Comparison of Vonoprazan and Low-Dose Amoxicillin Dual Therapy with Bismuth-Containing Quadruple Therapy for Naïve Helicobacter pylori Eradication: A Single-Center, Open-Label, Randomized Control Trial
Abstract
1. Introduction
2. Methods
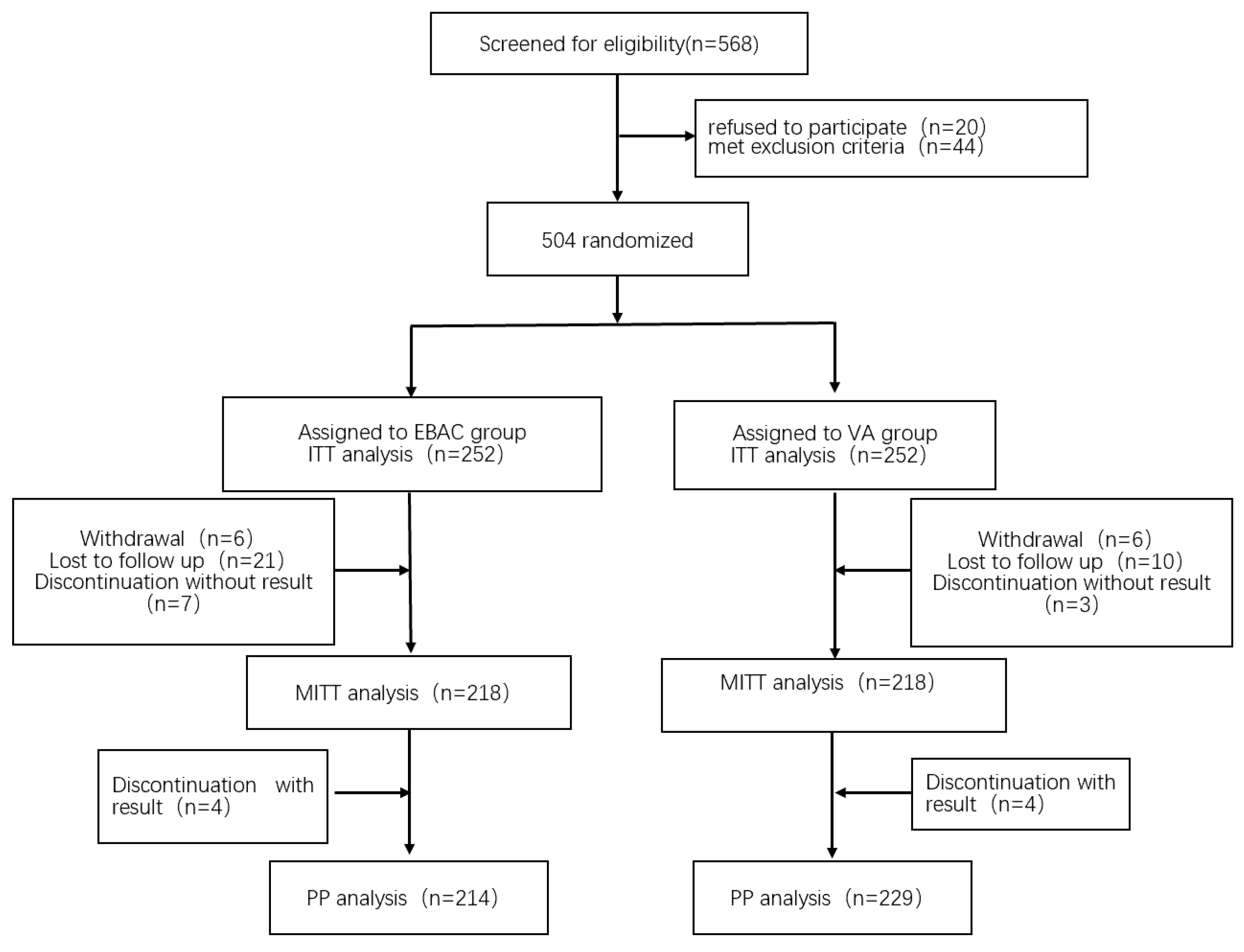
2.1. Study Design and Participants
2.2. Trial Assessments
2.3. Randomisation and Interventions
2.4. Sample Size Calculation and Statistical Analysis
3. Results
3.1. Baseline Data and Clinical Characteristics of Patients
3.2. Comparison of Eradication Rates
3.3. Logistic Multivariate Analysis of Factors Affecting Eradication Rate
3.4. Adverse Reactions and Patient Compliance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Registration Number
References
- Watts, G. Nobel prize is awarded to doctors who discovered H. pylori. BMJ 2005, 331, 795. [Google Scholar] [CrossRef]
- Mezmale, L.; Coelho, L.G.; Bordin, D.; Leja, M. Review: Epidemiology of Helicobacter pylori. Helicobacter 2020, 25 (Suppl. S1), e12734. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori infection—The Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Z.; Xie, Y.; Lu, H.; Cheng, H.; Zeng, Z.R.; Zhou, L.Y.; Chen, Y.; Wang, J.B.; Du, Y.Q.; Lu, N.H. Fifth Chinese National Consensus Report on the management of Helicobacter pylori infection. Helicobacter 2018, 23, e12475. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Ota, H.; Okuda, M.; Kikuchi, S.; Satoh, K.; Shimoyama, T.; Suzuki, H.; Handa, O.; Furuta, T.; Mabe, K.; et al. Guidelines for the management of Helicobacter pylori infection in Japan: 2016 Revised Edition. Helicobacter 2019, 24, e12597. [Google Scholar] [CrossRef] [PubMed]
- Chey, W.D.; Howden, C.W.; Moss, S.F.; Morgan, D.R.; Greer, K.B.; Grover, S.; Shah, S.C. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am. J. Gastroenterol. 2024, 119, 1730–1753. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C.; et al. Management of Helicobacter pylori infection: The Maastricht VI/Florence consensus report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef]
- Li, M.; Oshima, T.; Horikawa, T.; Tozawa, K.; Tomita, T.; Fukui, H.; Watari, J.; Miwa, H. Systematic review with meta-analysis: Vonoprazan, a potent acid blocker, is superior to proton-pump inhibitors for eradication of clarithromycin-resistant strains of Helicobacter pylori. Helicobacter 2018, 23, e12495. [Google Scholar] [CrossRef]
- Rokkas, T.; Gisbert, J.P.; Malfertheiner, P.; Niv, Y.; Gasbarrini, A.; Leja, M.; Megraud, F.; O’Morain, C.; Graham, D.Y. Comparative Effectiveness of Multiple Different First-Line Treatment Regimens for Helicobacter pylori Infection: A Network Meta-analysis. Gastroenterology 2021, 161, 495–507.e494. [Google Scholar] [CrossRef]
- Shinozaki, S.; Kobayashi, Y.; Osawa, H.; Sakamoto, H.; Hayashi, Y.; Lefor, A.K.; Yamamoto, H. Effectiveness and Safety of Vonoprazan versus Proton Pump Inhibitors for Second-Line Helicobacter pylori Eradication Therapy: Systematic Review and Meta-Analysis. Digestion 2021, 102, 319–325. [Google Scholar] [CrossRef]
- Ju, K.P.; Kong, Q.Z.; Li, Y.Y.; Li, Y.Q. Low-dose or high-dose amoxicillin in vonoprazan-based dual therapy for Helicobacter pylori eradication? A systematic review and meta-analysis. Helicobacter 2024, 29, e13054. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.; Ebrahimtabar, F.; Zamani, V.; Miller, W.H.; Alizadeh-Navaei, R.; Shokri-Shirvani, J.; Derakhshan, M.H. Systematic review with meta-analysis: The worldwide prevalence of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2018, 47, 868–876. [Google Scholar] [CrossRef]
- Li, Y.; Choi, H.; Leung, K.; Jiang, F.; Graham, D.Y.; Leung, W.K. Global prevalence of Helicobacter pylori infection between 1980 and 2022: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2023, 8, 553–564. [Google Scholar] [CrossRef]
- Zhou, X.Z.; Lyu, N.H.; Zhu, H.Y.; Cai, Q.C.; Kong, X.Y.; Xie, P.; Zhou, L.Y.; Ding, S.Z.; Li, Z.S.; Du, Y.Q. Large-scale, national, family-based epidemiological study on Helicobacter pylori infection in China: The time to change practice for related disease prevention. Gut 2023, 72, 855–869. [Google Scholar] [CrossRef]
- Yu, Y.; Xue, J.; Lin, F.; Liu, D.; Zhang, W.; Ru, S.; Jiang, F. Global Primary Antibiotic Resistance Rate of Helicobacter pylori in Recent 10 years: A Systematic Review and Meta-Analysis. Helicobacter 2024, 29, e13103. [Google Scholar] [CrossRef]
- Savoldi, A.; Carrara, E.; Graham, D.Y.; Conti, M.; Tacconelli, E. Prevalence of Antibiotic Resistance in Helicobacter pylori: A Systematic Review and Meta-analysis in World Health Organization Regions. Gastroenterology 2018, 155, 1372–1382.e1317. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.C.; El-Omar, E.M.; Kuo, Y.T.; Wu, J.Y.; Chen, M.J.; Chen, C.C.; Fang, Y.J.; Leow, A.H.R.; Lu, H.; Lin, J.T.; et al. Primary antibiotic resistance of Helicobacter pylori in the Asia-Pacific region between 1990 and 2022: An updated systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2024, 9, 56–67. [Google Scholar] [CrossRef]
- Saxena, A.; Mukhopadhyay, A.K.; Nandi, S.P. Helicobacter pylori: Perturbation and restoration of gut microbiome. J. Biosci. 2020, 45, 110. [Google Scholar] [CrossRef]
- Wang, Y.; Du, J.; Zhang, D.; Jin, C.; Chen, J.; Wang, Z.; Mei, T.; Fu, K.; Qian, Q.; Pang, T. Primary antibiotic resistance in Helicobacter pylori in China: A systematic review and meta-analysis. J. Glob. Antimicrob. Resist. 2023, 34, 30–38. [Google Scholar] [CrossRef]
- Yang, J.C.; Lin, C.J.; Wang, H.L.; Chen, J.D.; Kao, J.Y.; Shun, C.T.; Lu, C.W.; Lin, B.R.; Shieh, M.J.; Chang, M.C.; et al. High-dose dual therapy is superior to standard first-line or rescue therapy for Helicobacter pylori infection. Clin. Gastroenterol. Hepatol. 2015, 13, 895–905.e895. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.A.; Huang, H.K.; Chou, A.L.; Lin, H.J.; Feng, C.L.; Kuo, C.J.; Lai, C.H. Helicobacter pylori eradication with high-dose proton pump inhibitor-amoxicillin dual therapy: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2024, 63, 107159. [Google Scholar] [CrossRef]
- Li, C.; Shi, Y.; Suo, B.; Tian, X.; Zhou, L.; Song, Z. PPI-amoxicillin dual therapy four times daily is superior to guidelines recommended regimens in the Helicobacter pylori eradication therapy within Asia: A systematic review and meta-analysis. Helicobacter 2021, 26, e12816. [Google Scholar] [CrossRef]
- Jiang, G.; Luo, M.; Zheng, P.; Cong, Y.; Feng, Y.; Zhou, F. Eradication rate and safety of vonoprazan-amoxicillin dual therapy for Helicobacter pylori eradication: A randomized controlled trial. Scand. J. Gastroenterol. 2024, 59, 1229–1233. [Google Scholar] [CrossRef]
- Liu, L.; Shi, H.; Shi, Y.; Wang, A.; Guo, N.; Li, F.; Nahata, M.C. Vonoprazan-based therapies versus PPI-based therapies in patients with H. pylori infection: Systematic review and meta-analyses of randomized controlled trials. Helicobacter 2024, 29, e13094. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sun, D.; Kou, L.; Jia, L.; Hao, J.; Zhou, J.; Zheng, W.; Gao, F.; Chen, X. Vonoprazan-amoxicillin dual therapy with different amoxicillin dosages for treatment-naive patients of Helicobacter pylori infection in China: A prospective, randomized controlled study. Eur. J. Gastroenterol. Hepatol. 2024, 36, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Chen, H.W.; Wan, Y.; Su, P.Z.; Yu, J.; Liu, J.J.; Lu, Y.; Zhang, M.; Yao, J.Y.; Zhi, M. Combination of vonoprazan and amoxicillin as the first-line Helicobacter pylori eradication therapy: A multicenter, prospective, randomized, parallel-controlled study. Clin. Exp. Med. 2023, 23, 4011–4019. [Google Scholar] [CrossRef]
- Shih, C.A.; Wu, D.C.; Shie, C.B.; Hsu, P.I. Dual Therapies Containing an Antibiotic Plus a Proton Pump Inhibitor or Vonoprazan for Helicobacter pylori Infection: A Systematic Review. Microorganisms 2025, 13, 715. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.E.; Zhang, S.J.; Liu, Y.; Yao, S.Y.; Zhang, S.X.; Liu, X.M.; Liang, L.X.; Wang, F. Amoxicillin high-dose dual therapy for Helicobacter pylori primary eradication: Proton pump inhibitor and potassium-competitive acid blocker, which’s better? World J. Gastroenterol. 2025, 31, 100863. [Google Scholar] [CrossRef]
- Cheung, K.S.; Lyu, T.; Deng, Z.; Han, S.; Ni, L.; Wu, J.; Tan, J.T.; Qin, J.; Ng, H.Y.; Leung, W.K.; et al. Vonoprazan Dual or Triple Therapy Versus Bismuth-Quadruple Therapy as First-Line Therapy for Helicobacter pylori Infection: A Three-Arm, Randomized Clinical Trial. Helicobacter 2024, 29, e13133. [Google Scholar] [CrossRef]
- Chey, W.D.; Mégraud, F.; Laine, L.; López, L.J.; Hunt, B.J.; Howden, C.W. Vonoprazan Triple and Dual Therapy for Helicobacter pylori Infection in the United States and Europe: Randomized Clinical Trial. Gastroenterology 2022, 163, 608–619. [Google Scholar] [CrossRef]
- Gao, W.; Li, J.W.; Ye, H.; Zhang, X.Z.; Liu, J.X.; Cheng, H. Real-world evidence on the efficacy and safety of vonoprazan-amoxicillin dual therapy for Helicobacter pylori treatment in elderly patients. World J. Gastroenterol. 2025, 31, 101463. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.G.; Mei, Y.Z.; Jiang, X.; Zheng, A.J.; Ding, Y.B. Vonoprazan-amoxicillin dual therapy for Helicobacter pylori eradication: A systematic review and meta-analysis of randomized controlled trials. Saudi J. Gastroenterol. 2023, 29, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.S.; Li, W.J.; Dang, Y.N.; Li, L.R.; Xu, X.B.; Yuan, L.; Zhang, W.F.; Yang, Z.; Gao, X.; Zhang, M.; et al. Ten-Day Vonoprazan-Amoxicillin Dual Therapy as a First-Line Treatment of Helicobacter pylori Infection Compared With Bismuth-Containing Quadruple Therapy. Am. J. Gastroenterol. 2023, 118, 627–634. [Google Scholar] [CrossRef]
- Graham, D.Y.; Lu, H.; Yamaoka, Y. A report card to grade Helicobacter pylori therapy. Helicobacter 2007, 12, 275–278. [Google Scholar] [CrossRef]
- Shekeban, Y.M.; Hamdy, N.A.; Header, D.A.; Ahmed, S.M.; Helmy, M.M. Vonoprazan-based therapy versus standard regimen for Helicobacter pylori infection management in Egypt: An open-label randomized controlled trial. Sci. Rep. 2025, 15, 15989. [Google Scholar] [CrossRef]
- Graham, D.Y. Why the Vonoprazan Helicobacter pylori Therapies in the US-European Trial Produced Unacceptable Cure Rates. Dig. Dis. Sci. 2023, 68, 1691–1697. [Google Scholar] [CrossRef] [PubMed]
| EBAC (n = 252) | VA (n = 252) | p | ||
|---|---|---|---|---|
| Age (years, Median (P75–P25)) | 40.00 (50.75, 34.00) | 40.00 (52.00, 32.00) | −0.919 | 0.358 |
| 0.143 | 0.931 | |||
| 159 (63.1%) | 160 (63.5%) | ||
| 65 (25.8%) | 62 (24.6%) | ||
| 28 (11.1%) | 30 (11.9%) | ||
| Gender (male, n%) | 133 (51.8%) | 119 (47.2%) | 0.032 | 0.858 |
| BMI (Median (P75–P25)) | 24.20 (26.20, 22.03) | 24.20 (26.40, 21.50) | −0.580 | 0.562 |
| 1.174 | 0.759 | |||
| 8 (3.2%) | 10 (4.0%) | ||
| 106 (42.1%) | 111 (44.0%) | ||
| 104 (41.3%) | 93 (36.9%) | ||
| 34 (13.5%) | 38 (15.1%) | ||
| Cigarette smoking | 40 (15.9%) | 44 (17.5%) | 3.193 | 0.203 |
| Alcohol drinking | 70 (27.8%) | 64 (25.4%) | 0.366 | 0.545 |
| Family history of gastric cancer | 10 (4.1%) | 6 (2.4%) | 1.033 | 0.310 |
| Symptoms | 11.010 | 0.201 | ||
| 95 (37.7%) | 82 (32.5%) | ||
| 59 (23.4%) | 89 (35.3%) | ||
| 44 (17.5%) | 53 (21.0%) | ||
| 35 (13.9%) | 38 (15.1%) | ||
| 24 (9.5%) | 21 (8.3%) | ||
| 24 (9.5%) | 19 (7.5%) | ||
| 11 (4.4%) | 13 (5.2%) | ||
| 7 (2.8%) | 11 (4.4%) | ||
| 6 (2.4%) | 2 (0.8%) | ||
| Diagnosis with endoscopy | 2.330 | 0.312 | ||
| 96 (38.1%) | 111 (44.0%) | ||
| 9 (3.6%) | 11 (4.4%) | ||
| 147 (58.3%) | 130 (51.6%) | ||
| Chronic disease | 4.926 | 0.295 | ||
| 28 (11.1%) | 31 (12.3%) | ||
| 9 (3.6%) | 13 (5.2%) | ||
| 4 (1.6%) | 9 (3.6%) | ||
| 1 (0.4%) | 1 (0.4%) | ||
| 5 (2.0%) | 2 (0.8%) |
| Eradication Rates | EBAC Group | VA Group | p | Rate Difference (95% Cl) |
|---|---|---|---|---|
| ITT | 85.7% (216/252) | 79.4% (200/252) | 0.060 | 0.0635 |
| 95% CI | [81.4%, 90.1%] | [74.3%, 84.4%] | [−0.58%, 13.23%] | |
| mITT | 92.7% (216/233) | 91.7% (200/218) | 0.714 | 0.0096 |
| 95% CI | [89.3%, 96.1%] | [88.1%, 95.4%] | [−4.39%, 6.45%] | |
| PP | 93.0% (213/229) | 92.1% (197/214) | 0.712 | 0.0096 |
| 95% CI | [89.7%, 96.3%] | [88.4%, 95.7%] | [−4.36%, 6.42%] |
| Multivariate Analysis | |||
|---|---|---|---|
| OR | 95% CI | p | |
| Age (years) | 0.650 | ||
| <45 | 1 | ||
| 45–59 | 1.227 | (0.435–3.466) | 0.699 |
| ≥60 | 0.844 | (0.313–2.277) | 0.738 |
| BMI | 0.610 | ||
| <18.5 | 1 | ||
| 18.5–23.9 | 1.080 | (0.195–5.987) | 0.930 |
| 24.0–27.9 | 1.067 | (0.427–2.668) | 0.889 |
| ≥28.0 | 1.553 | (0.645–3.741) | 0.326 |
| Gender | |||
| male | 1 | ||
| female | 1.372 | (0.724–2.602) | 0.332 |
| Cigarette smoking | |||
| no | 1 | ||
| yes | 2.224 | (0.855–5.783) | 0.101 |
| Alcohol drinking | |||
| no | 1 | ||
| yes | 1.181 | (0.565–2.471) | 0.658 |
| Family history of gastric cancer | |||
| no | 1 | ||
| yes | 0.442 | (0.114–1.706) | 0.236 |
| Diagnosis with endoscopy | 0.960 | ||
| No endoscopy | 1 | ||
| Chronic gastritis | 1.182 | (0.279–5.000) | 0.820 |
| Peptic ulcer | 1.109 | (0.262–4.693) | 0.889 |
| Adherence | |||
| <80% | 1 | ||
| ≥80% | 17.557 | (5.678–54.287) | <0.001 |
| Group | |||
| EBAC group | 1 | ||
| VA group | 0.579 | (0.330–1.013) | 0.055 |
| EBAC (n = 246) | VA (n = 246) | p | |
|---|---|---|---|
| Total, n/N (%) | 105/246 (42.7%) | 67/246 (27.2%) | <0.001 |
| AE grade | 0.767 | ||
| Mild | 96 (91.4%) | 59 (88.1%) | |
| Moderate | 8 (7.6%) | 7 (10.4%) | |
| Severe | 1 (1.0%) | 1 (1.5%) | |
| AE variety | |||
| Bitter taste | 69 | 2 | |
| Diarrhea | 19 | 13 | |
| Abdominal discomfort | 16 | 28 | |
| Nausea | 16 | 10 | |
| Rash | 9 | 5 | |
| Vomiting | 7 | 1 | |
| Hunger sensation | 2 | 8 | |
| Dizziness and headache | 1 | 4 | |
| Decreased appetite | 1 | 3 | |
| Hyperhidrosis | 1 | 1 | |
| Tinnitus | 1 | 0 | |
| Constipation | 0 | 6 | |
| Pharyngeal discomfort | 0 | 3 | |
| Arthralgia and weakness | 0 | 2 | |
| Insomnia | 0 | 1 | |
| Discontinued due to AEs | 5/246 (2.0%) | 4/246 (1.6%) | 0.737 |
| Adherence, n/N (%) | 237/246 (96.3%) | 230/246 (93.5%) | 0.151 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, X.; Shi, Y.; Li, Y.; Lin, X. Comparison of Vonoprazan and Low-Dose Amoxicillin Dual Therapy with Bismuth-Containing Quadruple Therapy for Naïve Helicobacter pylori Eradication: A Single-Center, Open-Label, Randomized Control Trial. Antibiotics 2025, 14, 990. https://doi.org/10.3390/antibiotics14100990
Fan X, Shi Y, Li Y, Lin X. Comparison of Vonoprazan and Low-Dose Amoxicillin Dual Therapy with Bismuth-Containing Quadruple Therapy for Naïve Helicobacter pylori Eradication: A Single-Center, Open-Label, Randomized Control Trial. Antibiotics. 2025; 14(10):990. https://doi.org/10.3390/antibiotics14100990
Chicago/Turabian StyleFan, Xue, Yanyan Shi, Yuan Li, and Xiangchun Lin. 2025. "Comparison of Vonoprazan and Low-Dose Amoxicillin Dual Therapy with Bismuth-Containing Quadruple Therapy for Naïve Helicobacter pylori Eradication: A Single-Center, Open-Label, Randomized Control Trial" Antibiotics 14, no. 10: 990. https://doi.org/10.3390/antibiotics14100990
APA StyleFan, X., Shi, Y., Li, Y., & Lin, X. (2025). Comparison of Vonoprazan and Low-Dose Amoxicillin Dual Therapy with Bismuth-Containing Quadruple Therapy for Naïve Helicobacter pylori Eradication: A Single-Center, Open-Label, Randomized Control Trial. Antibiotics, 14(10), 990. https://doi.org/10.3390/antibiotics14100990








