Circulation of a Unique Klebsiella pneumoniae Clone, ST147 NDM-1/OXA-48, in Two Diverse Hospitals in Calabria (Italy)
Abstract
:1. Introduction
2. Results
2.1. Sample Characteristics
2.2. Resistome Analysis
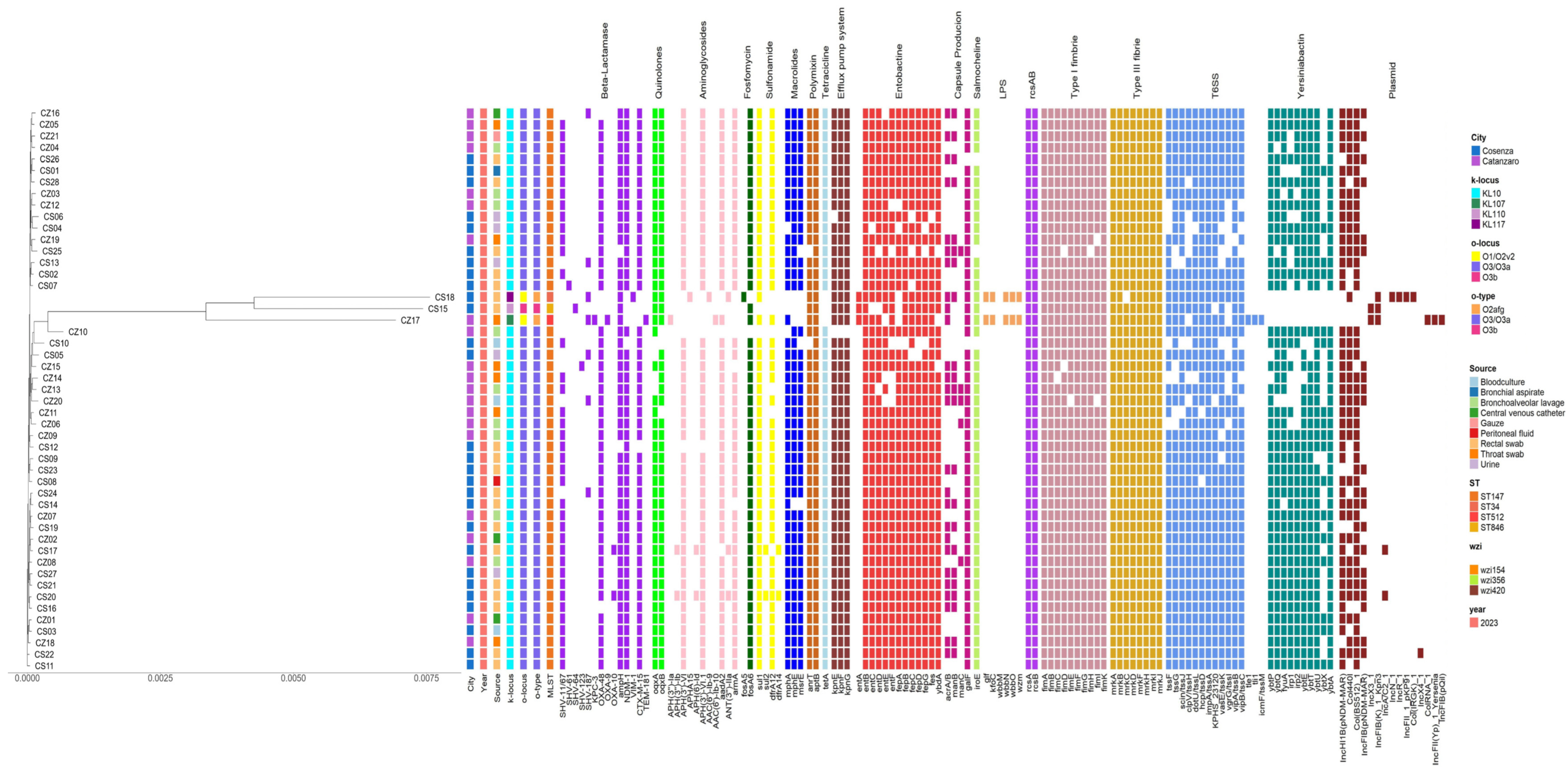
2.3. Virulome Analysis
2.4. Plasmid Analysis
2.5. Phylogenetic Tree
3. Discussion
3.1. Resistome
3.2. Virulome
3.3. Plasmids
3.4. Final Considerations
4. Materials and Methods
4.1. Sample Description and Isolation
4.2. Antibiotic Susceptibility Test (AST)
4.3. DNA Extraction
4.4. Sequencing
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, W.R.; Arias, C.A. ESKAPE pathogens: Antimicrobial resistance, epidemiology, clinical impact and therapeutics. Nat. Rev. Microbiol. 2024, 22, 598–616. [Google Scholar] [CrossRef]
- Wyres, K.L.; Holt, K.E. Klebsiella pneumoniae as a key trafficker of drug resistance genes from environmental to clinically important bacteria. Curr. Opin. Microbiol. 2018, 45, 131–139. [Google Scholar] [CrossRef]
- Arcari, G.; Cecilia, F.; Oliva, A.; Polani, R.; Raponi, G.; Sacco, F.; De Francesco, A.; Pugliese, F.; Carattoli, A. Genotypic Evolution of Klebsiella pneumoniae Sequence Type 512 during Ceftazidime/Avibactam, Meropenem/Vaborbactam, and Cefiderocol Treatment, Italy. Emerg. Infect. Dis. 2023, 29, 2266–2274. [Google Scholar] [CrossRef] [PubMed]
- Navon-Venezia, S.; Kondratyeva, K.; Carattoli, A. Klebsiella pneumoniae: A major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol. Rev. 2017, 41, 252–275. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, H.; Glasner, C.; Albiger, B.; Aanensen, D.M.; Tomlinson, C.T.; Andrasević, A.T.; Cantón, R.; Carmeli, Y.; Friedrich, A.W.; Giske, C.G.; et al. Occurrence of carbapenemase-producing Klebsiella pneumoniae and Escherichia coli in the European survey of carbapenemase-producing Enterobacteriaceae (EuSCAPE): A prospective, multinational study. Lancet Infect. Dis. 2017, 17, 153–163. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control (EU Body or Agency); World Health Organization. Antimicrobial Resistance Surveillance in Europe 2022: 2020 Data; Publications Office of the European Union: Luxembourg, 2022; ISBN 978-92-9498-552-1. Available online: https://data.europa.eu/doi/10.2900/112339 (accessed on 20 January 2025).
- Chudejova, K.; Kraftova, L.; Mattioni Marchetti, V.; Hrabak, J.; Papagiannitsis, C.C.; Bitar, I. Genetic Plurality of OXA/NDM-Encoding Features Characterized From Enterobacterales Recovered From Czech Hospitals. Front. Microbiol. 2021, 12, 641415. [Google Scholar] [CrossRef]
- Li, Y.; Kumar, S.; Zhang, L.; Wu, H.; Wu, H. Characteristics of antibiotic resistance mechanisms and genes of Klebsiella pneumoniae. Open Med. 2023, 18, 20230707. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Desai, S.; Passet, V.; Gajjar, D.; Brisse, S. Genomic evolution of the globally disseminated multidrug-resistant Klebsiella pneumoniae clonal group 147. Microb. Genom. 2022, 8, 000737. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z. Complex heatmap visualization. Imeta 2022, 1, e43. [Google Scholar] [CrossRef]
- Falcone, M.; Tiseo, G.; Galfo, V.; Giordano, C.; Leonildi, A.; Marciano, E.; De Simone, P.; Biancofiore, G.; Boggi, U.; Barnini, S.; et al. Bloodstream infections in patients with rectal colonization by Klebsiella pneumoniae producing different type of carbapenemases: A prospective, cohort study (CHIMERA study). Clin. Microbiol. Infect. 2022, 28, 298.e1–298.e7. [Google Scholar] [CrossRef]
- Fasciana, T.; Antonelli, A.; Bianco, G.; Lombardo, D.; Codda, G.; Roscetto, E.; Perez, M.; Lipari, D.; Arrigo, I.; Galia, E.; et al. Multicenter study on the prevalence of colonization due to carbapenem-resistant Enterobacterales strains before and during the first year of COVID-19, Italy 2018–2020. Front. Public Health 2023, 11, 1270924. [Google Scholar] [CrossRef] [PubMed]
- Pishtiwan, A.H.; Khadija, K.M. Prevalence of blaTEM, blaSHV, and blaCTX-M Genes among ESBL-Producing Klebsiella pneumoniae and Escherichia coli Isolated from Thalassemia Patients in Erbil, Iraq. Mediterr. J. Hematol. Infect. Dis. 2019, 11, e2019041. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Morgan, C.E.; Bonomo, R.A.; Yu, E.W. Cryo-EM Structures of the Klebsiella pneumoniae AcrB Multidrug Efflux Pump. mBio 2023, 14, e0065923. [Google Scholar] [CrossRef]
- Vinogradov, E.; Perry, M.B. Structural analysis of the core region of the lipopolysaccharides from eight serotypes of Klebsiella pneumoniae. Carbohydr. Res. 2001, 335, 291–296. [Google Scholar] [CrossRef]
- Schroll, C.; Barken, K.B.; Krogfelt, K.A.; Struve, C. Role of type 1 and type 3 fimbriae in Klebsiella pneumoniae biofilm formation. BMC Microbiol. 2010, 10, 179. [Google Scholar] [CrossRef]
- Talat, A.; Khan, F.; Khan, A.U. Genome analyses of colistin-resistant high-risk blaNDM-5 producing Klebsiella pneumoniae ST147 and Pseudomonas aeruginosa ST235 and ST357 in clinical settings. BMC Microbiol. 2024, 24, 174. [Google Scholar] [CrossRef]
- Ragupathi, N.K.D.; Bakthavatchalam, Y.D.; Mathur, P.; Pragasam, A.K.; Walia, K.; Ohri, V.C.; Veeraraghavan, B. Plasmid profiles among some ESKAPE pathogens in a tertiary care centre in south India. Indian J. Med. Res. 2019, 149, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Lorenzin, G.; Gona, F.; Battaglia, S.; Spitaleri, A.; Saluzzo, F.; Trovato, A.; di Marco, F.; Cichero, P.; Biancardi, A.; Nizzero, P.; et al. Detection of NDM-1/5 and OXA-48 co-producing extensively drug-resistant hypervirulent Klebsiella pneumoniae in Northern Italy. J. Glob. Antimicrob. Resist. 2022, 28, 146–150. [Google Scholar] [CrossRef]
- Villa, L.; Poirel, L.; Nordmann, P.; Carta, C.; Carattoli, A. Complete sequencing of an IncH plasmid carrying the blaNDM-1, blaCTX-M-15 and qnrB1 genes. J. Antimicrob. Chemother. 2012, 67, 1645–1650. [Google Scholar] [CrossRef] [PubMed]
- Mataseje, L.F.; Chen, L.; Peirano, G.; Fakharuddin, K.; Kreiswith, B.; Mulvey, M.; Pitout, J.D.D. Klebsiella pneumoniae ST147: And then there were three carbapenemases. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 1467–1472. [Google Scholar] [CrossRef] [PubMed]
- Ofosu-Appiah, F.; Acquah, E.E.; Mohammed, J.; Sakyi Addo, C.; Agbodzi, B.; Ofosu, D.A.S.; Myers, C.J.; Mohktar, Q.; Ampomah, O.-W.; Ablordey, A.; et al. Klebsiella pneumoniae ST147 harboring blaNDM-1, multidrug resistance and hypervirulence plasmids. Microbiol. Spectr. 2024, 12, e03017-23. [Google Scholar] [CrossRef] [PubMed]
- EUCAST. Clinical Breakpoints and Dosing of Antibiotics. Available online: https://www.eucast.org/clinical_breakpoints (accessed on 6 December 2024).
- Illumina. Local Run Manager Generate FASTQ Analysis Module Workflow Guide (Document No. 1000000003344); Illumina: San Diego, CA, USA, 2021. [Google Scholar]
- Bongiorno, D.; Bivona, D.A.; Cicino, C.; Trecarichi, E.M.; Russo, A.; Marascio, N.; Mezzatesta, M.L.; Musso, N.; Privitera, G.F.; Quirino, A.; et al. Omic insights into various ceftazidime-avibactam-resistant Klebsiella pneumoniae isolates from two southern Italian regions. Front. Cell Infect. Microbiol. 2022, 12, 1010979. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Krueger, F. FelixKrueger/TrimGalore. (5 Dicembre 2024) Perl. [Online]. Available online: https://github.com/FelixKrueger/TrimGalore (accessed on 6 December 2024).
- Lam, M.M.C.; Wick, R.R.; Watts, S.C.; Cerdeira, L.T.; Wyres, K.L.; Holt, K.E. A genomic surveillance framework and genotyping tool for Klebsiella pneumoniae and its related species complex. Nat. Commun. 2021, 12, 4188. [Google Scholar] [CrossRef]
- Wyres, K.L.; Wick, R.R.; Gorrie, C.; Jenney, A.; Follador, R.; Thomson, N.R.; Holt, K.E. Identification of Klebsiella capsule synthesis loci from whole genome data. Microb. Genom. 2016, 2, e000102. [Google Scholar] [CrossRef] [PubMed]
- Petit, R.A.; Read, T.D. Bactopia: A Flexible Pipeline for Complete Analysis of Bacterial Genomes. mSystems 2020, 5, e00190-20. [Google Scholar] [CrossRef]
- Carattoli, A.; Zankari, E.; García-Fernández, A.; Voldby Larsen, M.; Lund, O.; Villa, L.; Møller Aarestrup, F.; Hasman, H. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef] [PubMed]
- Yu, G. Data Integration, Manipulation and Visualization of Phylogenetic Trees; Chapman and Hall: Boca Raton, FL, USA; CRC: New York, NY, USA, 2022; ISBN 978-1-00-327924-2. [Google Scholar] [CrossRef]
- Xu, S.; Li, L.; Luo, X.; Chen, M.; Tang, W.; Zhan, L.; Dai, Z.; Lam, T.T.; Guan, Y.; Yu, G. Ggtree: A serialized data object for visualization of a phylogenetic tree and annotation data. iMeta 2022, 1, e56. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicitra, E.; Terrana, M.; Bongiorno, D.; Dodaro, S.; Greco, F.; Greco, S.; Marascio, N.; Mauro, M.V.; Pantanella, M.; Privitera, G.F.; et al. Circulation of a Unique Klebsiella pneumoniae Clone, ST147 NDM-1/OXA-48, in Two Diverse Hospitals in Calabria (Italy). Antibiotics 2025, 14, 128. https://doi.org/10.3390/antibiotics14020128
Nicitra E, Terrana M, Bongiorno D, Dodaro S, Greco F, Greco S, Marascio N, Mauro MV, Pantanella M, Privitera GF, et al. Circulation of a Unique Klebsiella pneumoniae Clone, ST147 NDM-1/OXA-48, in Two Diverse Hospitals in Calabria (Italy). Antibiotics. 2025; 14(2):128. https://doi.org/10.3390/antibiotics14020128
Chicago/Turabian StyleNicitra, Emanuele, Morena Terrana, Dafne Bongiorno, Saveria Dodaro, Francesca Greco, Sonia Greco, Nadia Marascio, Maria Vittoria Mauro, Marta Pantanella, Grete Francesca Privitera, and et al. 2025. "Circulation of a Unique Klebsiella pneumoniae Clone, ST147 NDM-1/OXA-48, in Two Diverse Hospitals in Calabria (Italy)" Antibiotics 14, no. 2: 128. https://doi.org/10.3390/antibiotics14020128
APA StyleNicitra, E., Terrana, M., Bongiorno, D., Dodaro, S., Greco, F., Greco, S., Marascio, N., Mauro, M. V., Pantanella, M., Privitera, G. F., Quirino, A., Serapide, F., Trecarichi, E. M., Vangeli, V., Mastroianni, A., Matera, G., Russo, A., & Stefani, S. (2025). Circulation of a Unique Klebsiella pneumoniae Clone, ST147 NDM-1/OXA-48, in Two Diverse Hospitals in Calabria (Italy). Antibiotics, 14(2), 128. https://doi.org/10.3390/antibiotics14020128









