Green Silver Nanoparticles: An Antibacterial Mechanism
Abstract
:1. Introduction
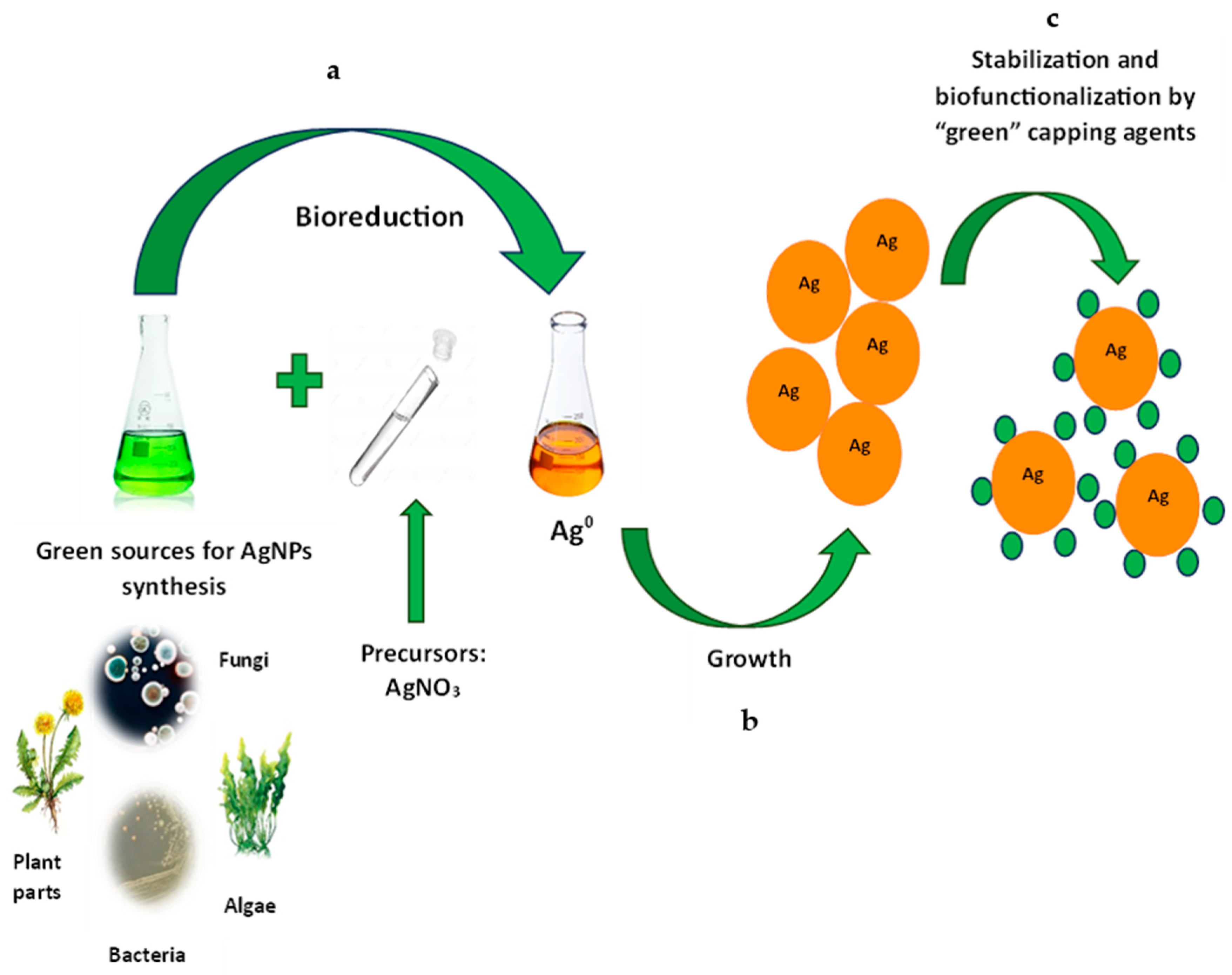
2. The Mechanism of Synthesis
2.1. By Bacteria
2.2. By Fungi
2.3. By Algae
2.4. By Plants
| Plant | Source | Shape | Size, nm | Ref. |
|---|---|---|---|---|
| Euporbia serpens | Leaves | spherical | 30–80 | [38] |
| C. fragrans | Leaves | spherical | 48 | [39] |
| S. hispanica | Seeds | spherical | 7 | [40] |
| Swertia paniculata | Aerial parts | spherical | 31–44 | [41] |
| T. arjuna | Bark | spherical | 2–100 | [42] |
| Atropa belladonna | Mother tincture | spherical | 15–20 | [43] |
| E. camaldulensis | Leaves | spherical | 100 | [42] |
| Camellia sinesis | Leaves | spherical | 10–16 | [44] |
| Litchi chinensis | Leaves | spherical | 40–50 | [45] |
| Ferula persica | Aerial parts | spherical | 15 | [46] |
| Buddleja globosa | Leaves | spherical | 16 | [47] |
| Linum usitatissimum | Leaves | spherical | ~47 | [48] |
| Phyllanthus amarus | Leaves | flower-like | 30–42 | [49] |
| Ricinus communis | Roots, Leaves | spherical | 29 37 | [50] |
| Momordica charantia | Leaves | spherical | 16 | [51] |
| Protium serratum | Leaves | spherical | ~74 | [52] |
| Citrus maxima | Peel | spherical | 4–11 | [53] |
| A. reticulata | Leaves | cubic | 6.5–8.13 | [54] |
| E. abyssinica | Leaves | spherical | 8.4–10 | [55] |
| Wedelia urticifolia | Flower | spherical | 30 | [57] |
| Moringa oleifera | Leaves | spherical | 10–25 | [58] |
| Prosopis juliflora | Bark | spherical | ~55 | [59] |
| Lepechinia meyenii | Leaves | spherical | 40–60 | [60] |
| Crocus haussknechtii Bois | Bulbs | spherical | 10–15 | [61] |
| Teucrium stocksianum | Aerial parts | spherical | 61 | [62] |
| Azadirachta indica (Neem plant) | Leaves | spherical | 22–30 | [63] |
| Loranthus pulverulentus | Leaves | spherical | 8–15 | [64] |
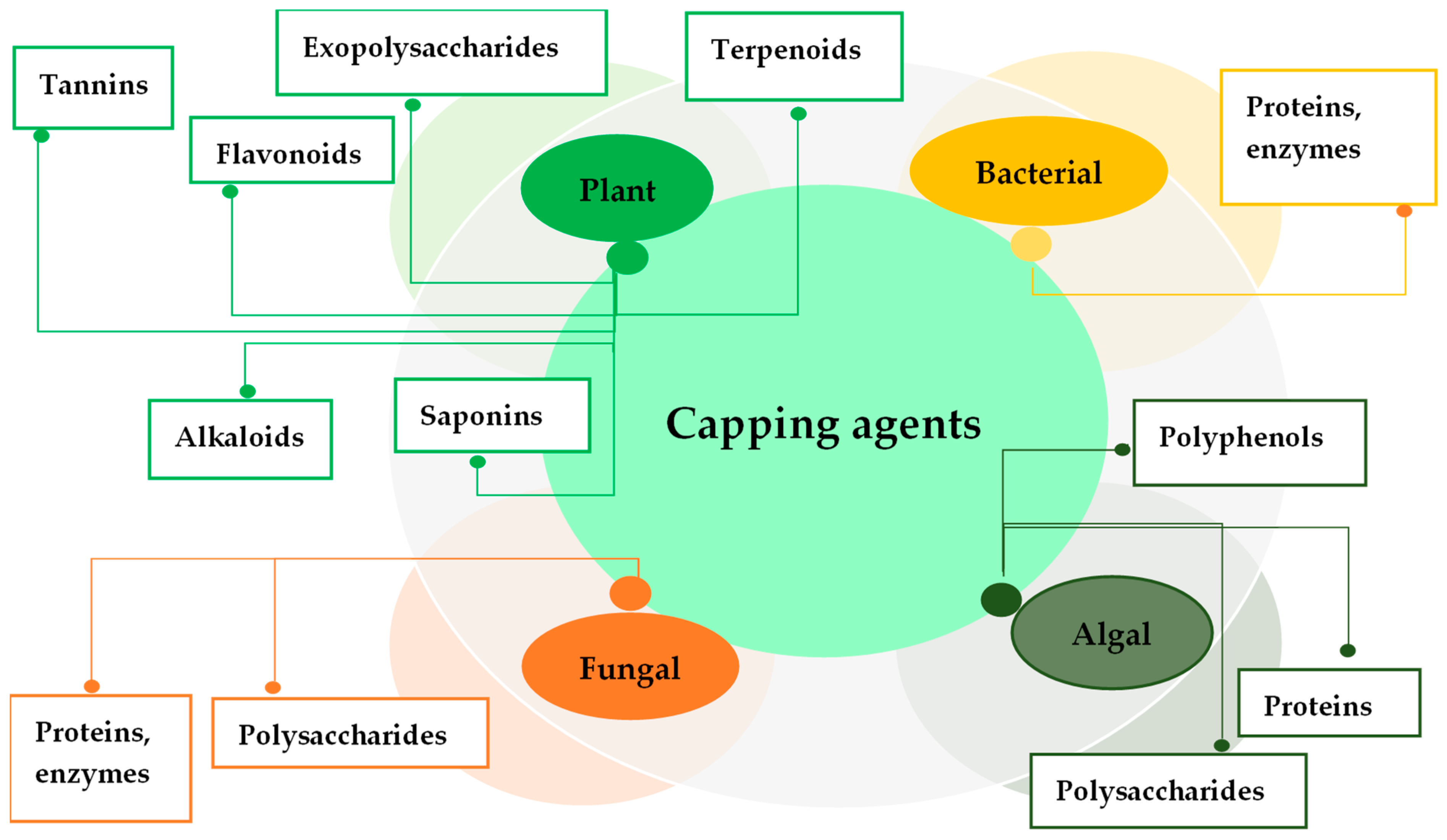
2.5. Capping Agents
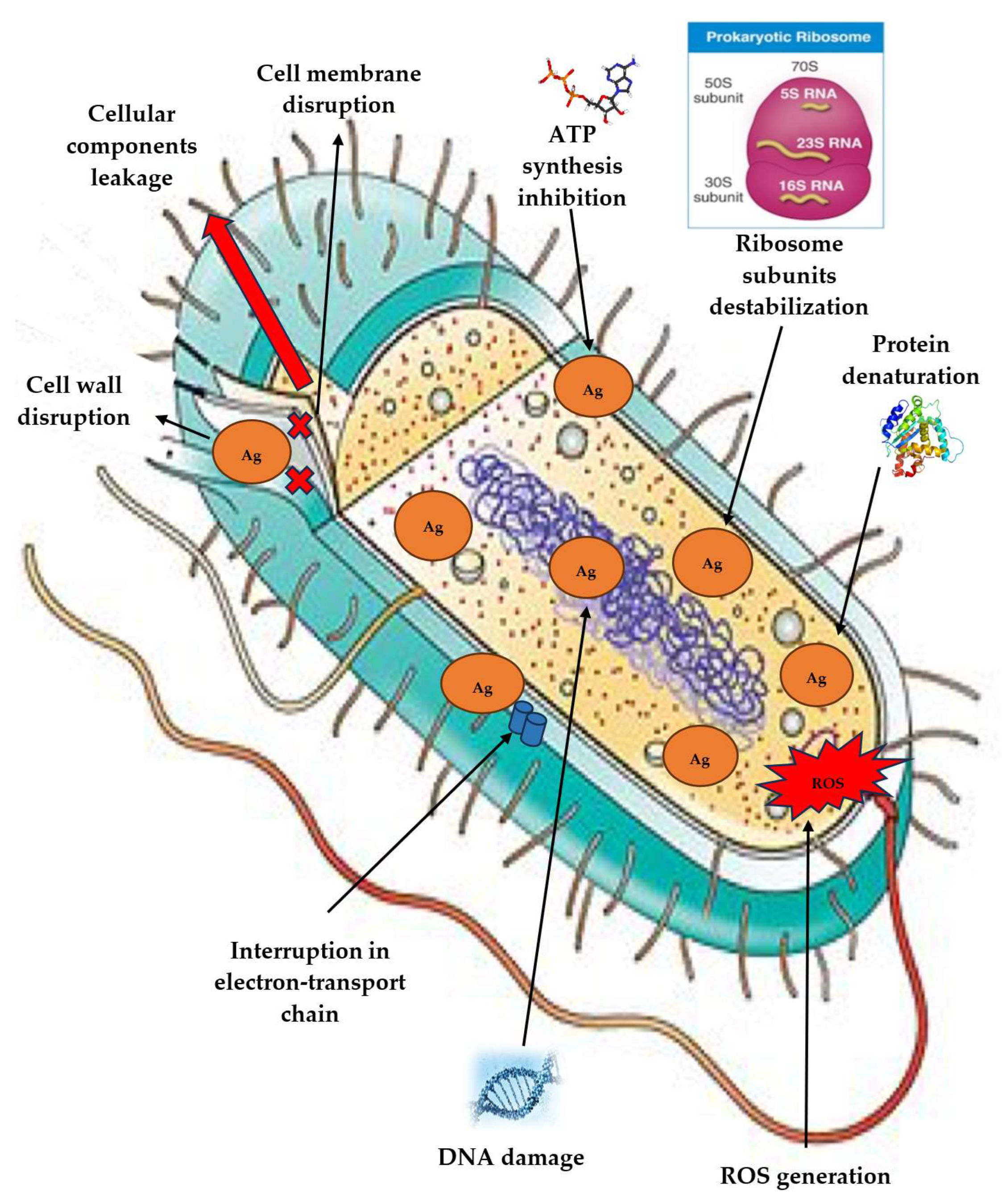
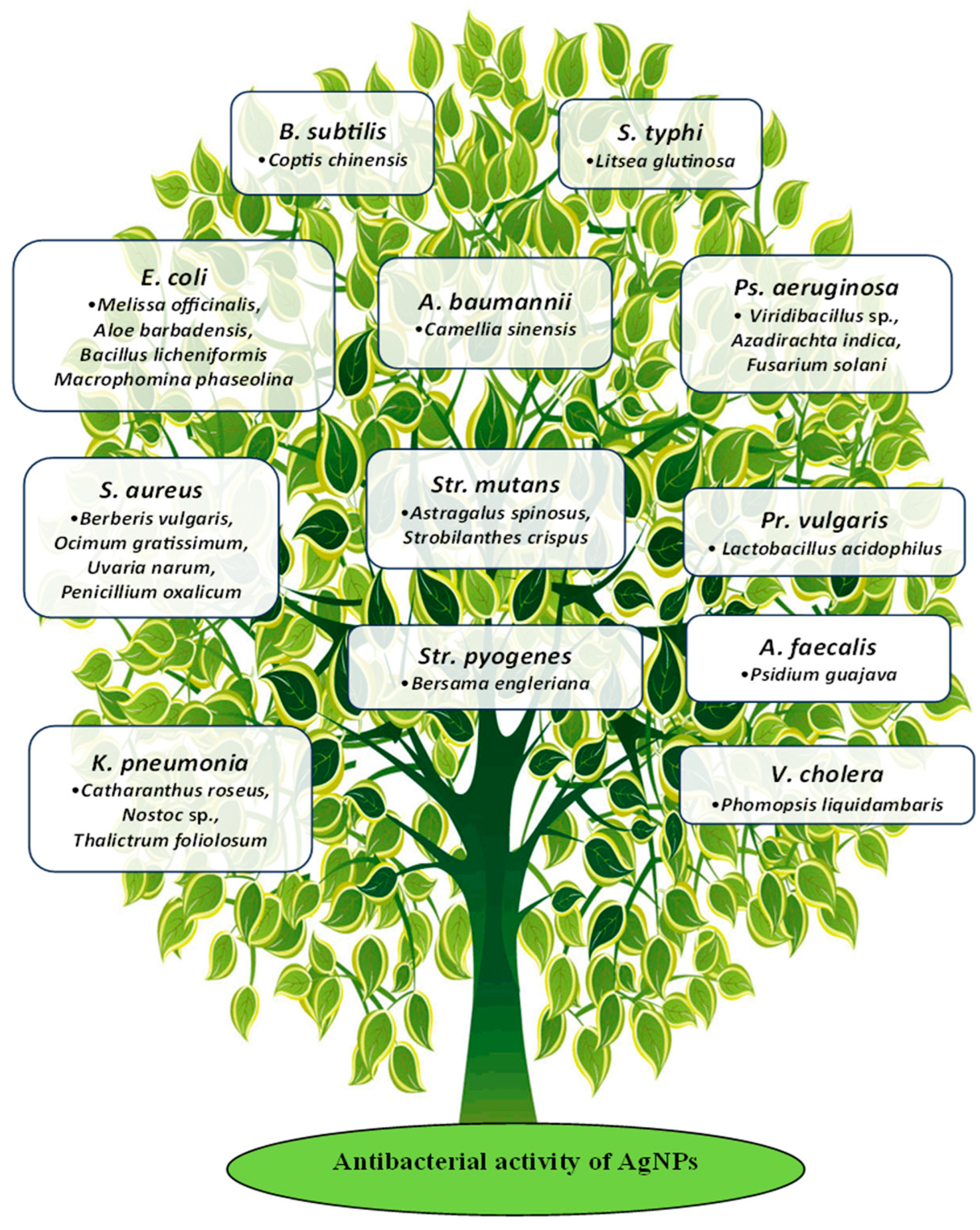
3. Mechanism of Action
3.1. Antibiofilm Activity
3.2. Anti-Quorum Sensing Activity
4. Toxicity
4.1. In Vitro
4.2. In Vivo
5. Conclusions
Funding
Conflicts of Interest
References
- Mikhailov, O.V.; Mikhailova, E.O. Elemental silver nanoparticles. Biosynthesis and bio application. Materials 2019, 12, 3177. [Google Scholar] [CrossRef] [PubMed]
- Okaiyeto, K.; Omotuyi, I.O.; Oguntibeju, O.O. Global mapping of research trends on antibacterial activity of green silver nanoparticles. Plant Sci. Today 2022, 9, 105–118. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Silver nanoparticles: Mechanism of action and probable bio-application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Upadhyay, L.S.B.; Kerketta, A.; Vasanth, D. Extracellular Synthesis of Silver Nanoparticles Using a Novel Bacterial Strain Kocuria rhizophila BR-1: Process Optimization and Evaluation of Antibacterial Activity. BioNanoScience 2022, 12, 423–438. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Malarkodi, C. In Vitro Antibacterial Activity and Mechanism of Silver Nanoparticles against Foodborne Pathogens. Bioinorg. Chem. Appl. 2014, 2014, 581890. [Google Scholar] [CrossRef]
- Saeed, S.; Iqbal, A.; Ashraf, M.A. Bacterial-mediated synthesis of silver nanoparticles and their significant effect against pathogens. Environ. Sci. Pollut. Res. Int. 2020, 27, 37347–37356. [Google Scholar] [CrossRef]
- Syame, S.M.; Mansour, A.S.; Khalaf, D.D.; Ibrahim, E.S.; Gaber, E.S. Green Synthesis of Silver Nanoparticles Using Lactic Acid Bacteria: Assessment of Antimicrobial Activity. World Vet. J. 2020, 10, 625–633. [Google Scholar] [CrossRef]
- Saleem, S.; Iqbal, A.; Hasnain, S. Bacterial mediated silver nanoparticles and their efficacy against MRSA. Trop. Biomed. 2020, 37, 482–488. [Google Scholar]
- Divya, M.; Kiran, G.S.; Selvin, J. Biogenic synthesis and effect of silver nanoparticles (AgNPs) to combat catheter-related urinary tract infections. Biocatal. Agric. Biotechnol. 2019, 18, 101037. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Malarkodi, C.; Vanaja, M.; Annadurai, G. Anticancer and enhanced antimicrobial activity of biosynthesizd silver nanoparticles against clinical pathogens. J. Mol. Struct. 2016, 1116, 165–173. [Google Scholar] [CrossRef]
- Peiris, M.M.K.; Fernando, S.S.N.; Jayaweera, P.M.; Arachchi, N.D.H.; Guansekara, T.D.C.P. Comparison of Antimicrobial Properties of Silver Nanoparticles Synthesized from Selected Bacteria. Indian J. Microbiol. 2018, 58, 301–311. [Google Scholar] [CrossRef] [PubMed]
- El-Baghdady, K.Z.; El-Shatoury, E.H.; Abdullah, O.M.; Khalil, M.M.H. Biogenic production of silver nanoparticles by Enterobacter cloacae Ism26. Turk. J. Biol. 2018, 42, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Solís-Sandí, I.; Cordero-Fuentes, S.; Pereira-Reyes, R.; Vega-Baudrit, J.R.; Batista-Menezes, D.; Montes de Oca-Vásquez, G. Optimization of the biosynthesis of silver nanoparticles using bacterial extracts and their antimicrobial potential. Biotechnol. Rep. 2023, 40, e00816. [Google Scholar] [CrossRef]
- Chung, D.; Jung, J.J.; Kim, J.Y.H.; Kim, K.W.; Kwon, Y.M. Aggregatimonas sangjinii gen. nov., sp. nov., a novel silver nanoparticle synthesizing bacterium belonging to the family Flavobacteriaceae. Antonie Van Leeuwenhoek 2022, 115, 325–335. [Google Scholar] [CrossRef]
- Li, S.; Niu, Y.; Chen, H.; He, P. Complete genome sequence of an Arctic Ocean bacterium Shewanella sp. Arc9-LZ with capacity of synthesizing silver nanoparticles in darkness. Mar. Genom. 2021, 56, 100808. [Google Scholar] [CrossRef]
- Esmail, R.; Afshar, A.; Morteza, M.; Abolfaz, A.; Esmail, E.A. Synthesis of silver nanoparticles with high efficiency and tability by culture supernatant of Bacillus ROM6 isolated from Zarshouran gold mine and evaluating its antibacterial effects. BMC Microbiol. 2022, 22, 97. [Google Scholar] [CrossRef]
- Wagi, S.; Ahmed, A. Green production of AgNPs and their phytostimulatory impact. Green Process. Synth. 2019, 8, 885–894. [Google Scholar] [CrossRef]
- Tariq, M.; Mohammad, K.N.; Ahmed, B.; Siddiqui, M.A.; Lee, J. Biological Synthesis of Silver Nanoparticles and Prospects in Plant Disease Management. Molecules 2022, 27, 4754. [Google Scholar] [CrossRef]
- Ibrahim, S.; Ahmad, Z.; Manzoor, M.Z.; Mujahid, M.; Faheem, Z.; Adnan, A. Optimization for biogenic microbial synthesis of silver nanoparticles through response surface methodology, characterization, their antimicrobial, antioxidant, and catalytic potential. Sci. Rep. 2021, 11, 770. [Google Scholar] [CrossRef]
- Saleh, M.N.; Alwan, S.K. Bio-synthesis of silver nanoparticles from bacteria Klebsiella pneumonia: Their characterization and antibacterial studies. J. Phys. Conf. Ser. 2020, 1664, 012115. [Google Scholar] [CrossRef]
- Verma, A.K.; Kumar, P. On Recent Developments in Biosynthesis and Application of Au and Ag Nanoparticles from Biological Systems. J. Nanotechnol. 2022, 2022, 5560244. [Google Scholar] [CrossRef]
- Hermosilla, E.; Díaz, M.; Vera, J.; Seabra, A.B.; Tortella, G.; Parada, J.; Rubilar, O. Molecular Weight Identification of Compounds Involved in the Fungal Synthesis of AgNPs: Effect on Antimicrobial and Photocatalytic Activity. Antibiotics 2022, 11, 622. [Google Scholar] [CrossRef] [PubMed]
- Guilger-Casagrande, M.; Germano-Costa, T.; Bilesky-José, N.; Pasquoto-Stigliani, T.; Carvalho, L.; Fraceto, L.F.; de Lima, R. Influence of the Capping of Biogenic Silver Nanoparticles on Their Toxicity and Mechanism of Action towards Sclerotinia Sclerotiorum. J. Nanobiotechnol. 2021, 19, 53. [Google Scholar] [CrossRef]
- Manjunath Hulikere, M.; Chandrashekhar, G.J. Characterization, antioxidant and antimicrobial activity of silver nanoparticles synthesized using marine endophytic fungus-Cladosporium cladosporioides. Process Biochem. 2019, 82, 199–204. [Google Scholar] [CrossRef]
- Gupta, P.; Rai, N.; Verma, A.; Saikia, D.; Singh, S.P.; Kumar, R.; Singh, S.K.; Kumar, D.; Gautam, V. Green-Based Approach to Synthesize Silver Nanoparticles Using the Fungal Endophyte Penicillium oxalicum and Their Antimicrobial, Antioxidant, and In Vitro Anticancer Potential. ACS Omega 2022, 7, 46653–46673. [Google Scholar] [CrossRef]
- El Sayed, M.T.; El-Sayed, A.S.A. Biocidal Activity of Metal Nanoparticles Synthesized by Fusarium solani Against Multidrug-Resistant Bacteria and Mycotoxigenic fungi. J. Microbiol. Biotechnol. 2020, 30, 226–236. [Google Scholar] [CrossRef]
- Jameel, M.S.; Aziz, A.A.; Dheyab, M.A.; Khaniabadi, P.M.; Kareem, A.A.; Alrosan, M.; Ali, A.T.; Rabeea, M.A.; Mehrdel, B. Mycosynthesis of ultrasonically-assisted uniform cubic silver nanoparticles by isolated phenols from Agaricus bisporus and its antibacterial activity. Surf. Interfaces 2022, 29, 101774. [Google Scholar] [CrossRef]
- Gade, A.; Gaikwad, S.; Duranm, N.; Rai, M. Screening of different species of Phoma for Synthesis of Silver nanoparticles. Biotechnol. Appl. Biochem. 2013, 60, 482–493. [Google Scholar] [CrossRef]
- Kumari, R.M.; Kumar, V.; Kumar, M.; Pareek, N.; Nimesh, S. Assessment of antibacterial and anticancer capability of silver nanoparticles extracellularly biosynthesized using Aspergillus terreus. Nano Express 2020, 1, 030011. [Google Scholar] [CrossRef]
- EL-Zawawy, N.A.; Abou-Zeid, A.M.; Beltagy, D.M.; Hantera, N.H.; Hoda, S. Nouh Microbial Cell Factories Mycosynthesis of silver nanoparticles from endophytic Aspergillus flavipes AUMC 15772: Ovat-statistical optimization, characterization and biological activities. Microb. Cell Fact. 2023, 22, 228. [Google Scholar] [CrossRef]
- Chugh, D.; Viswamalya, V.S.; Das, B. Green synthesis of silver nanoparticles with algae and the importance of capping agents in the process. J. Genet. Eng. Biotechnol. 2021, 19, 126. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-Y.; Saratale, R.G.; Shinde, S.; Syed, A.; Ameen, F.; Ghodake, G. Green synthesis of silver nanoparticles using Laminaria japonica extract: Characterization and seedling growth assessment. J. Clean. Prod. 2018, 172, 2910–2918. [Google Scholar] [CrossRef]
- El-Naggar, N.E.A.; Hussein, M.H.; Shaaban-Dessuuki, S.A.; Dalal, S.R. Production, extraction and characterization of Chlorella vulgaris soluble polysaccharides and their applications in AgNPs biosynthesis and biostimulation of plant growth. Sci. Rep. 2020, 10, 3011. [Google Scholar] [CrossRef] [PubMed]
- Hamida, R.S.; Ali, M.A.; Mugren, N.; Al-Zaban, M.I. Planophila laetevirens-Mediated Synthesis of Silver Nanoparticles: Optimization, Characterization, and Anticancer and Antibacterial Potentials. ACS Omega 2023, 8, 29169–29188. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Mohan, L.; Anand, R.; Bharadvaja, N. Chlorella minutissima-assisted silver nanoparticles synthesis and evaluation of its antibacterial activity. Syst. Microbiol. Biomanuf. 2024, 4, 230–239. [Google Scholar] [CrossRef]
- Alharbi, N.S.; Alsubhi, N.S.; Felimban, A.I. Green synthesis of silver nanoparticles using medicinal plants: Characterization and application. J. Radiat. Res. Appl. Sci. 2022, 15, 109–124. [Google Scholar] [CrossRef]
- Fahim, M.; Shahzaib, A.; Nishat, N.; Jahan, A.; Bha, T.A.; Inam, A. Green synthesis of silver nanoparticles: A comprehensive review of methods, in-fluencing factors, and applications. JCIS Open 2024, 16, 100125. [Google Scholar] [CrossRef]
- Ahmad, N.; Fozia; Jabeen, M.; Ul Haq, Z.; Ahmad, I.; Wahab, A.; Ul Islam, Z.; Ullah, R.; Bari, A.; Abdel-Daim, M.M.; et al. Green Fabrication of Silver Nanoparticles using Euphorbia serpens Kunth Aqueous Extract, Their Characterization, and Investigation of Its In Vitro Antioxidative, Antimicrobial, Insecticidal, and Cytotoxic Activities. Biomed. Res. Int. 2022, 2022, 5562849. [Google Scholar] [CrossRef]
- Nguyen, L.A.T.; Mai, B.V.; Nguyen, D.V.; Nguyen, N.Q.T.; Pham, V.V.; Pham, T.L.M.; Le, H.T. Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines. Green Process. Synth. 2023, 12, 20230024. [Google Scholar] [CrossRef]
- Hernández-Morales, L.; Espinoza-Gómez, H.; Flores-López, L.Z.; Sotelo-Barrera, E.L.; Núñez-Rivera, A.; Cadena-Nav, R.D.; Alonso-Núñez, G.; Espinoza, K.A. Study of the green synthesis of silver nanoparticles using a natural extract of dark or white Salvia hispanica L. seeds and their antibacterial application. Appl. Surf. Sci. 2019, 489, 952–961. [Google Scholar] [CrossRef]
- Ahluwalia, V.; Elumalai, S.; Kumar, V.; Kumar, S.; Sangwan, R.S. Nano silver particle synthesis using Swertia paniculata herbal extract and its antimicrobial activity. Microb. Pathog. 2018, 114, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Q.; Gupta, N.; Kumar, A.; Nimesh, S. Antibacterial efficacy of silver nanoparticles synthesized employing Terminalia arjuna bark extract. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Ghosal, K.; Jana, N.K.; Mukherjee, A.; Basak, P. Green synthesis and characterization of silver nanoparticles using belladonna mother tincture and its efficacy as a potential antibacterial and anti-inflammatory agent. Mater. Chem. Phys. 2019, 228, 310–317. [Google Scholar] [CrossRef]
- Liaqat, N.; Jahan, N.; Khalil-ur-Rahman; Anwar, T.; Qureshi, H. Green synthesized silver nanoparticles: Optimization, characterization, antimicrobial activity, and cytotoxicity study by hemolysis assay. Front. Chem. 2022, 10, 952006. [Google Scholar] [CrossRef]
- Kaur, R.; Avti, P.; Kumar, V.; Kumar, R. Effect of various synthesis parameters on the stability of size controlled green synthesis of silver nanoparticles. Nano Express 2021, 2, 020005. [Google Scholar] [CrossRef]
- Hashemi, Z.; Mohammadyan, M.; Naderi, S.; Fakhar, M.; Biparva, P.; Akhtari, J.; Ebrahimzadeh, M.A. Green synthesis of silver nanoparticles using Ferula persica extract (Fp-NPs): Characterization, antibacterial, antileishmanial, and in vitro anticancer activities. Mater. Today Commun. 2021, 27, 102264. [Google Scholar] [CrossRef]
- Carmona, E.R.; Benito, N.; Plaza, T.; Recio-Sánchez, G. Green synthesis of silver nanoparticles by using leaf extracts from the endemic Buddleja globosa hope. Green Chem. Lett. Rev. 2017, 10, 250–256. [Google Scholar] [CrossRef]
- Alzubaidi, A.K.; Al-Kaabi, W.J.; Ali, A.A.; Albukhaty, S.; Al-Karagoly, H.; Sulaiman, G.M.; Asiri, M.; Khane, Y. Green Synthesis and Characterization of Silver Nanoparticles Using Flaxseed Extract and Evaluation of Their Antibacterial and Antioxidant Activities. Appl. Sci. 2023, 13, 2182. [Google Scholar] [CrossRef]
- Ajitha, B.; Ashok Kumar Reddy, Y.; Jeon, H.-J.; Ahn, C.W. Synthesis of silver nanoparticles in an eco-friendly way using Phyllanthus amarus leaf extract: Antimicrobial and catalytic activity. Adv. Powder Technol. 2018, 29, 86–93. [Google Scholar] [CrossRef]
- Gul, A.; Fozia; Shaheen, A.; Ahmad, I.; Khattak, B.; Ahmad, M.; Ullah, R.; Bari, A.; Ali, S.S.; Alobaid, A.; et al. Green Synthesis, Characterization, Enzyme Inhibition, Antimicrobial Potential, and Cytotoxic Activity of Plant Mediated Silver Nanoparticle Using Ricinus communis Leaf and Root Extracts. Biomolecules 2021, 11, 206. [Google Scholar] [CrossRef]
- Ajitha, B.; Ashok Kumar Reddy, Y.; Sreedhara Reddy, P. Biosynthesis of silver nanoparticles using Momordica charantia leaf broth: Evaluation of their innate antimicrobial and catalytic activities. J. Photochem. Photobiol. B 2015, 146, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, Y.K.; Panda, S.K.; Bastia, A.K.; Mohanta, T.K. Biosynthesis of Silver Nanoparticles from Protium serratum and Investigation of their Potential Impacts on Food Safety and Control. Front. Microbiol. 2017, 8, 626. [Google Scholar] [CrossRef] [PubMed]
- Huo, C.; Khoshnamvand, M.; Liu, P.; Yuan, C.-G.; Cao, W. Eco-friendly approach for biosynthesis of silver nanoparticles using Citrus maxima peel extract and their characterization, catalytic, antioxidant and antimicrobial characteristics. Mater. Res. Express 2019, 6, 015010. [Google Scholar] [CrossRef]
- Parthiban, E.; Manivannan, N.; Ramanibai, R.; Mathivanan, N. Green synthesis of silver-nanoparticles from Annona reticulata leaves aqueous extract and its mosquito larvicidal and anti-microbial activity on human pathogens. Biotechnol. Rep. 2018, 21, e00297. [Google Scholar] [CrossRef]
- Mukaratirwa-Muchanyereyi, N.; Gusha, C.; Mujuru, M.; Guyo, U.; Nyoni, S. Synthesis of silver nanoparticles using plant extracts from Erythrina abyssinica aerial parts and assessment of their anti-bacterial and anti-oxidant activities. Results Chem. 2022, 4, 100402. [Google Scholar] [CrossRef]
- Mihailović, V.; Srećković, N.; Nedić, Z.P.; Dimitrijević, S.; Matić, M.; Obradović, A.; Selaković, D.; Rosić, G.; Katanić Stanković, J.S. Green Synthesis of Silver Nanoparticles Using Salvia verticillate and Filipendula ulmaria Extracts: Optimization of Synthesis, Biological Activities, and Catalytic Properties. Molecules 2023, 28, 808. [Google Scholar] [CrossRef]
- Rather, M.Y.; Shincy, M.; Sundarapandian, S. Silver nanoparticles synthesis using Wedelia urticifolia (Blume) DC. flower extract: Characterization and antibacterial activity evaluation. Microsc. Res. Tech. 2020, 83, 1085–1094. [Google Scholar] [CrossRef]
- Asif, M.; Yasmin, R.; Asif, R.; Ambreen, A.; Mustafa, M.; Umbreen, S. Green Synthesis of Silver Nanoparticles (AgNPs), Structural Characterization, and their Antibacterial Potential. Dose Response 2022, 20, 15593258221088709. [Google Scholar] [CrossRef]
- Arya, G.; Kumari, R.M.; Gupta, N.; Kumar, A.; Chandra, R.; Nimesh, S. Green synthesis of silver nanoparticles using Prosopis juliflora bark extract: Reaction optimization, antimicrobial and catalytic activities. Artif. Cells Nanomed. Biotechnol. 2018, 46, 985–993. [Google Scholar] [CrossRef]
- Laime-Oviedo, L.A.; Soncco-Ccahui, A.A.; Peralta-Alarcon, G.; Arenas-Chávez, C.A.; Pineda-Tapia, J.L.; Díaz-Rosado, J.C.; Alvarez-Risco, A.; Del-Aguila-Arcentales, S.; Davies, N.M.; Yáñez, J.A.; et al. Optimization of Synthesis of Silver Nanoparticles Conjugated with Lepechinia meyenii (Salvia) Using Plackett-Burman Design and Response Surface Methodology—Preliminary Antibacterial Activity. Processes 2022, 10, 1727. [Google Scholar] [CrossRef]
- Mosaviniya, M.; Kikhavani, T.; Tanzifi, M.; Tavakkoli Yaraki, M.; Tajbakhsh, P.; Lajevardi, A. Facile green synthesis of silver nanoparticles using Crocus Haussknechtii Bois bulb extract: Catalytic activity and antibacterial properties. Colloids Interface Sci. Commun. 2019, 33, 100211. [Google Scholar] [CrossRef]
- Rehman, I.; Gondal, H.Y.; Zamir, R.; Al-Hussain, S.A.; Batool, F.; Irfan, A.; Noreen, S.; Roheen, T.; Nisar, M.; Zaki, M.E.A. Green Synthesis: The Antibacterial and Photocatalytic Potential of Silver Nanoparticles Using Extract of Teucrium stocksianum. Nanomaterials 2023, 13, 1343. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.; Ahmed, S.; Abbasi, A.; Khan, M.T.; Subhan, M.; Bukhari, N.A.; Hatamleh, A.A.; Abdelsalam, N.A. Plant mediated fabrication of silver nanoparticles, process optimization, and impact on tomato plant. Sci. Rep. 2023, 13, 18048. [Google Scholar] [CrossRef]
- Subhani, M.A.; Irshad, M.; Nazir, M.; Hafeez, M.; Ali, S. Synthesis and antibacterial potential of Loranthus pulverulentus conjugated silver nanoparticles Microsc. Res. Tech. 2022, 85, 3530–3540. [Google Scholar] [CrossRef]
- Quinteros, M.Q.; Bonilla, J.O.; Alborés, S.V.; Villegas, L.V.; Páez, P.L. Biogenic nanoparticles: Synthesis, stability and biocompatibility mediated by proteins of Pseudomonas aeruginosa. Colloids Surf. B Biointerfaces 2019, 184, 110517. [Google Scholar] [CrossRef]
- Alwhibi, M.S.; Soliman, D.S.; Awad, M.A.; Alangery, A.B.; Al Dehaish, H.; Alwasel, Y.A. Green synthesis of silver nanoparticles: Characterization and its potential biomedical applications. Green Process. Synth. 2021, 10, 412–420. [Google Scholar] [CrossRef]
- Singh, P.; Mijakovic, I. Antibacterial Effect of Silver Nanoparticles Is Stronger If the Production Host and the Targeted Pathogen Are Closely Related. Biomedicines 2022, 10, 628. [Google Scholar] [CrossRef]
- Majoumouo, M.S.; Tincho, M.B.; Yimta, Y.D.; Adekiya, T.A.; Aruleba, R.T.; Ayawei, N.; Boyom, F.F.; Morris, T. Biosynthesis of Silver Nanoparticles Using Bersama engleriana Fruits Extracts and Their Potential Inhibitory Effect on Resistant Bacteria. Crystals 2022, 12, 1010. [Google Scholar] [CrossRef]
- Vazquez-Rodriguez, A.; Vasto-Anzaldo, X.G.; Leon-Buitimea, A.; Zarate, X.; Morones-Ramirez, J.R. Antibacterial and Antibiofilm Activity of Biosynthesized Silver Nanoparticles Coated with Exopolysaccharides Obtained from Rhodotorula mucilaginosa. IEEE Trans. Nanobiosci. 2020, 19, 498–503. [Google Scholar] [CrossRef]
- Akshaya, T.; Aravind, M.; Manoj Kumar, S.; Divya, B. Evaluation of In-vitro antibacterial activity against gram-negative bacteria using silver nanoparticles synthesized from Dypsis lutescens leaf extract. J. Chil. Chem. Soc. 2022, 67, 2. [Google Scholar] [CrossRef]
- Urnukhsaikhan, E.; Bold, B.E.; Gunbileg, A.; Sukhbaatar, N.; Mishig-Ochir, T. Antibacterial activity and characteristics of silver nanoparticles biosynthesized from Carduus crispus. Sci. Rep. 2021, 11, 21047. [Google Scholar] [CrossRef] [PubMed]
- Al-Otibi, F.; Alkhudhair, S.K.; Alharbi, R.I.; Al-Askar, A.A.; Aljowaie, R.M.; Al-Shehri, S. The Antimicrobial Activities of Silver Nanoparticles from Aqueous Extract of Grape Seeds against Pathogenic Bacteria and Fungi. Molecules 2021, 26, 6081. [Google Scholar] [CrossRef] [PubMed]
- Kemala, P.; Idroes, R.; Khairan, K.; Ramli, M.; Jalil, Z.; Idroes, G.M.; Tallei, T.E.; Helwani, Z.; Safitri, E.; Iqhrammullah, M.; et al. Green Synthesis and Antimicrobial Activities of Silver Nanoparticles Using Calotropis gigantea from IeSeu-Um Geothermal Area, Aceh Province, Indonesia. Molecules 2022, 27, 5310. [Google Scholar] [CrossRef]
- Hassan Afandy, H.; Sabir, D.K.; Aziz, S.B. Antibacterial Activity of the Green Synthesized Plasmonic Silver Nanoparticles with Crystalline Structure against Gram-Positive and Gram-Negative Bacteria. Nanomaterials 2023, 13, 1327. [Google Scholar] [CrossRef]
- Elsebaie, E.M.; El-Wakeil, N.H.M.; Khalil, A.M.M.; Bahnasy, R.M.; Asker, G.A.; El-Hassnin, M.F.; Ibraheim, S.S.; El-Farsy, M.F.A.; Faramawy, A.A.; Essa, R.Y.; et al. Silver Nanoparticle Synthesis by Rumex vesicarius Extract and Its Applicability against Foodborne Pathogens. Foods 2023, 12, 1746. [Google Scholar] [CrossRef]
- Jose, R.A.; Devina Merin, D.; Arulananth, T.S.; Shaik, N. Characterization Analysis of Silver Nanoparticles Synthesized from Chaetoceros calcitrans. J. Nanomater. 2022, 2022, 4056551. [Google Scholar] [CrossRef]
- Balciunaitiene, A.; Puzeryte, V.; Radenkovs, V.; Krasnova, I.; Memvanga, P.B.; Viskelis, P.; Streimikyte, P.; Viskelis, J. Sustainable–Green Synthesis of Silver Nanoparticles Using Aqueous Hyssopus officinalis and Calendula officinalis Extracts and Their Antioxidant and Antibacterial Activities. Molecules 2022, 27, 7700. [Google Scholar] [CrossRef]
- Qais, F.A.; Shafiq, A.; Khan, H.M.; Husain, F.M.; Khan, R.A.; Alenazi, B.; Alsalme, A.; Ahmad, I. Antibacterial Effect of Silver Nanoparticles Synthesized Using Murraya koenigii (L.) against Multidrug-Resistant Pathogens. Bioinorg. Chem. Appl. 2019, 2019, 4649506. [Google Scholar] [CrossRef]
- Tesfaye, M.; Gonfa, Y.; Tadesse, G.; Temesgen, T.; Periyasamy, S. Green synthesis of silver nanoparticles using Vernonia amygdalina plant extract and its antimicrobial activities. Heliyon 2023, 9, e17356. [Google Scholar] [CrossRef]
- Parlinska-Wojtan, M.; Kus-Liskiewicz, M.; Depciuch, J.; Sadik, O. Green synthesis and antibacterial effects of aqueous colloidal solutions of silver nanoparticles using camomile terpenoids as a combined reducing and capping agent. Bioprocess Biosyst. Eng. 2016, 39, 1213–1223. [Google Scholar] [CrossRef]
- Dilbar, S.; Sher, H.; Ali, A.; Ullah, Z. Biological synthesis of Ag-nanoparticles using Stachys parviflora and its inhibitory potential against Xanthomonas campestris. S. Afr. J. Bot. 2023, 157, 409–422. [Google Scholar] [CrossRef]
- Ferreyra Maillard, A.P.V.; Gonçalves, S.; Santos, N.C.; López de Mishima, B.A.; Dalmasso, P.R.; Hollmann, A. Studies on interaction of green silver nanoparticles with whole bacteria by surface characterization techniques. BBA-Biomembr. 2019, 1861, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Mijakovic, I. Strong Antimicrobial Activity of Silver Nanoparticles Obtained by the Green Synthesis in Viridibacillus sp. Extracts. Front. Microbiol. 2022, 13, 820048. [Google Scholar] [CrossRef] [PubMed]
- Chinnasamy, G.; Chandrasekharan, S.; Koh, T.W.; Bhatnagar, S. Synthesis, Characterization, Antibacterial and Wound Healing Efficacy of Silver Nanoparticles from Azadirachta indica. Front. Microbiol. 2021, 12, 611560. [Google Scholar] [CrossRef]
- Dhas, S.T.; Sowmiya, P.; Parthasarathy, K.; Natarajan, A.; Narendrakumar, G.; Kumar, R.; Samrot, A.V.; Riyaz, S.U.M.; Ganesh, V.K.; Karthick, V.; et al. In vitro antibacterial activity of biosynthesized silver nanoparticles against gram negative bacteria. Inorg. Nano-Met. Chem. 2024, 54, 332–341. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, J.; Yin, L.; Liu, J.; Chen, C. Silver nanoparticles: From in vitro green synthesis to in vivo biological effects in plants. Adv. Agrochem. 2023, 2, 313–323. [Google Scholar] [CrossRef]
- Hanafiah, R.M.; Ghafar, S.A.A.; Lim, V.; Musa, S.N.A.; Yakop, F.; Anuar, A.H.H. Green synthesis, characterisation and antibacterial activities of Strobilanthes crispus-mediated silver nanoparticles (SC-AGNPS) against selected bacteria. Artif. Cells Nanomed. Biotechnol. 2023, 51, 549–559. [Google Scholar] [CrossRef]
- Arokiyaraj, S.; Vincent, S.; Saravanan, M.; Lee, Y.; Oh, Y.K.; Kim, K.H. Green synthesis of silver nanoparticles using Rheum palmatum root extract and their antibacterial activity against Staphylococcus aureus and Pseudomonas aeruginosa. Artif. Cells Nanomed. Biotechnol. 2017, 45, 372–379. [Google Scholar] [CrossRef]
- Hamida, R.S.; Ali, M.A.; Goda, D.A.; Khalilad, M.I.; Redhwan, A. Cytotoxic effect of green silver nanoparticles against ampicillin-resistant Klebsiella pneumoniae. RSC Adv. 2020, 10, 21136. [Google Scholar] [CrossRef]
- Campo-Beleño, C.; Villamizar-Gallardo, R.A.; López-Jácome, L.E.; González, E.E.; Muñoz-Carranza, S.; Franco, B.; Morales-Espinosa, R.; Coria-Jimenez, R.; Franco-Cendejas, R.; Hernández-Durán, M.; et al. Biologically synthesized silver nanoparticles as potent antibacterial effective against multidrug-resistant Pseudomonas aeruginosa. Lett. Appl. Microbiol. 2022, 75, 680–688. [Google Scholar] [CrossRef]
- Behravan, M.; Hossein Panahi, A.; Naghizadeh, A.; Ziaee, M.; Mahdavi, R.; Mirzapour, A. Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int. J. Biol. Macromol. 2019, 124, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Yakoup, A.Y.; Kamel, A.G.; Elbermawy, Y.; Abdelsattar, A.S.; El-Shibiny, A. Characterization, antibacterial, and cytotoxic activities of silver nanoparticles using the whole biofilm layer as a macromolecule in biosynthesis. Sci. Rep. 2024, 14, 364. [Google Scholar] [CrossRef] [PubMed]
- Anuj, S.A.; Gajera, H.P.; Hirpara, D.G.; Golakiya, B.A. Bacterial membrane destabilization with cationic particles of nano-silver to combat efflux-mediated antibiotic resistance in Gram-negative bacteria. Life Sci. 2019, 230, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Ardra Lekshmi, A.; Sharath, M.; Raj, B.D.; Pramod, P.; Shyam, A.; Smitha Chandran, S. Bio-mediated synthesis of silver nanoparticles using Cotinus coggygria extract and their multifaceted applications. Inorg. Chem. Commun. 2023, 153, 110852. [Google Scholar] [CrossRef]
- Weiming, G.; Quanfeng, H.; Jianxia, S.; Dan, L.; Xuejuan, D. Green synthesis of silver nanoparticles using banana flower extract, and their antibacterial activity. Int. Food Res. J. 2023, 30, 613–625. [Google Scholar] [CrossRef]
- Ghabban, H.; Alnomasy, S.F.; Almohammed, H.; Al Idriss, O.M.; Rabea, S.; Eltahir, Y. Antibacterial, Cytotoxic, and Cellular Mechanisms of Green Synthesized Silver Nanoparticles against Some Cariogenic Bacteria (Streptococcus mutans and Actinomyces viscosus). J. Nanomater. 2022, 2022, 9721736. [Google Scholar] [CrossRef]
- Dong, Y.; Zhu, H.; Shen, Y.; Zhang, W.; Zhang, L. Antibacterial activity of silver nanoparticles of different particle size against Vibrio Natriegens. PLoS ONE 2019, 14, e0222322. [Google Scholar] [CrossRef]
- Aygun, A.; Ozdemir, S.; Gulcan, M.; Cellat, K.; Sen, F. Synthesis and Characterization of Reishi Mushroom-mediated Green Synthesis of Silver Nanoparticles for the Biochemical Applications. J. Pharm. Biomed. Anal. 2020, 178, 112970. [Google Scholar] [CrossRef]
- Laib, I.; Ali Boutlelis, D.; Boudebia, Q. Green synthesis of silver nanoparticles using Helianthemum lippii extracts (Hl-NPs): Characterization, antioxidant and antibacterial activities, and study of interaction with DNA. J. Organomet. Chem. 2023, 986, 122619. [Google Scholar] [CrossRef]
- Hazarika, S.N.; Gupta, K.; Shamin, K.N.A.M.; Bhardwaj, P.; Boruah, R.; Yadav, K.K.; Naglot, A.; Deb, P.; Mandal, M.; Doley, R. One-pot facile green synthesis of biocidal silver nanoparticles. Mater. Res. Express 2016, 3, 075401. [Google Scholar] [CrossRef]
- Komal; Sonia; Kukreti, S.; Kaushik, M. Exploring the potential of environment friendly silver nanoparticles for DNA interaction: Physicochemical approach. J. Photochem. Photobiol. B 2019, 194, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; He, B.; Liu, L.; Qu, G.; Shi, J.; Hu, L.; Jiang, G. Antibacterial mechanism of silver nanoparticles in Pseudomonas aeruginosa: Proteomics approach. Metallomics 2018, 10, 557. [Google Scholar] [CrossRef] [PubMed]
- Slavin, Y.N.; Ivanova, K.; Hoyo, J.; Perelshtein, I.; Owen, G.; Haegert, A.; Lin, Y.-Y.; LeBihan, S.; Gedanken, A.; Häfeli, U.O.; et al. Novel Lignin-Capped Silver Nanoparticles against Multidrug-Resistant Bacteria. ACS Appl. Mater. Interfaces 2021, 13, 22098–22109. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, E.; Abdel Rafea, M.; Mustapha, N.; Sultan, R.; Hafez, E. Silver nanoparticles synthesized by probiotic bacteria and antibacterial role in resistant bacteria. AMB Express 2023, 13, 140. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Silver nanoparticles as an antimicrobial agent: A case study on Staphylococcus aureus and Escherichia coli as models for Gram-positive and Gram-negative bacteria. J. Gen. Appl. Microbiol. 2017, 63, 36–43. [Google Scholar] [CrossRef]
- Rautela, A.; Rani, J.; Debnath (Das), M. Green synthesis of silver nanoparticles from Tectona grandis seeds extract: Characterization and mechanism of antimicrobial action on different microorganisms. J. Anal. Sci. Technol. 2019, 10, 5. [Google Scholar] [CrossRef]
- Arsene, M.M.J.; Podoprigora, I.V.; Marukhlenko, A.; Morozova, M.; Linda Davares, A.K.; Carime, B.Z.; Gizinger, O.A.; Yashina, N.V.; Zhigunova, A.V.; Smolyakova, L.A.; et al. Antimicrobial activity of phytofabricated silver nanoparticles using Carica papaya L. against Gram-negative bacteria. Vet. World 2023, 16, 1301–1311. [Google Scholar] [CrossRef]
- Wypij, M.; Jędrzejewski, T.; Trzcińska-Wencel, J.; Ostrowski, M.; Rai, M.; Golińska, P. Green Synthesized Silver Nanoparticles: Antibacterial and Anticancer Activities, Biocompatibility, and Analyses of Surface-Attached Proteins. Front. Microbiol. 2021, 12, 632505. [Google Scholar] [CrossRef]
- Raza, S.; Wdowiak, M.; Grotek, M.; Adamkiewicz, W.; Nikiforow, K.; Mente, P.; Paczesny, Y. Enhancing the antimicrobial activity of silver nanoparticles against ESKAPE bacteria and emerging fungal pathogens by using tea extracts. Nanoscale Adv. 2023, 5, 5786–5798. [Google Scholar] [CrossRef]
- Bari, A.K.; Belalekar, T.S.; Poojary, A.; Rohra, S. Combination drug strategies for bio-film eradication using synthetic and natural agents in KAPE pathogens. Front. Cell. Infect. Microbiol. 2023, 17, 1155699. [Google Scholar] [CrossRef]
- Scandorieiro, S.; Teixeira, F.M.M.B.; Nogueira, M.C.L.; Panagio, L.A.; de Oliveira, A.G.; Durán, N.; Nakazato, G.; Kobayashi, R.K.T. Antibiofilm Effect of Biogenic Silver Nanoparticles Combined with Oregano Derivatives against Carbapenem-Resistant Klebsiella pneumoniae. Antibiotics 2023, 12, 756. [Google Scholar] [CrossRef] [PubMed]
- Verduzco-Chavira, K.; Vallejo-Cardona, A.A.; González-Garibay, A.S.; Torres-González, O.R.; Sánchez-Hernández, I.M.; Flores-Fernández, J.M.; Padilla-Camberos, E. Antibacterial and Antibiofilm Activity of Chemically and Biologically Synthesized Silver Nanoparticles. Antibiotics 2023, 12, 1084. [Google Scholar] [CrossRef]
- Barabadi, H.; Mojab, F.; Vahidi, H.; Marashi, B.; Talank, N.; Hosseini, O.; Saravanan, M. Green synthesis, characterization, antibacterial and biofilm inhibitory activity of silver nanoparticles compared to commercial silver nanoparticles. Inorg. Chem. Commun. 2021, 129, 108647. [Google Scholar] [CrossRef]
- Qais, F.A.; Shafiq, A.; Ahmad, I.; Husain, F.M.; Khan, R.A.; Hassan, I. Green synthesis of silver nanoparticles using Carum copticum: Assessment of its quorum sensing and biofilm inhibitory potential against gram negative bacterial pathogens. Microb. Pathog. 2020, 144, 104172. [Google Scholar] [CrossRef] [PubMed]
- Selem, E.; Mekky, A.F.; Hassanein, W.A.; Reda, F.M.; Selim, Y.A. Antibacterial and antibiofilm effects of silver nanoparticles against the uropathogen Escherichia coli U12. Saudi J. Biol. Sci. 2022, 29, 103457. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Khan, H.M.; Husain, F.M.; Ahmad, R.; Qais, F.A.; Khan, M.A.; Jalal, M.; Tayyaba, U.; Ali, S.G.; Singh, A.; et al. Antibacterial and antibiofilm activity of Abroma augusta stabilized silver (Ag) nanoparticles against drug-resistant clinical pathogens. Front. Mol. Biosci. 2023, 10, 1292509. [Google Scholar] [CrossRef]
- Rajivgandhi, G.N.; Ramachandran, G.; Maruthupandy, M.; Manoharan, N.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Almana, T.N.; Li, W.-J. Anti-oxidant, anti-bacterial and anti-biofilm activity of biosynthesized silver nanoparticles using Gracilaria corticata against biofilm producing K. pneumoniae. Colloids Surf. A 2020, 600, 124830. [Google Scholar] [CrossRef]
- Kumar, S.; Khan, H.M.; Khan, M.A.; Jalal, M.; Ahamad, S.; Shahid, M.; Husain, F.M.; Arshad, M.; Adil, M. Broad-spectrum antibacterial and antibiofilm activity of biogenic silver nanoparticles synthesized from leaf extract of Phyllanthus niruri. J. King Saud Univ. Sci. 2023, 35, 102904. [Google Scholar] [CrossRef]
- Masum, M.M.I.; Siddiqa, M.M.; Ali, K.A.; Zhang, Y.; Abdallah, Y.; Ibrahim, E.; Qiu, W.; Yan, C.; Li, B. Biogenic Synthesis of Silver Nanoparticles Using Phyllanthus emblica Fruit Extract and Its Inhibitory Action Against the Pathogen Acidovorax oryzae Strain RS-2 of Rice Bacterial Brown Stripe. Front. Microbiol. 2019, 10, 820. [Google Scholar] [CrossRef]
- Elshaer, S.; Shaaban, M.I. Elshaer and Shaaban Antibiofilm activity of biosynthesized silver and copper nanoparticles using Streptomyces S29. AMB Express 2023, 13, 139. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Mousavi, S.M.A.; Moeinizadeh, M.; Aghajanidelavar, M.; Rajabi, S.; Mirshekar, M. Evaluation of biosynthesized silver nanoparticles effects on expression levels of virulence and biofilm-related genes of multidrug-resistant Klebsiella pneumoniae isolates. J. Basic Microbiol. 2023, 63, 632–645. [Google Scholar] [CrossRef] [PubMed]
- Hetta, H.F.; Al-Kadmy, I.M.S.; Khazaal, S.S. Antibiofilm and antivirulence potential of silver nanoparticles against multidrug-resistant Acinetobacter baumannii. Sci. Rep. 2021, 11, 10751. [Google Scholar] [CrossRef] [PubMed]
- Prem, P.; Naveenkumar, S.; Kamaraj, C.; Ragavendran, C.; Priyadharsan, A.; Manimaran, K.; Alharbi, N.S.; Rarokar, N.; Cherian, T.; Sugumar, V.; et al. Valeriana jatamansi root extract a potent source for biosynthesis of silver nanoparticles and their biomedical applications, and photocatalytic decomposition. Green Chem. Lett. Rev. 2024, 17, 2305142. [Google Scholar] [CrossRef]
- Shah, S.; Gaikwad, S.; Nagar, S.; Kulshrestha, S.; Vaidya, V.; Nawani, N.; Pawar, S. Biofilm Inhibition and Anti-Quorum Sensing Activity of Phytosynthesized Silver Nanoparticles against the Nosocomial Pathogen Pseudomonas aeruginosa. Biofouling 2019, 35, 34–49. [Google Scholar] [CrossRef]
- Awadelkareem, A.M.; Siddiqui, A.J.; Noumi, E.; Ashraf, S.A.; Hadi, S.; Snoussi, M.; Badraoui, R.; Bardakci, F.; Ashraf, M.S.; Danciu, C.; et al. Biosynthesized Silver Nanoparticles Derived from Probiotic Lactobacillus rhamnosus (AgNPs-LR) Targeting Biofilm Formation and Quorum Sensing-Mediated Virulence Factors. Antibiotics 2023, 12, 986. [Google Scholar] [CrossRef]
- Haris, Z.; Ahmad, I. Green synthesis of silver nanoparticles using Moringa oleifera and its efficacy against gram-negative bacteria targeting quorum sensing and biofilms. J. Umm Al-Qura Univ. Appli. Sci. 2024, 10, 156–167. [Google Scholar] [CrossRef]
- Adu, O.T.; Mohamed, F.; Naidoo, Y.; Adu, T.S.; Chenia, H.; Dewir, Y.H.; Rihan, H. Green Synthesis of Silver Nanoparticles from Diospyros villosa Extracts and Evaluation of Antioxidant, Antimicrobial and Anti-Quorum Sensing Potential. Plants 2022, 11, 2514. [Google Scholar] [CrossRef]
- Anju, S.; Sarada, J. Quorum Quenching Potential of Biogenic Silver Nanoparticles against Chromobacterium violaceum 4212. J. Pure Appl. Microbiol. 2022, 16, 2173–2196. [Google Scholar] [CrossRef]
- Rambaran, N.; Naidoo, Y.; Mohamed, F.; Chenia, H.Y.; Baijnath, H. Antibacterial and Anti-Quorum Sensing Properties of Silver Nanoparticles Phytosynthesized Using Embelia ruminata. Plants 2024, 13, 168. [Google Scholar] [CrossRef]
- Kant Jha, A.; Zamani, S.; Kumar, S. Green synthesis and characterization of silver nanoparticles using Pteris vitata extract and their therapeutic activities. Biotechnol. Appl. Bioc. 2022, 69, 1653–1662. [Google Scholar] [CrossRef]
- Wu, K.; Li, H.; Cui, X.; Feng, R.; Chen, W.; Jiang, Y.; Tang, C.; Wang, Y.; Wang, Y.; Shen, X.; et al. Mutagenesis and Resistance Development of Bacteria Challenged by Silver Nanoparticles. Antimicrob. Agents Chemother. 2022, 66, e0062822. [Google Scholar] [CrossRef] [PubMed]
- Poli, J.P.; Guinoiseau, E.; de Rocca Serra, D.; Sutour, S.; Paoli, M.; Tomi, F.; Quilichini, Y.; Berti, L.; Lorenzi, V. Anti-Quorum Sensing Activity of 12 Essential Oils on chromobacterium violaceum and Specific Action of cis-cis-p-Menthenolide from Corsican Mentha suaveolens ssp. Insularis. Molecules 2018, 23, 2125. [Google Scholar] [CrossRef] [PubMed]
- Jahan, I.; Erci, F.; Isildak, I. Microwave-Assisted Green Synthesis of Non-Cytotoxic Silver Nanoparticles Using the Aqueous Extract of Rosa santana (rose) Petals and Their Antimicrobial Activity. Anal. Lett. 2019, 52, 1860–1873. [Google Scholar] [CrossRef]
- Jalilian, F.; Chahardoli, A.; Sadrjavadi, K.; Fattahi, A.; Shokoohinia, Y. Green synthesized silver nanoparticle from Allium ampeloprasum aqueous extract: Characterization, antioxidant activities, antibacterial and cytotoxicity effects. Adv. Powder Technol. 2020, 31, 1323–1332. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Anbu, S.; Alegria, E.C.B.A.; Fernandes, A.R.; Baptistad, P.V.; Mendes, R.; Matias, A.S.; Mendes, M.; Guedes da Silvaa, M.F.C.; Pombeiro, A.J.L. Evaluation of cell toxicity and DNA and protein binding of green synthesized silver nanoparticles. Biomed. Pharmacother. 2018, 101, 137–144. [Google Scholar] [CrossRef]
- Saravanan, M.; Arokiyaraj, S.; Lakshmi, T.; Pugazhendhi, A. Synthesis of silver nanoparticles from Phenerochaete chrysosporium (MTCC-787) and their antibacterial activity against human pathogenic bacteria. Microb. Pathog. 2018, 117, 68–72. [Google Scholar] [CrossRef]
- Kubavat, K.; Trivedi, P.; Ansari, H. Green synthesis of silver nanoparticles using dietary antioxidant rutin and its biological contour. Beni-Suef Univ. J. Basic. Appl. Sci. 2022, 11, 115. [Google Scholar] [CrossRef]
- Mussin, J.; Robles-Botero, V.; Casañas-Pimentel, R.; Rojas, F.; Angiolella, L.; San Martín-Martínez, E.; Giusiano, G. Antimicrobial and cytotoxic activity of green synthesis silver nanoparticles targeting skin and soft tissue infectious agents. Sci. Rep. 2021, 11, 14566. [Google Scholar] [CrossRef]
- Garibo, D.; Borbón-Nuñez, H.A.; de León, J.N.D.; Mendoza, E.G.; Estrada, I.; Toledano-Magaña, Y.; Tiznado, H.; Ovalle-Marroquin, M.; Soto-Ramos, A.G.; Blanco, A.; et al. Green synthesis of silver nanoparticles using Lysiloma acapulcensis exhibit high-antimicrobial activity. Sci. Rep. 2020, 10, 12805. [Google Scholar] [CrossRef]
- Srećković, N.Z.; Nedić, Z.P.; Monti, D.M.; D’Elia, L.; Dimitrijević, S.B.; Mihailović, N.R.; Katanić Stanković, J.S.; Mihailović, V.B. Biosynthesis of Silver Nanoparticles Using Salvia pratensis L. Aerial Part and Root Extracts: Bioactivity, Biocompatibility, and Catalytic Potential. Molecules 2023, 28, 1387. [Google Scholar] [CrossRef]
- Govindappa, M.; Tejashree, S.; Thanuja, V.; Hemashekhar, B.; Srinivas, C.; Nasif, O.; Raghavendra, V.B. Pomegranate fruit fleshy pericarp mediated silver nanoparticles possessing antimicrobial, antibiofilm formation, antioxidant, biocompatibility and anticancer activity. J. Drug Deliv. Technol. 2020, 61, 102289. [Google Scholar] [CrossRef]
- Mohammed, A.E.; Al-Megrin, W.A. Biological Potential of Silver Nanoparticles Mediated by Leucophyllum frutescens and Russelia equisetiformis Extracts. Nanomaterials 2021, 11, 2098. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elhady, H.M.; Ashor, M.A.; Hazem, A.; Saleh, F.M.; Selim, S.; El Nahhas, N.; Abdel-Hafez, S.H.; Sayed, S.; Hassan, E.A. Biosynthesis and Characterization of Extracellular Silver Nanoparticles from Streptomyces aizuneusis: Antimicrobial, Anti Larval, and Anticancer Activities. Molecules 2022, 27, 212. [Google Scholar] [CrossRef] [PubMed]
- Radzikowska-Büchner, E.; Flieger, W.; Pasieczna-Patkowska, S.; Franus, W.; Panek, R.; Korona-Głowniak, I.; Suśniak, K.; Rajtar, B.; Świątek, Ł.; Żuk, N.; et al. Antimicrobial and Apoptotic Efficacy of Plant-Mediated Silver Nanoparticles. Molecules 2023, 28, 5519. [Google Scholar] [CrossRef]
- Khan, Q.; Yousafzai, A.M. Plant based synthesis of silver nanoparticles, antimicrobial efficiency, and toxicological assessment using freshwater fish (Cyprinus carpio). Microsc. Res. Tech. 2024, 87, 53–64. [Google Scholar] [CrossRef]
- Ajaykumar, A.P.; Mathew, A.; Chandni, A.P.; Varma, S.R.; Jayaraj, K.N.; Sabira, O.; Rasheed, V.A.; Binitha, V.S.; Swaminathan, T.R.; Basheer, V.S.; et al. Green Synthesis of Silver Nanoparticles Using the Leaf Extract of the Medicinal Plant, Uvaria narum and Its Antibacterial, Antiangiogenic, Anticancer and Catalytic Properties. Antibiotics 2023, 12, 564. [Google Scholar] [CrossRef]
- Sellami, H.; Khan, S.A.; Ahmad, I.; Alarfaj, A.A.; Hirad, A.H.; Al-Sabri, A.E. Green Synthesis of Silver Nanoparticles Using Olea europaea Leaf Extract for Their Enhanced Antibacterial, Antioxidant, Cytotoxic and Biocompatibility Applications. Int. J. Mol. Sci. 2021, 22, 12562. [Google Scholar] [CrossRef]
- Zhang, H.; Jacob, J.A.; Jiang, Z.; Xu, S.; Sun, K.; Zhong, Z.; Varadharaju, N.; Shanmugam, A. Hepatoprotective effect of silver nanoparticles synthesized using aqueous leaf extract of Rhizophora apiculata. Int. J. Nanomed. 2019, 14, 3517–3524. [Google Scholar] [CrossRef]
- Biruntha, M.; Selvi, B.K.; Paul, J.A.J.; Sivakumar, P.; Rajamanikandan, S.; Prabhu, D. Evaluation of hepato and renal protective effect of synthesized nanoparticles using Tinospora cordifolia leaf extract. Mater. Lett. 2022, 312, 131642. [Google Scholar] [CrossRef]
- Escárcega-González, C.E.; Garza-Cervantes, J.A.; Vázquez-Rodríguez, A.; Zulem Montelongo-Peralta, L.; Treviño-González, M.T.; Barriga Castro, E.D.; Saucedo-Salazar, E.M.; Chávez Morales, R.M.; Regalado Soto, D.I.; Treviño González, F.M.; et al. In vivo antimicrobial activity of silver nanoparticles produced via a green chemistry synthesis using Acacia rigidula as a reducing and capping agent. Int. J. Nanomed. 2018, 13, 2349–2363. [Google Scholar] [CrossRef]

| Microorganism | Type of Synthesis | Shape | Size, nm | Ref. |
|---|---|---|---|---|
| K. rhizophila | Extracellular | spherical | 10–200 | [4] |
| Planomicrobium sp. | Extracellular | spherical | 1–10 | [5] |
| Exiguobacterium aurantiacumm | Extracellular | spherical | 5–50 | [6] |
| Lactobacillus brevis | Extracellular | spherical, polyhedral | 5–40 | [7] |
| Bacillus sp. | Extracellular | spherical | 10–60 | [8] |
| Enterococcus sp. | Extracellular | spherical | 10–80 | [10] |
| A. faecalis | Extracellular | spherical | 30–50 | [10] |
| Pseudomonas aeruginosa | Extracellular | spherical | ~11 | [11] |
| Enterobacter cloacae | Intracellular | spherical | 7–25 | [3] |
| Cupriavidus necator, B. megaterium, and B. subtilis | Extra- and Intracellular | spherical | 20.8–118.4 | [13] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhailova, E.O. Green Silver Nanoparticles: An Antibacterial Mechanism. Antibiotics 2025, 14, 5. https://doi.org/10.3390/antibiotics14010005
Mikhailova EO. Green Silver Nanoparticles: An Antibacterial Mechanism. Antibiotics. 2025; 14(1):5. https://doi.org/10.3390/antibiotics14010005
Chicago/Turabian StyleMikhailova, Ekaterina O. 2025. "Green Silver Nanoparticles: An Antibacterial Mechanism" Antibiotics 14, no. 1: 5. https://doi.org/10.3390/antibiotics14010005
APA StyleMikhailova, E. O. (2025). Green Silver Nanoparticles: An Antibacterial Mechanism. Antibiotics, 14(1), 5. https://doi.org/10.3390/antibiotics14010005








