Glucose Supplementation Enhances the Bactericidal Effect of Penicillin and Gentamicin on Streptococcus sanguinis Persisters
Abstract
1. Introduction
2. Results
2.1. Minimum Inhibitory Concentration (MIC) of PCG and GM Against S. sanguinis
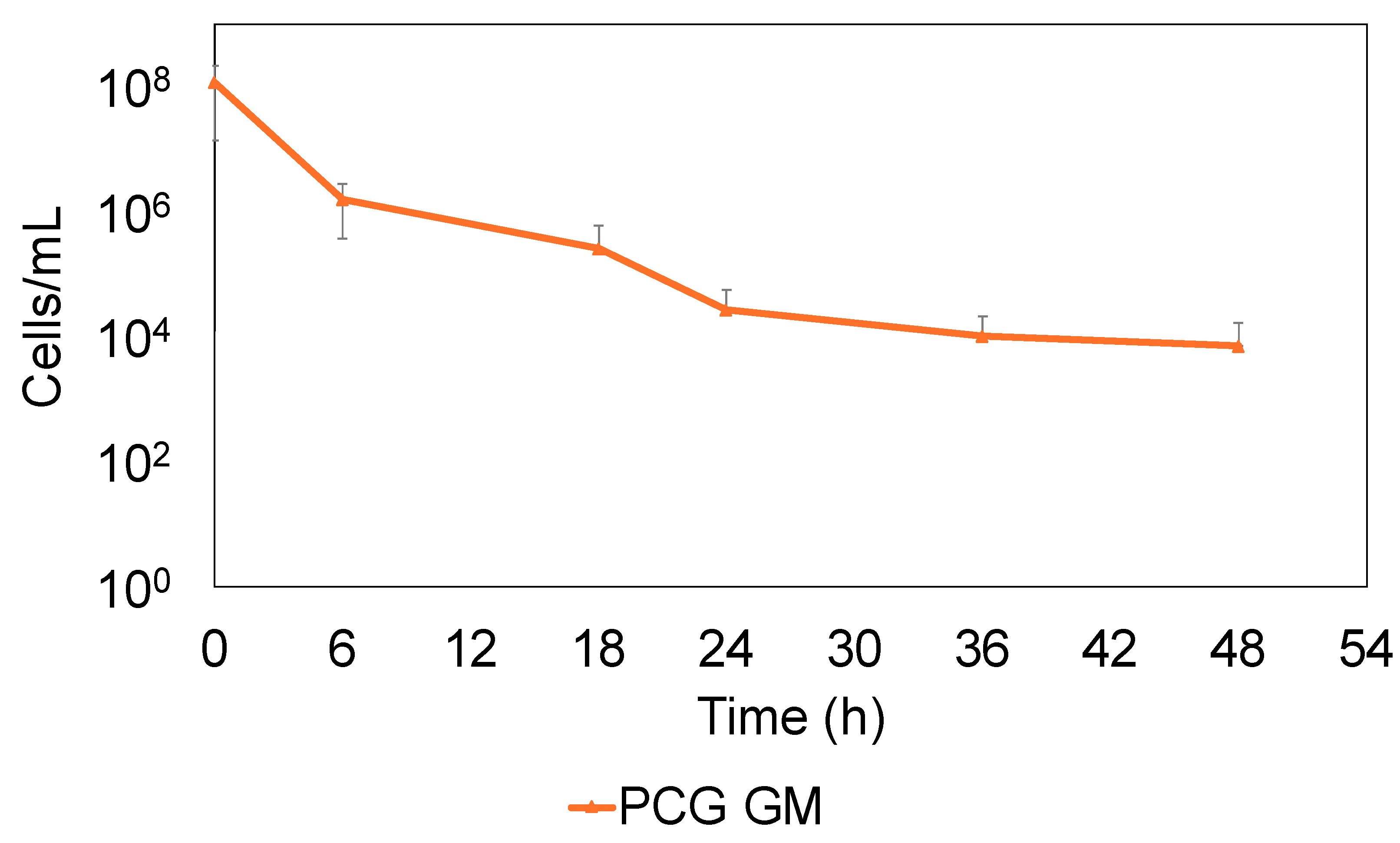
2.2. S. sanguinis Continues to Survive by Forming Persisters Against PCG and GM
2.3. S. sanguinis That Survived PCG/GM Is Not Drug-Resistant But Persister
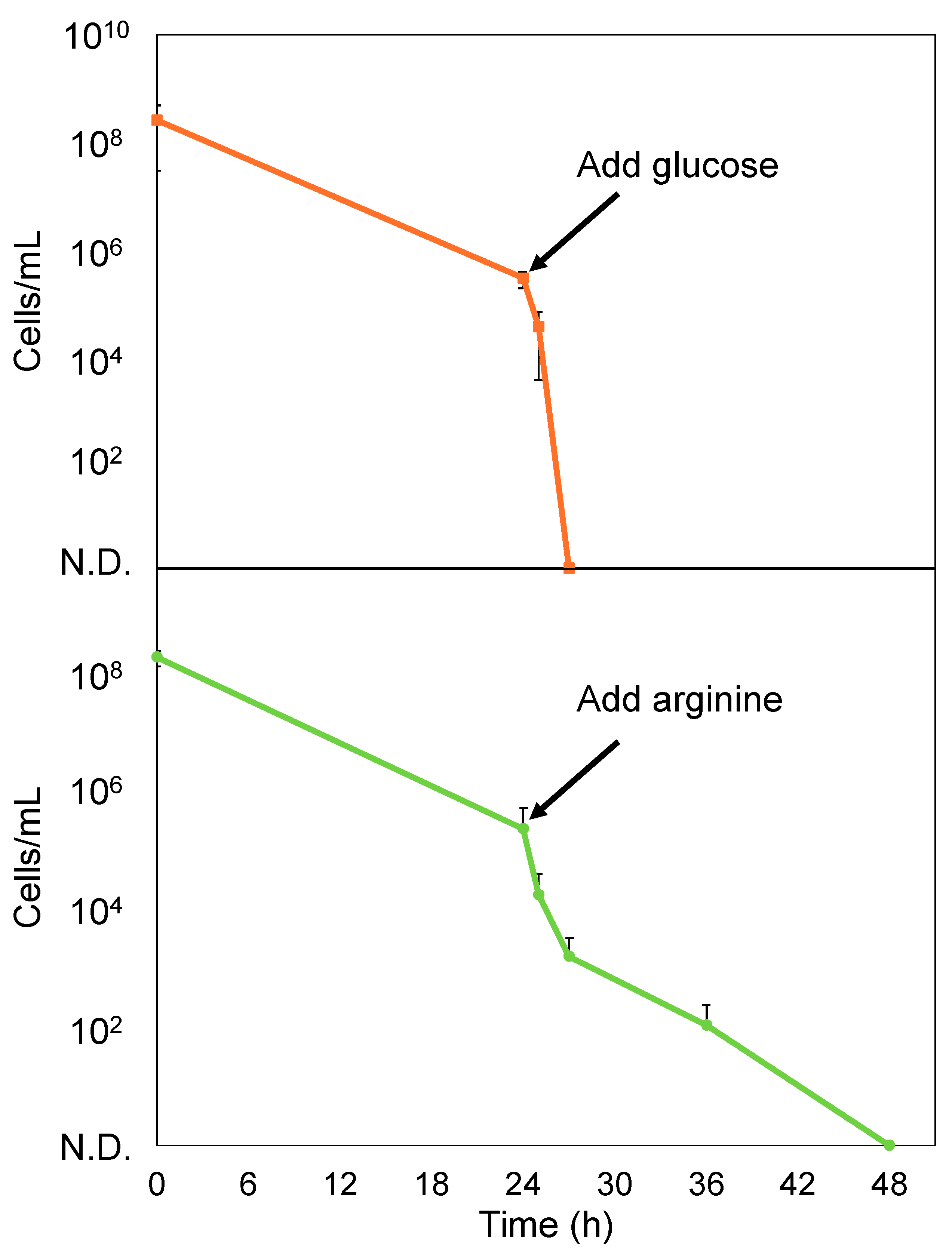
2.4. Glucose Enhances the Bactericidal Effect of PCG/GM Against S. sanguinis Persisters
3. Discussion
3.1. Persisters Play a Crucial Role in the Treatment of IE
3.2. Study Limitations
4. Materials and Methods
4.1. Microbial Strains and Culture Medium
4.2. MIC Measurement
4.3. Bactericidal Effect of PCG and GM Against S. sanguinis
4.4. Confirmation of Persistence
4.5. Effect of Glucose and Arginine on S. sanguinis Persisters
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mokdad, A.H.; Mensah, G.A.; Krish, V.; Glenn, S.D.; Miller-Petrie, M.K.; Lopez, A.D.; Murray, C.J.L. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Del Giudice, C.; Vaia, E.; Liccardo, D.; Marzano, F.; Valletta, A.; Spagnuolo, G.; Ferrara, N.; Rengo, C.; Cannavo, A.; Rengo, G. Infective endocarditis: A focus on oral microbiota. Microorganisms 2021, 9, 1218. [Google Scholar] [CrossRef] [PubMed]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef] [PubMed]
- Caufield, P.W.; Dasanayake, A.P.; Li, Y.; Pan, Y.; Hsu, J.; Hardin, J.M. Natural history of Streptococcus sanguinis in the oral cavity of infants: Evidence for a discrete window of infectivity. Infect. Immun. 2000, 68, 4018–4023. [Google Scholar] [CrossRef] [PubMed]
- Kuramitsu, H.K.; He, X.; Lux, R.; Anderson, M.H.; Shi, W. Interspecies interactions within oral microbial communities. Microbiol. Mol. Biol. Rev. 2007, 71, 653–670. [Google Scholar] [CrossRef]
- Zhu, B.; Macleod, L.C.; Kitten, T.; Xu, P. Streptococcus sanguinis biofilm formation & interaction with oral pathogens. Future Microbiol. 2018, 13, 915–932. [Google Scholar]
- Baker, S.P.; Nulton, T.J.; Kitten, T. Genomic, phenotypic, and virulence analysis of Streptococcus sanguinis oral and infective-endocarditis isolates. Infect. Immun. 2019, 87, 10.1128/iai.00703-18. [Google Scholar] [CrossRef] [PubMed]
- Rajani, R.; Klein, J.L. Infective endocarditis: A contemporary update. Clin. Med. 2020, 20, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, D.R.; Corey, G.R.; Hoen, B.; Miró, J.M.; Fowler, V.G.; Bayer, A.S.; Karchmer, A.W.; Olaison, L.; Pappas, P.A.; Moreillon, P. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: The International Collaboration on Endocarditis–Prospective Cohort Study. Arch. Intern. Med. 2009, 169, 463–473. [Google Scholar] [CrossRef]
- Lockhart, P.B.; Brennan, M.T.; Sasser, H.C.; Fox, P.C.; Paster, B.J.; Bahrani-Mougeot, F.K. Bacteremia associated with toothbrushing and dental extraction. Circulation 2008, 117, 3118–3125. [Google Scholar] [CrossRef]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.-P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B. 2015 ESC guidelines for the management of infective endocarditis: The task force for the management of infective endocarditis of the European Society of Cardiology (ESC) endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar] [PubMed]
- Calderón-Parra, J.; Kestler, M.; Ramos-Martínez, A.; Bouza, E.; Valerio, M.; de Alarcón, A.; Luque, R.; Goenaga, M.Á.; Echeverría, T.; Fariñas, M.C.; et al. Clinical factors associated with reinfection versus relapse in infective endocarditis: Prospective cohort study. J. Clin. Med. 2021, 10, 748. [Google Scholar] [CrossRef]
- Hobby, G.L.; Meyer, K.; Chaffee, E. Observations on the Mechanism of Action of Penicillin. Proc. Soc. Exp. Biol. Med. 1942, 50, 281–285. [Google Scholar] [CrossRef]
- Lewis, K. Persister cells: Molecular mechanisms related to antibiotic tolerance. In Antibiotic Resistance; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2012; Volume 211, pp. 121–133. [Google Scholar]
- Wood, T.K.; Song, S.; Yamasaki, R. Ribosome dependence of persister cell formation and resuscitation. J. Microbiol. 2019, 57, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Kaldalu, N.; Hauryliuk, V.; Turnbull, K.J.; La Mensa, A.; Putrinš, M.; Tenson, T. In vitro studies of persister cells. Microbiol. Mol. Biol. Rev. 2020, 84, 10.1128/mmbr.00070-20. [Google Scholar] [CrossRef] [PubMed]
- Balaban, N.Q.; Helaine, S.; Lewis, K.; Ackermann, M.; Aldridge, B.; Andersson, D.I.; Brynildsen, M.P.; Bumann, D.; Camilli, A.; Collins, J.J.; et al. Definitions and guidelines for research on antibiotic persistence. Nat. Rev. Microbiol. 2019, 17, 441–448. [Google Scholar] [CrossRef]
- Huemer, M.; Mairpady Shambat, S.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic resistance and persistence—Implications for human health and treatment perspectives. EMBO Rep. 2020, 21, e51034. [Google Scholar] [CrossRef] [PubMed]
- Moyed, H.S.; Bertrand, K.P. hipA, a newly recognized gene of Escherichia coli K-12 that affects frequency of persistence after inhibition of murein synthesis. J. Bacteriol. 1983, 155, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.A.; Gollan, B.; Helaine, S. Persistent bacterial infections and persister cells. Nat. Rev. Microbiol. 2017, 15, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Grassi, L.; Di Luca, M.; Maisetta, G.; Rinaldi, A.C.; Esin, S.; Trampuz, A.; Batoni, G. Generation of persister cells of Pseudomonas aeruginosa and Staphylococcus aureus by chemical treatment and evaluation of their susceptibility to membrane-targeting agents. Front. Microbiol. 2017, 8, 1917. [Google Scholar] [CrossRef]
- Yamasaki, R.; Song, S.; Benedik, M.J.; Wood, T.K. Persister Cells Resuscitate Using Membrane Sensors that Activate Chemotaxis, Lower cAMP Levels, and Revive Ribosomes. iScience 2020, 23, 100792. [Google Scholar] [CrossRef]
- Zhang, W.; Yamasaki, R.; Song, S.; Wood, T.K. Interkingdom signal indole inhibits Pseudomonas aeruginosa persister cell waking. J. Appl. Microbiol. 2019, 127, 1768–1775. [Google Scholar] [CrossRef] [PubMed]
- Leung, V.; Ajdic, D.; Koyanagi, S.; Lévesque, C.M. The formation of Streptococcus mutans persisters induced by the quorum-sensing peptide pheromone is affected by the LexA regulator. J. Bacteriol. 2015, 197, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Fung, Y.M.E.; Yin, X.; Seneviratne, C.J.; Che, C.M.; Jin, L. Controlled cellular redox, repressive hemin utilization and adaptive stress responses are crucial to metronidazole tolerance of Porphyromonas gingivalis persisters. J. Clin. Periodontol. 2018, 45, 1211–1221. [Google Scholar] [CrossRef]
- Nakamura, Y.; Watanabe, K.; Yoshioka, Y.; Ariyoshi, W.; Yamasaki, R. Persister Cell Formation and Elevated lsrA and lsrC Gene Expression upon Hydrogen Peroxide Exposure in a Periodontal Pathogen Aggregatibacter actinomycetemcomitans. Microorganisms 2023, 11, 1402. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, S.; Ohara, T.; Ashihara, K.; Izumi, C.; Iwanaga, S.; Eishi, K.; Okita, Y.; Daimon, M.; Kimura, T.; Toyoda, K.; et al. JCS 2017 guideline on prevention and treatment of infective endocarditis. Circ. J. 2019, 83, 1767–1809. [Google Scholar] [CrossRef]
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler Jr, V.G.; Tleyjeh, I.M.; Rybak, M.J.; Barsic, B.; Lockhart, P.B.; Gewitz, M.H.; Levison, M.E. Infective endocarditis in adults: Diagnosis, antimicrobial therapy, and management of complications: A scientific statement for healthcare professionals from the American Heart Association. Circulation 2015, 132, 1435–1486. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.R.; Brynildsen, M.P.; Collins, J.J. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature 2011, 473, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, M.M. Potential Uses of Arginine in Dentistry. Adv. Dent. Res. 2018, 29, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Freckmann, G.; Hagenlocher, S.; Baumstark, A.; Jendrike, N.; Gillen, R.C.; Rossner, K.; Haug, C. Continuous glucose profiles in healthy subjects under everyday life conditions and after different meals. J. Diabetes Sci. Technol. 2007, 1, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Pericas, J.M.; Nathavitharana, R.; Garcia-de-la-Maria, C.; Falces, C.; Ambrosioni, J.; Almela, M.; Garcia-Gonzalez, J.; Quintana, E.; Marco, F.; Moreno, A.; et al. Endocarditis Caused by Highly Penicillin-Resistant Viridans Group Streptococci: Still Room for Vancomycin-Based Regimens. Antimicrob. Agents Chemother. 2019, 63, e01113. [Google Scholar] [CrossRef]
- Matsuo, T.; Mori, N.; Sakurai, A.; Kanie, T.; Mikami, Y.; Uehara, Y.; Furukawa, K. Effectiveness of daptomycin against infective endocarditis caused by highly penicillin-resistant viridans group streptococci. IDCases 2021, 24, e01113. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler Jr, V.G. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, N.; Wood, T.L.; Martinez-Vazquez, M.; Garcia-Contreras, R.; Wood, T.K. DNA-crosslinker cisplatin eradicates bacterial persister cells. Biotechnol. Bioeng. 2016, 113, 1984–1992. [Google Scholar] [CrossRef]
- Kwan, B.W.; Chowdhury, N.; Wood, T.K. Combatting bacterial infections by killing persister cells with mitomycin C. Environ. Microbiol. 2015, 17, 4406–4414. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee; ElSayed, N.A.; Aleppo, G.; Bannuru, R.R.; Bruemmer, D.; Collins, B.S.; Ekhlaspour, L.; Gaglia, J.L.; Hilliard, M.E.; Johnson, E.L.; et al. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2024. Diabetes Care 2024, 47, S20–S42. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martin, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Yoshida, Y.; Konno, H.; Nagano, K.; Abiko, Y.; Nakamura, Y.; Tanaka, Y.; Yoshimura, F. The influence of a glucosyltransferase, encoded by gtfP, on biofilm formation by Streptococcus sanguinis in a dual-species model. APMIS 2014, 122, 951–960. [Google Scholar] [CrossRef]
- Kilian, M.; Mikkelsen, L.; Henrichsen, J. Taxonomic Study of Viridans Streptococci: Description of Streptococcus gordonii sp. nov. and Emended Descriptions of Streptococcus sanguis (White and Niven 1946), Streptococcus oralis (Bridge and Sneath 1982), and Streptococcus mitis (Andrewes and Horder 1906). Int. J. Syst. Evol. Microbiol. 1989, 39, 471–484. [Google Scholar] [CrossRef]
- Nadler, S.B.; Hidalgo, J.U.; Bloch, T. Prediction of blood volume in normal human adults. Surgery 1962, 51, 224–232. [Google Scholar] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takada, K.; Yoshioka, Y.; Morikawa, K.; Ariyoshi, W.; Yamasaki, R. Glucose Supplementation Enhances the Bactericidal Effect of Penicillin and Gentamicin on Streptococcus sanguinis Persisters. Antibiotics 2025, 14, 36. https://doi.org/10.3390/antibiotics14010036
Takada K, Yoshioka Y, Morikawa K, Ariyoshi W, Yamasaki R. Glucose Supplementation Enhances the Bactericidal Effect of Penicillin and Gentamicin on Streptococcus sanguinis Persisters. Antibiotics. 2025; 14(1):36. https://doi.org/10.3390/antibiotics14010036
Chicago/Turabian StyleTakada, Kazuya, Yoshie Yoshioka, Kazumasa Morikawa, Wataru Ariyoshi, and Ryota Yamasaki. 2025. "Glucose Supplementation Enhances the Bactericidal Effect of Penicillin and Gentamicin on Streptococcus sanguinis Persisters" Antibiotics 14, no. 1: 36. https://doi.org/10.3390/antibiotics14010036
APA StyleTakada, K., Yoshioka, Y., Morikawa, K., Ariyoshi, W., & Yamasaki, R. (2025). Glucose Supplementation Enhances the Bactericidal Effect of Penicillin and Gentamicin on Streptococcus sanguinis Persisters. Antibiotics, 14(1), 36. https://doi.org/10.3390/antibiotics14010036





