Irrigation Solutions in the Biofilm Microenvironment In Vitro and Ex Vivo" Antibiotics 14, no. 1: 25. https://doi.org/10.3390/antibiotics14010025
APA StyleHonegger, A. L., Schweizer, T. A., Achermann, Y., & Bosshard, P. P. (2025). Antimicrobial Efficacy of Five Wound Irrigation Solutions in the Biofilm Microenvironment In Vitro and Ex Vivo. Antibiotics, 14(1), 25. https://doi.org/10.3390/antibiotics14010025







