Harnessing Non-Antibiotic Strategies to Counter Multidrug-Resistant Clinical Pathogens with Special Reference to Antimicrobial Peptides and Their Coatings
Abstract
:1. Introduction
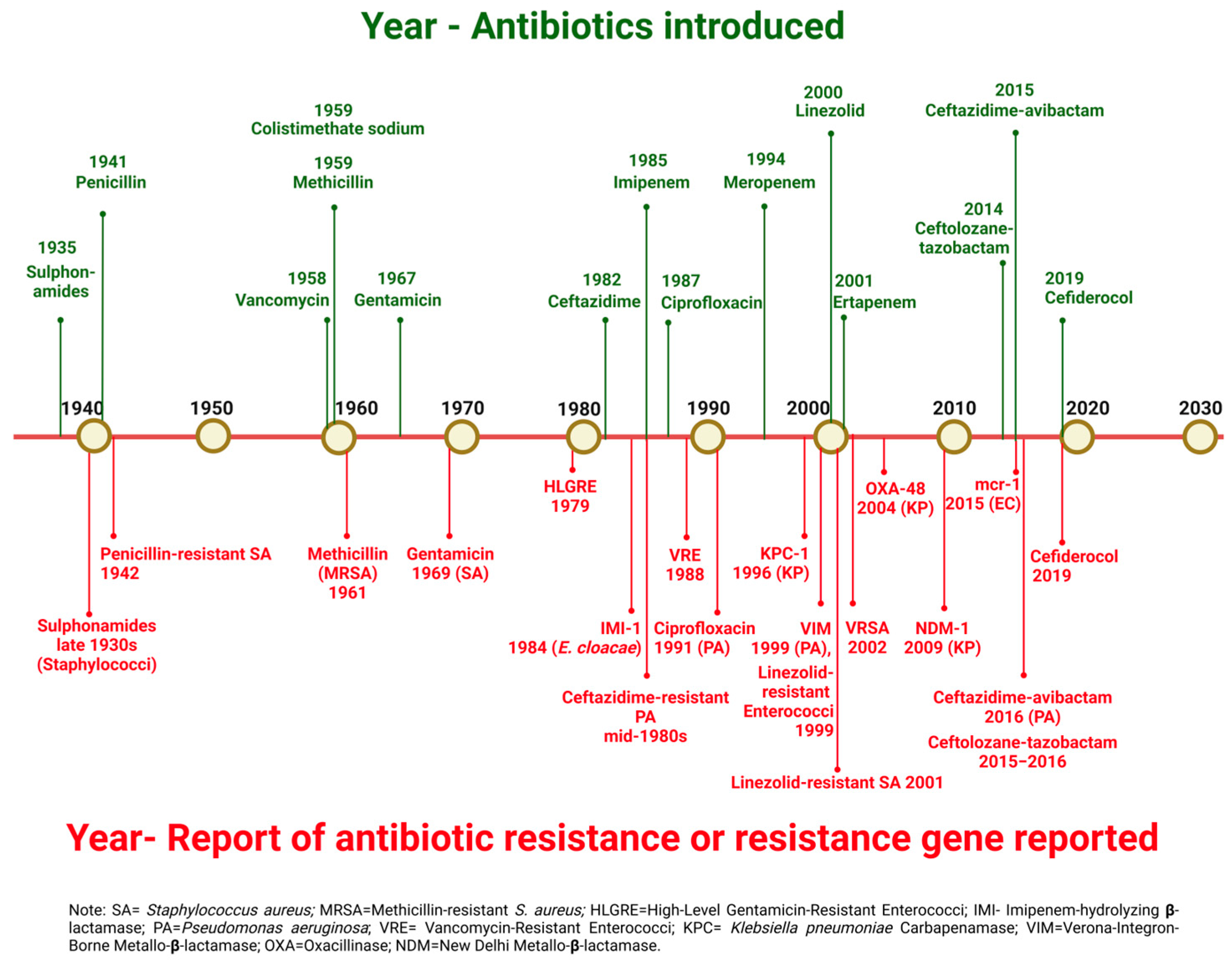
2. Antimicrobial Resistance in Bacteria
2.1. Phenotypic Resistance to Antimicrobials
2.2. Genetic Basis of Resistance to Antimicrobials
2.2.1. Intrinsic Resistance
2.2.2. Acquired Resistance
2.3. The Burden of Antimicrobial Resistance
3. Alternative Non-Antibiotic Approaches for the Prevention and Control of MDR Pathogens
3.1. Bacteriophage Therapy
3.2. Probiotics
3.3. Immunotherapies
3.4. Photodynamic Therapy
3.5. Essential Oils
3.6. Nanoparticles
3.7. Antimicrobial Peptides (AMPs)
Challenges in the Clinical Translation of AMPs
4. AMP Mimetics and Strategies to Enhance AMP Activity
4.1. Peptide Engineering and Chemical Modifications
4.1.1. Peptide Cyclisation
4.1.2. Non-Canonical Amino Acid Substitution
4.1.3. Peptoid-Based Mimetics
4.1.4. Lipidation
4.1.5. Conjugation with Functional Groups
4.2. Use of AMP Combinations
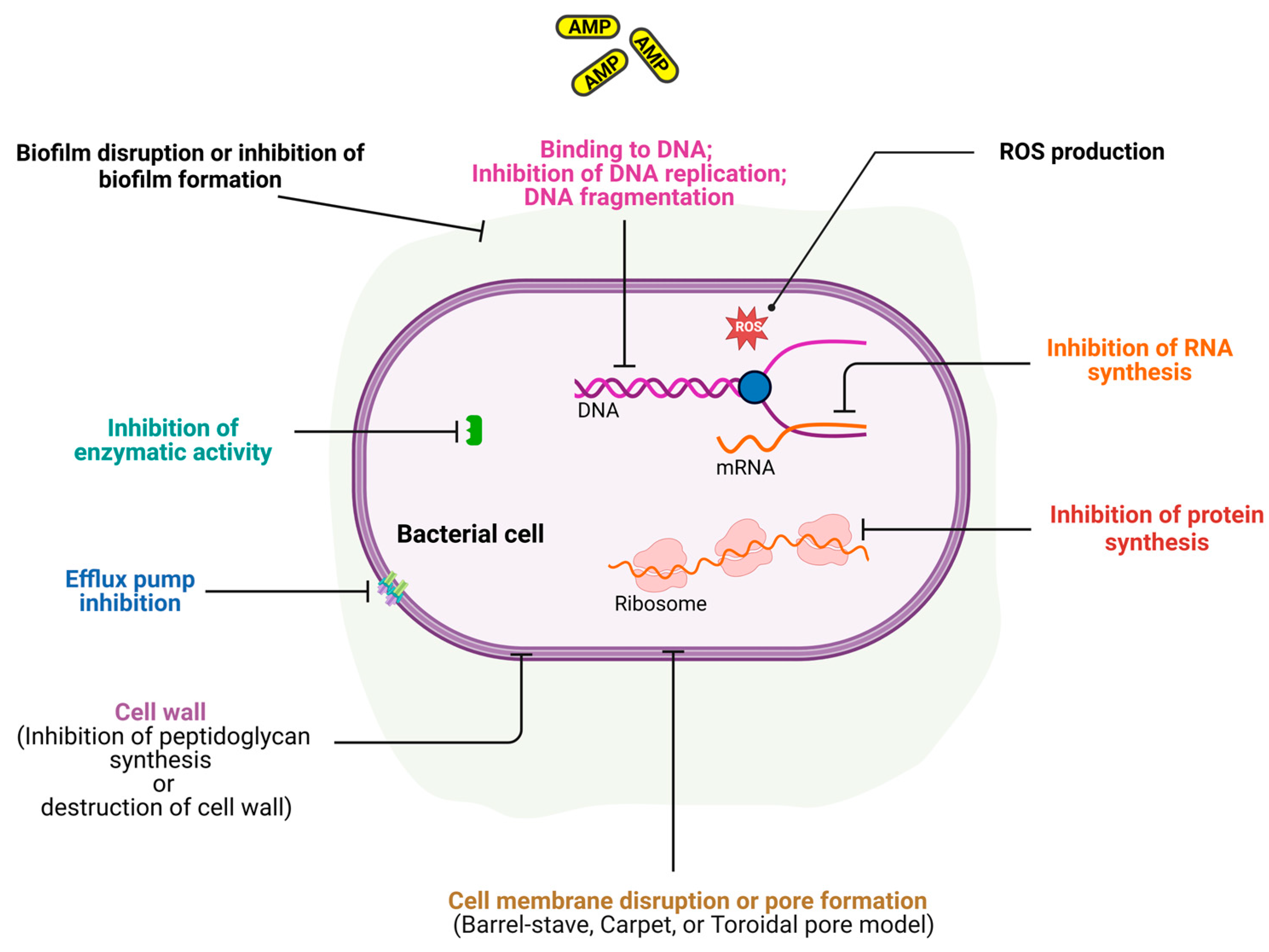
5. Mechanisms of Action of AMPs
5.1. Membrane Disruption
5.2. Intracellular Targeting
5.3. Immunomodulatory Effects
6. Reduction in Virulence Factors by Peptidomimetics
7. Antifungal Peptides and Their Mimetics
8. Antiviral Peptides and Their Mimetics
- (a)
- Disrupting the viral envelope or membrane: Many AMPs target the viral lipid membrane, destabilising its structure and preventing infection. This is particularly effective against enveloped viruses such as HIV [169], influenza [170], coronaviruses [171,172], hepatitis C [173,174], etc. For non-enveloped viruses like the BK virus, peptides can target the protein membrane, causing virion aggregation [175].
- (b)
- Inhibition of viral entry: Some AMPs block virus attachment and fusion with host cells by targeting virus or cell receptors or coreceptors, thereby preventing the attachment and fusion thereby initiating the infection [176].
- (c)
- (d)
9. Antiparasitic Peptides and Their Mimetics
10. Overview of Antimicrobial Coatings Using AMPs
10.1. Antiviral Coatings Using AMPs and Peptidomimetics
10.2. Antibacterial Coatings Using AMPs and Peptidomimetics
11. Additional Strategies for Attachment and Functionalisation
11.1. Non-Covalent Attachment Strategies
11.2. Electrostatics Layer-by-Layer Deposition
11.3. Photo Crosslinking
11.4. Polymer Brush Grafting
11.5. Silanes
11.6. Plasma Surfaces
11.7. Chemical Linkers
11.8. Michael Addition
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ho, C.S.; Wong, C.T.; Aung, T.T.; Lakshminarayanan, R.; Mehta, J.S.; Rauz, S.; McNally, A.; Kintses, B.; Peacock, S.J.; de la Fuente-Nunez, C. Antimicrobial resistance: A concise update. Lancet Microbe, 2024; ahead of print. [Google Scholar] [CrossRef]
- WHO. Antimicrobial Resistance and Primary Health Care. Available online: https://www.who.int/en/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 2 November 2024).
- Ferdinand, A.S.; McEwan, C.; Lin, C.; Betham, K.; Kandan, K.; Tamolsaian, G.; Pugeva, B.; McKenzie, J.; Browning, G.; Gilkerson, J. Development of a cross-sectoral antimicrobial resistance capability assessment framework. BMJ Glob. Health 2024, 9, e013280. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.; Carmeli, Y.; Falagas, M.; Giske, C.; Harbarth, S.; Hindler, J.; Kahlmeter, G.; Olsson-Liljequist, B.J.C.M.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Čivljak, R.; Giannella, M.; Di Bella, S.; Petrosillo, N. Could chloramphenicol be used against ESKAPE pathogens? A review of in vitro data in the literature from the 21st century. Expert Rev. Anti-Infect. Ther. 2014, 12, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.; Volova, T.; Prudnikova, S.V.; Shumilova, A.A.; Perianova, O.V.; Zharkov, S.M.; Kuzmin, A.; Olga, K.; Bogdan, K.; Shidlovskiy, I.P. Bio-hybridization of nanobactericides with cellulose films for effective treatment against members of ESKAPE multi-drug-resistant pathogens. Appl. Nanosci. 2018, 8, 1101–1110. [Google Scholar] [CrossRef]
- Darby, E.M.; Trampari, E.; Siasat, P.; Gaya, M.S.; Alav, I.; Webber, M.A.; Blair, J.M.A. Molecular mechanisms of antibiotic resistance revisited. Nat. Rev. Microbiol. 2023, 21, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Bellido, F.; Vladoianu, I.R.; Auckenthaler, R.; Suter, S.; Wacker, P.; Then, R.L.; Pechere, J.C. Permeability and penicillin-binding protein alterations in Salmonella muenchen: Stepwise resistance acquired during beta-lactam therapy. Antimicrob. Agents Chemother. 1989, 33, 1113–1115. [Google Scholar] [CrossRef] [PubMed]
- Tooke, C.L.; Hinchliffe, P.; Bragginton, E.C.; Colenso, C.K.; Hirvonen, V.H.; Takebayashi, Y.; Spencer, J. β-Lactamases and β-Lactamase Inhibitors in the 21st Century. J. Mol. Biol. 2019, 431, 3472–3500. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Miyoshi-Akiyama, T.; Shimada, K.; Dahal, R.K.; Mishra, S.K.; Ohara, H.; Kirikae, T.; Pokhrel, B.M. A Novel 6′-N-Aminoglycoside Acetyltransferase, AAC(6′)-Ial, from a Clinical Isolate of Serratia marcescens. Microb. Drug Resist. 2015, 22, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y.; Wachino, J.I.; Arakawa, Y. Aminoglycoside Resistance: The Emergence of Acquired 16S Ribosomal RNA Methyltransferases. Infect. Dis. Clin. N. Am. 2016, 30, 523–537. [Google Scholar] [CrossRef]
- Mah, T.-F.C.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Dahal, A.; Shrestha, K.; Karki, R.; Bhattarai, S.; Aryal, S.; Deo, S.K.; Regmi, B.; Willcox, M.; Mishra, S.K. Antimicrobial Resistance and Biofilm Production in Uropathogens from Renal Disease Patients Admitted to Tribhuvan University Teaching Hospital, Nepal. J. Clin. Pharm. Ther. 2023, 2023, 4867817. [Google Scholar] [CrossRef]
- Brauner, A.; Fridman, O.; Gefen, O.; Balaban, N.Q. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat. Rev. Microbiol. 2016, 14, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Balaban, N.Q.; Helaine, S.; Lewis, K.; Ackermann, M.; Aldridge, B.; Andersson, D.I.; Brynildsen, M.P.; Bumann, D.; Camilli, A.; Collins, J.J. Definitions and guidelines for research on antibiotic persistence. Nat. Rev. Microbiol. 2019, 17, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Bassler, B.L. Surviving as a community: Antibiotic tolerance and persistence in bacterial biofilms. Cell Host Microbe 2019, 26, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.R.; Lobritz, M.A.; Collins, J.J. Microbial persistence and the road to drug resistance. Cell Host Microbe 2013, 13, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Corona, F.; Martinez, J.L. Phenotypic resistance to antibiotics. Antibiotics 2013, 2, 237–255. [Google Scholar] [CrossRef] [PubMed]
- Peterson, E.; Kaur, P. Antibiotic resistance mechanisms in bacteria: Relationships between resistance determinants of antibiotic producers, environmental bacteria, and clinical pathogens. Front. Microbiol. 2018, 9, 2928. [Google Scholar] [CrossRef]
- Martinez, J.L. General principles of antibiotic resistance in bacteria. Drug Discov. Today Technol. 2014, 11, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Cox, G.; Wright, G.D. Intrinsic antibiotic resistance: Mechanisms, origins, challenges and solutions. Int. J. Med. Microbiol. 2013, 303, 287–292. [Google Scholar] [CrossRef]
- Hawkey, P.M. The origins and molecular basis of antibiotic resistance. BMJ 1998, 317, 657–660. [Google Scholar] [CrossRef]
- Urban-Chmiel, R.; Marek, A.; Stępień-Pyśniak, D.; Wieczorek, K.; Dec, M.; Nowaczek, A.; Osek, J. Antibiotic resistance in bacteria—A review. Antibiotics 2022, 11, 1079. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10, S122–S129. [Google Scholar] [CrossRef] [PubMed]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 481–511. [Google Scholar] [CrossRef] [PubMed]
- Poudel, A.N.; Zhu, S.; Cooper, N.; Little, P.; Tarrant, C.; Hickman, M.; Yao, G. The economic burden of antibiotic resistance: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0285170. [Google Scholar] [CrossRef] [PubMed]
- Balasegaram, M.; Outterson, K.; Røttingen, J.-A. The time to address the antibiotic pipeline and access crisis is now. Lancet 2024, 404, 1385–1387. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Lamichhane, S.; Basnet, B.B.; Dahal, A.; Awal, B.K.; Mishra, S.K. In vitro antimicrobial synergy testing of extensively drug-resistant clinical isolates at an organ transplant center in Nepal. Infect. Drug Resist. 2021, 14, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Anastassopoulou, C.; Ferous, S.; Petsimeri, A.; Gioula, G.; Tsakris, A. Phage-Based Therapy in Combination with Antibiotics: A Promising Alternative against Multidrug-Resistant Gram-Negative Pathogens. Pathogens 2024, 13, 896. [Google Scholar] [CrossRef] [PubMed]
- Domingo-Calap, P.; Delgado-Martínez, J. Bacteriophages: Protagonists of a post-antibiotic era. Antibiotics 2018, 7, 66. [Google Scholar] [CrossRef]
- Pirnay, J.-P.; Verbeken, G.; Ceyssens, P.-J.; Huys, I.; De Vos, D.; Ameloot, C.; Fauconnier, A. The magistral phage. Viruses 2018, 10, 64. [Google Scholar] [CrossRef]
- Pallavali, R.R.; Degati, V.L.; Lomada, D.; Reddy, M.C.; Durbaka, V.R.P. Isolation and in vitro evaluation of bacteriophages against MDR-bacterial isolates from septic wound infections. PLoS ONE 2017, 12, e0179245. [Google Scholar] [CrossRef]
- Dvorackova, M.; Ruzicka, F.; Benesik, M.; Pantucek, R.; Dvorakova-Heroldova, M. Antimicrobial effect of commercial phage preparation Stafal (R) on biofilm and planktonic forms of methicillin-resistant Staphylococcus aureus. Folia Microbiol. 2019, 64, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; ur Rahman, S.; Das, C.R. Isolation, characterization and efficacy of phage MJ2 against biofilm forming multi-drug resistant Enterobacter cloacae. Folia Microbiol. 2019, 64, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Mulani, M.; Kamble, E.; Kumkar, S.N.; Tawre, M.; Pardesi, K. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: A review. Front Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Markoishvili, K.; Tsitlanadze, G.; Katsarava, R.; Glenn, J.; Morris, M., Jr.; Sulakvelidze, A. A novel sustained-release matrix based on biodegradable poly (ester amide) s and impregnated with bacteriophages and an antibiotic shows promise in management of infected venous stasis ulcers and other poorly healing wounds. Int. J. Dermatol. 2002, 41, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Pirnay, J.-P.; Djebara, S.; Steurs, G.; Griselain, J.; Cochez, C.; De Soir, S.; Glonti, T.; Spiessens, A.; Vanden Berghe, E.; Green, S. Personalized bacteriophage therapy outcomes for 100 consecutive cases: A multicentre, multinational, retrospective observational study. Nat. Microbiol. 2024, 9, 1434–1453. [Google Scholar] [CrossRef] [PubMed]
- Kolenda, C.; Jourdan, J.; Roussel-Gaillard, T.; Medina, M.; Laurent, F. Phage susceptibility testing methods or ‘phagograms’: Where do we stand and where should we go? J. Antimicrob. Chemother. 2024, 79, 2742–2749. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.R.; Sardi, J.d.C.O.; de Souza Pitangui, N.; Roque, S.M.; da Silva, A.C.B.; Rosalen, P.L. Probiotics as an alternative antimicrobial therapy: Current reality and future directions. J. Funct. Foods 2020, 73, 104080. [Google Scholar] [CrossRef]
- Shokri, D.; Khorasgani, M.R.; Mohkam, M.; Fatemi, S.M.; Ghasemi, Y.; Taheri-Kafrani, A. The inhibition effect of lactobacilli against growth and biofilm formation of Pseudomonas aeruginosa. Probiotics Antimicrob. Proteins 2018, 10, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Raheem, A.; Liang, L.; Zhang, G.; Cui, S. Modulatory effects of probiotics during pathogenic infections with emphasis on immune regulation. Front. Immunol. 2021, 12, 616713. [Google Scholar] [CrossRef]
- Kuwelker, K.; Langeland, N.; Löhr, I.H.; Gidion, J.; Manyahi, J.; Moyo, S.J.; Blomberg, B.; Klingenberg, C. Use of probiotics to reduce infections and death and prevent colonization with extended-spectrum beta-lactamase (ESBL)-producing bacteria among newborn infants in Tanzania (ProRIDE Trial): Study protocol for a randomized controlled clinical trial. Trials 2021, 22, 312. [Google Scholar] [CrossRef] [PubMed]
- Neidhöfer, C.; Rathore, K.; Parčina, M.; Sieber, M.A. ESKAPEE pathogen biofilm control on surfaces with probiotic Lactobacillaceae and Bacillus species. Antibiotics 2023, 12, 871. [Google Scholar] [CrossRef] [PubMed]
- Karacaer, F.; Hamed, I.; Özogul, F.; Glew, R.H.; Özcengiz, D. The function of probiotics on the treatment of ventilator-associated pneumonia (VAP): Facts and gaps. J. Med. Microbiol. 2017, 66, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Merenstein, D.; Pot, B.; Leyer, G.; Ouwehand, A.C.; Preidis, G.A.; Elkins, C.A.; Hill, C.; Lewis, Z.T.; Shane, A.L.; Zmora, N. Emerging issues in probiotic safety: 2023 perspectives. Gut Microbes 2023, 15, 2185034. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, D.; Nundalall, T.; Cingo, S.; Mungra, N.; Karaan, M.; Naran, K.; Barth, S. Recent advances in immunotherapies against infectious diseases. Immunother. Adv. 2021, 1, ltaa007. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, T.R.; Wells, T.J.; Souza-Fonseca-Guimaraes, F. Towards efficient immunotherapy for bacterial infection. Trends Microbiol. 2022, 30, 158–169. [Google Scholar] [CrossRef]
- Ali, S.O.; Yu, X.Q.; Robbie, G.J.; Wu, Y.; Shoemaker, K.; Yu, L.; DiGiandomenico, A.; Keller, A.E.; Anude, C.; Hernandez-Illas, M.; et al. Phase 1 study of MEDI3902, an investigational anti–Pseudomonas aeruginosa PcrV and Psl bispecific human monoclonal antibody, in healthy adults. Clin. Microbiol. Infect. 2019, 25, 629.e1–629.e6. [Google Scholar] [CrossRef]
- Jain, R.; Beckett, V.V.; Konstan, M.W.; Accurso, F.J.; Burns, J.L.; Mayer-Hamblett, N.; Milla, C.; VanDevanter, D.R.; Chmiel, J.F. KB001-A, a novel anti-inflammatory, found to be safe and well-tolerated in cystic fibrosis patients infected with Pseudomonas aeruginosa. J. Cyst. Fibros. 2018, 17, 484–491. [Google Scholar] [CrossRef]
- François, B.; Mercier, E.; Gonzalez, C.; Asehnoune, K.; Nseir, S.; Fiancette, M.; Desachy, A.; Plantefève, G.; Meziani, F.; de Lame, P.-A.; et al. Safety and tolerability of a single administration of AR-301, a human monoclonal antibody, in ICU patients with severe pneumonia caused by Staphylococcus aureus: First-in-human trial. Intensive Care Med. 2018, 44, 1787–1796. [Google Scholar] [CrossRef]
- Bekeredjian-Ding, I. Challenges for Clinical Development of Vaccines for Prevention of Hospital-Acquired Bacterial Infections. Front. Immunol. 2020, 11, 1755. [Google Scholar] [CrossRef]
- Lee, W.-H.; Choi, H.-I.; Hong, S.-W.; Kim, K.-s.; Gho, Y.S.; Jeon, S.G. Vaccination with Klebsiella pneumoniae-derived extracellular vesicles protects against bacteria-induced lethality via both humoral and cellular immunity. Exp. Mol. Med. 2015, 47, e183. [Google Scholar] [CrossRef]
- Mai, B.; Gao, Y.; Li, M.; Wang, X.; Zhang, K.; Liu, Q.; Xu, C.; Wang, P. Photodynamic antimicrobial chemotherapy for Staphylococcus aureus and multidrug-resistant bacterial burn infection in vitro and in vivo. Int. J. Nanomed. 2017, 12, 5915–5931. [Google Scholar] [CrossRef]
- Yang, M.-Y.; Chang, K.-C.; Chen, L.-Y.; Wang, P.-C.; Chou, C.-C.; Wu, Z.-B.; Hu, A. Blue light irradiation triggers the antimicrobial potential of ZnO nanoparticles on drug-resistant Acinetobacter baumannii. J. Photochem. Photobiol. B Biol. 2018, 180, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A. Photodynamic Therapy in the Inactivation of Microorganisms. Antibiotics 2020, 9, 138. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy—What we know and what we don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef] [PubMed]
- Kling, S.; Hufschmid, F.S.; Torres-Netto, E.A.; Randleman, J.B.; Willcox, M.; Zbinden, R.; Hafezi, F. High fluence increases the antibacterial efficacy of PACK cross-linking. Cornea 2020, 39, 1020–1026. [Google Scholar] [CrossRef]
- Halstead Fenella, D.; Thwaite Joanne, E.; Burt, R.; Laws Thomas, R.; Raguse, M.; Moeller, R.; Webber Mark, A.; Oppenheim Beryl, A. Antibacterial Activity of Blue Light against Nosocomial Wound Pathogens Growing Planktonically and as Mature Biofilms. Appl. Environ. Microbiol. 2016, 82, 4006–4016. [Google Scholar] [CrossRef] [PubMed]
- Sueoka, K.; Chikama, T.; Latief, M.A.; Ko, J.-A.; Kiuchi, Y.; Sakaguchi, T.; Obana, A. Time-dependent antimicrobial effect of photodynamic therapy with TONS 504 on Pseudomonas aeruginosa. Lasers Med. Sci. 2018, 33, 1455–1460. [Google Scholar] [CrossRef]
- Woźniak, A.; Kruszewska, B.; Pierański, M.K.; Rychłowski, M.; Grinholc, M. Antimicrobial Photodynamic Inactivation Affects the Antibiotic Susceptibility of Enterococcus spp. Clinical Isolates in Biofilm and Planktonic Cultures. Biomolecules 2021, 11, 693. [Google Scholar] [CrossRef]
- Buchovec, I.; Vyčaitė, E.; Badokas, K.; Sužiedelienė, E.; Bagdonas, S. Application of Antimicrobial Photodynamic Therapy for Inactivation of Acinetobacter baumannii Biofilms. Int. J. Mol. Sci. 2023, 24, 722. [Google Scholar] [CrossRef]
- da Silva, L.C.N.; da Silva, M.V.; Correia, M.T.d.S. Editorial: New Frontiers in the Search of Antimicrobials Agents from Natural Products. Front. Microbiol. 2017, 8, 210. [Google Scholar] [CrossRef] [PubMed]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef]
- Vasconcelos, N.G.; Croda, J.; Simionatto, S. Antibacterial mechanisms of cinnamon and its constituents: A review. Microb. Pathog. 2018, 120, 198–203. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef]
- Panda, S.K.; Buroni, S.; Swain, S.S.; Bonacorsi, A.; da Fonseca Amorim, E.A.; Kulshrestha, M.; da Silva, L.C.N.; Tiwari, V. Recent advances to combat ESKAPE pathogens with special reference to essential oils. Front. Microbiol. 2022, 13, 1029098. [Google Scholar] [CrossRef] [PubMed]
- Artini, M.; Patsilinakos, A.; Papa, R.; Božović, M.; Sabatino, M.; Garzoli, S.; Vrenna, G.; Tilotta, M.; Pepi, F.; Ragno, R.; et al. Antimicrobial and Antibiofilm Activity and Machine Learning Classification Analysis of Essential Oils from Different Mediterranean Plants against Pseudomonas aeruginosa. Molecules 2018, 23, 482. [Google Scholar] [CrossRef] [PubMed]
- Husain, F.M.; Ahmad, I.; Asif, M.; Tahseen, Q. Influence of clove oil on certain quorum-sensing-regulated functions and biofilm of Pseudomonas aeruginosa and Aeromonas hydrophila. J. Biosci. 2013, 38, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Husain, F.M.; Ahmad, I.; Khan, M.S.; Ahmad, E.; Tahseen, Q.; Khan, M.S.; Alshabib, N.A. Sub-MICs of Mentha piperita essential oil and menthol inhibits AHL mediated quorum sensing and biofilm of Gram-negative bacteria. Front. Microbiol. 2015, 6, 420. [Google Scholar] [CrossRef]
- Yadav, M.K.; Chae, S.-W.; Im, G.J.; Chung, J.-W.; Song, J.-J. Eugenol: A Phyto-Compound Effective against Methicillin-Resistant and Methicillin-Sensitive Staphylococcus aureus Clinical Strain Biofilms. PLoS ONE 2015, 10, e0119564. [Google Scholar] [CrossRef] [PubMed]
- Kalaiselvan, P.; Yasir, M.; Kuppusamy, R.; Willcox, M.; Vijay, A.K. Ability of essential oil vapours to reduce numbers of culturable aerosolised coronavirus, bacteria and fungi. Antibiotics 2022, 11, 393. [Google Scholar] [CrossRef]
- Yadav, P.; Shrestha, S.; Basyal, D.; Tiwari, A.; Sah, R.; Sah, A.K.; Yadav, B.; Willcox, M.; Mishra, S.K. Characterization and Biofilm Inhibition of Multidrug-Resistant Acinetobacter baumannii Isolates. Int. J. Microbiol. 2024, 2024, 5749982. [Google Scholar] [CrossRef]
- Pattnaik, S.; Mishra, M.; Naik, P.K. Computational Approaches for the Inhibition of ESKAPE Pathogens. In ESKAPE Pathogens: Detection, Mechanisms and Treatment Strategies; Springer: Berlin/Heidelberg, Germany, 2024; pp. 503–544. [Google Scholar]
- Hajipour, M.J.; Fromm, K.M.; Akbar Ashkarran, A.; Jimenez de Aberasturi, D.; Larramendi, I.R.d.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Mukherjee, A.; Bose, S.; Shaoo, A.; Das, S.K. Nanotechnology based therapeutic approaches: An advanced strategy to target the biofilm of ESKAPE pathogens. Mater. Adv. 2023, 4, 2544–2572. [Google Scholar] [CrossRef]
- Mondal, S.K.; Chakraborty, S.; Manna, S.; Mandal, S.M. Antimicrobial nanoparticles: Current landscape and future challenges. RSC Pharm. 2024, 1, 388–402. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Jemal, K.; Sandeep, B.; Pola, S. Synthesis, characterization, and evaluation of the antibacterial activity of Allophylus serratus leaf and leaf derived callus extracts mediated silver nanoparticles. J. Nanomater. 2017, 2017, 4213275. [Google Scholar] [CrossRef]
- Safawo, T.; Sandeep, B.; Pola, S.; Tadesse, A. Synthesis and characterization of zinc oxide nanoparticles using tuber extract of anchote (Coccinia abyssinica (Lam.) Cong.) for antimicrobial and antioxidant activity assessment. OpenNano 2018, 3, 56–63. [Google Scholar] [CrossRef]
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Dyčka, F.; Šebela, M.; Prucek, R.; Tomanec, O. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nanotechnol. 2018, 13, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Dar, M.A.; Gul, R.; Karuppiah, P.; Al-Dhabi, N.A.; Alfadda, A.A. Antibacterial Activity of Cerium Oxide Nanoparticles against ESKAPE Pathogens. Crystals 2022, 12, 179. [Google Scholar] [CrossRef]
- Shkodenko, L.; Kassirov, I.; Koshel, E. Metal oxide nanoparticles against bacterial biofilms: Perspectives and limitations. Microorganisms 2020, 8, 1545. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.-Y.; Ko, W.-C.; Hsueh, P.-R. Nanoparticles in the treatment of infections caused by multidrug-resistant organisms. Front. Pharmacol. 2019, 10, 1153. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antimicrobial Peptides of Multicellular Organisms: My Perspective. Adv. Exp. Med. Biol. 2019, 1117, 3–6. [Google Scholar] [CrossRef]
- van Hoek, M.L. Diversity in Host Defense Antimicrobial Peptides. In Host Defense Peptides and Their Potential as Therapeutic Agents; Epand, R.M., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 3–26. [Google Scholar] [CrossRef]
- Hancock, R.E.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Rončević, T.; Puizina, J.; Tossi, A. Antimicrobial peptides as anti-infective agents in pre-post-antibiotic era? Int. J. Mol. Sci. 2019, 20, 5713. [Google Scholar] [CrossRef]
- Howell, M.; Wenc, A.K.; Donaghy, C.M.; Wasche, D.V.; Abissi, I.; Naing, M.D.; Pierce, S.; Angeles-Boza, A.M. Exploring synergy and its role in antimicrobial peptide biology. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2022; Volume 663, pp. 99–130. [Google Scholar]
- Browne, K.; Chakraborty, S.; Chen, R.; Willcox, M.D.; Black, D.S.; Walsh, W.R.; Kumar, N. A New Era of Antibiotics: The Clinical Potential of Antimicrobial Peptides. Int. J. Mol. Sci. 2020, 21, 7047. [Google Scholar] [CrossRef] [PubMed]
- Moravej, H.; Moravej, Z.; Yazdanparast, M.; Heiat, M.; Mirhosseini, A.; Moosazadeh Moghaddam, M.; Mirnejad, R. Antimicrobial Peptides: Features, Action, and Their Resistance Mechanisms in Bacteria. Microb. Drug Resist. 2018, 24, 747–767. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919. [Google Scholar]
- Rodríguez-Rojas, A.; Rolff, J. Antimicrobial activity of cationic antimicrobial peptides against stationary phase bacteria. Front. Microbiol. 2022, 13, 1029084. [Google Scholar] [CrossRef]
- Shao, C.; Zhu, Y.; Lai, Z.; Tan, P.; Shan, A. Antimicrobial peptides with protease stability: Progress and perspective. Future Med. Chem. 2019, 11, 2047–2050. [Google Scholar] [CrossRef] [PubMed]
- Garcia Maset, R.n.; Hapeshi, A.; Hall, S.; Dalgliesh, R.M.; Harrison, F.; Perrier, S. Evaluation of the antimicrobial activity in host-mimicking media and in vivo toxicity of antimicrobial polymers as functional mimics of AMPs. ACS Appl. Mater. Interfaces 2022, 14, 32855–32868. [Google Scholar] [CrossRef]
- Dijksteel, G.S.; Ulrich, M.M.W.; Middelkoop, E.; Boekema, B. Review: Lessons Learned From Clinical Trials Using Antimicrobial Peptides (AMPs). Front. Microbiol. 2021, 12, 616979. [Google Scholar] [CrossRef] [PubMed]
- Enninful, G.N.; Kuppusamy, R.; Tiburu, E.K.; Kumar, N.; Willcox, M.D. Non-canonical amino acid bioincorporation into antimicrobial peptides and its challenges. J. Pept. Sci. 2024, 30, e3560. [Google Scholar] [CrossRef] [PubMed]
- Cresti, L.; Cappello, G.; Pini, A. Antimicrobial Peptides towards Clinical Application—A Long History to Be Concluded. Int. J. Mol. Sci. 2024, 25, 4870. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.P.; Tripathi, A.K.; Thakur, A.K. Innovative Strategies and Methodologies in Antimicrobial Peptide Design. J. Funct. Biomater. 2024, 15, 320. [Google Scholar] [CrossRef] [PubMed]
- Svenson, J.; Molchanova, N.; Schroeder, C.I. Antimicrobial Peptide Mimics for Clinical Use: Does Size Matter? Front. Immunol. 2022, 13, 915368. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, R.; Willcox, M.; Black, D.S.; Kumar, N. Short cationic peptidomimetic antimicrobials. Antibiotics 2019, 8, 44. [Google Scholar] [CrossRef]
- Rotem, S.; Mor, A. Antimicrobial peptide mimics for improved therapeutic properties. Biochim. Biophys. Acta 2009, 1788, 1582–1592. [Google Scholar] [CrossRef] [PubMed]
- Gunasekera, S.; Muhammad, T.; Strömstedt, A.A.; Rosengren, K.J.; Göransson, U. Backbone Cyclization and Dimerization of LL-37-Derived Peptides Enhance Antimicrobial Activity and Proteolytic Stability. Front. Microbiol. 2020, 11, 168. [Google Scholar] [CrossRef]
- Dathe, M.; Nikolenko, H.; Klose, J.; Bienert, M. Cyclization increases the antimicrobial activity and selectivity of arginine- and tryptophan-containing hexapeptides. Biochemistry 2004, 43, 9140–9150. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Chau, J.K.; Perry, N.A.; de Boer, L.; Zaat, S.A.; Vogel, H.J. Serum stabilities of short tryptophan- and arginine-rich antimicrobial peptide analogs. PLoS ONE 2010, 5, e12684. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K. Control of cell selectivity of antimicrobial peptides. Biochim. Biophys. Acta (BBA)-Biomembr. 2009, 1788, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Su, P.Y.; Shih, C. Improvement of in vivo antimicrobial activity of HBcARD peptides by D-arginine replacement. Appl. Microbiol. Biotechnol. 2016, 100, 9125–9132. [Google Scholar] [CrossRef] [PubMed]
- Falciani, C.; Lozzi, L.; Pollini, S.; Luca, V.; Carnicelli, V.; Brunetti, J.; Lelli, B.; Bindi, S.; Scali, S.; Di Giulio, A.; et al. Isomerization of an antimicrobial peptide broadens antimicrobial spectrum to gram-positive bacterial pathogens. PLoS ONE 2012, 7, e46259. [Google Scholar] [CrossRef] [PubMed]
- Maji, K.; Haldar, D. 1-(2-aminophenyl)-1H-1, 2, 3-triazole-4-carboxylic acid: Activity against Gram-positive and Gram-negative pathogens including Vibrio cholerae. R. Soc. Open Sci. 2017, 4, 170684. [Google Scholar] [CrossRef] [PubMed]
- Horne, W.S.; Johnson, L.M.; Ketas, T.J.; Klasse, P.J.; Lu, M.; Moore, J.P.; Gellman, S.H. Structural and biological mimicry of protein surface recognition by alpha/beta-peptide foldamers. Proc. Natl. Acad. Sci. USA 2009, 106, 14751–14756. [Google Scholar] [CrossRef]
- Goodson, B.; Ehrhardt, A.; Ng, S.; Nuss, J.; Johnson, K.; Giedlin, M.; Yamamoto, R.; Moos, W.H.; Krebber, A.; Ladner, M. Characterization of novel antimicrobial peptoids. Antimicrob. Agents Chemother. 1999, 43, 1429–1434. [Google Scholar] [CrossRef]
- Sara, M.; Yasir, M.; Kalaiselvan, P.; Hui, A.; Kuppusamy, R.; Kumar, N.; Chakraborty, S.; Yu, T.T.; Wong, E.H.H.; Molchanova, N.; et al. The activity of antimicrobial peptoids against multidrug-resistant ocular pathogens. Cont. Lens Anterior Eye 2024, 47, 102124. [Google Scholar] [CrossRef]
- Godballe, T.; Nilsson, L.L.; Petersen, P.D.; Jenssen, H. Antimicrobial β-peptides and α-peptoids. Chem. Biol. Drug Des. 2011, 77, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Molchanova, N.; Hansen, P.R.; Damborg, P.; Nielsen, H.M.; Franzyk, H. Lysine-Based alpha-Peptide/beta-Peptoid Peptidomimetics: Influence of Hydrophobicity, Fluorination, and Distribution of Cationic Charge on Antimicrobial Activity and Cytotoxicity. ChemMedChem 2017, 12, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Appella, D.H.; Christianson, L.A.; Karle, I.L.; Powell, D.R.; Gellman, S.H. β-Peptide foldamers: Robust helix formation in a new family of β-amino acid oligomers. J. Am. Chem. Soc. 1996, 118, 13071–13072. [Google Scholar] [CrossRef]
- Zuckermann, R.N.; Kerr, J.M.; Kent, S.B.; Moos, W.H. Efficient method for the preparation of peptoids [oligo (N-substituted glycines)] by submonomer solid-phase synthesis. J. Am. Chem. Soc. 1992, 114, 10646–10647. [Google Scholar] [CrossRef]
- Chongsiriwatana, N.P.; Miller, T.M.; Wetzler, M.; Vakulenko, S.; Karlsson, A.J.; Palecek, S.P.; Mobashery, S.; Barron, A.E. Short alkylated peptoid mimics of antimicrobial lipopeptides. Antimicrob. Agents Chemother. 2011, 55, 417–420. [Google Scholar] [CrossRef]
- Lebedev, M.; Benjamin, A.B.; Kumar, S.; Molchanova, N.; Lin, J.S.; Koster, K.J.; Leibowitz, J.L.; Barron, A.E.; Cirillo, J.D. Antiviral Effect of Antimicrobial Peptoid TM9 and Murine Model of Respiratory Coronavirus Infection. Pharmaceutics 2024, 16, 464. [Google Scholar] [CrossRef]
- Brown, N.J.; Lin, J.S.; Barron, A.E. Helical side chain chemistry of a peptoid-based SP-C analogue: Balancing structural rigidity and biomimicry. Biopolymers 2019, 110, e23277. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.W.; Sanborn, T.J.; Zuckermann, R.N.; Barron, A.E. Peptoid oligomers with alpha-chiral, aromatic side chains: Effects of chain length on secondary structure. J. Am. Chem. Soc. 2001, 123, 2958–2963. [Google Scholar] [CrossRef] [PubMed]
- Dohm, M.T.; Kapoor, R.; Barron, A.E. Peptoids: Bio-inspired polymers as potential pharmaceuticals. Curr. Pharm. Des. 2011, 17, 2732–2747. [Google Scholar] [CrossRef]
- Amerikova, M.; Pencheva El-Tibi, I.; Maslarska, V.; Bozhanov, S.; Tachkov, K. Antimicrobial activity, mechanism of action, and methods for stabilisation of defensins as new therapeutic agents. Biotechnol. Biotechnol. Equip. 2019, 33, 671–682. [Google Scholar] [CrossRef]
- Rounds, T.; Straus, S.K. Lipidation of antimicrobial peptides as a design strategy for future alternatives to antibiotics. Int. J. Mol. Sci. 2020, 21, 9692. [Google Scholar] [CrossRef]
- Manteghi, R.; Pallagi, E.; Olajos, G.; Csóka, I. Pegylation and formulation strategy of Anti-Microbial Peptide (AMP) according to the quality by design approach. Eur. J. Pharm. Sci. 2020, 144, 105197. [Google Scholar] [CrossRef] [PubMed]
- Benincasa, M.; Zahariev, S.; Pelillo, C.; Milan, A.; Gennaro, R.; Scocchi, M. PEGylation of the peptide Bac7 (1–35) reduces renal clearance while retaining antibacterial activity and bacterial cell penetration capacity. Eur. J. Med. Chem. 2015, 95, 210–219. [Google Scholar] [CrossRef]
- Tang, Z.; Ma, Q.; Chen, X.; Chen, T.; Ying, Y.; Xi, X.; Wang, L.; Ma, C.; Shaw, C.; Zhou, M. Recent advances and challenges in nanodelivery systems for antimicrobial peptides (AMPs). Antibiotics 2021, 10, 990. [Google Scholar] [CrossRef] [PubMed]
- Makowski, M.; Silva, Í.C.; Pais do Amaral, C.; Gonçalves, S.; Santos, N.C. Advances in lipid and metal nanoparticles for antimicrobial peptide delivery. Pharmaceutics 2019, 11, 588. [Google Scholar] [CrossRef]
- Wu, C.-L.; Peng, K.-L.; Yip, B.-S.; Chih, Y.-H.; Cheng, J.-W. Boosting synergistic effects of short antimicrobial peptides with conventional antibiotics against resistant bacteria. Front. Microbiol. 2021, 12, 747760. [Google Scholar] [CrossRef] [PubMed]
- Taheri-Araghi, S. Synergistic action of antimicrobial peptides and antibiotics: Current understanding and future directions. Front. Microbiol. 2024, 15, 1390765. [Google Scholar] [CrossRef] [PubMed]
- Sheard, D.E.; O’Brien-Simpson, N.M.; Wade, J.D.; Separovic, F. Combating bacterial resistance by combination of antibiotics with antimicrobial peptides. Pure Appl. Chem. 2019, 91, 199–209. [Google Scholar] [CrossRef]
- Morroni, G.; Sante, L.D.; Simonetti, O.; Brescini, L.; Kamysz, W.; Kamysz, E.; Mingoia, M.; Brenciani, A.; Giovanetti, E.; Bagnarelli, P. Synergistic effect of antimicrobial peptide LL-37 and colistin combination against multidrug-resistant Escherichia coli isolates. Future Microbiol. 2021, 16, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Soren, O.; Brinch, K.S.; Patel, D.; Liu, Y.; Liu, A.; Coates, A.; Hu, Y. Antimicrobial Peptide Novicidin Synergizes with Rifampin, Ceftriaxone, and Ceftazidime against Antibiotic-Resistant Enterobacteriaceae In Vitro. Antimicrob. Agents Chemother. 2015, 59, 6233–6240. [Google Scholar] [CrossRef] [PubMed]
- Chongsiriwatana, N.P.; Wetzler, M.; Barron, A.E. Functional synergy between antimicrobial peptoids and peptides against Gram-negative bacteria. Antimicrob. Agents Chemother. 2011, 55, 5399–5402. [Google Scholar] [CrossRef]
- Corrêa, J.A.F.; Evangelista, A.G.; de Melo Nazareth, T.; Luciano, F.B.J.M. Fundamentals on the molecular mechanism of action of antimicrobial peptides. Materalia 2019, 8, 100494. [Google Scholar] [CrossRef]
- Malanovic, N.; Lohner, K. Gram-positive bacterial cell envelopes: The impact on the activity of antimicrobial peptides. Biochim. Biophys. Acta (BBA)-Biomembr. 2016, 1858, 936–946. [Google Scholar] [CrossRef]
- Ciumac, D.; Gong, H.; Hu, X.; Lu, J.R. Membrane targeting cationic antimicrobial peptides. J. Colloid Interface Sci. 2019, 537, 163–185. [Google Scholar] [CrossRef] [PubMed]
- Herrera, A.I.; Tomich, J.M.; Prakash, O. Membrane interacting peptides: A review. Curr. Protein Pept. Sci. 2016, 17, 827–841. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W. Action of antimicrobial peptides: Two-state model. Biochemistry 2000, 39, 8347–8352. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Choudhary, P.; Singh, S. Antimicrobial peptides: Mechanism of action. Insights Antimicrob. Pept. 2022, 23, 1417. [Google Scholar]
- Dean, R.; O’brien, L.; Thwaite, J.; Fox, M.; Atkins, H.; Ulaeto, D. A carpet-based mechanism for direct antimicrobial peptide activity against vaccinia virus membranes. Peptides 2010, 31, 1966–1972. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, D.; Leontiadou, H.; Mark, A.E.; Marrink, S.-J. Toroidal pores formed by antimicrobial peptides show significant disorder. Biochim. Biophys. Acta (BBA)-Biomembr. 2008, 1778, 2308–2317. [Google Scholar] [CrossRef]
- Mihajlovic, M.; Lazaridis, T. Antimicrobial peptides bind more strongly to membrane pores. Biochim. Biophys. Acta (BBA)-Biomembr. 2010, 1798, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial peptides: Classification, design, application and research progress in multiple fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Chikushi, A.; Tougu, S.; Imura, Y.; Nishida, M.; Yano, Y.; Matsuzaki, K. Membrane translocation mechanism of the antimicrobial peptide buforin 2. Biochemistry 2004, 43, 15610–15616. [Google Scholar] [CrossRef]
- Nicolas, P. Multifunctional host defense peptides: Intracellular-targeting antimicrobial peptides. FEBS J. 2009, 276, 6483–6496. [Google Scholar] [CrossRef] [PubMed]
- Haney, E.F.; Hancock, R.E. Peptide design for antimicrobial and immunomodulatory applications. Pept. Sci. 2013, 100, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Guryanova, S.V.; Ovchinnikova, T.V. Immunomodulatory and allergenic properties of antimicrobial peptides. Int. J. Mol. Sci. 2022, 23, 2499. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-J.; Wang, A.H.; Jennings, M.P. Discovery of virulence factors of pathogenic bacteria. Curr. Opin. Chem. Biol. 2008, 12, 93–101. [Google Scholar] [CrossRef]
- Alegbeleye, O.O.; Singleton, I.; Sant’Ana, A.S. Sources and contamination routes of microbial pathogens to fresh produce during field cultivation: A review. Food Microbiol. 2018, 73, 177–208. [Google Scholar] [CrossRef] [PubMed]
- Martínez, O.F.; Duque, H.M.; Franco, O.L. Peptidomimetics as potential anti-virulence drugs against resistant bacterial pathogens. Front. Microbiol. 2022, 13, 831037. [Google Scholar] [CrossRef]
- Rokas, A. Evolution of the human pathogenic lifestyle in fungi. Nat. Microbiol. 2022, 7, 607–619. [Google Scholar] [CrossRef]
- Denning, D.W. Global incidence and mortality of severe fungal disease. Lancet Infect. Dis. 2024, 24, e428–e438. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.E. Current concepts in antifungal pharmacology. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2011; pp. 805–817. [Google Scholar]
- Sun, S.; Hoy, M.J.; Heitman, J. Fungal pathogens. Curr. Biol. 2020, 30, R1163–R1169. [Google Scholar] [CrossRef] [PubMed]
- Struyfs, C.; Cammue, B.P.; Thevissen, K. Membrane-interacting antifungal peptides. Front. Cell Dev. Biol. 2021, 9, 649875. [Google Scholar] [CrossRef] [PubMed]
- Buda De Cesare, G.; Cristy, S.A.; Garsin, D.A.; Lorenz, M.C. Antimicrobial peptides: A new frontier in antifungal therapy. mBio 2020, 11. [Google Scholar] [CrossRef]
- Efremenko, E.; Aslanli, A.; Stepanov, N.; Senko, O.; Maslova, O. Various biomimetics, including peptides as antifungals. Biomimetics 2023, 8, 513. [Google Scholar] [CrossRef] [PubMed]
- Casalini, G.; Giacomelli, A.; Antinori, S. The WHO fungal priority pathogens list: A crucial reappraisal to review the prioritisation. Lancet Microbe 2024, 5, 717–724. [Google Scholar] [CrossRef]
- Carmo, A.; Rocha, M.; Pereirinha, P.; Tomé, R.; Costa, E. Antifungals: From pharmacokinetics to clinical practice. Antibiotics 2023, 12, 884. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.G.; Felipe, M.S. Candida albicans and antifungal peptides. Infect. Dis. Ther. 2023, 12, 2631–2648. [Google Scholar] [CrossRef]
- Ul Haq, I.; Maryam, S.; Shyntum, D.Y.; Khan, T.A.; Li, F. Exploring the frontiers of therapeutic breadth of antifungal peptides: A new avenue in antifungal drugs. J. Ind. Microbiol. Biotechnol. 2024, 51, kuae018. [Google Scholar] [CrossRef] [PubMed]
- Spicer, S.K.; Subramani, A.; Aguila, A.L.; Green, R.M.; McClelland, E.E.; Bicker, K.L. Toward a clinical antifungal peptoid: Investigations into the therapeutic potential of AEC5. Biopolymers 2019, 110, e23276. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Cole, N.; Kumar, N.; Willcox, M.D. Broad spectrum antimicrobial activity of melimine covalently bound to contact lenses. Investig. Ophthalmol. Vis. Sci. 2013, 54, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Vilas Boas, L.C.P.; Campos, M.L.; Berlanda, R.L.A.; de Carvalho Neves, N.; Franco, O.L. Antiviral peptides as promising therapeutic drugs. Cell Mol. Life Sci. 2019, 76, 3525–3542. [Google Scholar] [CrossRef]
- Urmi, U.L.; Vijay, A.K.; Kuppusamy, R.; Islam, S.; Willcox, M.D.P. A review of the antiviral activity of cationic antimicrobial peptides. Peptides 2023, 166, 171024. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Xu, S.; da Silva-Júnior, E.F.; Liu, X.; Zhan, P. Medicinal chemistry insights into antiviral peptidomimetics. Drug Discov. Today 2023, 28, 103468. [Google Scholar] [CrossRef] [PubMed]
- Lorin, C.; Saidi, H.; Belaid, A.; Zairi, A.; Baleux, F.; Hocini, H.; Bélec, L.; Hani, K.; Tangy, F. The antimicrobial peptide dermaseptin S4 inhibits HIV-1 infectivity in vitro. Virology 2005, 334, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhao, Z.; Zhou, D.; Chen, Y.; Hong, W.; Cao, L.; Yang, J.; Zhang, Y.; Shi, W.; Cao, Z. Virucidal activity of a scorpion venom peptide variant mucroporin-M1 against measles, SARS-CoV and influenza H5N1 viruses. Peptides 2011, 32, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; To, K.K.; Sze, K.-H.; Yung, T.T.-M.; Bian, M.; Lam, H.; Yeung, M.L.; Li, C.; Chu, H.; Yuen, K.-Y. A broad-spectrum virus-and host-targeting peptide against respiratory viruses including influenza virus and SARS-CoV-2. Nat. Commun. 2020, 11, 4252. [Google Scholar] [CrossRef]
- Chianese, A.; Zannella, C.; Monti, A.; De Filippis, A.; Doti, N.; Franci, G.; Galdiero, M. The Broad-Spectrum Antiviral Potential of the Amphibian Peptide AR-23. Int. J. Mol. Sci. 2022, 23, 883. [Google Scholar] [CrossRef] [PubMed]
- Batista, M.N.; Sanches, P.R.d.S.; Carneiro, B.M.; Braga, A.C.S.; Campos, G.R.F.; Cilli, E.M.; Rahal, P. GA-Hecate antiviral properties on HCV whole cycle represent a new antiviral class and open the door for the development of broad spectrum antivirals. Sci. Rep. 2018, 8, 14329. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Sun, F.; Li, S.; Gao, M.; Wang, L.; Sarhan, M.; Abdel-Rahman, M.A.; Li, W.; Kwok, H.F.; Wu, Y. Inhibitory activity of a scorpion defensin BmKDfsin3 against Hepatitis C virus. Antibiotics 2020, 9, 33. [Google Scholar] [CrossRef]
- Dugan, A.S.; Maginnis, M.S.; Jordan, J.A.; Gasparovic, M.L.; Manley, K.; Page, R.; Williams, G.; Porter, E.; O’Hara, B.A.; Atwood, W.J. Human α-defensins inhibit BK virus infection by aggregating virions and blocking binding to host cells. J. Biol. Chem. 2008, 283, 31125–31132. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Owen, S.M.; Rudolph, D.L.; Cole, A.M.; Hong, T.; Waring, A.J.; Lal, R.B.; Lehrer, R.I. Activity of α-and θ-defensins against primary isolates of HIV-1. J. Immunol. 2004, 173, 515–520. [Google Scholar] [CrossRef]
- Steinstraesser, L.; Tippler, B.; Mertens, J.; Lamme, E.; Homann, H.-H.; Lehnhardt, M.; Wildner, O.; Steinau, H.-U.; Überla, K. Inhibition of early steps in the lentiviral replication cycle by cathelicidin host defense peptides. Retrovirology 2005, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Wachinger, M.; Kleinschmidt, A.; Winder, D.; von Pechmann, N.; Ludvigsen, A.; Neumann, M.; Holle, R.; Salmons, B.; Erfle, V.; Brack-Werner, R. Antimicrobial peptides melittin and cecropin inhibit replication of human immunodeficiency virus 1 by suppressing viral gene expression. J. Gen. Virol. 1998, 79, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Wohlford-Lenane, C.L.; Meyerholz, D.K.; Perlman, S.; Zhou, H.; Tran, D.; Selsted, M.E.; McCray, P.B., Jr. Rhesus theta-defensin prevents death in a mouse model of severe acute respiratory syndrome coronavirus pulmonary disease. J. Virol. 2009, 83, 11385–11390. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Dai, Y.; Fu, Y.; Wang, K.; Yang, Y.; Li, M.; Xu, W.; Wei, L. Cathelicidin antimicrobial peptides suppress EV71 infection via regulating antiviral response and inhibiting viral binding. Antivir. Res. 2021, 187, 105021. [Google Scholar] [CrossRef]
- Urmi, U.L.; Attard, S.; Vijay, A.K.; Willcox, M.D.; Kumar, N.; Islam, S.; Kuppusamy, R. Antiviral Activity of Anthranilamide Peptidomimetics against Herpes Simplex Virus 1 and a Coronavirus. Antibiotics 2023, 12, 1436. [Google Scholar] [CrossRef]
- Urmi, U.L.; Vijay, A.K.; Willcox, M.D.; Attard, S.; Enninful, G.; Kumar, N.; Islam, S.; Kuppusamy, R. Exploring the efficacy of peptides and mimics against Influenza A Virus, Adenovirus, and murine norovirus. Int. J. Mol. Sci. 2024, 25, 7030. [Google Scholar] [CrossRef] [PubMed]
- Diamond, G.; Molchanova, N.; Herlan, C.; Fortkort, J.A.; Lin, J.S.; Figgins, E.; Bopp, N.; Ryan, L.K.; Chung, D.; Adcock, R.S.; et al. Potent Antiviral Activity against HSV-1 and SARS-CoV-2 by Antimicrobial Peptoids. Pharmaceuticals 2021, 14, 304. [Google Scholar] [CrossRef] [PubMed]
- Tate, P.M.; Mastrodomenico, V.; Cunha, C.; McClure, J.; Barron, A.E.; Diamond, G.; Mounce, B.C.; Kirshenbaum, K. Peptidomimetic Oligomers Targeting Membrane Phosphatidylserine Exhibit Broad Antiviral Activity. ACS Infect. Dis. 2023, 9, 1508–1522. [Google Scholar] [CrossRef]
- Niu, Y.; Wu, H.; Varani, G.; Cai, J. γ-AApeptides bind to RNA by mimicking RNA-binding proteins. Org. Biomol. Chem. 2011, 9, 6604–6609. [Google Scholar] [CrossRef]
- Naghavi, M.; Mestrovic, T.; Gray, A.; Hayoon, A.G.; Swetschinski, L.R.; Aguilar, G.R.; Weaver, N.D.; Ikuta, K.S.; Chung, E.; Wool, E.E. Global burden associated with 85 pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Infect. Dis. 2024, 24, 868–895. [Google Scholar] [CrossRef] [PubMed]
- Milne, G.; Webster, J.P.; Walker, M. Toxoplasma gondii: An underestimated threat? Trends Parasitol. 2020, 36, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Rayamajhee, B.; Willcox, M.; Henriquez, F.L.; Vijay, A.K.; Petsoglou, C.; Shrestha, G.S.; Peguda, H.K.; Carnt, N. The role of naturally acquired intracellular Pseudomonas aeruginosa in the development of Acanthamoeba keratitis in an animal model. PLoS Negl. Trop. Dis. 2024, 18, e0011878. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Fernández, N.; Anacleto-Santos, J.; Casarrubias-Tabarez, B.; López-Pérez, T.d.J.; Rojas-Lemus, M.; López-Valdez, N.; Fortoul, T.I. Bioactive Peptides against Human Apicomplexan Parasites. Antibiotics 2022, 11, 1658. [Google Scholar] [CrossRef]
- El-Dirany, R.; Shahrour, H.; Dirany, Z.; Abdel-Sater, F.; Gonzalez-Gaitano, G.; Brandenburg, K.; Martinez de Tejada, G.; Nguewa, P.A. Activity of anti-microbial peptides (AMPs) against Leishmania and other parasites: An overview. Biomolecules 2021, 11, 984. [Google Scholar] [CrossRef]
- Peguda, H.K.; Carnt, N.A.; Gu, Z.; Kumar, N.; Willcox, M.D.; Kuppusamy, R. The Anti-Amoebic Activity of a Peptidomimetic against Acanthamoeba castellanii. Microorganisms 2022, 10, 2377. [Google Scholar] [CrossRef]
- Peguda, H.K.; Lakshminarayanan, R.; Carnt, N.A.; Gu, Z.; Willcox, M.D. The Activity of Polyhomoarginine against Acanthamoeba castellanii. Biology 2022, 11, 1726. [Google Scholar] [CrossRef]
- Mishra, S.K.; Hui, A.; Willcox, M. Biofilm and medical device-associated infections. In Global Infection Prevention and Management in Healthcare, 1st ed.; Advanced Meetings Solutions: Carrollton, TX, USA, 2024; Volume 2, pp. 168–180. [Google Scholar]
- Sun, Z.; Ma, L.; Sun, X.; Sloan, A.J.; O’Brien-Simpson, N.M.; Li, W. The overview of antimicrobial peptide-coated implants against oral bacterial infections. Aggregate 2023, 4, e309. [Google Scholar] [CrossRef]
- Tiwari, A.; Sharma, P.; Vishwamitra, B.; Singh, G. Review on surface treatment for implant infection via gentamicin and antibiotic releasing coatings. Coatings 2021, 11, 1006. [Google Scholar] [CrossRef]
- Rakowska, P.D.; Tiddia, M.; Faruqui, N.; Bankier, C.; Pei, Y.; Pollard, A.J.; Zhang, J.; Gilmore, I.S. Antiviral surfaces and coatings and their mechanisms of action. Commun. Mater. 2021, 2, 53. [Google Scholar] [CrossRef]
- Hu, T.; Agazani, O.; Nir, S.; Cohen, M.; Pan, S.; Reches, M. Antiviral Activity of Peptide-Based Assemblies. ACS Appl. Mater. Interfaces 2021, 13, 48469–48477. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Dutta, D.; Hossain, K.R.; Chen, R.; Ho, K.K.K.; Kuppusamy, R.; Clarke, R.J.; Kumar, N.; Willcox, M.D.P. Mechanism of Action of Surface Immobilized Antimicrobial Peptides Against Pseudomonas aeruginosa. Front. Microbiol. 2019, 10, 3053. [Google Scholar] [CrossRef] [PubMed]
- Gamna, F.; Cochis, A.; Mojsoska, B.; Kumar, A.; Rimondini, L.; Spriano, S. Nano-topography and functionalization with the synthetic peptoid GN2-Npm9 as a strategy for antibacterial and biocompatible titanium implants. Heliyon 2024, 10, e24246. [Google Scholar] [CrossRef] [PubMed]
- Statz, A.R.; Park, J.P.; Chongsiriwatana, N.P.; Barron, A.E.; Messersmith, P.B. Surface-immobilised antimicrobial peptoids. Biofouling 2008, 24, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Lo, J.C.; Yan, M.; Yang, X.; Brooks, D.E.; Hancock, R.E.; Lange, D.; Kizhakkedathu, J.N. Anti-adhesive antimicrobial peptide coating prevents catheter associated infection in a mouse urinary infection model. Biomaterials 2017, 116, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Aronson, M.R.; Ali Akbari Ghavimi, S.; Gehret, P.M.; Jacobs, I.N.; Gottardi, R. Drug-Eluting Endotracheal Tubes for Preventing Bacterial Inflammation in Subglottic Stenosis. Laryngoscope 2022, 132, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- Willcox, M.D.; Chen, R.; Kalaiselvan, P.; Yasir, M.; Rasul, R.; Kumar, N.; Dutta, D. The development of an antimicrobial contact lens–from the laboratory to the clinic. Curr. Protein Pept. Sci. 2020, 21, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.; Yasir, M.; Dutta, D.; Eswaramoorthy, N.; Suchowerska, N.; Willcox, M.; McKenzie, D.R. Single Step Plasma Process for Covalent Binding of Antimicrobial Peptides on Catheters To Suppress Bacterial Adhesion. ACS Appl. Bio Mater. 2019, 2, 5739–5748. [Google Scholar] [CrossRef]
- Yao, Q.; Zhang, J.; Pan, G.; Chen, B. Mussel-inspired clickable antibacterial peptide coating on ureteral stents for encrustation prevention. ACS Appl. Mater. Interfaces 2022, 14, 36473–36486. [Google Scholar] [CrossRef] [PubMed]
- Chhablani, J.; Nayak, S.; Jindal, A.; Motukupally, S.R.; Mathai, A.; Jalali, S.; Pappuru, R.R.; Sharma, S.; Das, T.; Flynn, H.W. Scleral buckle infections: Microbiological spectrum and antimicrobial susceptibility. J. Ophthalmic Inflamm. Infect. 2013, 3, 67. [Google Scholar] [CrossRef]
- Cheng, K.H.; Leung, S.L.; Hoekman, H.W.; Beekhuis, W.H.; Mulder, P.G.; Geerards, A.J.; Kijlstra, A. Incidence of contact-lens-associated microbial keratitis and its related morbidity. Lancet 1999, 354, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Kumar, N.; Willcox, M.D.P. Antimicrobial activity of four cationic peptides immobilised to poly-hydroxyethylmethacrylate. Biofouling 2016, 32, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Vijay, A.K.; Kumar, N.; Willcox, M.D. Melimine-Coated Antimicrobial Contact Lenses Reduce Microbial Keratitis in an Animal Model. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5616–5624. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Kuppusamy, R.; Roohani, I.; Walsh, W.R.; Willcox, M.D.P.; Kumar, N.; Chen, R.X. Antibacterial peptidomimetic and characterization of its efficacy as an antibacterial and biocompatible coating for bioceramic-based bone substitutes. Mater. Adv. 2021, 2, 6369–6379. [Google Scholar] [CrossRef]
- Dutta, D.; Willcox, M.D. Antimicrobial contact lenses and lens cases: A review. Eye Contact Lens 2014, 40, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Browne, K.; Kuppusamy, R.; Chen, R.; Willcox, M.D.; Walsh, W.R.; Black, D.S.; Kumar, N. Bioinspired polydopamine coatings facilitate attachment of antimicrobial peptidomimetics with broad-spectrum antibacterial activity. Int. J. Mol. Sci. 2022, 23, 2952. [Google Scholar] [CrossRef]
- Kruse, H.V.; Chakraborty, S.; Chen, R.; Kumar, N.; Yasir, M.; Lewin, W.T.; Suchowerska, N.; Willcox, M.D.; McKenzie, D.R. Protecting Orthopaedic Implants from Infection: Antimicrobial Peptide Mel4 Is Non-Toxic to Bone Cells and Reduces Bacterial Colonisation When Bound to Plasma Ion-Implanted 3D-Printed PAEK Polymers. Cells 2024, 13, 656. [Google Scholar] [CrossRef]
- Lim, K.; Chua, R.R.Y.; Ho, B.; Tambyah, P.A.; Hadinoto, K.; Leong, S.S.J. Development of a catheter functionalized by a polydopamine peptide coating with antimicrobial and antibiofilm properties. Acta Biomater. 2015, 15, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Yang, W.; Hu, Y.; Luo, Z.; Li, J.; Hou, Y.; Liu, Y.; Cai, K. Surface functionalization of titanium substrates with cecropin B to improve their cytocompatibility and reduce inflammation responses. Colloids Surf. B Biointerfaces 2013, 110, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Willcox, M.; Hume, E.; Aliwarga, Y.; Kumar, N.; Cole, N. A novel cationic-peptide coating for the prevention of microbial colonization on contact lenses. J. Appl. Microbiol. 2008, 105, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Cole, N.; Hume, E.B.; Vijay, A.K.; Sankaridurg, P.; Kumar, N.; Willcox, M.D. In vivo performance of melimine as an antimicrobial coating for contact lenses in models of CLARE and CLPU. Investig. Ophthalmol. Vis. Sci. 2010, 51, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Sara, M.; Chakraborty, S.; Chen, R.; Palms, D.; Katsifis, G.; Li, Z.; Farajikhah, S.; Massedupally, V.; Hui, A.; Wong, E.H. The effect of immobilisation strategies on the ability of peptoids to reduce the adhesion of P. aeruginosa strains to contact lenses. Exp. Eye Res. 2025, 250, 110149. [Google Scholar] [CrossRef] [PubMed]
- Hotaling, N.A.; Tang, L.; Irvine, D.J.; Babensee, J.E. Biomaterial strategies for immunomodulation. Annu. Rev. Biomed. Eng. 2015, 17, 317–349. [Google Scholar] [CrossRef]
- Montz, B.J.; Emrick, T. Building structured, functional materials inspired by nature: Using peptides, peptoids, and polymerizations. J. Polym. Sci. 2024, 62, 3597–3628. [Google Scholar] [CrossRef]
- Lombardi, L.; Falanga, A.; Del Genio, V.; Galdiero, S. A new hope: Self-assembling peptides with antimicrobial activity. Pharmaceutics 2019, 11, 166. [Google Scholar] [CrossRef]
- Liu, Y.; He, T.; Gao, C. Surface modification of poly (ethylene terephthalate) via hydrolysis and layer-by-layer assembly of chitosan and chondroitin sulfate to construct cytocompatible layer for human endothelial cells. Colloids Surf. B Biointerfaces 2005, 46, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, L.P.; Borges, J.; Rodrigues, J.M.; Mano, J.F. Unveiling the Assembly of Neutral Marine Polysaccharides into Electrostatic-Driven Layer-by-Layer Bioassemblies by Chemical Functionalization. Mar. Drugs 2023, 21, 92. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, K.; Toyoda, T.; Fukui, Y. Preparation of bionanocapsules by the layer-by-layer deposition of polypeptides onto a liposome. Macromolecules 2007, 40, 5122–5128. [Google Scholar] [CrossRef]
- Lichter, J.A.; Van Vliet, K.J.; Rubner, M.F. Design of antibacterial surfaces and interfaces: Polyelectrolyte multilayers as a multifunctional platform. Macromolecules 2009, 42, 8573–8586. [Google Scholar] [CrossRef]
- Andrea, A.; Molchanova, N.; Jenssen, H. Antibiofilm peptides and peptidomimetics with focus on surface immobilization. Biomolecules 2018, 8, 27. [Google Scholar] [CrossRef]
- Pierau, L.; Versace, D.-L. Light and hydrogels: A new generation of antimicrobial materials. Materials 2021, 14, 787. [Google Scholar] [CrossRef]
- Chen, X.; Ayres, N. Synthesis of low grafting density molecular brush from a poly (N-alkyl urea peptoid) backbone. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 3030–3037. [Google Scholar] [CrossRef]
- Lau, K.H.A.; Ren, C.; Sileika, T.S.; Park, S.H.; Szleifer, I.; Messersmith, P.B. Surface-grafted polysarcosine as a peptoid antifouling polymer brush. Langmuir 2012, 28, 16099–16107. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, S.; Sharma, S.; Saha, S. Infection resistant surface coatings by polymer brushes: Strategies to construct and applications. ACS Appl. Bio Mater. 2022, 5, 1364–1390. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Feng, E.; Liao, Y.; Liu, H.; Tang, R.; Tan, Y. Brush-modified hydrogels: Preparations, properties, and applications. Chem. Mater. 2022, 34, 6210–6231. [Google Scholar] [CrossRef]
- Statz, A.R.; Barron, A.E.; Messersmith, P.B. Protein, cell and bacterial fouling resistance of polypeptoid-modified surfaces: Effect of side-chain chemistry. Soft Matter 2008, 4, 131–139. [Google Scholar] [CrossRef]
- Lau, K.H.A.; Sileika, T.S.; Park, S.H.; Sousa, A.M.; Burch, P.; Szleifer, I.; Messersmith, P.B. Molecular Design of Antifouling Polymer Brushes Using Sequence-Specific Peptoids. Adv. Mater. Interfaces 2015, 2, 1400225. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Yang, C.; Wang, Y.; Alexander, C.A.; Yi, G.; Zhang, Y.; Qin, X.; Yang, Y.Y. Silane-functionalized polyionenes-coated cotton fabrics with potent antimicrobial and antiviral activities. Biomaterials 2022, 284, 121470. [Google Scholar] [CrossRef]
- Mohorčič, M.; Jerman, I.; Zorko, M.; Butinar, L.; Orel, B.; Jerala, R.; Friedrich, J. Surface with antimicrobial activity obtained through silane coating with covalently bound polymyxin B. J. Mater. Sci. Mater. Med. 2010, 21, 2775–2782. [Google Scholar] [CrossRef] [PubMed]
- Gomathi, N.; Sureshkumar, A.; Neogi, S. RF plasma-treated polymers for biomedical applications. Curr. Sci. 2008, 94, 1478–1486. [Google Scholar]
- Kondyurin, A.; Naseri, P.; Fisher, K.; McKenzie, D.R.; Bilek, M.M. Mechanisms for surface energy changes observed in plasma immersion ion implanted polyethylene: The roles of free radicals and oxygen-containing groups. Polym. Degrad. Stab. 2009, 94, 638–646. [Google Scholar] [CrossRef]
- Liston, E.; Martinu, L.; Wertheimer, M.R. Plasma surface modification of polymers for improved adhesion: A critical review. J. Adhes. Sci. Technol. 1993, 7, 1091–1127. [Google Scholar] [CrossRef]
- Salvagni, E.; Garcia, C.; Manresa, A.; Muller-Sanchez, C.; Reina, M.; Rodriguez-Abreu, C.; Garcia-Celma, M.J.; Esquena, J. Short and ultrashort antimicrobial peptides anchored onto soft commercial contact lenses inhibit bacterial adhesion. Colloids Surf. B Biointerfaces 2020, 196, 111283. [Google Scholar] [CrossRef] [PubMed]
- Jarach, N.; Zuckerman, R.; Naveh, N.; Dodiuk, H.; Kenig, S. Bio-and water-based reversible covalent bonds containing polymers (vitrimers) and their relevance to adhesives–a critical review. Prog. Adhes. Adhes. 2021, 6, 587–619. [Google Scholar]
- Fairbanks, B.D.; Macdougall, L.J.; Mavila, S.; Sinha, J.; Kirkpatrick, B.E.; Anseth, K.S.; Bowman, C.N. Photoclick chemistry: A bright idea. Chem. Rev. 2021, 121, 6915–6990. [Google Scholar] [CrossRef]
- Musgrave, C.S.A.; Fang, F. Contact lens materials: A materials science perspective. Materials 2019, 12, 261. [Google Scholar] [CrossRef] [PubMed]


| Antimicrobial Approaches | Advantages | Limitations |
|---|---|---|
| Bacteriophages |
|
|
| Probiotics |
|
|
| Immunotherapies |
|
|
| Photodynamic therapies |
|
|
| Essential oils |
|
|
| Nanoparticles |
|
|
| Antimicrobial peptides |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, S.K.; Akter, T.; Urmi, U.L.; Enninful, G.; Sara, M.; Shen, J.; Suresh, D.; Zheng, L.; Mekonen, E.S.; Rayamajhee, B.; et al. Harnessing Non-Antibiotic Strategies to Counter Multidrug-Resistant Clinical Pathogens with Special Reference to Antimicrobial Peptides and Their Coatings. Antibiotics 2025, 14, 57. https://doi.org/10.3390/antibiotics14010057
Mishra SK, Akter T, Urmi UL, Enninful G, Sara M, Shen J, Suresh D, Zheng L, Mekonen ES, Rayamajhee B, et al. Harnessing Non-Antibiotic Strategies to Counter Multidrug-Resistant Clinical Pathogens with Special Reference to Antimicrobial Peptides and Their Coatings. Antibiotics. 2025; 14(1):57. https://doi.org/10.3390/antibiotics14010057
Chicago/Turabian StyleMishra, Shyam Kumar, Tanzina Akter, Umme Laila Urmi, George Enninful, Manjulatha Sara, Jiawei Shen, Dittu Suresh, Liangjun Zheng, Elias Shiferaw Mekonen, Binod Rayamajhee, and et al. 2025. "Harnessing Non-Antibiotic Strategies to Counter Multidrug-Resistant Clinical Pathogens with Special Reference to Antimicrobial Peptides and Their Coatings" Antibiotics 14, no. 1: 57. https://doi.org/10.3390/antibiotics14010057
APA StyleMishra, S. K., Akter, T., Urmi, U. L., Enninful, G., Sara, M., Shen, J., Suresh, D., Zheng, L., Mekonen, E. S., Rayamajhee, B., Labricciosa, F. M., Sartelli, M., & Willcox, M. (2025). Harnessing Non-Antibiotic Strategies to Counter Multidrug-Resistant Clinical Pathogens with Special Reference to Antimicrobial Peptides and Their Coatings. Antibiotics, 14(1), 57. https://doi.org/10.3390/antibiotics14010057









