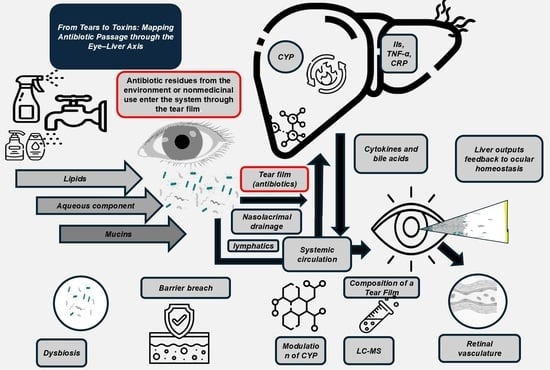
From Tears to Toxins: Mapping Antibiotic Passage Through the Eye–Liver Axis
Abstract
1. Methodology of the Literature Review
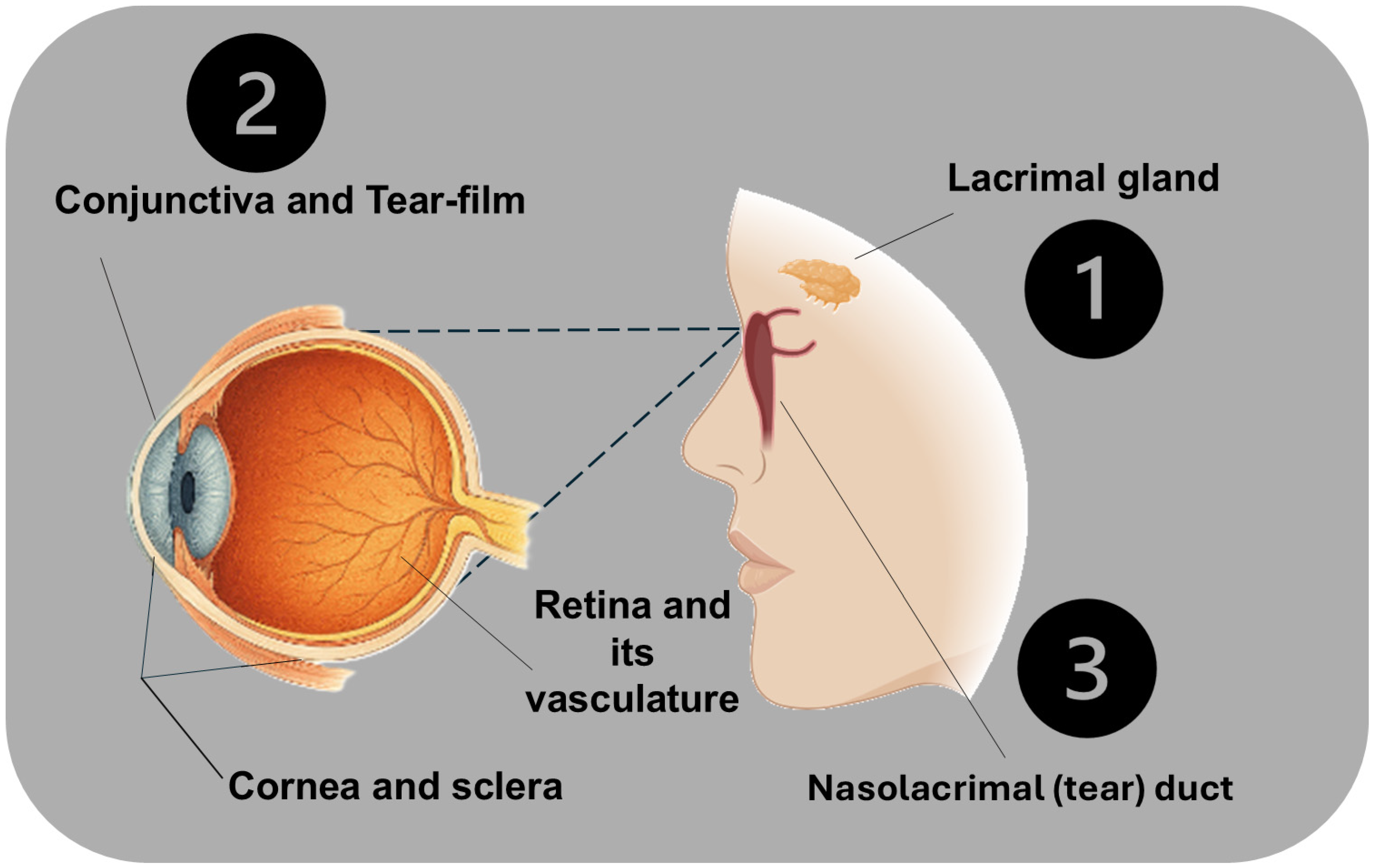
2. Ocular Surface: A Gateway Beyond Vision
2.1. Anatomy and Exposure
2.2. Microbial Modulation
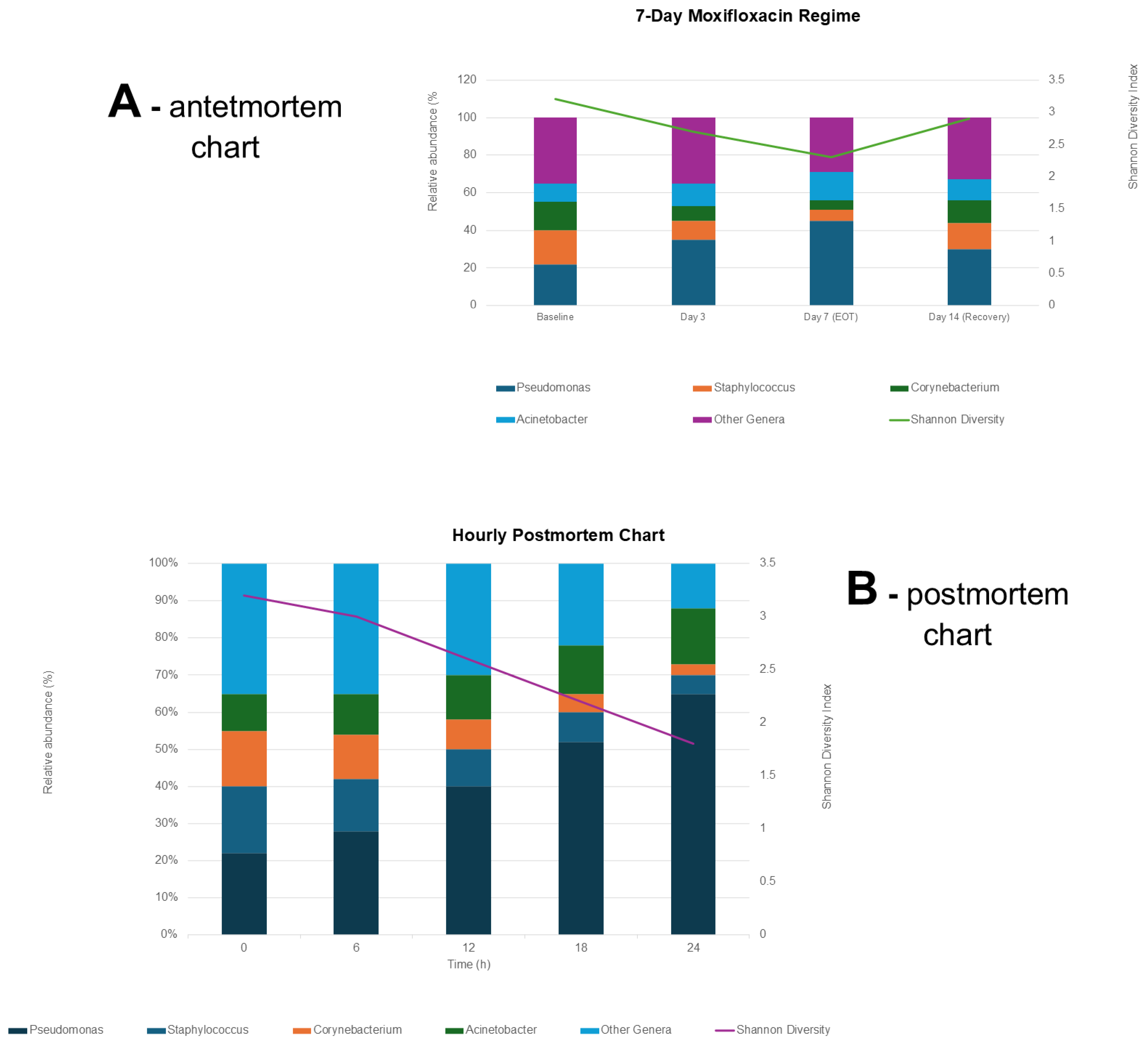
2.3. Postmortem Ocular Microbiota Shifts
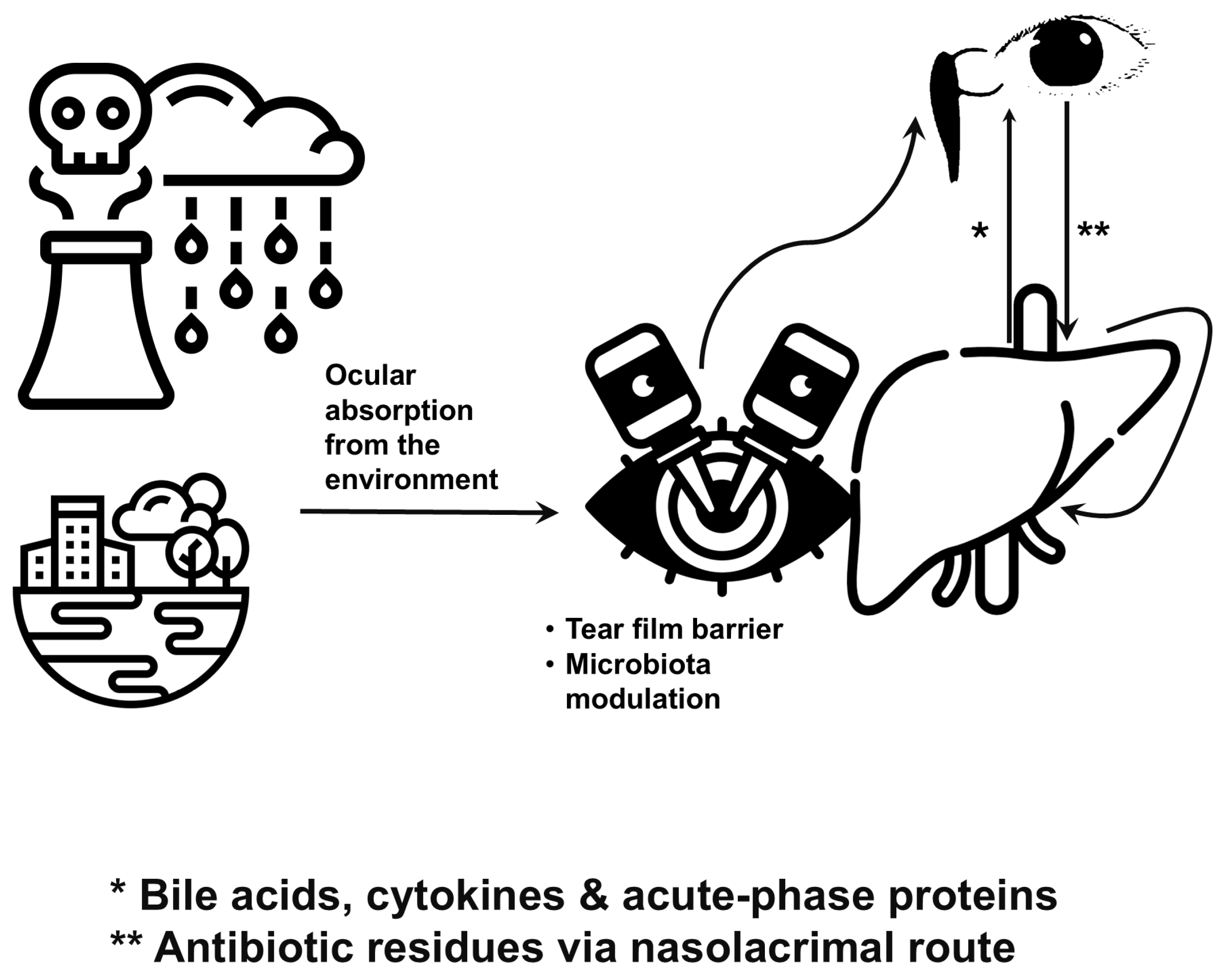
3. Liver–Eye Axis: A Metabolic and Immunological Highway
3.1. Correlations Through the Lens of Ocular Surface Microbiota
- Positive correlations of ALT, AST, GGT, ALP, TBIL, Complement C3, and CRP with tear-film thickness imply that systemic inflammation or mild hepatic injury coincides with tear-film expansion [75].
- A thicker tear-film can dilute antimicrobial peptides and disrupt nutrient gradients that normally sustain commensal bacteria, fostering dysbiosis [42].
- Subclinical inflammation on the ocular surface can release cytokines that traverse to the retina, triggering glial activation and subtle RPE hypertrophy, which in turn may further disturb immune surveillance at the conjunctiva [78].
- Elevated liver enzymes and acute-phase proteins often accompany antibiotic residues that have traversed the nasolacrimal system—antibiotics themselves are potent modulators of microbial diversity [79].
- Complement C3’s positive correlation underscores an immune-driven culling of certain bacterial taxa, while CRP’s similar trend highlights low-grade inflammation that may perpetuate microbial dysbiosis and contribute to systemic immune modulation [80].
3.2. Forensic and Clinical Implications for Ocular Surface Microbiota
- Biomarker-Guided Microbiome Profiling: In cases of unexplained ocular surface disease, pairing tear-film thickness/OCT-derived RPE measures with 16S rRNA sequencing can pinpoint dysbiotic signatures.
- Nonmedicinal Antibiotic Surveillance: Trace antibiotic detection in tears, together with elevated liver enzymes, flags environmental exposures that may covertly reshape the ocular microbiome and compromise barrier integrity.
4. Antibiotics and Eye Microbiota
5. Nonmedicinal Antibiotic Use: Mechanisms and Implications
6. Emerging Research and Therapeutic Potential
6.1. CRISPR-Based Approaches
6.2. Nanoparticle-Based Scavengers
6.3. Probiotic and Microbiota-Centric Therapies
6.4. RNA Interference Platforms
7. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full Term/Meaning |
| 16S rRNA | 16S ribosomal RNA |
| ALB | Albumin |
| ALP | Alkaline phosphatase |
| ALT | Alanine aminotransferase |
| AMR | Antimicrobial resistance |
| ANOVA | Analysis of variance |
| AST | Aspartate aminotransferase |
| CV | Coefficient of variation |
| CRP | C-reactive protein |
| CYP | Cytochrome P450 |
| GGT | Gamma-glutamyl transferase |
| DDD | defined daily dose |
| IL6 | Interleukin-6 |
| ISOSRPE | Inner/outer segment—retinal pigment epithelium thickness |
| LOD | Limit of detection |
| LCMS | Liquid chromatography–mass spectrometry |
| LCMS/MS | Liquid chromatography–tandem mass spectrometry |
| LFTs | Liver function tests |
| LPS | Lipopolysaccharide |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| NAFLD | Nonalcoholic fatty liver disease |
| NGS | Next-generation sequencing |
| OCT | Optical coherence tomography |
| PCR | Polymerase chain reaction |
| PMI | Postmortem interval |
| QC | Quality control |
| RPE | Retinal pigment epithelium |
| RT | Room temperature |
| t½ | Half-life |
| T_max | Time to maximum concentration |
| TBIL | Total bilirubin |
| TP | Total protein |
| µL | Microlitre |
| °C | Degree Celsius |
References
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Cook, D.A. Systematic and Nonsystematic Reviews: Choosing an Approach. In Healthcare Simulation Research; Springer: Berlin/Heidelberg, Germany, 2019; pp. 55–60. [Google Scholar]
- Abbey, A.M.; Yoo, S.H. Corneal Anatomy and Optics. In Corneal Topography; CRC Press: Boca Raton, FL, USA, 2024; pp. 11–19. [Google Scholar]
- Croker, C. Ocular Surface-Tear Film Interface. 2024. Available online: https://synapse.org.za/cpd-activities/croker-ocular-surface-2.pdf (accessed on 3 September 2025).
- Jiao, X.; Wang, C.; Li, H.; Wang, T.; Li, X.; Deng, A.; Li, Z. Redefining tear film Biology: Immune regulation, multi-omics integration, and systemic disease interfaces. Exp. Eye Res. 2025, 259, 110574. [Google Scholar] [CrossRef]
- Galletti, J.G.; de Paiva, C.S. The ocular surface immune system through the eyes of aging. Ocul. Surf. 2021, 20, 139–162. [Google Scholar] [CrossRef]
- Koh, S.; Rao, S.K.; Srinivas, S.P.; Tong, L.; Young, A.L. Evaluation of ocular surface and tear function-A review of current approaches for dry eye. Indian J. Ophthalmol. 2022, 70, 1883–1891. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Duan, C.; Yang, Y.; Wang, Z.; Tan, C.; Han, C.; Hou, X. Insights into the liver-eyes connections, from epidemiological, mechanical studies to clinical translation. J. Transl. Med. 2023, 21, 712. [Google Scholar] [CrossRef]
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Amin, M.M.; Sayed, S. Ocular Drug Delivery: A Comprehensive Review. AAPS PharmSciTech 2023, 24, 66. [Google Scholar] [CrossRef]
- Loscher, M.; Seiz, C.; Hurst, J.; Schnichels, S. Topical Drug Delivery to the Posterior Segment of the Eye. Pharmaceutics 2022, 14, 134. [Google Scholar] [CrossRef]
- Mofidfar, M.; Abdi, B.; Ahadian, S.; Mostafavi, E.; Desai, T.A.; Abbasi, F.; Sun, Y.; Manche, E.E.; Ta, C.N.; Flowers, C.W. Drug delivery to the anterior segment of the eye: A review of current and future treatment strategies. Int. J. Pharm. 2021, 607, 120924. [Google Scholar] [CrossRef]
- Sanap, A.R. A Review on Ophthalmic Drug Delivery System. Res. J. Sci. Technol. 2024, 16, 79–86. [Google Scholar] [CrossRef]
- Maharana, P.K.; Sharma, N. Applied Anatomy and Physiology of the Cornea. In Mastering Corneal Surgery; CRC Press: Boca Raton, FL, USA, 2024; pp. 3–9. [Google Scholar]
- Santana, C.P.; Matter, B.A.; Patil, M.A.; Silva-Cunha, A.; Kompella, U.B. Corneal Permeability and Uptake of Twenty-Five Drugs: Species Comparison and Quantitative Structure-Permeability Relationships. Pharmaceutics 2023, 15, 1646. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.M.; Borroni, D.; Liu, R.; Zhao, Y.; Zhang, J.; Lim, J.; Ma, B.; Romano, V.; Qi, H.; Ferdousi, M.; et al. An artificial intelligence-based deep learning algorithm for the diagnosis of diabetic neuropathy using corneal confocal microscopy: A development and validation study. Diabetologia 2020, 63, 419–430. [Google Scholar] [CrossRef]
- Chang, A.Y.; Purt, B. Biochemistry, Tear Film. Available online: https://www.ncbi.nlm.nih.gov/books/NBK572136/ (accessed on 3 September 2025).
- Dosmar, E.; Walsh, J.; Doyel, M.; Bussett, K.; Oladipupo, A.; Amer, S.; Goebel, K. Targeting Ocular Drug Delivery: An Examination of Local Anatomy and Current Approaches. Bioengineering 2022, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Obeng, E.M.; Hodge, C.; You, J. Microplastic pollution: A review of specific blood-tissue barrier breaches and health effects. Environ. Pollut. 2025, 376, 126416. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, F. Anatomy and Physiology of the Nasolacrimal Ducts. In Atlas of Lacrimal Surgery; Weber, R.K., Keerl, R., Schaefer, S.D., Della Rocca, R.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–13. [Google Scholar]
- Gupta, N. An Overview of Nasolacrimal Duct (NLD) Encountered in Different Situations; Identification, Prevention and Management of NLD Injuries. In Endoscopic Dacryocystorhinostomy; Gupta, N., Ed.; Springer: Singapore, 2021; pp. 215–222. [Google Scholar]
- Wang, L.; Zhou, M.B.; Zhang, H. The Emerging Role of Topical Ocular Drugs to Target the Posterior Eye. Ophthalmol. Ther. 2021, 10, 465–494. [Google Scholar] [CrossRef]
- Petrillo, F.; Pignataro, D.; Lavano, M.A.; Santella, B.; Folliero, V.; Zannella, C.; Astarita, C.; Gagliano, C.; Franci, G.; Avitabile, T.; et al. Current Evidence on the Ocular Surface Microbiota and Related Diseases. Microorganisms 2020, 8, 1033. [Google Scholar] [CrossRef]
- Patangia, D.V.; Anthony Ryan, C.; Dempsey, E.; Paul Ross, R.; Stanton, C. Impact of antibiotics on the human microbiome and consequences for host health. Microbiologyopen 2022, 11, e1260. [Google Scholar] [CrossRef]
- Zhang, R.; Ding, N.; Feng, X.; Liao, W. The gut microbiome, immune modulation, and cognitive decline: Insights on the gut-brain axis. Front. Immunol. 2025, 16, 1529958. [Google Scholar] [CrossRef]
- Fu, L.; Qian, Y.; Shang, Z.; Sun, X.; Kong, X.; Gao, Y. Antibiotics enhancing drug-induced liver injury assessed for causality using Roussel Uclaf Causality Assessment Method: Emerging role of gut microbiota dysbiosis. Front. Med. 2022, 9, 972518. [Google Scholar] [CrossRef]
- Cusumano, G.; Flores, G.A.; Venanzoni, R.; Angelini, P. The Impact of Antibiotic Therapy on Intestinal Microbiota: Dysbiosis, Antibiotic Resistance, and Restoration Strategies. Antibiotics 2025, 14, 371. [Google Scholar] [CrossRef]
- Jiao, X.; Li, Y.; Hu, Y.; Yan, R.; Fu, T.; Liu, J.; Li, Z. Antibiotic-induced dysbiosis of the ocular microbiome affects corneal circadian rhythmic activity in mice. Mucosal Immunol. 2025, 18, 562–582. [Google Scholar] [CrossRef]
- Chen, G.; Shi, F.; Yin, W.; Guo, Y.; Liu, A.; Shuai, J.; Sun, J. Gut microbiota dysbiosis: The potential mechanisms by which alcohol disrupts gut and brain functions. Front. Microbiol. 2022, 13, 916765. [Google Scholar] [CrossRef] [PubMed]
- Tariq, F.; Hehar, N.K.; Chigbu, D.I. The Ocular Surface Microbiome in Homeostasis and Dysbiosis. Microorganisms 2025, 13, 1992. [Google Scholar] [CrossRef] [PubMed]
- Mitamura, Y.; Ogulur, I.; Pat, Y.; Rinaldi, A.O.; Ardicli, O.; Cevhertas, L.; Bruggen, M.C.; Traidl-Hoffmann, C.; Akdis, M.; Akdis, C.A. Dysregulation of the epithelial barrier by environmental and other exogenous factors. Contact Dermat. 2021, 85, 615–626. [Google Scholar] [CrossRef]
- Singh, N.; Diebold, Y.; Sahu, S.K.; Leonardi, A. Epithelial barrier dysfunction in ocular allergy. Allergy 2022, 77, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Y.; Rudd Zhong Manis, J.; Ronczkowski, N.M.; Bui, T.; Oxenrider, A.; Jadeja, R.N.; Thounaojam, M.C. Unveiling the gut-eye axis: How microbial metabolites influence ocular health and disease. Front. Med. 2024, 11, 1377186. [Google Scholar] [CrossRef]
- Chassaing, B.; Etienne-Mesmin, L.; Gewirtz, A.T. Microbiota-liver axis in hepatic disease. Hepatology 2014, 59, 328–339. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, J.; Wang, L.; Zhang, C.; Zhang, Y.; Feng, Y. Exploring the hepatic-ophthalmic axis through immune modulation and cellular dynamics in diabetic retinopathy and non-alcoholic fatty liver disease. Hum. Genom. 2025, 19, 19. [Google Scholar] [CrossRef]
- Sakoulas, G. Do Antibiotics Diminish Innate Immunity by Attenuating Toll-Like Receptor Activity? NEJM J. Watch. 2022, NA55209. [Google Scholar]
- Wicherska-Pawłowska, K.; Wróbel, T.; Rybka, J. Toll-Like Receptors (TLRs), NOD-Like Receptors (NLRs), and RIG-I-Like Receptors (RLRs) in Innate Immunity. TLRs, NLRs, and RLRs Ligands as Immunotherapeutic Agents for Hematopoietic Diseases. Int. J. Mol. Sci. 2021, 22, 13397. [Google Scholar] [CrossRef]
- Niederreiter, L.; Tilg, H. Cytokines and fatty liver diseases. Liver Res. 2018, 2, 14–20. [Google Scholar] [CrossRef]
- Dabrowska, A.; Wilczynski, B.; Mastalerz, J.; Kucharczyk, J.; Kulbacka, J.; Szewczyk, A.; Rembialkowska, N. The Impact of Liver Failure on the Immune System. Int. J. Mol. Sci. 2024, 25, 9522. [Google Scholar] [CrossRef]
- Periman, L.M.; Perez, V.L.; Saban, D.R.; Lin, M.C.; Neri, P. The Immunological Basis of Dry Eye Disease and Current Topical Treatment Options. J. Ocul. Pharmacol. Ther. 2020, 36, 137–146. [Google Scholar] [CrossRef]
- Rusanac, A.; Skibola, Z.; Matijasic, M.; Cipcic Paljetak, H.; Peric, M. Microbiome-Based Products: Therapeutic Potential for Inflammatory Skin Diseases. Int. J. Mol. Sci. 2025, 26, 6745. [Google Scholar] [CrossRef]
- Rusciano, D. Restoring Ocular Microbiota Balance: A New Bioprinted Approach to Treating Anterior Segment Diseases. Front. Biosci. (Landmark Ed.) 2025, 30, 28268. [Google Scholar] [CrossRef]
- Enriquez-de-Salamanca, A.; Calder, V.; Gao, J.; Galatowicz, G.; Garcia-Vazquez, C.; Fernandez, I.; Stern, M.E.; Diebold, Y.; Calonge, M. Cytokine responses by conjunctival epithelial cells: An in vitro model of ocular inflammation. Cytokine 2008, 44, 160–167. [Google Scholar] [CrossRef]
- Duan, Y.; Pan, X.; Luo, J.; Xiao, X.; Li, J.; Bestman, P.L.; Luo, M. Association of Inflammatory Cytokines with Non-Alcoholic Fatty Liver Disease. Front. Immunol. 2022, 13, 880298. [Google Scholar] [CrossRef]
- Gachon, F.; Bugianesi, E.; Castelnuovo, G.; Oster, H.; Pendergast, J.S.; Montagnese, S. Potential bidirectional communication between the liver and the central circadian clock in MASLD. NPJ Metab. Health Dis. 2025, 3, 15. [Google Scholar] [CrossRef]
- Fu, Y.; Yi, Y.; Fan, Y.; Shang, R. Cytochrome P450 inhibition potential and initial genotoxic evaluation of 14-O-[(4,6-diaminopyrimidine-2-yl)thioacetyl] mutilin. Sci. Rep. 2020, 10, 13474. [Google Scholar] [CrossRef]
- Chowdhary, A.; Van Gelder, R.N.; Sundararajan, M. Methodologic Considerations for Studying the Ocular Surface Microbiome. Ophthalmol. Sci. 2023, 3, 100408. [Google Scholar] [CrossRef]
- Trojacka, E.; Izdebska, J.; Szaflik, J.; Przybek-Skrzypecka, J. The Ocular Microbiome: Micro-Steps Towards Macro-Shift in Targeted Treatment? A Comprehensive Review. Microorganisms 2024, 12, 2232. [Google Scholar] [CrossRef]
- Montenegro, Y.H.A.; Guimarães, S.E.F.; Araújo, E.G.L.d.; Ferreira, R.S.; Martin, E.R.d.A.; Marcelino, M.K.d.S.; Nascimento, D.d.Q.; Silva, G.C.d.L. Molecular Era of the Forensic Science. Braz. J. Biol. Sci. 2018, 5, 195–211. [Google Scholar] [CrossRef]
- Kaur, S.; Patel, B.C.K.; Collen, A.; Malhotra, R. The microbiome and the eye: A new era in ophthalmology. Eye 2025, 39, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Nioi, M.; Napoli, P.E.; Demontis, R.; Chighine, A.; De-Giorgio, F.; Grassi, S.; Scorcia, V.; Fossarello, M.; d’Aloja, E. The Influence of Eyelid Position and Environmental Conditions on the Corneal Changes in Early Postmortem Interval: A Prospective, Multicentric OCT Study. Diagnostics 2022, 12, 2169. [Google Scholar] [CrossRef]
- Padzińska-Pruszyńska, I.B.; Pruszyński, J.J.; Górczak, M.; Smolarska, A.; Kubiak, M.; Kucharzewska, P.; Szeliga, J.; Taciak, B.; Florczak, L.; Siedlecka, P. Use of microorganisms, insects, plants and soil in criminological research. Probl. Forensic Sci. 2023, 135, 217–237. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Zhang, X.; Gu, Q.; Wu, Y.; Tao, X.; Tian, T.; Pan, G.; Chu, M. The Potential Impact of Antibiotic Exposure on the Microbiome and Human Health. Microorganisms 2025, 13, 602. [Google Scholar] [CrossRef]
- Zilliox, M.J.; Bouchard, C.S. The Microbiome, Ocular Surface, and Corneal Disorders. Am. J. Pathol. 2023, 193, 1648–1661. [Google Scholar] [CrossRef] [PubMed]
- Ghita, A.M.; Iliescu, D.A.; Ghita, A.C.; Ilie, L.A. Susceptibility of Ocular Surface Bacteria to Various Antibiotic Agents in a Romanian Ophthalmology Clinic. Diagnostics 2023, 13, 3409. [Google Scholar] [CrossRef] [PubMed]
- de Miranda Goncalves, L.B.; Campos, M.; Barros, G.F.; da Veiga, G.L.; Silva, J.A.; Fonseca, F.L.A.; Gascon, T.M.; de Carvalho, S.S.; de Carvalho, A.K.R.; Fernandes, G.E.P.; et al. Analysis of topical conjunctival microbiotic cultures in patients treated with intravitreal injections using antibiotic prophylaxis with 0.3% ofloxacin eye drops. Int. J. Retin. Vitr. 2024, 10, 99. [Google Scholar] [CrossRef]
- Song, J.; Dong, H.; Wang, T.; Yu, H.; Yu, J.; Ma, S.; Song, X.; Sun, Q.; Xu, Y.; Liu, M. What is the impact of microbiota on dry eye: A literature review of the gut-eye axis. BMC Ophthalmol. 2024, 24, 262. [Google Scholar] [CrossRef]
- Wang, X.; Deng, K.; Zhang, P.; Chen, Q.; Magnuson, J.T.; Qiu, W.; Zhou, Y. Microplastic-mediated new mechanism of liver damage: From the perspective of the gut-liver axis. Sci. Total Environ. 2024, 919, 170962. [Google Scholar] [CrossRef] [PubMed]
- Martin Ask, N.; Leung, M.; Radhakrishnan, R.; Lobo, G.P. Vitamin A Transporters in Visual Function: A Mini Review on Membrane Receptors for Dietary Vitamin A Uptake, Storage, and Transport to the Eye. Nutrients 2021, 13, 3987. [Google Scholar] [CrossRef]
- McEldrew, E.P.; Lopez, M.J.; Milstein, H. Vitamin A. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Argikar, U.A.; Dumouchel, J.L.; Kramlinger, V.M.; Cirello, A.L.; Gunduz, M.; Dunne, C.E.; Sohal, B. Do We Need to Study Metabolism and Distribution in the Eye: Why, When, and Are We There Yet? J. Pharm. Sci. 2017, 106, 2276–2281. [Google Scholar] [CrossRef]
- Huang, S.; Xu, Y.; Luo, Y.; Wang, Z.; Li, F.; Qin, Z.; Ban, J. Enhanced Ocular Bioavailability and Prolonged Duration via Hydrophilic Surface Nanocomposite Vesicles for Topical Drug Administration. Pharmaceutics 2024, 16, 1496. [Google Scholar] [CrossRef] [PubMed]
- Pączek, S.; Zajkowska, M.; Mroczko, B. Pigment Epithelial-Derived Factor in Pancreatic and Liver Cancers—From Inflammation to Cancer. Biomedicines 2024, 12, 2260. [Google Scholar] [CrossRef]
- Mulfaul, K.; Mullin, N.K.; Giacalone, J.C.; Voigt, A.P.; DeVore, M.R.; Stone, E.M.; Tucker, B.A.; Mullins, R.F. Local factor H production by human choroidal endothelial cells mitigates complement deposition: Implications for macular degeneration. J. Pathol. 2022, 257, 29–38. [Google Scholar] [CrossRef]
- Li, J.H.; Hepworth, M.R.; O’Sullivan, T.E. Regulation of systemic metabolism by tissue-resident immune cell circuits. Immunity 2023, 56, 1168–1186. [Google Scholar] [CrossRef]
- Dai, S.; Liu, F.; Qin, Z.; Zhang, J.; Chen, J.; Ding, W.X.; Feng, D.; Ji, Y.; Qin, X. Kupffer cells promote T-cell hepatitis by producing CXCL10 and limiting liver sinusoidal endothelial cell permeability. Theranostics 2020, 10, 7163–7177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lu, S.; Li, Z. Extrahepatic factors in hepatic immune regulation. Front. Immunol. 2022, 13, 941721. [Google Scholar] [CrossRef]
- Orfanidou, M.; Polyzos, S.A. Retinopathy in Metabolic Dysfunction-Associated Steatotic Liver Disease. Medicina 2025, 61, 38. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Desai, D.T.; Kalaiselvan, P.; Dumpati, S.; Kuppusamy, R.; Masoudi, S.; Shah, D.O.; Willcox, M.D.P. Lipid-based eye drop formulations for the management of evaporative dry eyes. Cont. Lens Anterior Eye 2024, 47, 102154. [Google Scholar] [CrossRef]
- Yan, W.; Zhang, K.; Guo, J.; Xu, L. Bile acid-mediated gut-liver axis crosstalk: The role of nuclear receptor signaling in dynamic regulation of inflammatory networks. Front. Immunol. 2025, 16, 1595486. [Google Scholar] [CrossRef]
- Utakata, Y.; Miwa, T.; Hanai, T.; Aiba, M.; Unome, S.; Imai, K.; Shirakami, Y.; Takai, K.; Shimizu, M. Usefulness of Retinol-Binding Protein in Predicting Mortality in Patients with Chronic Liver Disease. JGH Open 2025, 9, e70087. [Google Scholar] [CrossRef]
- Harrell, C.R.; Feulner, L.; Djonov, V.; Pavlovic, D.; Volarevic, V. The Molecular Mechanisms Responsible for Tear Hyperosmolarity-Induced Pathological Changes in the Eyes of Dry Eye Disease Patients. Cells 2023, 12, 2755. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.R.; Volarevic, V. Ion Channels as Potential Drug Targets in Dry Eye Disease and Their Clinical Relevance: A Review. Cells 2024, 13, 2017. [Google Scholar] [CrossRef]
- Terhaar, H.M.; Allbaugh, R.A.; Mochel, J.P.; Sebbag, L. Serum albumin and total protein concentration in the tear film of horses with healthy or diseased eyes. Vet. Ophthalmol. 2021, 24, 20–27. [Google Scholar] [CrossRef]
- Wang, G.; Tang, C.; Wang, R.; Xu, C.; Shen, T.; Li, G. Inflammatory markers CRP and WBC as predictors of liver function impairment in multiple injury trauma patients: A repeated measures analysis. Front. Med. 2025, 12, 1570474. [Google Scholar] [CrossRef] [PubMed]
- Garhofer, G.; Dos Santos, V.A.; Stegmann, H.; Schmidl, D.; Adzhemian, N.; Werkmeister, R.M.; Schmetterer, L. The Association between Tear Film Thickness as Measured with OCT and Symptoms and Signs of Dry Eye Disease: A Pooled Analysis of 6 Clinical Trials. J. Clin. Med. 2020, 9, 3791. [Google Scholar] [CrossRef]
- Yoon, D.Y.; Hee Eun, J.; Hyon, J.Y. Correlation between tear film lipid layer thickness and transepidermal water loss from the ocular area in patients with dry eye disease and in healthy controls. PLoS ONE 2022, 17, e0270810. [Google Scholar] [CrossRef]
- Stinnett, G.S.; Kuo, C.H.; Ono, S.J. Impact of inflammasomes on the ocular surface. Curr. Opin. Allergy Clin. Immunol. 2024, 24, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Hong, S.; Jun, D.W.; Yoon, J.H.; Lee, K.N.; Lee, H.L.; Lee, O.Y.; Yoon, B.C.; Choi, H.S. Prevalence and clinical characteristics of antibiotics associated drug induced liver injury. Ann. Transl. Med. 2021, 9, 642. [Google Scholar] [CrossRef]
- Wu, M.; Jia, B.B.; Li, M.F. Complement C3 and Activated Fragment C3a Are Involved in Complement Activation and Anti-Bacterial Immunity. Front. Immunol. 2022, 13, 813173. [Google Scholar] [CrossRef]
- Asao, K.; Hashida, N. Overview of Microorganisms: Bacterial Microbiome, Mycobiome, Virome Identified Using Next-Generation Sequencing, and Their Application to Ophthalmic Diseases. Microorganisms 2025, 13, 1300. [Google Scholar] [CrossRef] [PubMed]
- Shivaji, S.; Jayasudha, R.; Chakravarthy, S.K.; SaiAbhilash, C.R.; Sai Prashanthi, G.; Sharma, S.; Garg, P.; Murthy, S.I. Alterations in the conjunctival surface bacterial microbiome in bacterial keratitis patients. Exp. Eye Res. 2021, 203, 108418. [Google Scholar] [CrossRef] [PubMed]
- Varela-Fernandez, R.; Diaz-Tome, V.; Luaces-Rodriguez, A.; Conde-Penedo, A.; Garcia-Otero, X.; Luzardo-Alvarez, A.; Fernandez-Ferreiro, A.; Otero-Espinar, F.J. Drug Delivery to the Posterior Segment of the Eye: Biopharmaceutic and Pharmacokinetic Considerations. Pharmaceutics 2020, 12, 269. [Google Scholar] [CrossRef]
- Hemmati, M.A.; Monemi, M.; Asli, S.; Mohammadi, S.; Foroozanmehr, B.; Haghmorad, D.; Oksenych, V.; Eslami, M. Using New Technologies to Analyze Gut Microbiota and Predict Cancer Risk. Cells 2024, 13, 1987. [Google Scholar] [CrossRef]
- Oliveira, M.; Marszalek, K.; Kowalski, M.; Frolova, A.; Labaj, P.P.; Branicki, W.; Madureira-Carvalho, A.; da Silva, D.D.; Dinis-Oliveira, R.J. Sequencing Technologies in Forensic Microbiology: Current Trends and Advancements. Forensic Sci. 2024, 4, 523–545. [Google Scholar] [CrossRef]
- D’Urso, F.; Broccolo, F. Applications of Artificial Intelligence in Microbiome Analysis and Probiotic Interventions—An Overview and Perspective Based on the Current State of the Art. Appl. Sci. 2024, 14, 8627. [Google Scholar] [CrossRef]
- Seok, H.; Park, D.W. Role of biomarkers in antimicrobial stewardship: Physicians’ perspectives. Korean J. Intern. Med. 2024, 39, 413–429. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Tian, L.; Gu, X.; Chen, Y.; Ma, X.; Lin, S.; Li, Z.; Lou, Y.; Zheng, M. Characterization of the Ocular Surface Microbiome in Keratitis Patients after Repeated Ophthalmic Antibiotic Exposure. Microbiol. Spectr. 2022, 10, e0216221. [Google Scholar] [CrossRef]
- Julian, T.H.; Fitzgerald, T.; Eye, U.K.B.; Vision, C.; Birney, E.; Sergouniotis, P.I. Pigmentation and Retinal Pigment Epithelium Thickness: A Study of the Phenotypic and Genotypic Relationships Between Ocular and Extraocular Pigmented Tissues. Pigment. Cell Melanoma Res. 2025, 38, e70038. [Google Scholar] [CrossRef]
- Fong, P.Y.; Shih, K.C.; Lam, P.Y.; Chan, T.C.Y.; Jhanji, V.; Tong, L. Role of tear film biomarkers in the diagnosis and management of dry eye disease. Taiwan. J. Ophthalmol. 2019, 9, 150–159. [Google Scholar] [CrossRef]
- Mondal, H.; Kim, H.J.; Mohanto, N.; Jee, J.P. A Review on Dry Eye Disease Treatment: Recent Progress, Diagnostics, and Future Perspectives. Pharmaceutics 2023, 15, 990. [Google Scholar] [CrossRef]
- Ferraz, M.P. Antimicrobial Resistance: The Impact from and on Society According to One Health Approach. Societies 2024, 14, 187. [Google Scholar] [CrossRef]
- Sanz-Codina, M.; Nowak, H.; Zeitlinger, M. Host Biomarkers and Antibiotic Tissue Penetration in Sepsis: Insights from Moxifloxacin. Eur. J. Drug Metab. Pharmacokinet. 2025, 50, 289–294. [Google Scholar] [CrossRef]
- Klein, E.Y.; Impalli, I.; Poleon, S.; Denoel, P.; Cipriano, M.; Van Boeckel, T.P.; Pecetta, S.; Bloom, D.E.; Nandi, A. Global trends in antibiotic consumption during 2016–2023 and future projections through 2030. Proc. Natl. Acad. Sci. USA 2024, 121, e2411919121. [Google Scholar] [CrossRef]
- Piddock, L.J.; Alimi, Y.; Anderson, J.; de Felice, D.; Moore, C.E.; Røttingen, J.-A.; Skinner, H.; Beyer, P. Advancing global antibiotic research, development and access. Nat. Med. 2024, 30, 2432–2443. [Google Scholar] [CrossRef]
- Karnwal, A.; Jassim, A.Y.; Mohammed, A.A.; Al-Tawaha, A.R.M.S.; Selvaraj, M.; Malik, T. Addressing the global challenge of bacterial drug resistance: Insights, strategies, and future directions. Front. Microbiol. 2025, 16, 1517772. [Google Scholar] [CrossRef]
- Polianciuc, S.I.; Gurzau, A.E.; Kiss, B.; Stefan, M.G.; Loghin, F. Antibiotics in the environment: Causes and consequences. Med. Pharm. Rep. 2020, 93, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Vora, L.K.; Gholap, A.D.; Jetha, K.; Thakur, R.R.S.; Solanki, H.K.; Chavda, V.P. Artificial Intelligence in Pharmaceutical Technology and Drug Delivery Design. Pharmaceutics 2023, 15, 1916. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, C.; Khosya, R.; Thakur, K.; Mahajan, D.; Kumar, R.; Kumar, S.; Sharma, A.K. A systematic review of pesticide exposure, associated risks, and long-term human health impacts. Toxicol. Rep. 2024, 13, 101840. [Google Scholar] [CrossRef]
- Zhao, M.; Ma, J.; Li, M.; Zhang, Y.; Jiang, B.; Zhao, X.; Huai, C.; Shen, L.; Zhang, N.; He, L.; et al. Cytochrome P450 Enzymes and Drug Metabolism in Humans. Int. J. Mol. Sci. 2021, 22, 12808. [Google Scholar] [CrossRef]
- Le, J. Drug Metabolism. Available online: https://www.msdmanuals.com/home/drugs/administration-and-kinetics-of-drugs/drug-metabolism (accessed on 22 October 2025).
- Larsson, D.G.J.; Flach, C.F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef]
- La Rosa, M.C.; Maugeri, A.; Favara, G.; La Mastra, C.; Magnano San Lio, R.; Barchitta, M.; Agodi, A. The Impact of Wastewater on Antimicrobial Resistance: A Scoping Review of Transmission Pathways and Contributing Factors. Antibiotics 2025, 14, 131. [Google Scholar] [CrossRef]
- Rahman, L.; Hafejee, A.; Anantharanjit, R.; Wei, W.; Cordeiro, M.F. Accelerating precision ophthalmology: Recent advances. Expert. Rev. Precis. Med. Drug Dev. 2022, 7, 150–161. [Google Scholar] [CrossRef]
- Zhao, Q.; Wei, L.; Chen, Y. From bench to bedside: Developing CRISPR/Cas-based therapy for ocular diseases. Pharmacol. Res. 2025, 213, 107638. [Google Scholar] [CrossRef]
- Dubey, A.K.; Mostafavi, E. Biomaterials-mediated CRISPR/Cas9 delivery: Recent challenges and opportunities in gene therapy. Front. Chem. 2023, 11, 1259435. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.S.; Wong, C.T.H.; Aung, T.T.; Lakshminarayanan, R.; Mehta, J.S.; Rauz, S.; McNally, A.; Kintses, B.; Peacock, S.J.; de la Fuente-Nunez, C.; et al. Antimicrobial resistance: A concise update. Lancet Microbe 2025, 6, 100947. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.; Dehari, D.; Chaudhuri, A.; Kumar, D.N.; Kumar, D.; Singh, S.; Nath, G.; Agrawal, A.K. Recent advancements in nanotechnology-based bacteriophage delivery strategies against bacterial ocular infections. Microbiol. Res. 2023, 273, 127413. [Google Scholar] [CrossRef]
- Sneller, L.; Lin, C.; Price, A.; Kottilil, S.; Chua, J.V. RNA Interference Therapeutics for Chronic Hepatitis B: Progress, Challenges, and Future Prospects. Microorganisms 2024, 12, 599. [Google Scholar] [CrossRef]
- Yuan, T.H.; Yue, Z.S.; Zhang, G.H.; Wang, L.; Dou, G.R. Beyond the Liver: Liver-Eye Communication in Clinical and Experimental Aspects. Front. Mol. Biosci. 2021, 8, 823277. [Google Scholar] [CrossRef]
- Klodnicka, K.; Januszewski, J.; Tyc, H.; Michalska, A.; Forma, A.; Teresinska, B.; Rejdak, R.; Baj, J.; Dolar-Szczasny, J. From Pathophysiology to Innovative Therapies in Eye Diseases: A Brief Overview. Int. J. Mol. Sci. 2025, 26, 8496. [Google Scholar] [CrossRef]
- Altman, J.; Jones, G.; Ahmed, S.; Sharma, S.; Sharma, A. Tear Film MicroRNAs as Potential Biomarkers: A Review. Int. J. Mol. Sci. 2023, 24, 3694. [Google Scholar] [CrossRef]
- Magrelli, A.; O’Connor, D.J.; Stoyanova-Beninska, V. Editorial: The changing focus of regulatory frameworks around the globe and the opportunities for harmonization. Front. Med. 2025, 12, 1645278. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, B.; Li, X.; Li, M.; Wang, Y.; Dan, H.; Zhou, J.; Wei, Y.; Ge, K.; Li, P.; et al. The application and progression of CRISPR/Cas9 technology in ophthalmological diseases. Eye 2023, 37, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Tao, Y.; Liu, Y.; Li, J. Advances in delivery systems for CRISPR/Cas-mediated cancer treatment: A focus on viral vectors and extracellular vesicles. Front. Immunol. 2024, 15, 1444437. [Google Scholar] [CrossRef]
- Ana, R.D.; Fonseca, J.; Karczewski, J.; Silva, A.M.; Zielinska, A.; Souto, E.B. Lipid-Based Nanoparticulate Systems for the Ocular Delivery of Bioactives with Anti-Inflammatory Properties. Int. J. Mol. Sci. 2022, 23, 12102. [Google Scholar] [CrossRef]
- Habeebuddin, M.; Karnati, R.K.; Shiroorkar, P.N.; Nagaraja, S.; Asdaq, S.M.B.; Khalid Anwer, M.; Fattepur, S. Topical Probiotics: More Than a Skin Deep. Pharmaceutics 2022, 14, 557. [Google Scholar] [CrossRef] [PubMed]
- Hui, R.W.; Mak, L.Y.; Seto, W.K.; Yuen, M.F. Investigational RNA Interference Agents for Hepatitis B. BioDrugs 2025, 39, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Fontanellas, A.; Berraondo, P.; Urigo, F.; Jerico, D.; Martini, P.G.V.; Pastor, F.; Avila, M.A. RNA-based therapies in liver metabolic diseases. Gut 2025, 74, 1514–1527. [Google Scholar] [CrossRef] [PubMed]
- Traber, G.M.; Yu, A.M. RNAi-Based Therapeutics and Novel RNA Bioengineering Technologies. J. Pharmacol. Exp. Ther. 2023, 384, 133–154. [Google Scholar] [CrossRef]
- Hassan, M.S.A.-R.; Zhong, C.; Hassan, F.; Li, S.K. Targeting the Eye: RNA-Based Therapies, Interferences, and Delivery Strategies. Pharmaceutics 2025, 17, 1326. [Google Scholar] [CrossRef]
- Dziegielewska, M.; Tomczyk, M.; Wiater, A.; Woyton, A.; Junka, A. Targeting Ocular Biofilms with Plant-Derived Antimicrobials in the Era of Antibiotic Resistance. Molecules 2025, 30, 2863. [Google Scholar] [CrossRef] [PubMed]
- Parvin, N.; Joo, S.W.; Jung, J.H.; Mandal, T.K. Multimodal AI in Biomedicine: Pioneering the Future of Biomaterials, Diagnostics, and Personalized Healthcare. Nanomaterials 2025, 15, 895. [Google Scholar] [CrossRef] [PubMed]
- Puig-Castellví, F.; Pacheco-Tapia, R.; Deslande, M.; Jia, M.; Andrikopoulos, P.; Chechi, K.; Bonnefond, A.; Froguel, P.; Dumas, M.-E. Advances in the integration of metabolomics and metagenomics for human gut microbiome and their clinical applications. TrAC Trends Anal. Chem. 2023, 167, 117248. [Google Scholar] [CrossRef]
- Singh, A.V.; Chandrasekar, V.; Paudel, N.; Laux, P.; Luch, A.; Gemmati, D.; Tisato, V.; Prabhu, K.S.; Uddin, S.; Dakua, S.P. Integrative toxicogenomics: Advancing precision medicine and toxicology through artificial intelligence and OMICs technology. Biomed. Pharmacother. 2023, 163, 114784. [Google Scholar] [CrossRef]
| Parameter | Correlation Coefficient with Tear-Film Thickness | Correlation Coefficient with Retinal Pigment Epithelium (RPE) Thickness | Unit |
|---|---|---|---|
| ALT | 0.96 | 0.99 | U/L |
| AST | 0.96 | 0.99 | U/L |
| GGT | 0.96 | 0.99 | U/L |
| ALP | 0.96 | 0.99 | U/L |
| TBIL | 0.96 | 0.99 | mg/dL |
| TP | −0.94 | −0.97 | g/L |
| ALB | −0.94 | −0.97 | g/L |
| Complement C3 | 0.95 | 0.98 | g/L |
| CRP | 0.95 | 0.98 | mg/L |
| ISOSRPE thickness | −0.93 | −0.95 | µm |
| RPE thickness | 0.98 | µm | |
| Tear-film thickness | 0.981 | µm |
| Contents/Data Type | Why this Column Matters | Example Entry | |
|---|---|---|---|
| Application | Short name of the forensic/clinical use (text). | Quickly orients reader to the use case. | Detection of trace antibiotics. |
| Rationale | One-sentence biological or analytical justification (text). | Links application to mechanism or evidence. | Tear-film retains drugs when blood is degraded. |
| Sample/Matrix | Which specimen to collect (controlled vocabulary). | Guides sampling choice and downstream methods. | Tear film; conjunctival swab; ocular tissue. |
| Analytical method | Primary assay(s) and platform (text). | Specifies how the marker will be measured. | LC-MS/MS; 16S rRNA sequencing; OCT. |
| Typical detection window/stability | Timeframe and conditions (text + units). | Sets realistic expectations for when marker is informative. | 0–7 days post-exposure; stable at −20 °C. |
| Sensitivity/LOD | Typical limit of detection or sensitivity range (numeric or “unknown”). | Technical feasibility and comparability across labs. | ng/mL range (0.5–5 ng/mL). |
| Evidence level | Tiered scale (Preclinical/Observational/Validated/Speculative). | Conveys maturity and confidence for application. | Observational human data. |
| Sampling notes | Practical collection volume, technique, preservative (text). | Reduces preanalytical variability and contamination. | Capillary tear collection 5–10 µL; freeze in methanol. |
| Main limitations/caveats | Key confounders, stability issues, forensic/legal limits (text). | Prevents overinterpretation and informs study design. | Environmental contamination; postmortem degradation. |
| Forensic validity/PMI use | Yes/No/Partial + applicable PMI range (text). | Directly informs forensic utility and limitations. | Partial—informative 0–72 h under cool conditions. |
| Suggested QC/controls | Recommended quality controls or reference standards (text). | Ensures analytical reliability and reproducibility. | Spiked tear standards; blank swab controls. |
| References | Key supporting citations (numbered). | Directs the reader to evidence and methods. | [11,22,83] |
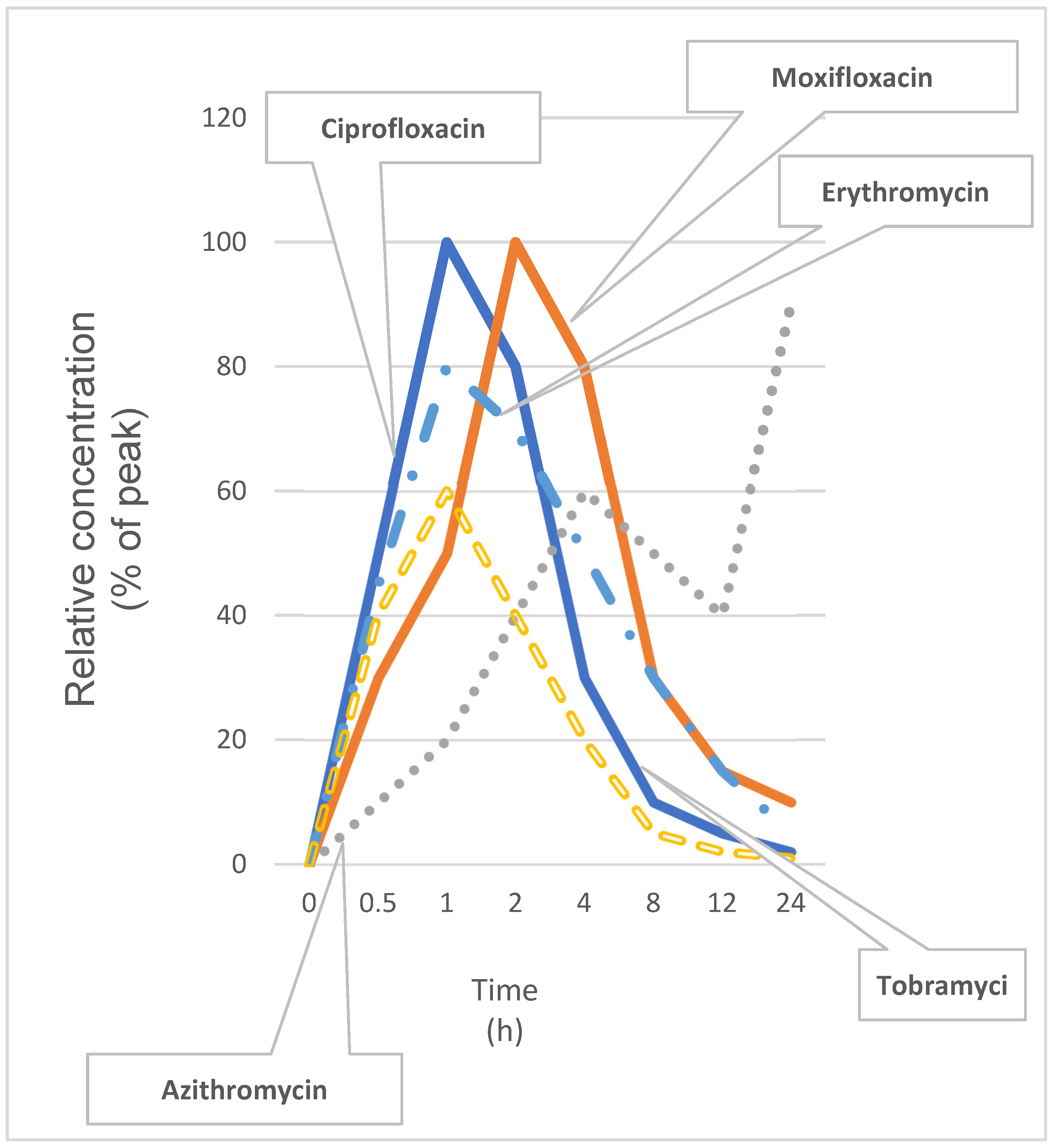
| Antibiotic | Ocular Surface Kinetics | Role in Liver–Eye Axis |
|---|---|---|
| Ciprofloxacin | Peak tear concentration within 1 h; half-life ~1.5 h. | Inhibits CYP1A2 in hepatocytes; shifts hepatic detox pathways and modulates ocular inflammatory tone. |
| Moxifloxacin | Peak in tears at ~2 h; half-life ~2.5 h; high corneal penetration. | Alters hepatic oxidative stress pathways; influences retinal immune cell activation. |
| Azithromycin | Prolonged tear-film residency (~24 h); strong tissue binding. | Activates Kupffer cells indirectly; reduces systemic IL-6 levels and reshapes ocular immune responses. |
| Tobramycin | Rapid absorption; half-life ~1.8 h; minimal systemic uptake. | Limited hepatic metabolism; low direct liver–eye signaling but may drive selection of resistant flora. |
| Erythromycin | Peak in ~1.5 h; half-life ~2 h; accumulates in conjunctiva. | Inhibits bile acid transporters; can provoke mild cholestasis and exacerbate ocular surface inflammation. |
| Antibiotic | Tmax (h) | t½ (h) | Key Liver–Eye Axis Role |
|---|---|---|---|
| Ciprofloxacin | 1 | 1.5 | Inhibits CYP1A2; modulates ocular inflammatory tone |
| Moxifloxacin | 2 | 2.5 | Alters hepatic oxidative stress; influences retinal immune activation |
| Azithromycin | 24 | 24 | Activates Kupffer cells; reduces systemic IL-6 |
| Tobramycin | 1 | 1.8 | Minimal hepatic metabolism; may drive selection of resistant flora |
| Erythromycin | 1.5 | 2 | Inhibits bile acid transporters; can exacerbate ocular inflammation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šoša, I. From Tears to Toxins: Mapping Antibiotic Passage Through the Eye–Liver Axis. Antibiotics 2025, 14, 1069. https://doi.org/10.3390/antibiotics14111069
Šoša I. From Tears to Toxins: Mapping Antibiotic Passage Through the Eye–Liver Axis. Antibiotics. 2025; 14(11):1069. https://doi.org/10.3390/antibiotics14111069
Chicago/Turabian StyleŠoša, Ivan. 2025. "From Tears to Toxins: Mapping Antibiotic Passage Through the Eye–Liver Axis" Antibiotics 14, no. 11: 1069. https://doi.org/10.3390/antibiotics14111069
APA StyleŠoša, I. (2025). From Tears to Toxins: Mapping Antibiotic Passage Through the Eye–Liver Axis. Antibiotics, 14(11), 1069. https://doi.org/10.3390/antibiotics14111069