Antimicrobial Susceptibility of Toxin-Producing Corynebacterium diphtheriae and C. ulcerans in Belgium
Abstract
1. Introduction
2. Results
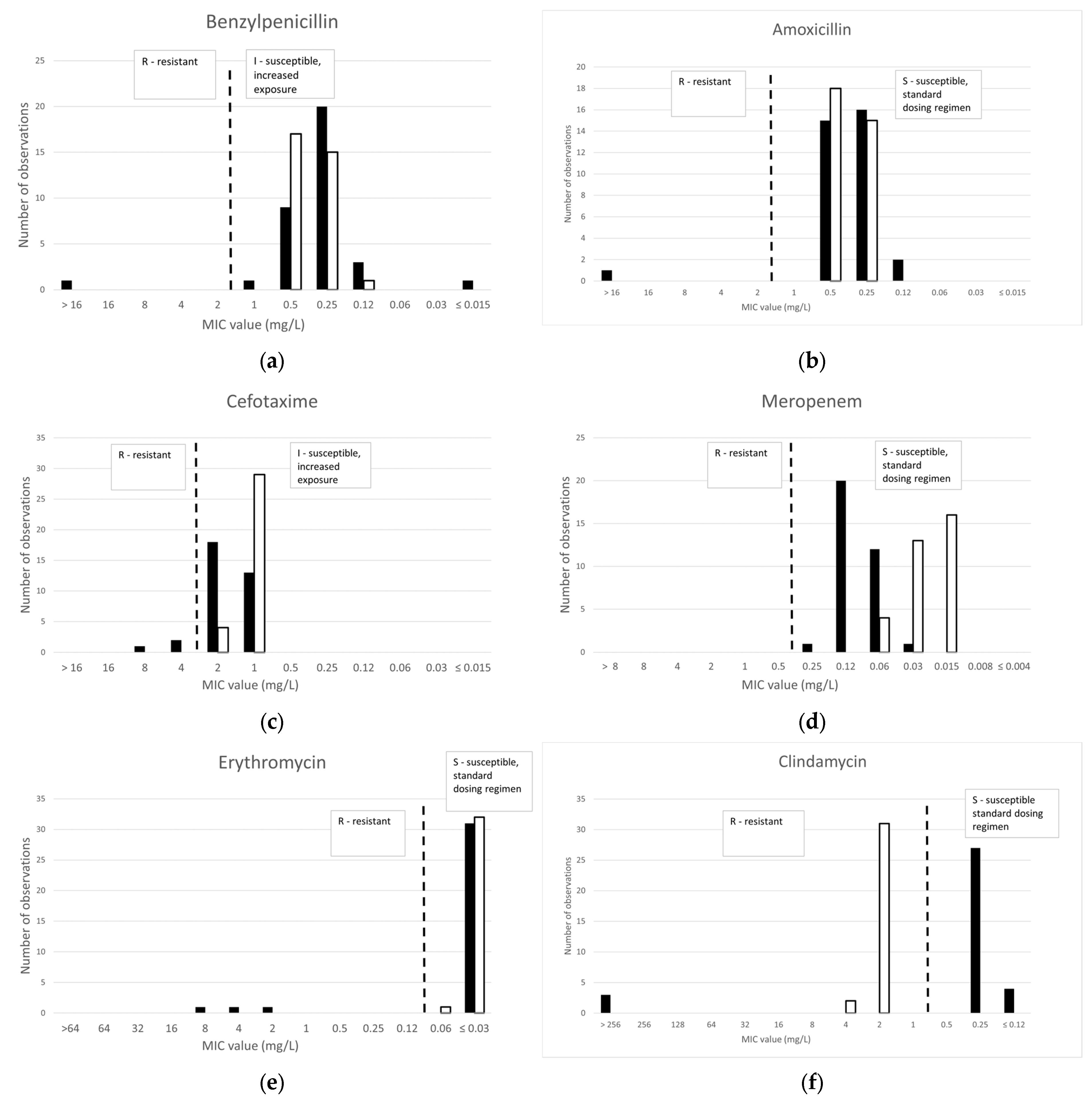
2.1. Broth Microdilution (BMD)
2.1.1. β-Lactams
2.1.2. Macrolides and Lincosamides
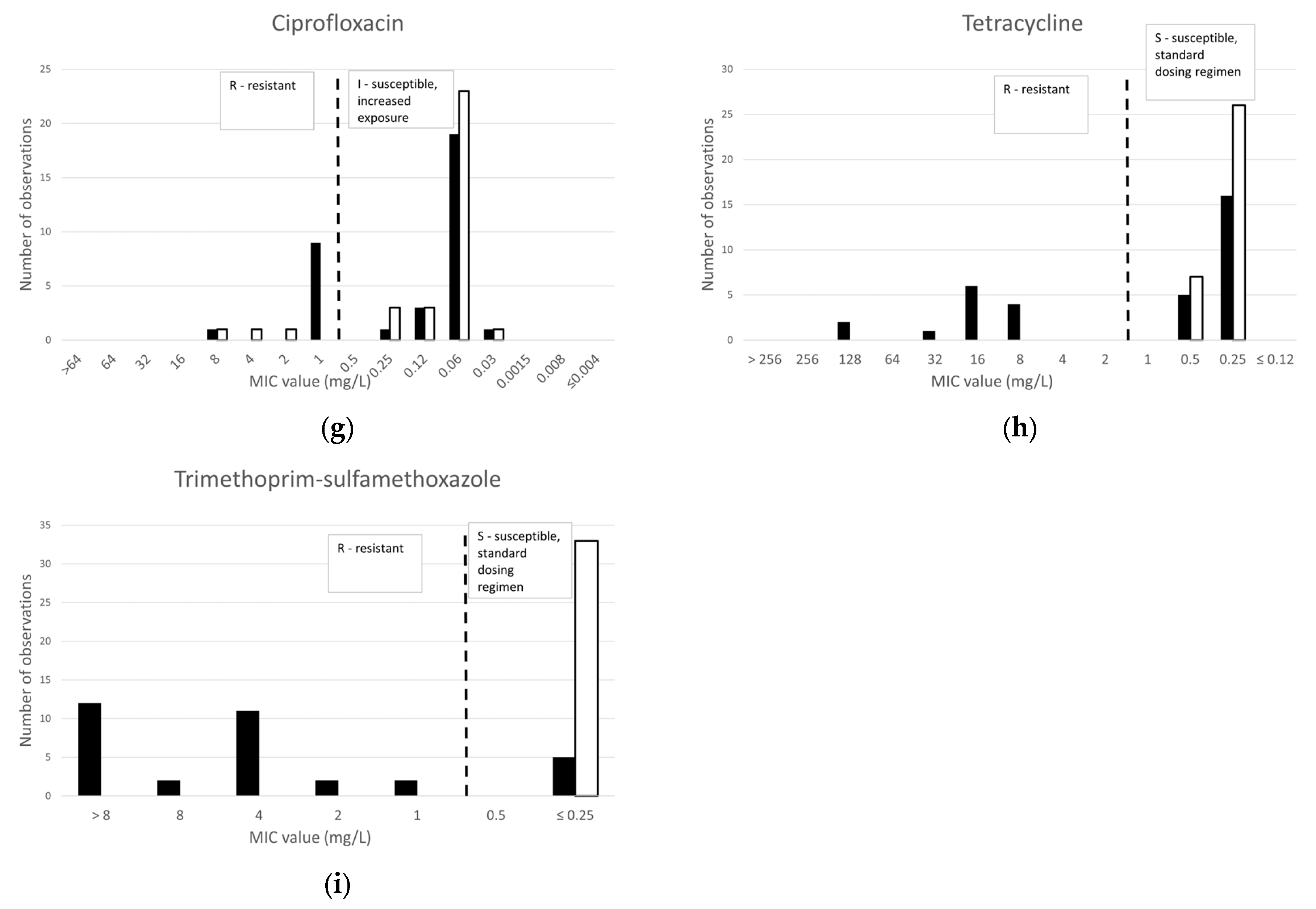
2.1.3. Other Antibiotics
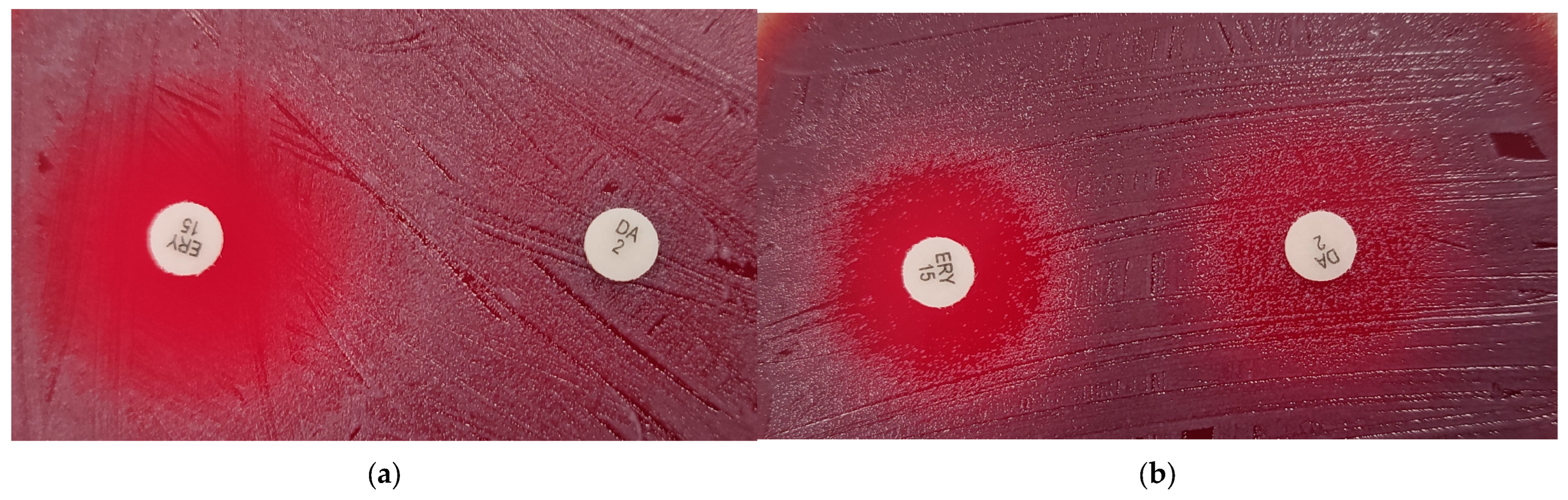
2.2. Disk Diffusion Susceptibility Testing
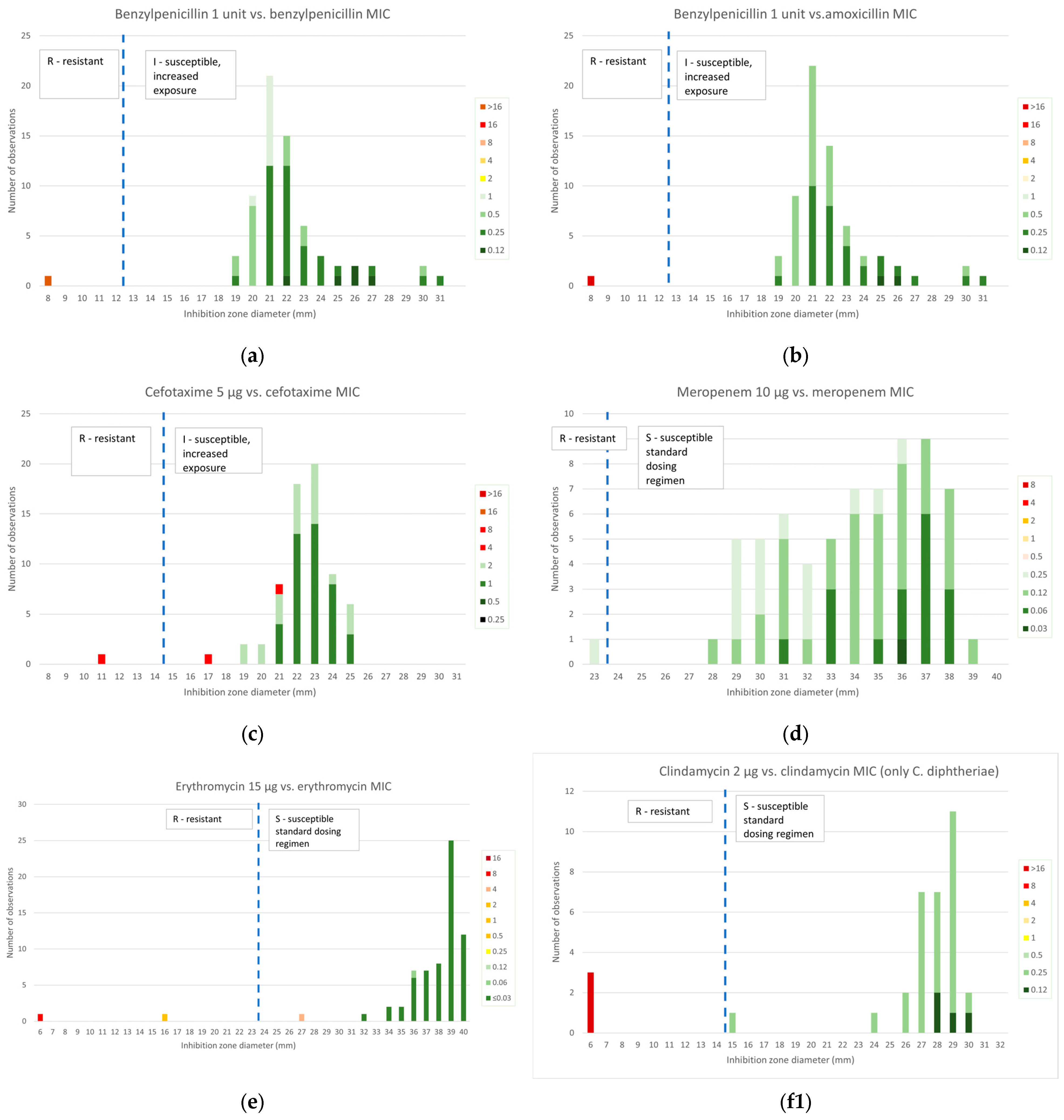
2.2.1. β-Lactams
2.2.2. Macrolides and Lincosamides
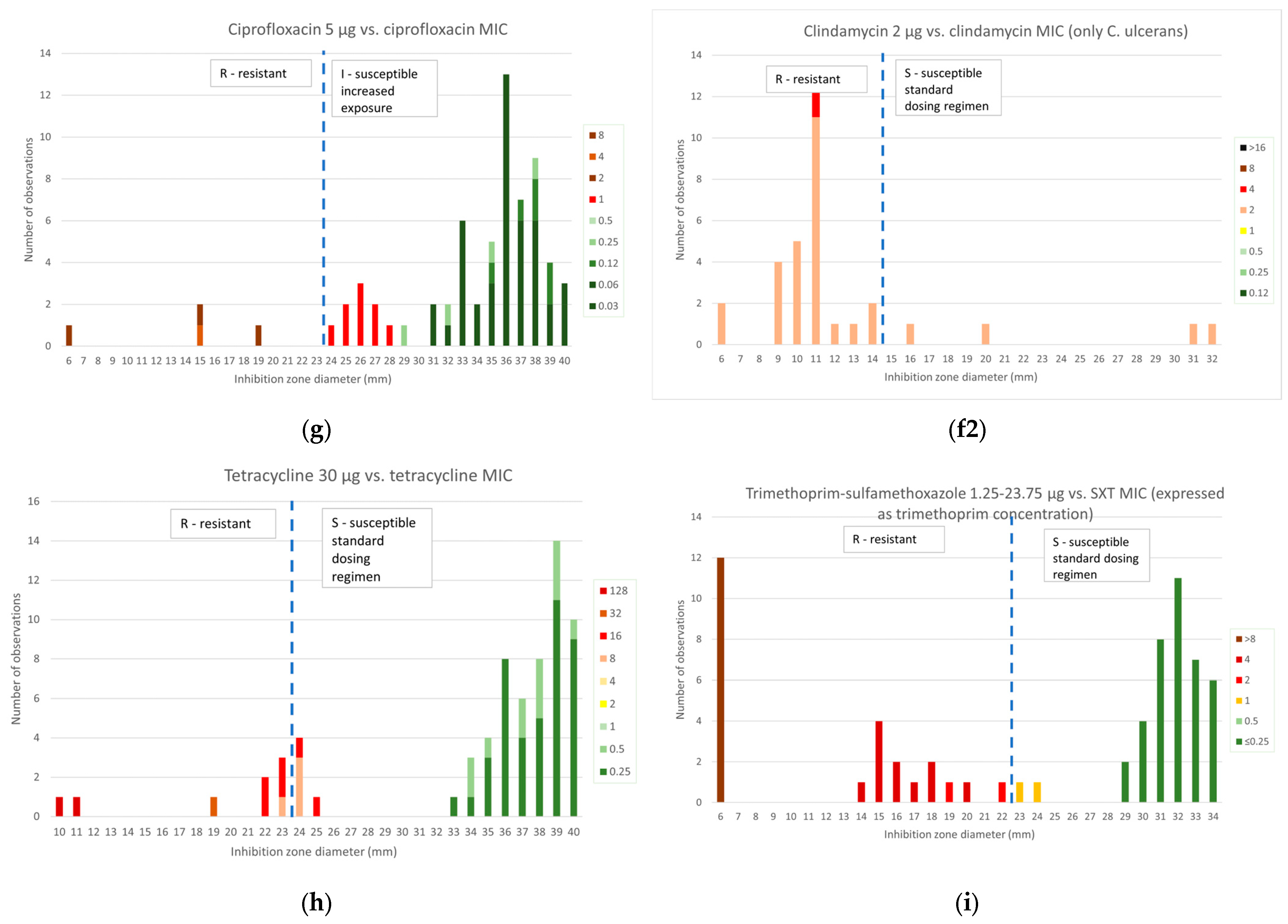
2.2.3. Other Antibiotics
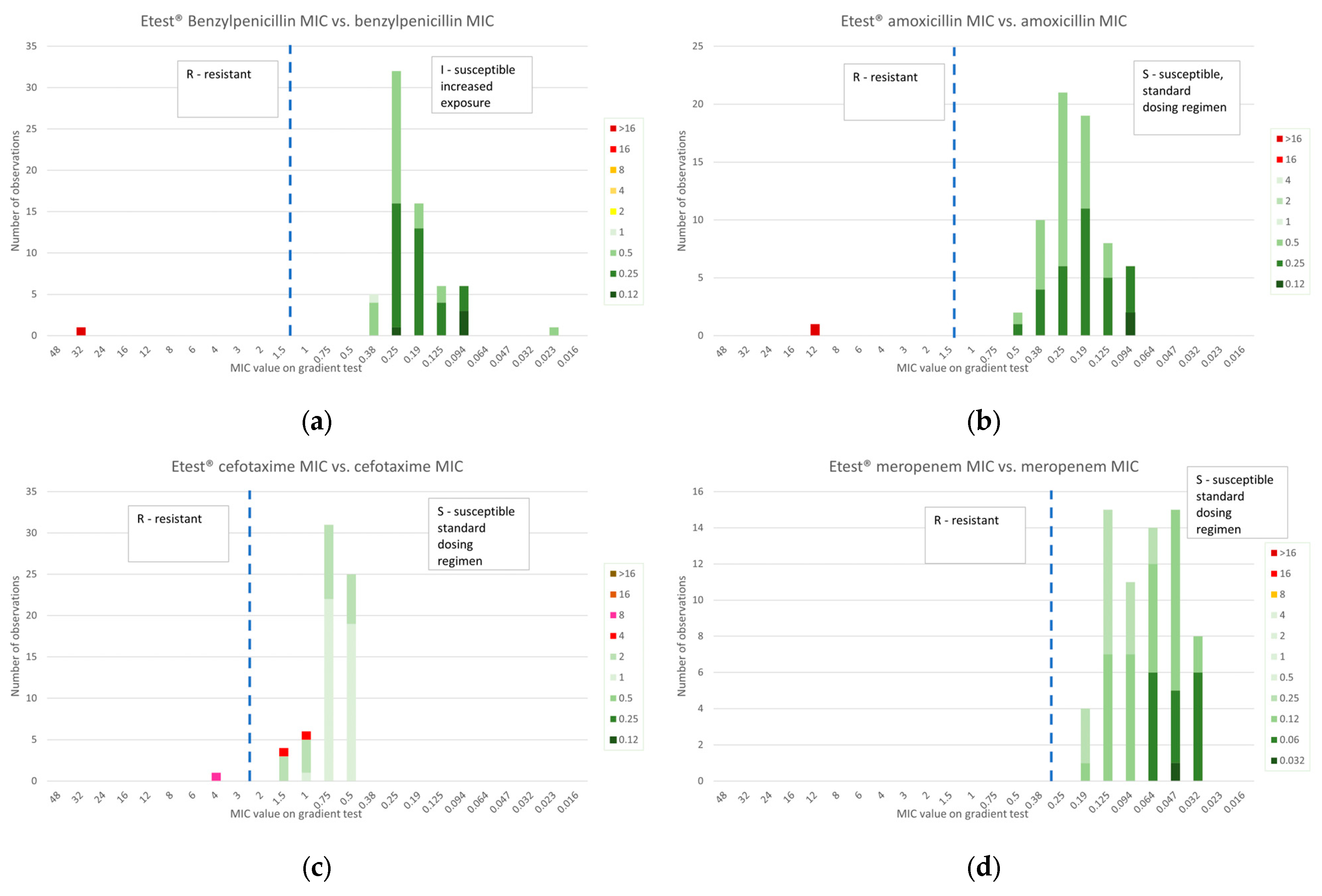
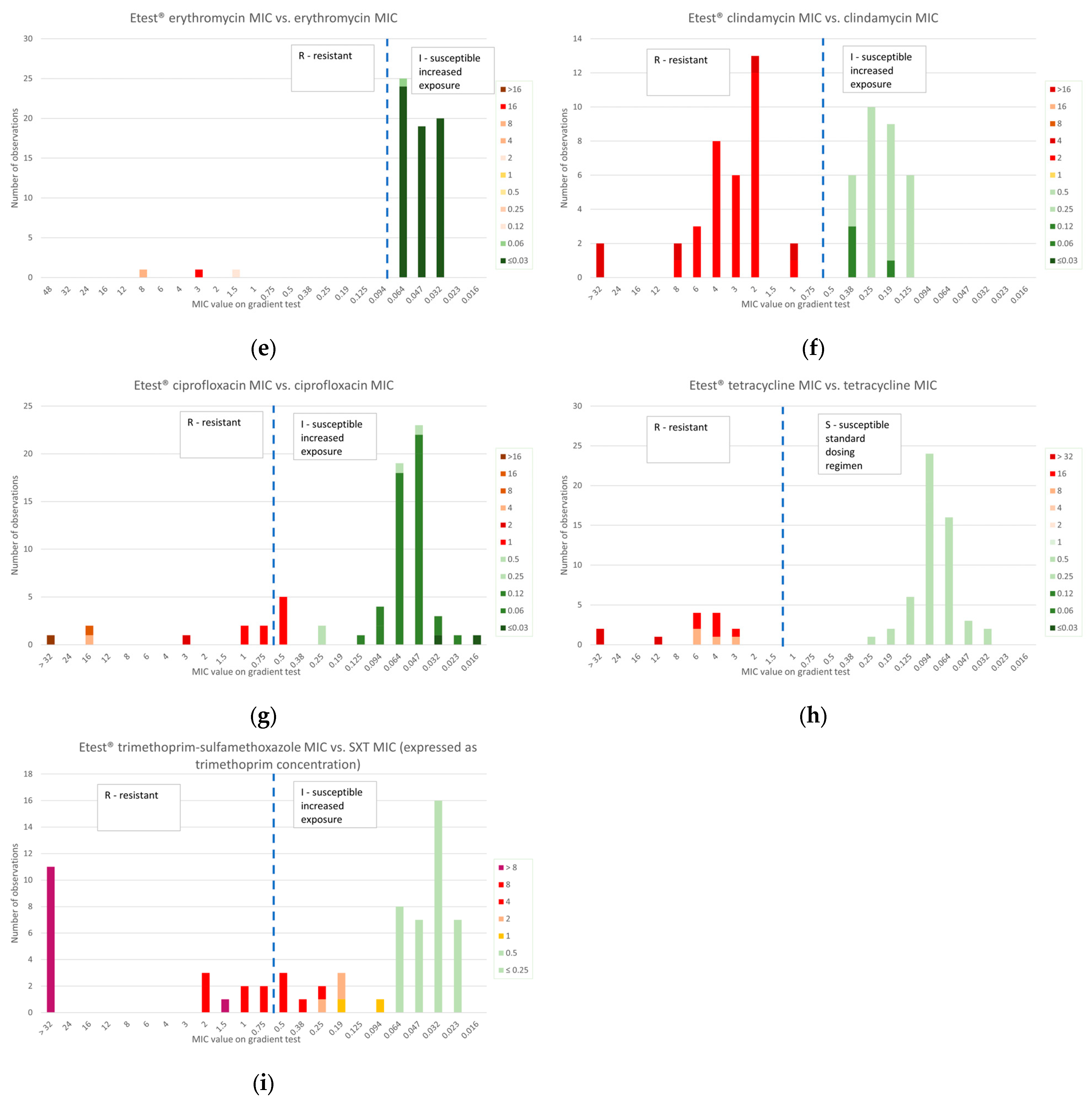
2.3. Gradient Diffusion Susceptibility Testing
2.3.1. β-Lactams
2.3.2. Macrolides and Lincosamides
2.3.3. Other Antibiotics
2.4. Whole-Genome Sequencing (WGS)
2.5. Comparison of Resistance Prevalence with Other European Studies
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burkovski, A. Proteomics of Toxigenic Corynebacteria. Proteomes 2024, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Saleeb, P.G. Corynebacterium diphtheriae (diphtheria). In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; Elsevier: Philadelphia, PA, USA, 2020; pp. 2426–2531. [Google Scholar]
- European Commission. Commission Implementing Decision (EU) 2018/945 of 22 June 2018 on the Communicable Diseases and Related Special Health Issues to Be Covered by Epidemiological Surveillance as Well as Relevant Case Definitions. 2018. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018D0945&from=EN#page=18 (accessed on 15 January 2025).
- Hoefer, A.; Seth-Smith, H.; Palma, F.; Schindler, S.; Freschi, L.; Dangel, A.; Berger, A.; D’Aeth, J.; Indra, A.; Fry, N.K.; et al. Phenotypic and genomic analysis of a large-scale Corynebacterium diphtheriae outbreak among migrant populations in Europe. NEJM, 2024; submitted. [Google Scholar] [CrossRef]
- ECDC. Surveillance Atlas of Infectious Diseases. 2024. Available online: https://atlas.ecdc.europa.eu/public/index.aspx (accessed on 15 January 2025).
- Jacquinet, S.; Martini, H.; Mangion, J.P.; Neusy, S.; Detollenaere, A.; Hammami, N.; Bruggeman, L.; Hoorelbeke, B.; Pierard, D.; Cornelissen, L. Outbreak of Corynebacterium diphtheriae among asylum seekers in Belgium in 2022: Operational challenges and lessons learnt. Eurosurveillance 2023, 28, 2300130. [Google Scholar] [CrossRef] [PubMed]
- ECDC. Scientific Panel on Childhood Immunisation Schedule: Diphtheria-Tetanus-Pertussis (DTP) Vaccination. 2009. Available online: https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/0911_GUI_Scientific_Panel_on_Childhood_Immunisation_DTP.pdf (accessed on 15 January 2024).
- Berbers, G.; van Gageldonk, P.; Kassteele, J.V.; Wiedermann, U.; Desombere, I.; Dalby, T.; Toubiana, J.; Tsiodras, S.; Ferencz, I.P.; Mullan, K.; et al. Team Circulation of pertussis and poor protection against diphtheria among middle-aged adults in 18 European countries. Nat. Commun. 2021, 12, 2871. [Google Scholar] [CrossRef] [PubMed]
- Hennart, M.; Panunzi, L.G.; Rodrigues, C.; Gaday, Q.; Baines, S.L.; Barros-Pinkelnig, M.; Carmi-Leroy, A.; Dazas, M.; Wehenkel, A.M.; Didelot, X.; et al. Population genomics and antimicrobial resistance in Corynebacterium diphtheriae. Genome Med. 2020, 12, 107. [Google Scholar] [CrossRef]
- Marosevic, D.V.; Berger, A.; Kahlmeter, G.; Payer, S.K.; Hormansdorfer, S.; Sing, A. Antimicrobial susceptibility of Corynebacterium diphtheriae and Corynebacterium ulcerans in Germany 2011–17. J. Antimicrob. Chemother. 2020, 75, 2885–2893. [Google Scholar] [CrossRef]
- Arcari, G.; Hennart, M.; Badell, E.; Brisse, S. Multidrug-resistant toxigenic Corynebacterium diphtheriae sublineage 453 with two novel resistance genomic islands. Microb. Genom. 2023, 9, 923. [Google Scholar] [CrossRef] [PubMed]
- Bremont, S.; Passet, V.; Hennart, M.; Fonteneau, L.; Toubiana, J.; Badell, E.; Brisse, S. Multidrug-resistant Corynebacterium diphtheriae in people with travel history from West Africa to France, March to September 2023. Eurosurveillance 2023, 28, 2300615. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.A.; Pimenta, F.P.; Santos, F.R.W.D.; Damasco, P.V.; Hirata Júnior, R.; Mattos-Guaraldi, A.L. Antimicrobial resistance among Brazilian Corynebacterium diphtheriae strains. Mem. Inst. Oswaldo Cruz 2008, 103, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Xiaoli, L.; Peng, Y.; Williams, M.M.; Lawrence, M.; Cassiday, P.K.; Aneke, J.S.; Pawloski, L.C.; Shil, S.R.; Rashid, M.O.; Bhowmik, P.; et al. Genomic characterization of cocirculating Corynebacterium diphtheriae and non-diphtheritic Corynebacterium species among forcibly displaced Myanmar nationals, 2017–2019. Microb. Genom. 2023, 9, 1085. [Google Scholar] [CrossRef]
- Paveenkittiporn, W.; Sripakdee, S.; Koobkratok, O.; Sangkitporn, S.; Kerdsin, A. Molecular epidemiology and antimicrobial susceptibility of outbreak-associated Corynebacterium diphtheriae in Thailand, 2012. Infect. Genet. Evol. 2019, 75, 104007. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; Badell, E.; Ahman, J.; Matuschek, E.; Zidane, N.; Kahlmeter, G.; Sing, A.; Brisse, S. Corynebacterium diphtheriae and Corynebacterium ulcerans: Development of EUCAST methods and generation of data on which to determine breakpoints. J. Antimicrob. Chemother. 2024, 79, 968–976. [Google Scholar] [CrossRef] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone diameters. Version 13.0, 2023, Version 14.0, 2024, Version 15.0, 2025. Available online: https://www.eucast.org/clinical_breakpoints (accessed on 15 January 2025).
- WHO. Clinical Management of Diphtheria: Guideline, 2 February 2024. Available online: https://iris.who.int/bitstream/handle/10665/375887/WHO-DIPH-Clinical-2024.1-eng.pdf (accessed on 15 January 2025).
- Conseil Supérieur dee la Santé. Prise en Charge des Infections à Corynebacetrium Ulcerans et Diphteriae 2019. Available online: https://www.hgr-css.be/file/download/f9d8d540-5ef3-4470-9016-e404c1aaf79f/oZFJ365ELYGMW4pVlLaRZbcYc9K6B6UPI86dFNF3cdA3d.pdf (accessed on 15 January 2025).
- Batista Araujo, M.R.; Bernardes Sousa, M.Â.; Seabra, L.F.; Caldeira, L.A.; Faria, C.D.; Bokermann, S.; Sant’Anna, L.O.; Dos Santos, L.S.; Mattos-Guaraldi, A.L. Cutaneous infection by non-diphtheria-toxin producing and penicillin-resistant Corynebacterium diphtheriae strain in a patient with diabetes mellitus. Access Microbiol. 2021, 3, 000284. [Google Scholar] [CrossRef] [PubMed]
- Benamrouche, N.; Hasnaoui, S.; Badell, E.; Guettou, B.; Lazri, M.; Guiso, N.; Rahal, K. Microbiological and molecular characterization of Corynebacterium diphtheriae isolated in Algeria between 1992 and 2015. Clin. Microbiol. Infect. 2016, 22, 1005.e1–1005.e7. [Google Scholar] [CrossRef]
- WHO. Disease Outbreak News: Diphtheria—Nigeria. 2023. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON485 (accessed on 15 January 2025).
- Antunes, N.T.; Fisher, J.F. Acquired Class D beta-Lactamases. Antibiotics 2014, 3, 398–434. [Google Scholar] [CrossRef]
- Coyle, M.B.; Minshew, B.H.; Bland, J.A.; Hsu, P.C. Erythromycin and clindamycin resistance in Corynebacterium diphtheriae from skin lesions. Antimicrob. Agents Chemother. 1979, 16, 525–527. [Google Scholar] [CrossRef] [PubMed][Green Version]
- EUCAST. Area of Technical Uncertainty (ATU) in Antimicrobial Susceptibility Testing. 2024. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Guidance_documents/Area_of_Technical_Uncertainty_-_guidance_v4_2024.pdf (accessed on 15 January 2025).
- EUCAST. Antimicrobial Susceptibility Testing EUCAST Disk Diffusion Method v. 12.0. 2024. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/2024_manuals/Manual_v_12.0_EUCAST_Disk_Test_2024.pdf (accessed on 15 January 2025).
- EUCAST. EUCAST Reading Guide for Broth Microdilution v.5.0. 2024. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/MIC_testing/Reading_guide_BMD_v_5.0_2024.pdf (accessed on 15 January 2025).
- EUCAST. Routine and Extended Internal Quality Control for MIC Determination and Disk Diffusion as Recommended by EUCAST v. 14.0 2024. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/QC/v_14.0_EUCAST_QC_tables_routine_and_extended_QC.pdf (accessed on 24 December 2024).
- Prjibelski, A.; Meleshko, D.; Lapidus, A.; Korobeynikov, A. Using SPAdes De Novo Assembler. Curr. Protoc. Bioinform. 2020, 70, e102. [Google Scholar] [CrossRef] [PubMed]
- Mikheenko, A.; Prjibelski, A.; Saveliev, V.; Antipov, D.; Gurevich, A. Versatile genome assembly evaluation with QUAST-LG. Bioinformatics 2018, 34, i142–i150. [Google Scholar] [CrossRef]
- Hennart, M.; Crestani, C.; Bridel, S.; Armatys, N.; Brémont, S.; Carmi-Leroy, A.; Landier, A.; Passet, V.; Fonteneau, L.; Vaux, S.; et al. A global Corynebacterium diphtheriae genomic framework sheds light on current diphtheria reemergence. Peer Community J. 2023, 3, e76. [Google Scholar] [CrossRef]
- Feldgarten, M.; Brover, V.; Gonzalez-Escalona, N.; Frye, J.G.; Haendiges, J.; Haft, D.H.; Hoffmann, M.; Pettengill, J.B.; Prasad, A.B.; Tillman, G.E.; et al. AMRFinderPlus and the Reference Gene Catalog facilitate examination of the genomic links among antimicrobial resistance, stress response, and virulence. Sci. Rep. 2021, 11, 12728. [Google Scholar] [CrossRef] [PubMed]
| Antimicrobial | ||||
|---|---|---|---|---|
| Agent | AMR Determinants 1 | nP/nR 2 | AMR Determinants 1 | nP/nS 3 |
| Benzylpenicillin | pbp2m | 1/1 | blaOXA-2 | 2/33 |
| Amoxicillin | pbp2m | 1/1 | blaOXA-2 | 2/33 |
| Cefotaxime | pbp2m | 1/3 | blaOXA-2 | 2/31 |
| Meropenem | / | none | ||
| Ciprofloxacin | gyrA_S89F + qacEΔ | 6/10 | qacEΔ, qacL | 1/24 |
| gyrA_S89F | 2/10 | |||
| qacEΔ | 2/10 | |||
| Erythromycin | ermX | 3/3 | none | |
| Clindamycin | ermX | 3/3 | none | |
| Tetracycline | tet(33) | 9/13 | none | |
| tet(33) + tet(W) | 2/13 | |||
| tet(65) | 1/13 | |||
| tet(Z) | 1/13 | |||
| Trimethoprim-sulfamethoxazole | sul1 | 17/29 | sul1 | 1/5 |
| sul1, dfrA1 | 9/29 | |||
| sul1, dfrA16 | 1/29 | |||
| none | 2/29 | |||
| Antibiotic | This Study (n = 34) | Marosevic et al. [10] (n = 298) | Berger et al. [16] (n = 200) |
|---|---|---|---|
| Benzylpenicillin | 3 | 3 | 5 |
| Amoxicillin | 3 | / | 3 |
| Cefotaxime (ceftriaxone *) | 3 | 6 | 1 |
| Meropenem | 0 | 0 | 1 |
| Erythromycin | 9 | 4 | 5 |
| Clindamycin | 9 | 2 | 3 |
| Ciprofloxacin | 29 | 9 | 4 |
| Tetracycline | 38 | / | 38 |
| Trimethoprim-sulfamethoxazole | 85 | / | 33 |
| Antibiotic | This Study (n = 33) | Marosevic et al. [10] (n = 123) | Berger et al. [16] (n = 200) |
|---|---|---|---|
| Benzylpenicillin | 0 | 0 | 0 |
| Amoxicillin | 0 | / | 0 |
| Cefotaxime (ceftriaxone *) | 9 | 0 | 0 |
| Meropenem | 0 | 1 | 1 |
| Erythromycin | 0 | 2 | 1 |
| Clindamycin | 100 | 84 | 93 |
| Ciprofloxacin | 9 | 3 | 4 |
| Tetracycline | 0 | / | 3 |
| Trimethoprim-sulfamethoxazole | 0 | / | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janssen, Z.; Martini, H.; Vanstokstraeten, R.; Vandoorslaer, K.; Wybo, I.; Honacker, E.V.; Piérard, D. Antimicrobial Susceptibility of Toxin-Producing Corynebacterium diphtheriae and C. ulcerans in Belgium. Antibiotics 2025, 14, 160. https://doi.org/10.3390/antibiotics14020160
Janssen Z, Martini H, Vanstokstraeten R, Vandoorslaer K, Wybo I, Honacker EV, Piérard D. Antimicrobial Susceptibility of Toxin-Producing Corynebacterium diphtheriae and C. ulcerans in Belgium. Antibiotics. 2025; 14(2):160. https://doi.org/10.3390/antibiotics14020160
Chicago/Turabian StyleJanssen, Zan, Helena Martini, Robin Vanstokstraeten, Kristof Vandoorslaer, Ingrid Wybo, Eveline Van Honacker, and Denis Piérard. 2025. "Antimicrobial Susceptibility of Toxin-Producing Corynebacterium diphtheriae and C. ulcerans in Belgium" Antibiotics 14, no. 2: 160. https://doi.org/10.3390/antibiotics14020160
APA StyleJanssen, Z., Martini, H., Vanstokstraeten, R., Vandoorslaer, K., Wybo, I., Honacker, E. V., & Piérard, D. (2025). Antimicrobial Susceptibility of Toxin-Producing Corynebacterium diphtheriae and C. ulcerans in Belgium. Antibiotics, 14(2), 160. https://doi.org/10.3390/antibiotics14020160






