Isolation of Dual-Active Drugs with Anticancer and Antibacterial Activities That Target Both Tubulin and FtsZ
Abstract
1. Introduction
2. Results
2.1. Minimum Inhibitory Concentration (MIC) Detection
2.2. Time–Killing Curve Determinations
2.3. Sa-FtsZ Expression and Purification
2.4. FtsZ Polymerization Assay
2.5. Sedimentation Assay
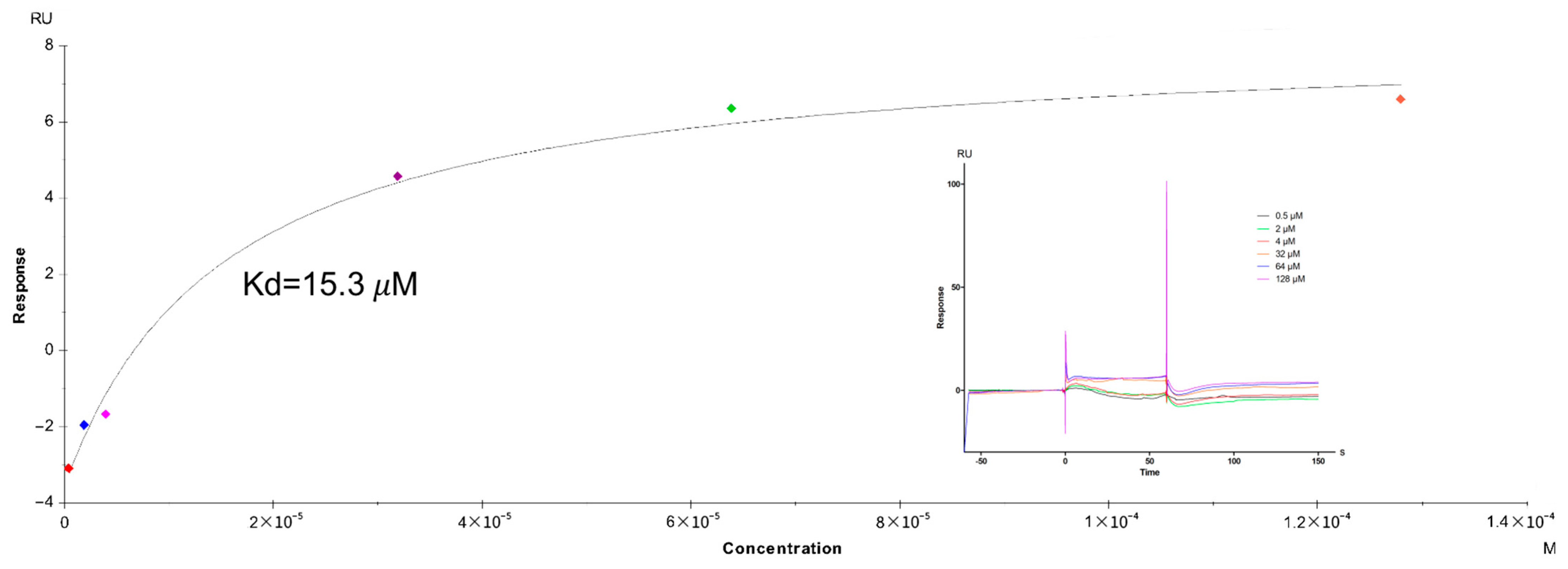
2.6. Affinity Assay by SPR Technology
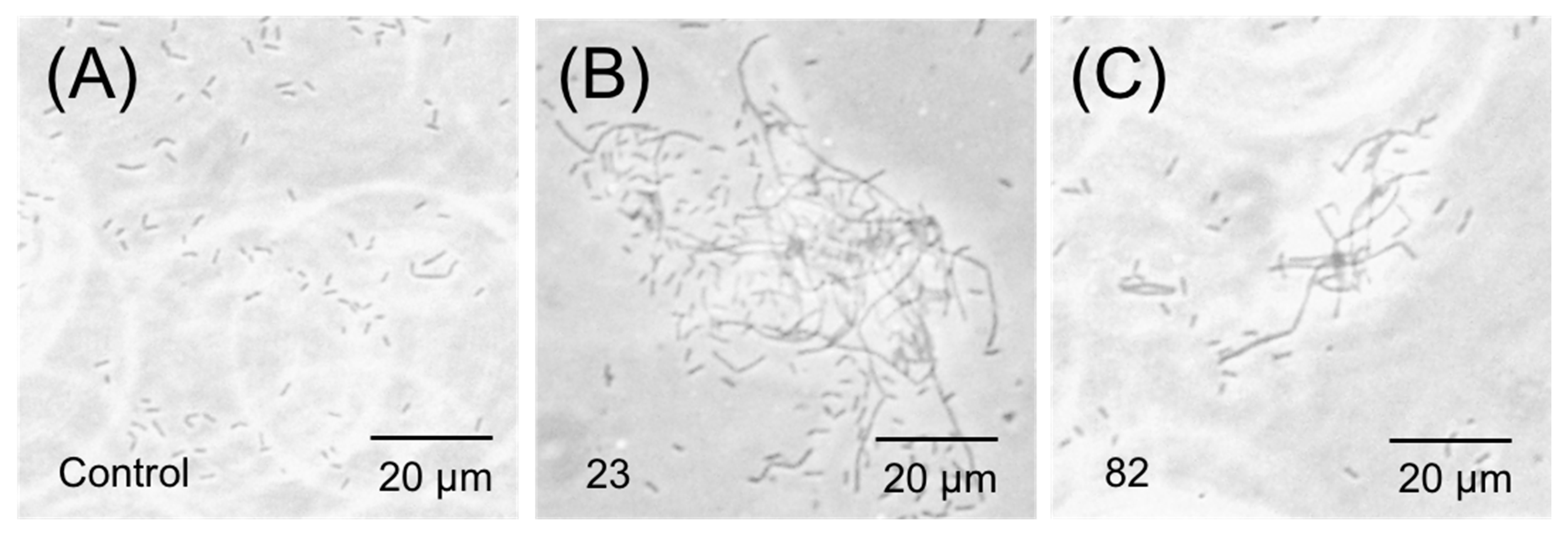
2.7. Phase-Contrast Microscopy
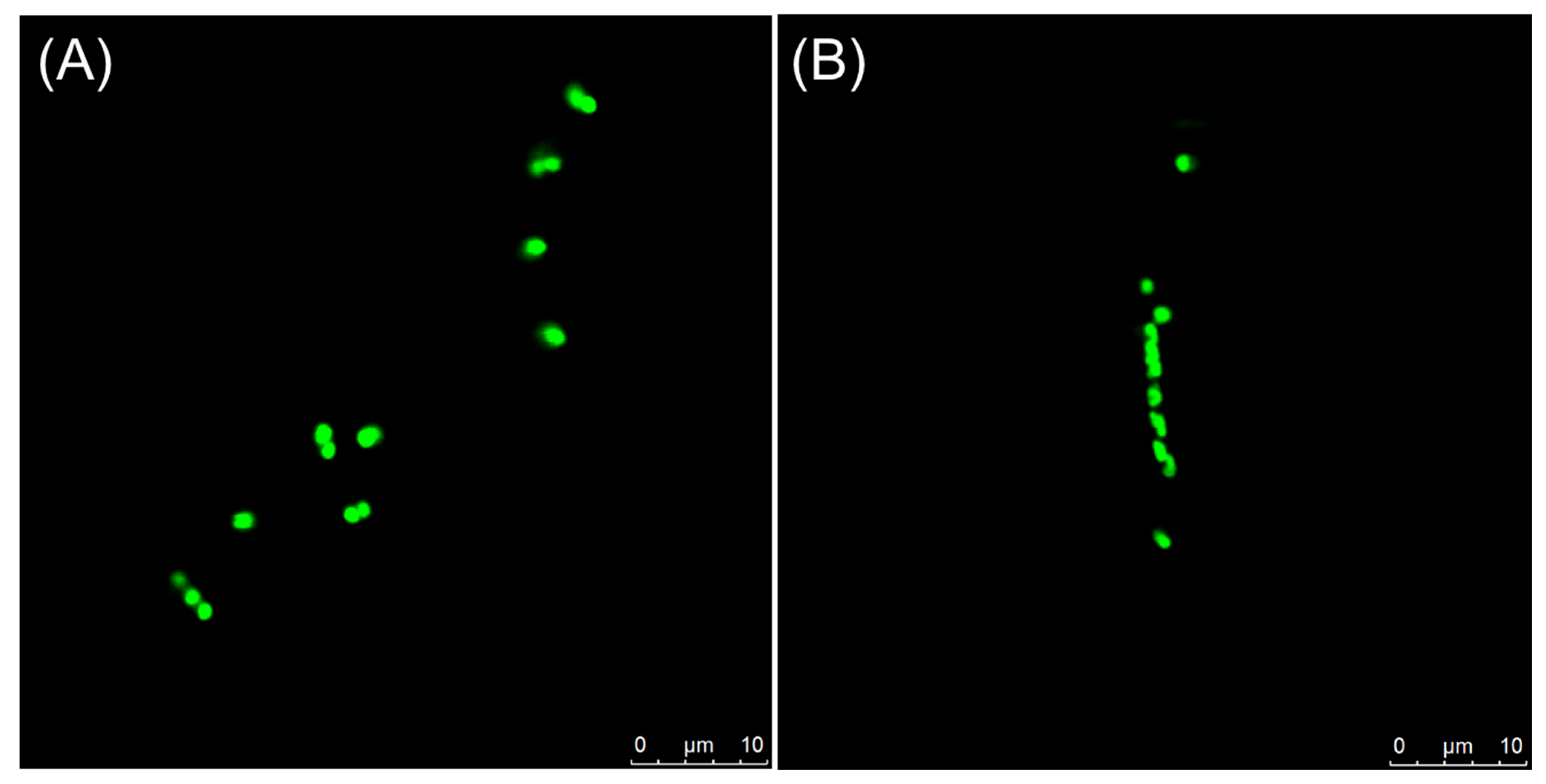
2.8. Visualization of Z-Ring in Bacterial Cells
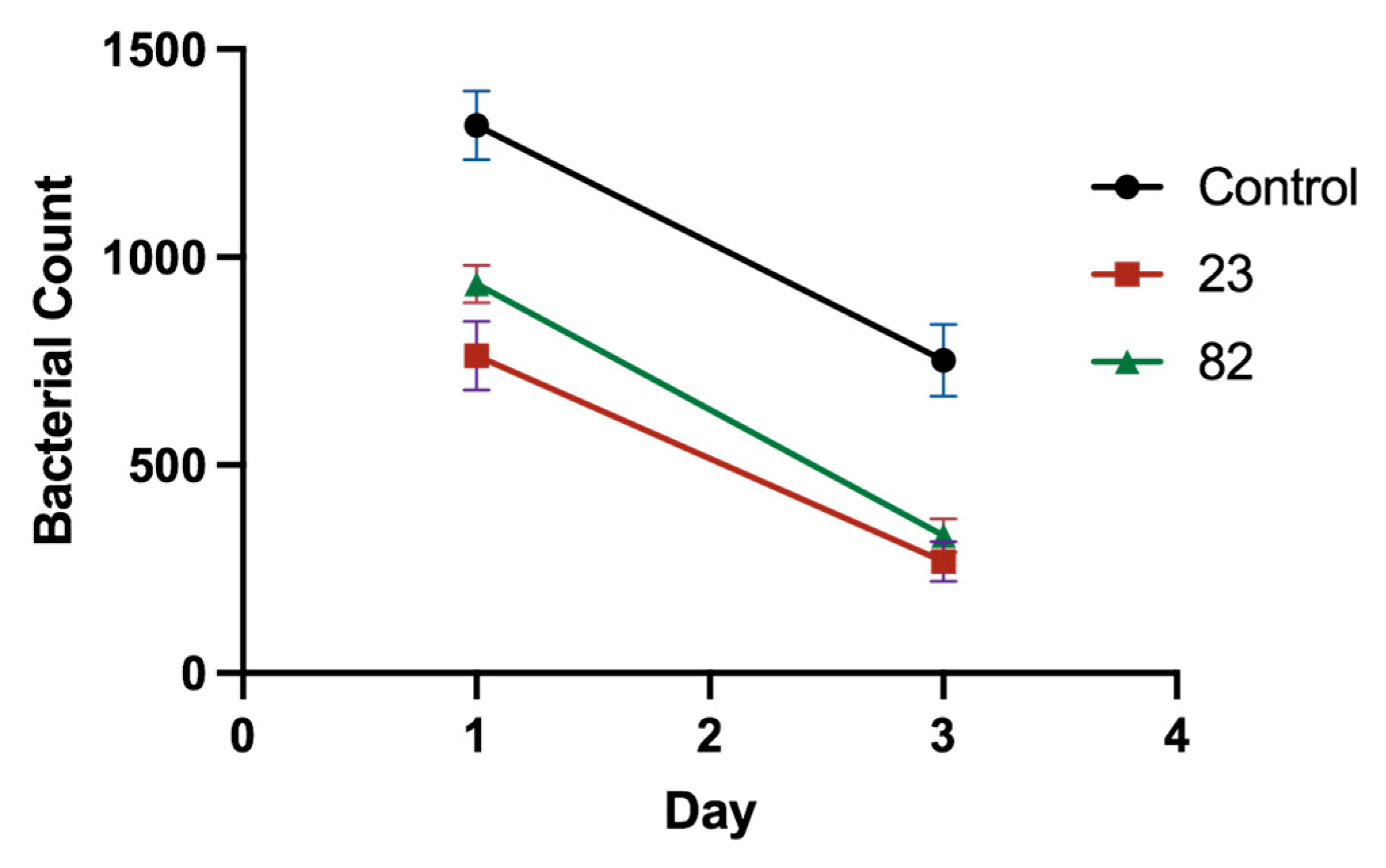
2.9. In Vivo Antibacterial Activity Testing
2.10. Antiproliferative Activities
2.11. Cytotoxicity
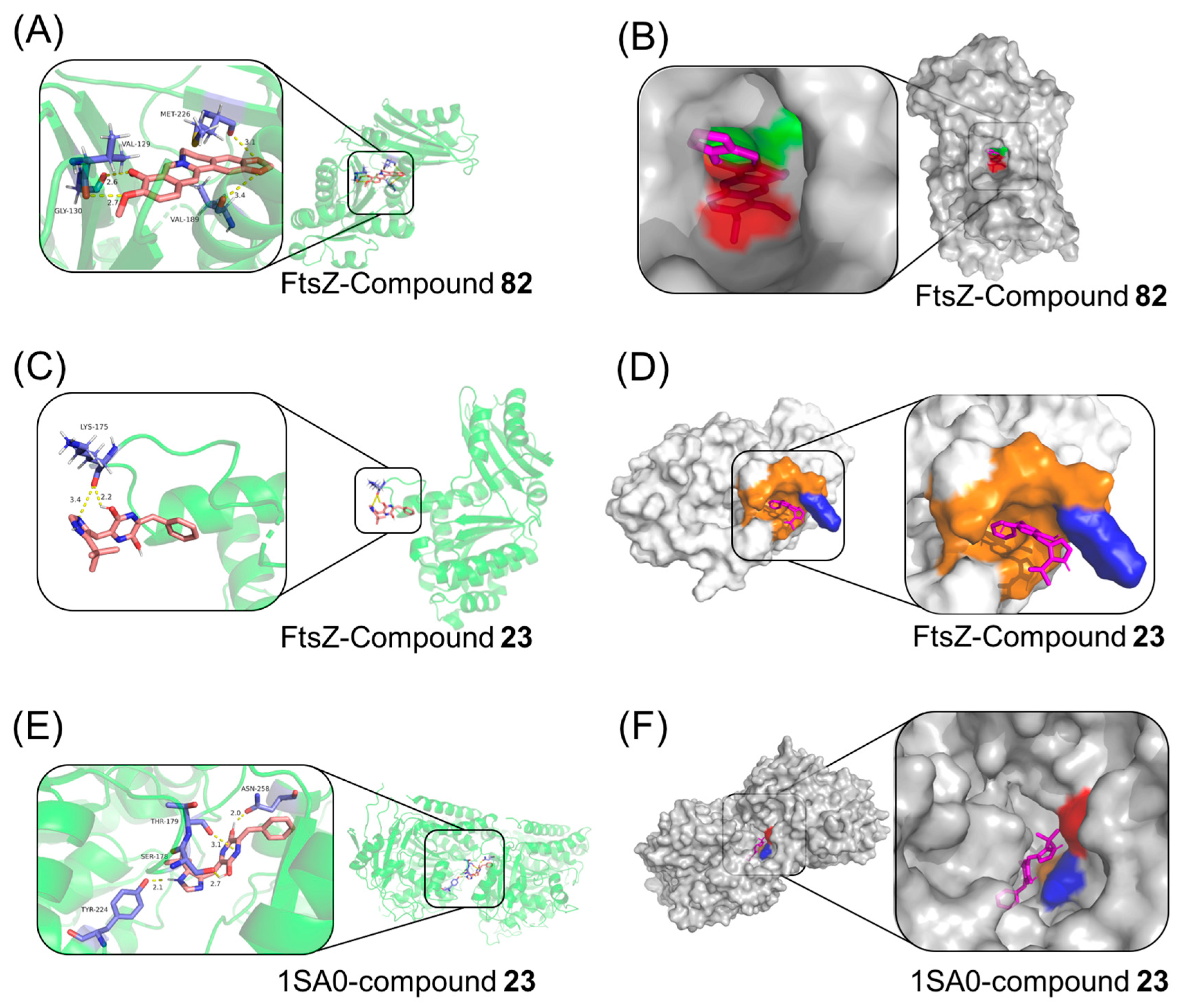
2.12. Molecular Docking Study
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Determination of Minimum Inhibitory Concentration (MIC)
4.2.2. Time–Killing Curve
4.2.3. Sa-FtsZ Expression and Purification
4.2.4. SDS-PAGE and Western Blot
4.2.5. FtsZ Polymerization Assay
4.2.6. Sedimentation Assay
4.2.7. Affinity Assay by SPR Technology
4.2.8. Phase-Contrast Microscopy
4.2.9. Visualization of Z-Ring in Bacterial Cells
4.2.10. In Vivo Antibacterial Activity Testing
4.2.11. Antiproliferative Activities
4.2.12. Effects of Tubulin Polymerization
4.2.13. Cytotoxicity
4.2.14. Molecular Docking Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chan, C.; Liu, B.; Bhuket, T.; Gish, R.G.; Wong, R. SAT-494-Global hepatocellular carcinoma mortality trends: An analysis of the world health organization cancer mortality database. J. Hepatol. 2019, 70, 851–852. [Google Scholar] [CrossRef]
- Hu, X.-L.; Xu, S.-T.; Wang, X.-C.; Hou, D.-N.; Chen, C.-C.; Song, Y.-L.; Yang, D. Prevalence of and risk factors for presenting initial respiratory symptoms in patients undergoing surgery for lung cancer. J. Cancer 2018, 9, 3515–3521. [Google Scholar] [CrossRef]
- Li, Y.J.; Yu, H.; Ke-Bin, Z.; Hospital, P.; County, Y.; Province, S. Progress of research on role of bacterial infections and their components in promoting occurrence, development and treatment of lung cancer. Chin. J. Nosocomiol. 2018, 28, 2716–2720. [Google Scholar]
- Masson, F.; Zaidman-Rémy, A.; Hedd, A. Antimicrobial peptides and cell processes tracking endosymbiont dynamics. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150298. [Google Scholar] [CrossRef]
- Kourbeti, I.S.; Vakis, A.F.; Ziakas, P.; Karabetsos, D.; Potolidis, E.; Christou, S.; Samonis, G. Infections in patients undergoing craniotomy: Risk factors associated with post-craniotomy meningitis. J. Neurosurg. 2015, 122, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Liu, J.; Ye, Q.L.; Zheng, Y. Risk factors and imaging findings of pulmonary infection in patients with advanced tumors. Chin. J. Prev. Med. 2020, 21, 60–63. [Google Scholar]
- Bao, Q.; Zhou, H.; Chen, X.; Yang, Q.; Zhou, J. Characteristics and Influencing Factors of Pathogenic Bacteria in Lung Cancer Chemotherapy Combined with Nosocomial Pulmonary Infection. Chin. J. Lung Cancer 2019, 22, 772–778. [Google Scholar]
- Wang, J.; Peng, Z.Y. Significance of NAP, NLR and CD64 in early diagnosis of patients with bacterial infection during chemotherapy. Chin. J. Microecol. 2020, 32, 3. [Google Scholar]
- Gu, L. Clinical treatment of severe pulmonary infection and electrolyte disturbance in elderly patients with advanced tumor. World’s Latest Med. Inf. Dig. 2017, 17, 217. [Google Scholar]
- Hu, Y.Y.; Wang, J.B. Clinical value of procalcitonin in guiding antimicrobial therapy in critically ill ICU patients. Chin. Foreign Women’s Health Res. 2016, 16, 162. [Google Scholar]
- Sun, J.T.; Jia, S.J.; Ju, G.F.; Rong, Y.; Wu, D. Effect analysis of prophylactic use of antibiotics in cancer patients with leukocyte value ≤ 1 × 109/L. Int. J. Oncol. 2016, 43, 103–105. [Google Scholar]
- Zhou, L.D. Clinical observation of the interaction between antitumor drugs and antibacterial drugs. Eval. Anal. Hosp. Drugs China 2016, 21, 58–59. [Google Scholar]
- Shen, S.Y.; Zhang, Z.L. Interaction of antitumor agents and antimicrobial agents. New Drug Clin. 1987, 40–43. [Google Scholar]
- Cheng, Z.; Lu, X.; Feng, B. A review of research progress of antitumor drugs based on tubulin targets. Transl. Cancer Res. 2020, 9, 4020–4027. [Google Scholar] [CrossRef] [PubMed]
- Kalra, A.P.; Eakins, B.B.; Patel, S.D.; Ciniero, G.; Tuszynski, J.A. All Wired Up: An Exploration of the Electrical Properties of Microtubules and Tubulin. ACS Nano 2020, 14, 16301–16320. [Google Scholar] [CrossRef]
- Oliva, M.A.; Prota, A.E.; Rodríguez-Salarichs, J.; Gu, W.; Díaz, J.F. Structural Basis of Noscapine Activation for Tubulin Binding. J. Med. Chem. 2020, 63, 8495–8501. [Google Scholar] [CrossRef]
- Rixel, V.H.V.; Ramu, V.; Auyeung, A.B.; Beztsinna, N.; Bonnet, S. Photo-uncaging of a ruthenium-caged microtubule-targeted rigidin analogue in hypoxic cancer cells and in a xenograft mouse model. J. Am. Chem. Soc. 2019, 141, 18444–18454. [Google Scholar] [CrossRef]
- Erickson, H.P.; Anderson, D.E.; Osawa, M. FtsZ in Bacterial Cytokinesis: Cytoskeleton and Force Generator All in One. Microbiol. Mol. Biol. Rev. Mmbr. 2010, 74, 504–528. [Google Scholar] [CrossRef] [PubMed]
- Aylett, C.H.S.; Lwe, J.; Amos, L.A. New Insights into the Mechanisms of Cytomotive Actin and Tubulin Filaments. Int. Rev. Cell Mol. Biol. 2011, 292, 1–71. [Google Scholar] [PubMed]
- Ingerson-Mahar, M.; Gitai, Z. A growing family: The expanding universe of the bacterial cytoskeleton. FEMS Microbiol. Rev. 2012, 36, 256–266. [Google Scholar] [CrossRef]
- Wang, Y.T.; Liu, L.T.; Hou, B.; Yao, C.M.; Wang, X.F.; Lu, B. Recent advances in studies on FtsZ inhibitors. Biochem. Pharmacol. 2024, 230, 116551–116561. [Google Scholar] [CrossRef]
- Li, T.; Wang, P.; Guo, W. Natural berberine-based chinese herb medicine assembled nanostructures with modified antibacterial application. ACS Nano 2019, 13, 6770–6781. [Google Scholar] [CrossRef]
- Huang, S.Y.; Zou, X.Q. Advances and challenges in protein-ligand docking. Int. J. Mol. Sci. 2010, 11, 3016–3034. [Google Scholar] [CrossRef]
- Hoda, M.; Zahra, M.; Maryam, Y.; Mohammad, H.; Ali, M.; Mehrdad, M.M.; Reza, M. Antimicrobial Peptides: Features, Action, and Their Resistance Mechanisms in Bacteria. Microb. Drug Resist. 2018, 24, 747–767. [Google Scholar] [CrossRef]
- Tyagi, A.; Agarwal, C.; Dwyer-Nield, L.D.; Singh, R.P.; Malkinson, A.M.; Agarwal, R. Silibinin modulates TNF- and IFN- mediated signaling to regulate COX2 and iNOS expression in tumorigenic mouse lung epithelial LM2 cells. Mol. Carcinog. 2012, 51, 832–842. [Google Scholar] [CrossRef]
- Bosch-Barrera, J.; Sais, E.; Cañete, N.; Marruecos, J.; Cuyàs, E.; Izquierdo, A.; Porta, R.; Haro, M.; Brunet, J.; Pedraza, S. Response of brain metastasis from lung cancer patients to an oral nutraceutical product containing silibinin. Oncotarget 2016, 7, 32006–32014. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, B.; Zhu, F. Epigallocatechin-3-gallate protects Kuruma shrimp Marsupeneaus japonicus from white spot syndrome virus and Vibrio alginolyticus. Fish Shellfish Immunol. 2018, 78, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yoon, I.S.; Park, D.H.; Kim, J.E.; Yoo, J.C.; Bae, M.S.; Oh, D.S.; Shim, J.H.; Choi, C.Y.; An, K.W.; Kim, E.I. Identification of the biologically active constituents of Camellia japonica leaf and anti-hyperuricemic effect invitro andinvivo. Int. J. Mol. Med. 2017, 39, 1613–1620. [Google Scholar] [CrossRef]
- Fazal, H.; Abbasi, B.H.; Ahmad, N.; Ali, M. Elicitation of Medicinally Important Antioxidant Secondary Metabolites with Silver and Gold Nanoparticles in Callus Cultures of Prunella vulgaris L. Appl. Biochem. Biotechnol. 2016, 180, 1076–1092. [Google Scholar] [CrossRef]
- David, J.H.; Neil, R.S.; Rebecca, U.; Greta, G.; James, M.B.; David, R.B.; Patrick, J.B.; Vladimir, V.B.; David, W.R.; Sveta, E.S.; et al. An inhibitor of FtsZ with potent and selective anti-staphylococcal activity. Science 2008, 321, 1673–1675. [Google Scholar]
- M07-A4; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Clinical & Laboratory Standards Institute: Wayne, IL, USA, 2000.
- Yang, Z.; Dongyang, X.; Lei, S.; Rujian, C.; Chunling, L.; He, Y. Association Between agr Type, Virulence Factors, Biofilm Formation and Antibiotic Resistance of Staphylococcus aureus Isolates From Pork Production. Front. Microbiol. 2018, 9, 1876–1887. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Y.; Du, R.L.; Cai, S.Y.; Liu, Z.H.; Fang, Z.Y.; Liu, T.; So, L.Y.; Lu, Y.J.; Sun, N.; Wong, K.Y. Study of Benzofuroquinolinium Derivatives as a New Class of Pothent Antibacterial Agent and the Mode of Inhibiton Targeting FtsZ. Front. Microbiol. 2018, 9, 1937–1947. [Google Scholar] [CrossRef] [PubMed]


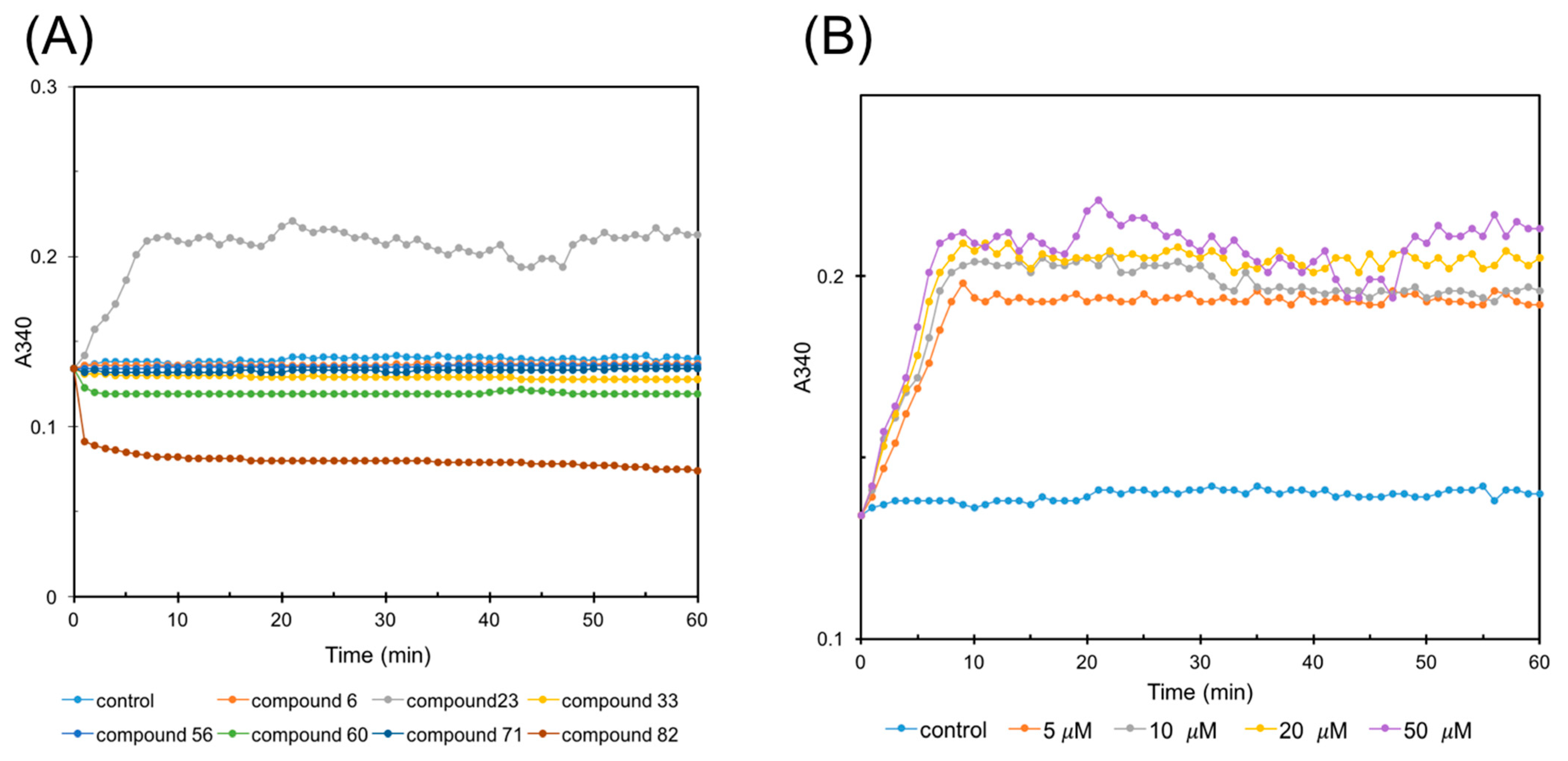
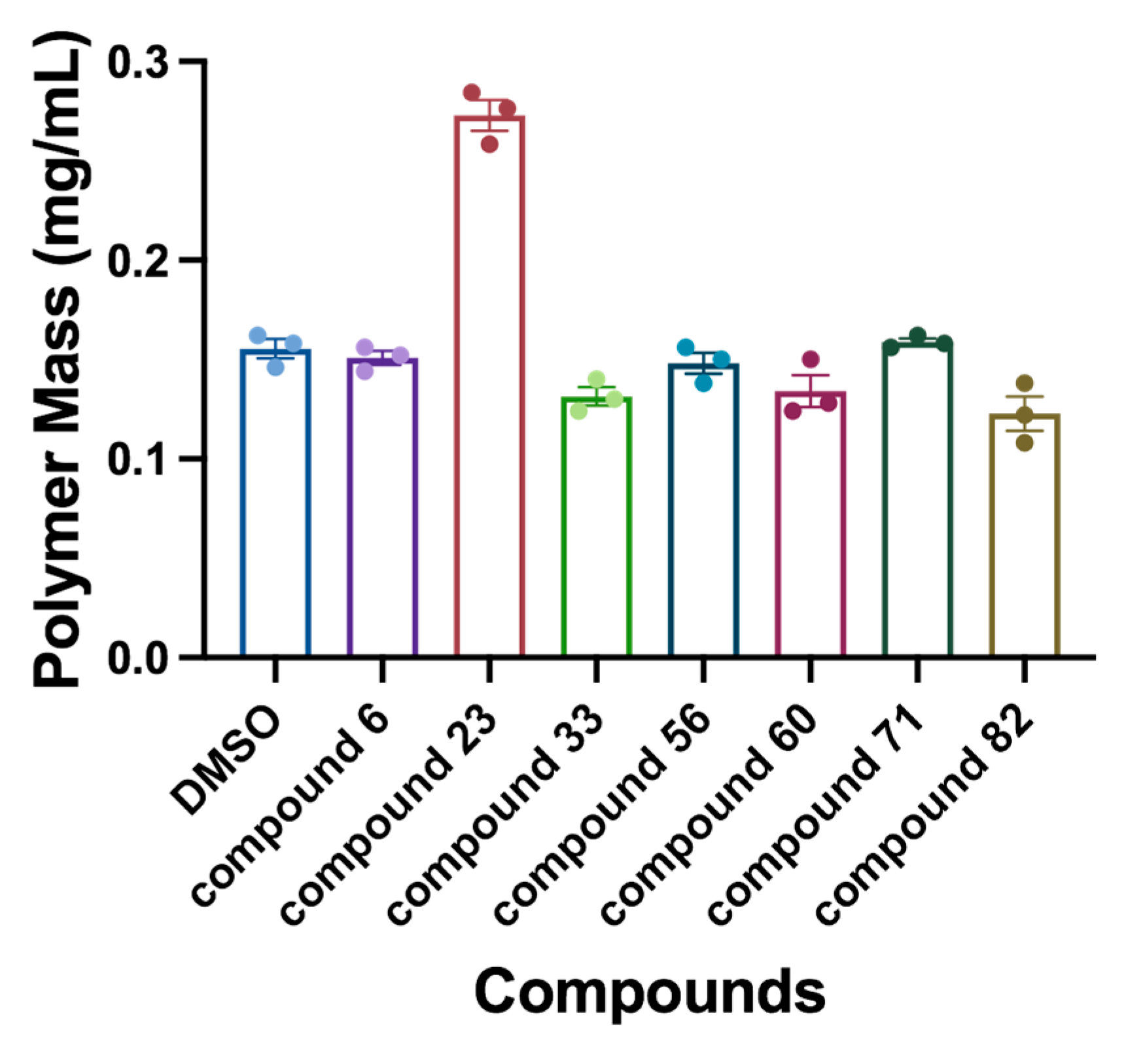


| Compounds | Structure | MIC (μM) | |||
|---|---|---|---|---|---|
| S. aureus (ATCC 6538) | B. subtilis (CICC 21164) | E. coli (ATCC 8739) | P. aeruginosa (DSM 939) | ||
| 6 | 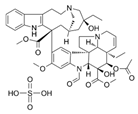 | 8 | 16 | 64 | 32 |
| 23 | 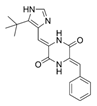 | 8 | 8 | 64 | 32 |
| 33 |  | 4 | 64 | 32 | 2 |
| 56 |  | 128 | 64 | 128 | 32 |
| 60 | 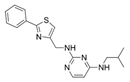 | 8 | 64 | 16 | 8 |
| 71 | 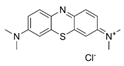 | 2 | 32 | 64 | 64 |
| 82 | 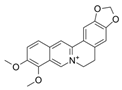 | 4 | 64 | 64 | 8 |
| Compound | KD (μM) | Rmax (RU) | Chi2 |
|---|---|---|---|
| 23 | 15.3 | 11.82 | 0.234 |
| Compounds | IC50 a ± SD b (μM) |
|---|---|
| Tubulin | |
| 23 | 0.02 ± 0.31 |
| 33 | 74.46 ± 0.25 |
| 56 | 38.66 ± 0.28 |
| 60 | 5.62 ± 0.12 |
| Colchicine c | 3.08 ± 0.13 |
| CA-4 c | 2.44 ± 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, X.; Yao, C.; Zhang, Y.; Liu, L.; Cao, Y.; Lu, B. Isolation of Dual-Active Drugs with Anticancer and Antibacterial Activities That Target Both Tubulin and FtsZ. Antibiotics 2025, 14, 1014. https://doi.org/10.3390/antibiotics14101014
Wang Y, Wang X, Yao C, Zhang Y, Liu L, Cao Y, Lu B. Isolation of Dual-Active Drugs with Anticancer and Antibacterial Activities That Target Both Tubulin and FtsZ. Antibiotics. 2025; 14(10):1014. https://doi.org/10.3390/antibiotics14101014
Chicago/Turabian StyleWang, Yanting, Xufang Wang, Chunmeng Yao, Yaliang Zhang, Lantian Liu, Yan Cao, and Bin Lu. 2025. "Isolation of Dual-Active Drugs with Anticancer and Antibacterial Activities That Target Both Tubulin and FtsZ" Antibiotics 14, no. 10: 1014. https://doi.org/10.3390/antibiotics14101014
APA StyleWang, Y., Wang, X., Yao, C., Zhang, Y., Liu, L., Cao, Y., & Lu, B. (2025). Isolation of Dual-Active Drugs with Anticancer and Antibacterial Activities That Target Both Tubulin and FtsZ. Antibiotics, 14(10), 1014. https://doi.org/10.3390/antibiotics14101014






