Abstract
Background/Objectives: Invasive pulmonary aspergillosis (IPA) represents a critical respiratory condition mainly caused by Aspergillus fumigatus and other ubiquitous species such as Aspergillus flavus, Aspergillus niger, and Aspergillus terreus. IPA clinical management has been complicated by diagnostic challenges and therapeutic difficulties due to antifungal intrinsic or secondary resistance episodes. Despite this assumption, few scientific data have been reported about possible antifungal drug combinations. Herein, we propose an experimental evaluation using isavuconazole/amphotericin B and isavuconazole/caspofungin in vitro synergy assays to investigate their combined activity on Aspergillus spp. IPA clinical isolates. Methods: We globally analyzed 55 Aspergillus spp. isolates, practicing the gradient test methods with single and combined antifungal drugs through the MIC Strip test (Liofilchem, Roseto degli Abruzzi, Italy). The collected MIC values were interpreted according to the EUCAST guidelines and classified as synergy, additivity, indifference, and antagonism cases through a FIC index calculation. A statistical analysis on species’ correlation with particular synergy testing results was finally provided. Results: Despite an interesting activity against A. fumigatus, isavuconazole/amphotericin B did not report statistical significance, obtaining a consistent antagonism percentage (43.6%). On the other hand, isavuconazole/caspofungin showed a promising in vitro synergic activity, except for A. flavus isolates. Conclusions: Synergy testing demonstrated a significant species-specific trend. Future studies should be focused on Aspergillus spp. isolates and antifungal in vitro synergy testing, aiming to discourage or recommend any specific antifungal combinations, depending on the isolated species.
1. Introduction
Invasive pulmonary aspergillosis currently represents a significant clinical challenge due to difficult microbiological workflows and frequent diagnostic delays [1,2,3].
Fungal culture remains the gold standard method to isolate Aspergillus species and provide complete antifungal susceptibility testing [4,5]. However, serological assays for galactomannan antigen detection and molecular tests may integrate imaging and clinical symptoms to define the aspergillosis diagnosis [6]. Recent antifungal resistance has enhanced the importance of always identifying and testing the antimicrobial susceptibility of fungal isolates. Aspergillosis therapy includes triazoles, amphotericin B, and echinocandins. Specifically, voriconazole, isavuconazole, and posaconazole are first-line agents in the case of pulmonary aspergillosis. Unfortunately, Aspergillus spp. isolates may express mutations in the sterol-demethylase gene cyp51A, reporting a reduction in the affinity between azole drugs and their targets [7]. Furthermore, the same isolates occasionally reveal efflux pumps overexpression, with a consequent reduction in azole concentrations within the fungal cell. Azole resistance generally depends on extensive agricultural azole usage or prolonged azole therapies within specific hospital settings [7]. The progressive azole resistance detection led to the intensification of amphotericin B insertion among aspergillosis therapeutic choices. In vitro evaluations demonstrated an intrinsic amphotericin B resistance among A. terreus isolates, even if the specific resistance mechanism still remains unclear. Moreover, the increase in amphotericin B prescription caused higher minimum inhibitory concentration (MIC) values to be detected among other Aspergillus species [7]. Echinocandin resistance depends on the glucan-synthase codifying genes FKS1 and FKS2, but it has rarely been detected among Aspergillus spp. isolates.
Refractory aspergillosis episodes have sometimes been treated through antifungal combination therapies, including azoles and echinocandins or azoles and amphotericin B [8,9]. The European Committee on Antimicrobial Susceptibility Testing (EUCAST) and the Clinical and Laboratory Standards Institute (CLSI) established the criteria to standardize antifungal susceptibility testing, reporting epidemiological cut-offs (ECOFFs) in the absence of clinical breakpoints (BPs). According to the same guidelines, the gradient test method may be performed to classify azoles, echinocandins, and amphotericin B MIC values for Aspergillus spp. The above-mentioned methods can also be used to test the in vitro synergic activity [10,11]. Recent literature data documented a potential in vitro synergy of isavuconazole and amphotericin B against different fungal isolates, including Aspergillus spp. isolates [12,13]. Additionally, Raffetin et al. [8] demonstrated similar results for caspofungin and isavuconazole combinations.
Herein, we propose an experimental in vitro study to address the challenge of identifying optimal antifungal combination therapy for invasive pulmonary aspergillosis, given increasing resistance among Aspergillus species and limited treatment options. We specifically investigate the synergic effects of isavuconazole-amphotericin B and isavuconazole-caspofungin on clinical isolates from invasive pulmonary aspergillosis episodes. Our evaluation aims to clarify which antifungal combinations may offer enhanced efficacy, depending on the isolated Aspergillus species. Moreover, it has the purpose of enriching literature data and general knowledge about antifungal combinations against molds.
2. Results
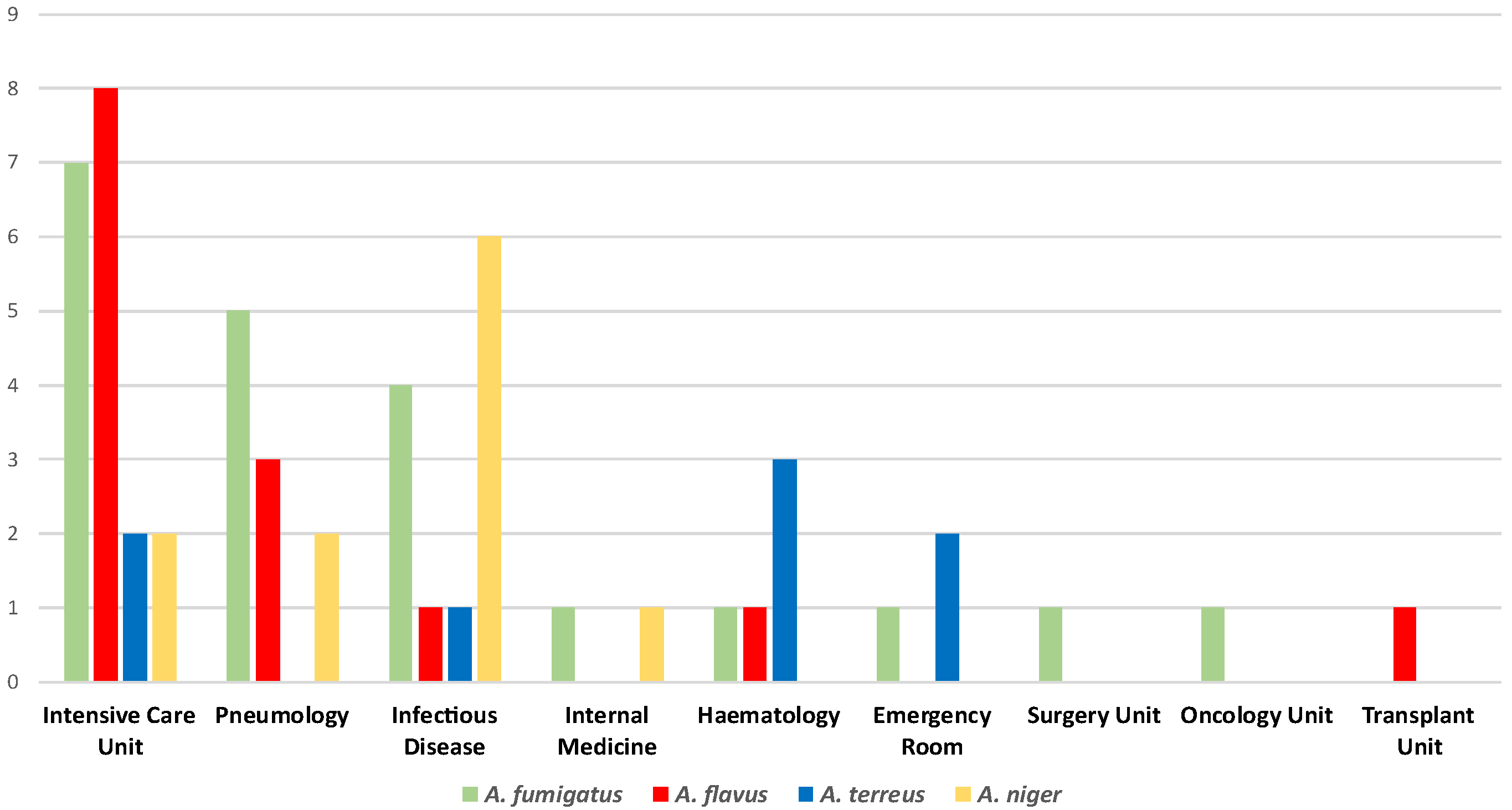
The experimental analysis globally collected 55 Aspergillus spp. isolates from respiratory samples such as bronchoalveolar lavage fluids, bronchial aspirates, and sputum samples. Figure 1 summarizes the Aspergillus spp. isolates distribution within the included hospital settings. According to our data, the Intensive Care Unit registered the highest Aspergillus spp. strains isolation rate (34.5%). Furthermore, this ward was the only one to report all the four main species (A. fumigatus, A. flavus, A. terreus, and A. niger), along with the Infectious Disease Unit, which showed a 21.8% isolation percentage. Pneumology revealed an 18.2% isolation rate, while 9.1% of the strains belonged to the Hematology Unit. Additionally, lower percentages emerged from the Emergency Room (5.4%) and the Internal Medicine Unit (3.63%). Finally, the Surgery, Oncology, and Transplant Units revealed the same isolation rates (1.8%).
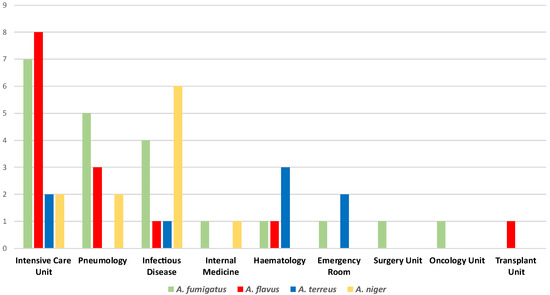
Figure 1.
Distribution of the Aspergillus spp. isolates within the included hospital units.
Aspergillus fumigatus sensu strictu was the most isolated fungal species (40%), followed by A. flavus sensu strictu (25.4%), A. niger sensu strictu (20%), and A. terreus sensu strictu (14.5%). Table 1 provides details about species, hospital wards, biological samples, and MIC values for all the tested antifungal drugs. Additionally, Table 2 indicates the isolates number for each species, along with the total isolates number. Furthermore, the same table illustrates the obtained MIC ranges and MIC90 for all the collected species, reporting the eventual probable resistance in the case of MIC values higher than clinical breakpoints, epidemiological cut-offs, or the latest tested antifungal concentrations. A. terreus demonstrated the highest amphotericin B resistance rate (87.5%) due to its intrinsic resistance to this antifungal drug. Moreover, the same species showed the highest caspofungin resistance percentage (62.5%).

Table 1.
MIC values of amphotericin B, caspofungin, and isavuconazole for all the tested isolates, along with hospital wards, isolated species, and biological samples.

Table 2.
MIC ranges of amphotericin B, caspofungin, and isavuconazole for all the tested isolates, along with percentages of probable resistant or resistant isolates according to ECOFF and BP interpretation.
On the other hand, A. fumigatus and A. niger rarely showed amphotericin B or caspofungin resistance. A. flavus did not express caspofungin resistance, but it reported a single case (7.1%) of amphotericin B resistance. Finally, isavuconazole MIC values were never elevated for the tested Aspergillus spp. isolates.
Table 3 summarizes the in vitro gathered results, including isavuconazole and amphotericin B synergy testing, together with statistical significance indications. Overall, this antifungal combination reported additivity (18.2%) and synergy (7.8%) cases, along with a consistent antagonism percentage (43.6%) and 30.9% of indifference episodes. As regards the species-specific results, A. fumigatus revealed the highest additivity (27.7%) rate, while A. flavus reported the highest antagonism percentage (71.4%). A. terreus interestingly revealed the most elevated indifference rate (50.0%). Finally, synergy percentages were similar for all the tested species. None of the cases showed a statistical significance between the identified species and the synergy assay result.

Table 3.
In vitro synergy testing results regarding isavuconazole and amphotericin B for all the included isolates.
Table 4 indicates the in vitro obtained results, including isavuconazole and caspofungin synergy testing, along with the statistical significance p-values. The total percentages document the same synergy rate (7.8%) as the previous antifungal combination, while the additivity episodes slightly increased (27.3). Antagonism cases (14.8%) appeared to be less numerous, whereas indifference percentage increased (43.6%) when compared to the other tested combinations. Remarkably, the comparison between the two tested antifungal combinations revealed a statistical significance (p < 0.0008) only with regard to antagonism, whose percentages were significantly higher in the case of isavuconazole and amphotericin B. On the other hand, indifference, additivity, and synergy did not show any significance. Speaking of species-specific details, A. flavus did not report additivity, while A. niger and A. terreus did not show antagonism cases. Additionally, A. terreus had a lot of indifference cases (75%), and A. niger reached the highest additivity rate (54.5%). Interestingly, synergy percentages were similar for all the tested species. Statistical significance emerged in A. flavus (p < 0.05), whose identification correlated with antagonism and indifference episodes. Furthermore, A. niger reported statistical significance (p < 0.05), mainly corresponding to additivity episodes.

Table 4.
In vitro synergy testing results regarding isavuconazole and caspofungin for all the included isolates.
3. Discussion
Invasive pulmonary aspergillosis is currently considered to be a fatal opportunistic infection, especially among critically ill patients. Unfortunately, the real IPA incidence is underestimated due to diagnostic complications and difficulties in distinguishing between colonization and infection episodes [14,15,16,17,18,19]. Scientific evidence has demonstrated that most IPA cases correspond to probable invasive aspergillosis, reporting a significant number of proven IPA only after post-mortem confirmations [19,20]. Despite triazoles’ first-line role in IPA treatment, azole resistance is increasing among Aspergillus spp. isolates. Consequently, echinocandins and amphotericin B have become alternative therapies or salvage therapies in specific cases. In vitro and in vivo studies had already furnished some proof about the importance of combining antifungal agents in IPA treatments. Those studies described data about azoles and echinocandins or azoles and amphotericin B combinations [13,14,15,16,17,18,19,20,21].
On these premises, we decided to focus our attention on in vitro synergy testing to extend the scientific literature about antifungal combinations on Aspergillus spp., studying species-specific response to the tested synergies. Our analysis highlighted the diffusion of four main Aspergillus spp., A. fumigatus, A. flavus, A. niger, and A. terreus. This information confirmed previously published epidemiological data about our geographical area [13,22,23]. The identified species diffused within specific hospital settings, reporting significant isolation rates among critically ill patients. Intensive care and hematological patients documented the highest Aspergillus spp. isolation percentages, probably due to their peculiar risk factors (corticosteroids’ usage, severe and prolonged neutropenia, mechanical ventilation, simultaneous respiratory viral or bacterial infections). All the tested isolates reported low MIC values of isavuconazole, underlining the importance of integrating this antifungal drug within susceptibility testing [24]. Moreover, isavuconazole has a compliant pharmacological profile due to there being no need to plan therapeutic drug monitoring (TDM), unlike with other azoles [25].
Regarding amphotericin B, the isolates reporting MIC values above the ECOFFs are increasing among all the main Aspergillus species, and previously published data already reported this trend [26]. On the one hand, most of the A. terreus strains exhibited an intrinsic resistance, while one isolate tested susceptible, probably due to antifungal tolerance mechanisms. Shahandashti et al. [27] documented a similar condition through in vitro studies investigating the minimum duration for killing 99% of the population (MDK99). Among our identified Aspergillus spp. isolates, a significant A. terreus percentage revealed elevated caspofungin MIC values, along with A. fumigatus and A. niger strains. Literature data already noticed a similar condition several years ago [28]. However, further analysis, including the minimum effective concentration (MEC) should be performed to confirm these preliminary susceptibility data.
Isavuconazole/amphotericin B combinations did not report any species-specific statistical significance, demonstrating concerning antagonism rates. Future in vivo confirmations may be interesting to evaluate the clinical impact of this discouraging preliminary in vitro data. On the other hand, statistical significances emerged from isavuconazole/caspofungin synergy assays, with positive results only for A. niger, with the promising additivity percentage. Previously published data documented possible azole/amphotericin B antagonism due to their action against the same target [29]. We justified some failures in synergy attempts by hypothesizing that azoles’ inhibition of sterols synthesis may interfere with amphotericin B target research.
Despite the similar synergy rates, isavuconazole/caspofungin revealed more in vitro effectiveness due to lower antagonism rates (14.8%) when compared to isavuconazole/amphotericin B combinations (antagonism percentage of 43.6%). Unfortunately, A. flavus complicated the overall deductions because of its concerning indifference and antagonism rates for both the tested combinations. On the basis of our preliminary results, antifungal combinations are not recommended for this species, while isavuconazole/caspofungin synergies may be a considerable option in the case of other species. Otherwise, A. niger did not report antagonism episodes and revealed a frequent additivity, suggesting the possibility of including isavuconazole/caspofungin regimens against this species. According to these assumptions, it is essential to always perform species identification to plan an effective antifungal treatment. Recent experimental studies showed the azoles’ capability to induce β-glucan accumulation and fungal membrane invaginations [30]. Echinocandins inhibit β-glucan synthesis, probably interfering with these events, leading us to hypothesize about synergy failures.
Despite the importance of observing percentages and statistical significance, we further analyzed some details about the MIC values for the included Aspergillus spp. strains. Specifically, the three A. fumigatus (13.6%) reporting elevated amphotericin B MIC values revealed a significant diminution after the isavuconazole addition during the synergy assay. Two isolates showed synergy (66.6%), while one showed additivity (33.3%) for the isavuconazole/amphotericin B combination. Conversely, the A. fumigatus strain showing a high caspofungin MIC value (4.5%) did not reveal advantages from the isavuconazole addition: the caspofungin MIC value decreased, but the assay resulted in indifference. These data suggest that isavuconazole/amphotericin B combinations may be effective in the case of A. fumigatus with amphotericin B high MIC values. One A. niger isolate with high amphotericin B MIC (9.1%) resulted in indifference for the isavuconazole/amphotericin B combination. Otherwise, the strain with a caspofungin high MIC value (9.1%) reported synergy for the isavuconazole/caspofungin association.
A. flavus did not reveal high caspofungin MIC values, but one strain (7.4%) exhibited an amphotericin-elevated MIC value. This strain had an antagonism result in the case of the isavuconazole/amphotericin B combination, enforcing the hypothesis to discourage antifungal combinations for this species. Regarding the seven A. terreus isolates reporting high amphotericin B MIC values (87.5%), the isavuconazole/amphotericin B combination did not often improve the initial condition, resulting in four cases (57.1%) of indifference, three cases (28.5%) of antagonism, and only one case (14.3%) of synergy. Five A. terreus isolates revealed elevated caspofungin MIC values (62.5%), demonstrating only indifference episodes for the isavuconazole/caspofungin combination. These data led us to hypothesize the use of single isavuconazole regimens in the case of A. terreus, due to elevated amphotericin B and caspofungin MIC values not always being related to successful synergy results.
Certainly, our experimental evaluation had some limitations. The retrospective analysis included a few isolates’ numbers for some species, especially regarding A. niger and A. terreus. This condition probably affected all the statistical considerations. It would be ideal to perform similar investigations, including the same number for all the tested species, but extending the sample size. Further studies should test the in vitro synergy effect by inserting antifungal molecules during different time intervals to test their effectiveness in the case of subsequent and not simultaneous action against the fungal strain.
We hypothesized this future perspective according to the frequent antifungal sequential therapy usage in the case of invasive fungal infections. Finally, advanced molecular protocols may be applied to further investigate Aspergillus spp. resistance patterns, aiming to explain the detected differences in synergy results within the same species.
4. Materials and Methods
4.1. General Information and Isolates Collection
We performed a retrospective analysis at the University Hospital Policlinico of Catania, encompassing the Laboratory Analysis Unit during a two-year (2022–2024) period. The study included Aspergillus spp. isolates from respiratory samples (bronchial aspirates, sputum samples, and bronchoalveolar lavage fluids). These samples were derived from intensive care, infectious disease, pneumology, internal medicine, surgery, emergency room, oncology, and transplant units. The study retrospectively classified patients with probable invasive pulmonary aspergillosis according to the European Organization for the Research and Treatment of Cancer (EORTC) [20,31,32]. Consequently, IPA cases derived from the presence of at least one host risk factor (neutropenia, transplants, immunosuppression), one clinical criterion (halo sign, air crescent sign, dense well-circumscribed lesions, lobar consolidation), and mycological evidence (positive microscopical and/or culture assays, galactomannan antigen detected in plasma or serum or bronchoalveolar lavage with the minimum index value of 1, duplicate positive molecular results on serum or plasma or bronchoalveolar lavage or whole blood). Cases documenting probable COVID-19-associated pulmonary aspergillosis (CAPA) were similarly categorized according to the specific CAPA diagnostic guidelines [33]. None of the gathered results had a clinical or therapeutic application. Furthermore, all the collected data were anonymized, and no patient-identifiable data was included within the study. According to these assumptions, ethical approvals were not mandatory for our local legislation. All the isolates were stored at −80 °C in sterile water after the routine diagnostic procedures. Consequently, they were restored and inoculated into R.P.M.I agar (Liofilchem® s.r.l., Roseto degli Abruzzi, Italy) to perform the experimental study.
4.2. Isolates Identification and Susceptibility Testing
The MALDI Biotyper® Sirius System (Bruker, Billerica, MA, USA) identified the grown filamentous fungi colonies according to the MBT Filamentous Fungi IVD Library Version 2022. The above-mentioned library allowed to sensu strictu (s.s.) Aspergillus isolates identification. The identified strains were subjected to isavuconazole, amphotericin B, and caspofungin susceptibility testing through the MIC Strip method and the gradient test principle [34,35]. The obtained MIC values were confirmed through the EUCAST broth microdilution guidelines on filamentous fungi [36]. We considered susceptible all the isolates reporting MIC values under or equal to the CBP, along with the isolates showing MIC values under the ECOFF. On the other hand, we classified resistant all the isolates showing MIC values over the CBP, along with the ones reporting MIC values under or equal to the ECOFF [37,38].
4.3. Synergy Assays
Isavuconazole/amphotericin B and isavuconazole/caspofungin were arranged to study synergy, following the manufacturer’s instructions [39].
The same instructions were established to perform all the single and combination assays on R.P.M.I. agar (Liofilchem® s.r.l., Roseto degli Abruzzi, Italy), incubating the inoculated plates for 24–48 h at 37 °C. Finally, the synergy results were managed by calculating the corresponding FIC index, which allowed for the categorization of the results as antagonism, indifference, synergy, or additivity cases [36]. Specifically, a FIC index equal to or lower than 0.5 indicated synergy, while antagonism emerged from a FIC index higher than 4. A FIC index higher than 0.5 but equal to or lower than 1 indicated additivity, whereas a value higher than 1 but equal to or lower than 4 was related to indifference. Antagonism reported a combination with less effect than the single antifungals. Indifference is derived from the total absence of interactions between the two molecules. Additivity occurred when the combination demonstrated a higher effect than the single compounds. Synergy emerged when the combination had a more intense effect than the simple addition of the two drugs [40].
4.4. Statistical Analysis
The MedCalc Statistical Software version 17.9.2 “http://www.medcalc.org” (Accessed on 28 August 2025) was used to perform a statistical analysis of Aspergillus species and synergy result correspondence. Moreover, the χ2 and Fisher’s exact test established the categorical variables as percentages. Our experimental protocol did not involve any direct action on human beings. The susceptibility analyses were related to biological samples and fungal isolates, which were not collected as supplementary specimens and derived from previous routine diagnostic procedures.
5. Conclusions
Invasive pulmonary aspergillosis treatment poses a significant clinical challenge. Certainly, caspofungin exhibits limited activity against Aspergillus spp. due to its fungistatic attitude. On the other hand, amphotericin B and isavuconazole extensively demonstrated efficacy against the same molds [3,4,5,6]. However, amphotericin B recently demonstrated an increase in potential antifungal resistance rates [41]. Isavuconazole reported encouraging in vitro and in vivo results against Aspergillus spp. [25,42], always showing low MIC values. Despite the concerning increase in aspergillosis treatment issues, scientific literature does not sufficiently document in vitro synergy studies. On this premise, in vivo antifungal combinations are often discouraged, excepting for specific cases such as fluconazole and amphotericin B usage in the case of cryptococcosis [43]. The main purpose of our study was to report microbiological susceptibility results regarding Aspergillus spp. clinical isolates.
Our results enhanced the importance of always performing identification and antifungal susceptibility assays for invasive molds isolates. Synergy testing demonstrated a significant species-specific trend, mainly underlining isavuconazole/amphotericin B in vitro effectiveness against A. fumigatus and the elevated isavuconazole/caspofungin antagonism rate for A. flavus. Future studies should be focused on Aspergillus spp. isolates and antifungal in vitro synergy testing, aiming to discourage or recommend any specific antifungal combinations depending on the isolated species.
Author Contributions
Conceptualization, L.T.; methodology, M.C. (Maddalena Calvo) and M.C. (Michelangelo Caruso); investigation, M.C. (Maddalena Calvo), A.A.T., and M.C. (Michelangelo Caruso); writing—original draft preparation, M.C. (Maddalena Calvo) and L.T.; writing—review and editing, L.T.; supervision, L.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
None of the gathered results had a clinical or therapeutic application. According to these assumptions, ethical approvals were not mandatory according to our local legislation.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the gathered results are included in the present manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ledoux, M.P.; Herbrecht, R. Invasive Pulmonary Aspergillosis. J. Fungi 2023, 9, 131. [Google Scholar] [CrossRef]
- Machado, M.; Fortún, J.; Muñoz, P. Invasive aspergillosis: A comprehensive review. Med. Clin. 2024, 163, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F.; Calandra, T. Pulmonary aspergillosis: Diagnosis and treatment. Eur. Respir. Rev. 2022, 31, 220114. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patterson, T.F.; Thompson, G.R.; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef] [PubMed]
- AMCLI ETS. Percorso Diagnostico “MICOSI PROFONDE E SISTEMICHE”—Rif. 2023-16, rev. 2023. Available online: https://amcli.it/percorsi-diagnostici-218/ (accessed on 6 September 2025).
- Schwarz, M.C.R.; Moskaluk, A.E.; Daniels, J.B.; VandeWoude, S.; Reynolds, M.M. Current Analytical Methods and Challenges for the Clinical Diagnosis of Invasive Pulmonary Aspergillosis Infection. J. Fungi 2024, 10, 829. [Google Scholar] [CrossRef]
- De Francesco, M.A. Drug-Resistant Aspergillus spp.: A Literature Review of Its Resistance Mechanisms and Its Prevalence in Europe. Pathogens 2023, 12, 1305. [Google Scholar] [CrossRef]
- Raffetin, A.; Courbin, V.; Jullien, V.; Dannaoui, E. In Vitro Combination of Isavuconazole with Echinocandins against Azole-Susceptible and -Resistant Aspergillus spp. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Katragkou, A.; McCarthy, M.; Meletiadis, J.; Petraitis, V.; Moradi, P.W.; Strauss, G.E.; Fouant, M.M.; Kovanda, L.L.; Petraitiene, R.; Roilides, E.; et al. In vitro combination of isavuconazole with micafungin or amphotericin B deoxycholate against medically important molds. Antimicrob. Agents Chemother. 2014, 58, 6934–6937. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antifungal Susceptibility Testing of Filamentous Fungi, 3rd ed.; CLSI supplement M38M51S; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2022. [Google Scholar]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 15.0. 2025. Available online: https://www.eucast.org (accessed on 5 January 2025).
- Ortalli, G.; Oliva, E.; Lo Cascio, G.; on behalf of the Medical Mycology Committee (CoSM)—Italian Association of Clinical Microbiologists (AMCLI); Farina, C. In Vitro Activity of Isavuconazole and Amphotericin B in Association against Mucorales. Pathogens 2023, 12, 948. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Calvo, M.; Lauricella, F.; Mellini, A.M.; Scalia, G.; Trovato, L. Isavuconazole and Amphotericin B Synergic Antifungal Activity: In Vitro Evaluation on Pulmonary Aspergillosis Molds Isolates. Antibiotics 2024, 13, 1005. [Google Scholar] [CrossRef]
- Douglas, A.P.; Smibert, O.C.; Bajel, A.; Halliday, C.L.; Lavee, O.; McMullan, B.; Yong, M.K.; van Hal, S.J.; Chen, S.C.-A.; the Australasian Antifungal Guidelines Steering Committee. Consensus guidelines for the diagnosis and management of invasive aspergillosis, 2021. Intern. Med. J. 2021, 51 (Suppl. S7), 143–176. [Google Scholar] [CrossRef] [PubMed]
- Dagenais, T.R.T.; Keller, N.P. Pathogenesis of Aspergillus fumigatus in Invasive Aspergillosis. Clin. Microbiol. Rev. 2009, 22, 447–465. [Google Scholar] [CrossRef] [PubMed]
- Franconi, I.; Rizzato, C.; Ghelardi, E.; Lupetti, A. Hospital distribution, seasonality, time trends and antifungal susceptibility profiles of all Aspergillus species isolated from clinical samples from 2015 to 2022 in a tertiary care hospital. BMC Microbiol. 2024, 24, 111. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Tiseo, G.; Falcone, M.; Menichetti, F. Pulmonary Aspergillosis: An Evo5. Russo A, Tiseo G, Falcone M, Menichetti F. Pulmonary Aspergillosis: An Evolving Challenge for Diagnosis and Treatment. Infect Dis Ther. 2020; 9:511-524lving Challenge for Diagnosis and Treatment. Infect. Dis. Ther. 2020, 9, 511–524. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Zhou, L.; Zhang, M.; Xu, Y. Epidemiology, drug susceptibility, and clinical risk factors in patients with invasive aspergillosis. Front. Public Health 2022, 10, 835092. [Google Scholar] [CrossRef]
- Elkhapery, A.; Fatima, M.; Soubani, A.O. Emerging Risk Factors for Invasive Pulmonary Aspergillosis: A Narrative Review. J. Fungi 2025, 11, 555. [Google Scholar] [CrossRef]
- Bassetti, M.; Azoulay, E.; Kullberg, B.-J.; Ruhnke, M.; Shoham, S.; Vazquez, J.; Giacobbe, D.R.; Calandra, T. EORTC/MSGERC Definitions of Invasive Fungal Diseases: Summary of Activities of the Intensive Care Unit Working Group. Clin. Infect. Dis. 2021, 72 (Suppl. S2), S121–S127. [Google Scholar] [CrossRef]
- Jenks, J.D.; Hoenigl, M. Treatment of Aspergillosis. J. Fungi 2018, 4, 98. [Google Scholar] [CrossRef]
- Trovato, L.; Scalia, G.; Domina, M.; Oliveri, S. Environmental Isolates of Multi-Azole-Resistant Aspergillus spp. in Southern Italy. J. Fungi 2018, 4, 131. [Google Scholar] [CrossRef]
- Trovato, L.; Calvo, M.; Palermo, C.I.; Scalia, G. The Role of Quantitative Real-Time PCR in the Invasive Pulmonary Aspergillosis Diagnosis: A Retrospective Study. Microorganisms 2025, 13, 409. [Google Scholar] [CrossRef]
- Buil, J.B.; Brüggemann, R.J.M.; E Wasmann, R.; Zoll, J.; Meis, J.F.; Melchers, W.J.G.; Mouton, J.W.; E Verweij, P. Isavuconazole susceptibility of clinical Aspergillus fumigatus isolates and feasibility of isavuconazole dose escalation to treat isolates with elevated MICs. J. Antimicrob. Chemother. 2018, 73, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.S.; Wiederhold, N.P.; Hakki, M.; Thompson, G.R. New Perspectives on Antimicrobial Agents: Isavuconazole. Antimicrob. Agents Chemother. 2022, 66, e00177-22. [Google Scholar] [CrossRef] [PubMed]
- Fakhim, H.; Badali, H.; Dannaoui, E.; Nasirian, M.; Jahangiri, F.; Raei, M.; Vaseghi, N.; Ahmadikia, K.; Vaezi, A. Trends in the Prevalence of Amphotericin B-Resistance (AmBR) among Clinical Isolates of Aspergillus Species. J. Mycol. Med. 2022, 32, 101310. [Google Scholar] [CrossRef] [PubMed]
- Vahedi-Shahandashti, R.; Dietl, A.M.; Binder, U.; Nagl, M.; Würzner, R.; Lass-Flörl, C. Aspergillus terreus and the Interplay with Amphotericin B: From Resistance to Tolerance? Antimicrob. Agents Chemother. 2022, 66, e0227421. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Zimbeck, A.J.; Baddley, J.W.; Marr, K.A.; Andes, D.R.; Walsh, T.J.; Kauffman, C.A.; Kontoyiannis, D.P.; Ito, J.I.; Pappas, P.G.; et al. In vitro echinocandin susceptibility of Aspergillus isolates from patients enrolled in the Transplant-Associated Infection Surveillance Network. Antimicrob. Agents Chemother. 2011, 55, 3944–3946. [Google Scholar] [CrossRef]
- Scheven, M.; Schwegler, F. Antagonistic interactions between azoles and amphotericin B with yeasts depend on azole lipophilia for special test conditions in vitro. Antimicrob. Agents Chemother. 1995, 39, 1779–1783. [Google Scholar] [CrossRef]
- Geißel, B.; Loiko, V.; Klugherz, I.; Zhu, Z.; Wagener, N.; Kurzai, O.; Hondel, C.A.M.J.J.v.D.; Wagener, J. Azole-induced cell wall carbohydrate patches kill Aspergillus fumigatus. Nat. Commun. 2018, 9, 3098. [Google Scholar] [CrossRef]
- Available online: https://c.peervoice.com/programs/140200557/downloads/PV_practiceaids_QVJ.pdf (accessed on 9 December 2024).
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koehler, P.; Bassetti, M.; Chakrabarti, A.; Chen, S.C.; Colombo, A.L.; Hoenigl, M.; Klimko, N.; Lass-Flörl, C.; Oladele, R.O.; Vinh, D.C.; et al. Defining and managing COVID-19-associated pulmonary aspergillosis: The 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect. Dis. 2021, 21, e149–e162. [Google Scholar] [CrossRef]
- Available online: https://www.liofilchem.net/login.area.mic/technical_sheets/MTS40.pdf (accessed on 3 January 2025).
- Available online: https://www.liofilchem.com/images/brochure/mic_test_strip_patent/MTS06.pdf (accessed on 3 January 2025).
- Guinea, J.; Meletiadis, J.; Arikan-Akdagli, S.; Muehlethaler, K.; Kahlmeter, G.; Arendrup, M.C. the Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST) EUCAST DEFINITIVE DOCUMENT E.DEF 9.4 Method for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Conidia Forming Moulds. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Files/EUCAST_EDef_9.4_method_for_susceptibility_testing_of_moulds.pdf (accessed on 20 September 2023).
- The European Committee on Antimicrobial Susceptibility Testing Breakpoint Tables for Interpretation of MICs for Antifungal Agents, Version 10.0. 2020. Available online: http://www.eucast.org/astoffungi/clinicalbreakpointsforantifungals/ (accessed on 20 September 2023).
- The European Committee on Antimicrobial Susceptibility Testing EUCAST Development Laboratories. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Network_labs/EDL/EUCAST_Development_Laboratories.pdf (accessed on 20 September 2023).
- Available online: https://www.liofilchem.net/login.area.mic/technical_sheets/MTS31.pdf (accessed on 24 September 2023).
- Roell, K.R.; Reif, D.M.; Motsinger-Reif, A.A. An Introduction to Terminology and Methodology of Chemical Synergy-Perspectives from Across Disciplines. Front. Pharmacol. 2017, 8, 158. [Google Scholar] [CrossRef]
- Kaya, A.; Kaya, S.Y.; Balkan, I.I.; Alkan, S.; Kurt, A.F.; Elverdi, T.; Urkmez, S.; Ongoren, S.; Aygun, G. Amphotericin B Resistant Aspergillus spp.: Report of Two Cases. Infect. Dis. Clin. Microbiol. 2024, 6, 343–348. [Google Scholar] [CrossRef]
- Trovato, L.; Scalia, G.; Palermo, C.I.; Costanzo, C.M.; Oliveri, S. Evaluation of isavuconazole MIC strips for susceptibility testing of Aspergillus and Scedosporium species. Med. Mycol. 2019, 57, 429–433. [Google Scholar] [CrossRef]
- Available online: https://www.idsociety.org/practice-guideline/Diagnosis-and-Management-of-Cryptococcosis/ (accessed on 27 September 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).