Abstract
Millions of people around the world rely on aquaculture as a major source of food. In the recent few years, probiotics have gained considerable attention as an alternative agent to antibiotics. They have been shown to play an important role in improving aquaculture species through different mechanisms, mainly disease management, improving their growth performance, and improving water quality. Consequently, this review aimed to identify the key areas of research in the global literature about the influence of probiotics on aquaculture based on the selected keywords “aquaculture” AND “probiotics” AND “growth performance” AND “disease resistance” (APGD). The meta-data of the published literature were extracted from the Web of Science (WoS) database on 23 December 2024, and then the co-authors, countries, and keywords were analyzed and visualized using VOSviewer (v. 1.6.20). The search found a remarkable number of documents, which included 175 APGD documents. The results of the bibliometric analysis of the global literature reveal a substantial increase in the publication of APGD documents from 2019 to 2024. Asia, particularly China (32.3% of documents), has emerged as a leader of APGD publications, followed by Iran (8.67%), India (8.01%), Malaysia (7.5%), and Spain (7.5%), respectively. Keyword analysis revealed the prevalence of disease resistance (length = 1793), probiotics (1348), aquaculture (1169), and growth performance (913) as the most impactful keywords based on the WoS database. This could indicate that most of the APGD documents were focused on disease resistance and probiotics relationships. In addition, an extensive review of the recent literature showed that probiotics have demonstrated a remarkable efficacy in improving the growth performance, feed utilization efficiency, disease prevention, and water quality management in various aquaculture species under different aquaculture systems when used as feed or water additives for 30–90 days. It can be concluded that Asia is the lead continent in aquaculture probiotics research, with a significant increase in APGD documents in the last 5 years. Probiotics played a major role in improving aquatic species. This research aims to provide valuable insight into the use of probiotics in aquaculture and highlights the need for further research to fully understand their benefits and mechanisms of action.
1. Introduction
According to the Food and Agriculture Organization (FAO) [1], the aquaculture industry constitutes a substantial food source for millions of individuals globally. A review of the fishery yearbook [2] indicates that approximately 89% of the global total fish production in 2024 was utilized directly for human consumption. However, the presence of pathogens has been observed, which can negatively impact fish production. These pathogens are predominantly gram-negative bacteria, including Aeromonas salmonicida, A. hydrophila, Vibrio harveyi, V. anguillarum, Flavobacterium psychrophilum, Pseudomonas fluorescens, Citrobacter freundii, and Yersinia ruckeri [3]. Less frequently, gram positive bacteria such as Streptococcus spp. are also involved. The combined effect of these pathogens and unfavorable environmental factors is deleterious to productivity, resulting in significant economic losses for farmers. Poor fish management practices, including excessive stock densities, overfeeding, and water contamination, also contribute to the occurrence and spread of pathogens in aquaculture environments [4,5]. Antibiotics are frequently utilized in aquaculture as a means of combating diseases and enhancing fish performance. However, the continuous use of antibiotics in aquaculture has increased the selective natural pressure on the microbial community, leading to the emergence of antibiotic-resistant bacterial strains that can disseminate widely [6]. These resistant bacteria can spread rapidly, negatively impacting aquaculture production, fish consumers, and the surrounding environment [7,8,9]. In the context of these concerns, probiotics are being explored as an alternative technique that can be used as feed or water additives to promote environmental sustainability and the aquaculture industry [10].
As posited by Gismondo et al. [11], the term “probiotic” is derived from two Greek words, “pro” and “bios”, which together signify “for life”. However, the initial definition of the term probiotic was put forth by Parker [12], who defined a probiotic microorganism as one that contributes to intestinal microbial balance. As new findings have emerged, several alternate definitions of probiotics have been proposed. According to FAO and the World Health Organization (WHO) [13], probiotics are “live microorganisms which, when administered in adequate amounts, confer a health benefit to the host”. Probiotics have been found to be effective in a variety of aquatic environments, including freshwater [14], marine water, and brackish water [15]. The most commonly used probiotics are from the lactic acid bacteria (LAB), including the genera Leuconostoc, Pediococcus, Lactococcus, Oenococcus, and Enterococcus [16].
A substantial body of research has demonstrated the efficacy of the use of probiotics in a diverse range of fish and aquatic organisms such as African catfish (Clarias gariepinus), rainbow trout (Oncorhynchus mykiss), Nile tilapia (Oreochromis niloticus), European sea bass (Dicentrarchus labrax), rohu (Labeo rohita), snook (Centropomus undecimalis), and Red seabream (Pagrus major) [17,18,19]. Probiotics confer benefits to their hosts via a variety of mechanisms, including the enhancement of growth parameters [20,21,22,23,24] and the reduction of disease incidence [22,25]. Several factors can influence the effectiveness of probiotics, including the animal host, the strain of probiotic, the dosage, and the specific environmental conditions of the aquatic system. For instance, different strains of Bacillus have been shown to exhibit strain-specific benefits, with some strains providing significant growth rates and immune benefits while others do not [26]. This highlights the importance of selecting appropriate probiotic strains that are tailored to the specific needs of the aquaculture species being cultured.
The present review aims to provide a bibliometric analysis of the trends in the utilization of probiotics in aquaculture, with the objective of identifying research gaps in the existing literature concerning the interaction between probiotics and aquaculture under various conditions. Moreover, the specific objectives of the current study were as follows: (A) to identify the key areas of focus of research about the influence of probiotics in aquaculture based on the selected keywords (aquaculture, probiotics, growth parameters, and disease resistance); (B) to identify specific effects of probiotics on the growth performance and feed utilization of aquaculture species; (C) to identify the impact of probiotics on disease prevention, water quality (nitrogenous materials), and the survival of aquaculture species; and (D) to identify the impact of the interaction between different strains of probiotics and synbiotics in aquaculture.
2. Materials and Methods
2.1. Research Questions
- What are the key areas of research, research volume, geographical distribution of the literature, co-authoring counties, and leading publishers of the studies that focus on the influence of probiotics in aquaculture? This investigation was based on a selection of keywords, including “aquaculture”, “probiotics”, “growth performance”, and “disease resistance”;
- What are the specific effects of probiotics on fish growth performance, fish feed utilization, and the survival rate of aquaculture species?
- What is the impact of probiotics on disease prevention, water quality (nitrogenous materials), and survival in aquaculture species?
- What is the impact of the interaction between different strains of probiotics and synbiotics in aquaculture?
2.2. Searching and Exclusion Strategy
A thorough investigation was conducted by examining the worldwide literature in the Web of Science (WoS) database. WoS was selected because of its reputation as the most comprehensive and widely used database of literature in the area of reviews and bibliometric analyses. The search was conducted using a combination of keywords including “aquaculture”, “probiotics”, “growth performance”, and “disease resistance”, with a time frame extending from 2008 to 2024. All documents originally written in English were included. In addition, all open access research articles or book chapters were included; however, other types of documents were excluded. The search yielded a substantial number of documents, which was 175 in total.
2.3. Bibliometric Analysis
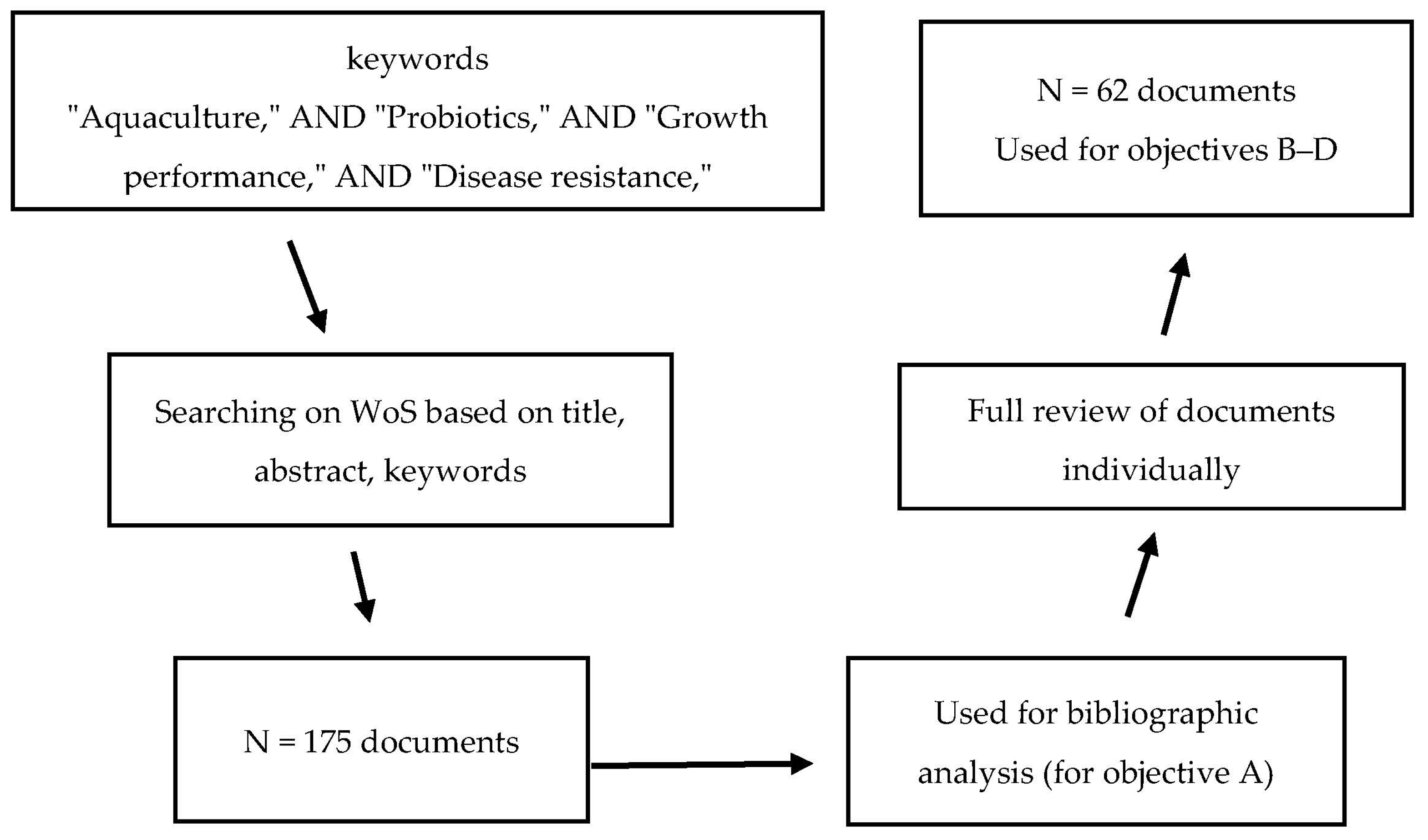
The first objective of this study (objective A) was to perform a screening of WoS based on titles, abstracts, and keywords. The obtained metadata comprised 175 research articles or book chapters that contained at least one of the search keywords. These 175 documents were then selected for bibliometric analysis (Figure 1). The bibliometric information, including names, countries of affiliation, document titles, keywords, publication dates, and publishers, were exported. Subsequently, co-occurrence (all keywords, significant keywords to the research question), co-authors’ countries, and total links were analyzed according to the method described by Van Eck and Waltman [27] using VOSviewer (v. 1.6.20) (CWTS, Leiden, The Netherlands). The results were then visualized as clusters to identify possible research gaps and to highlight knowledge limitations with relation to the regions (countries) where the research studies were conducted.
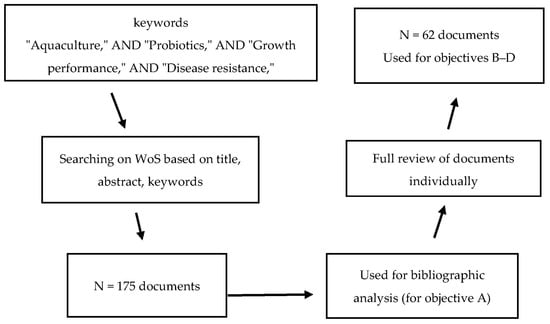
Figure 1.
Workflow screening, extraction, and processing of the obtained metadata from Web of Science database (WoS) from 2008–2024.
2.4. Screening and Data Extraction
Regarding this study’s objectives, data including country, probiotic species/strain, aquatic animal host, growth parameters, feed utilization indications, situation of disease incidence, gut integrity and microbiome of animal host, level of ammonia and toxic material in water, and activity of digestive enzymes were extracted directly from the results of reviewed articles or by using Web Plot Digitizer (v. 5.0) (Ankit Rohatgi, Austin, TX, USA) if the data presented as figures. The extracted results were then summarized, arranged in tables, and discussed.
3. Results and Discussions
3.1. Situation of the Scientific Documents Based on WoS Search on Aquaculture, Probiotics, Growth Performance and Disease Resistance (APGD)
3.1.1. Leading Countries on APGD Research
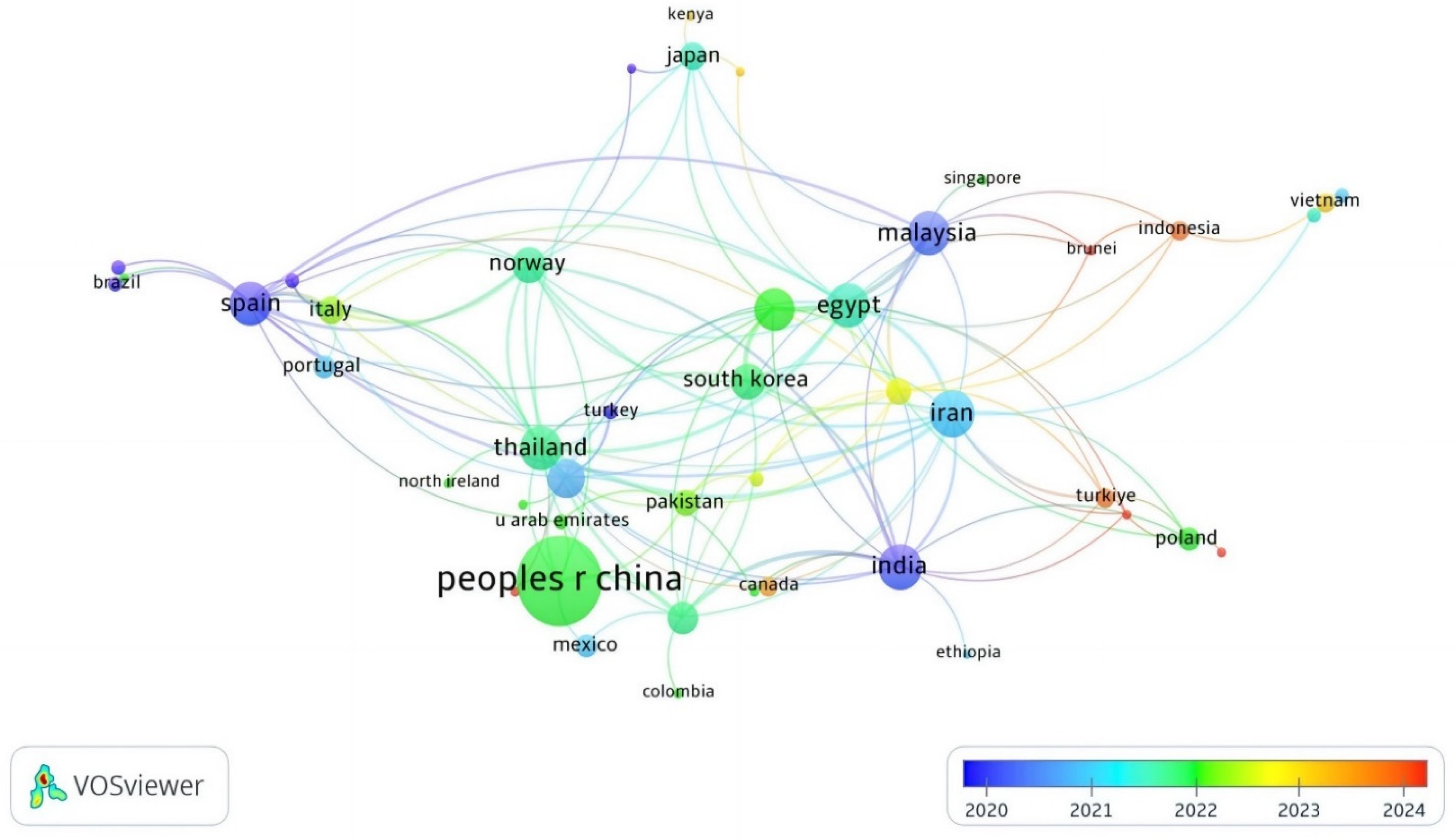
In total, 56 countries have published at least one research document on APGD based on the WoS database. In fact, 175 documents have been published by 57 countries. Asia was the leading continent for APGD-related documents, with co-authors of 85.1% of the documents published by the top 20 APGD countries (Table 1). China (nine total links) was the largest contributor with 56 APGD documents (Figure 2), representing around 32.3% of the total APGD documents published in the years 2008–2024 in the WoS database. In fact, China is the world’s largest producer of aquatic species, accounting for approximately 35% of the world’s fish and seafood production [28]. China’s aquaculture sector is characterized by its vast scale and diversity, producing more than 49,000,000 tons of fish in 2020. This diversity includes not only finfish but also mollusks and crustaceans, allowing for a wide range of products that cater to both domestic and international markets [28]. The top three contributors of APGD documents after China were Iran (15 documents), India (14 documents), and Malaysia (13 documents); the total links of these countries were 25, 22, and 17 for Iran, India, and Malaysia, respectively. The other leading countries with significant contributions to APGD documents were Spain, Thailand, Egypt, Bangladesh, the USA, Norway, Republic of Korea, Australia, Italy, Japan, Pakistan, Saudi Arabia, Poland, and Portugal, respectively (Table 1).

Table 1.
The top 20 co-authoring countries of APGD documents based on WoS, 2008–2024.
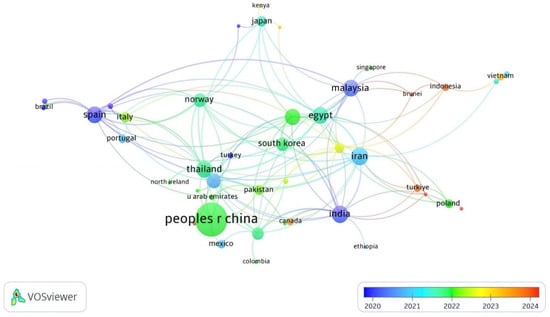
Figure 2.
Network visualization of co-authoring countries of APGD documents based on Web of Science (WoS) database, 2008–2024. APGD: aquaculture, probiotics, growth performance, and disease resistance.
3.1.2. Years of Publication and Publishing Entities
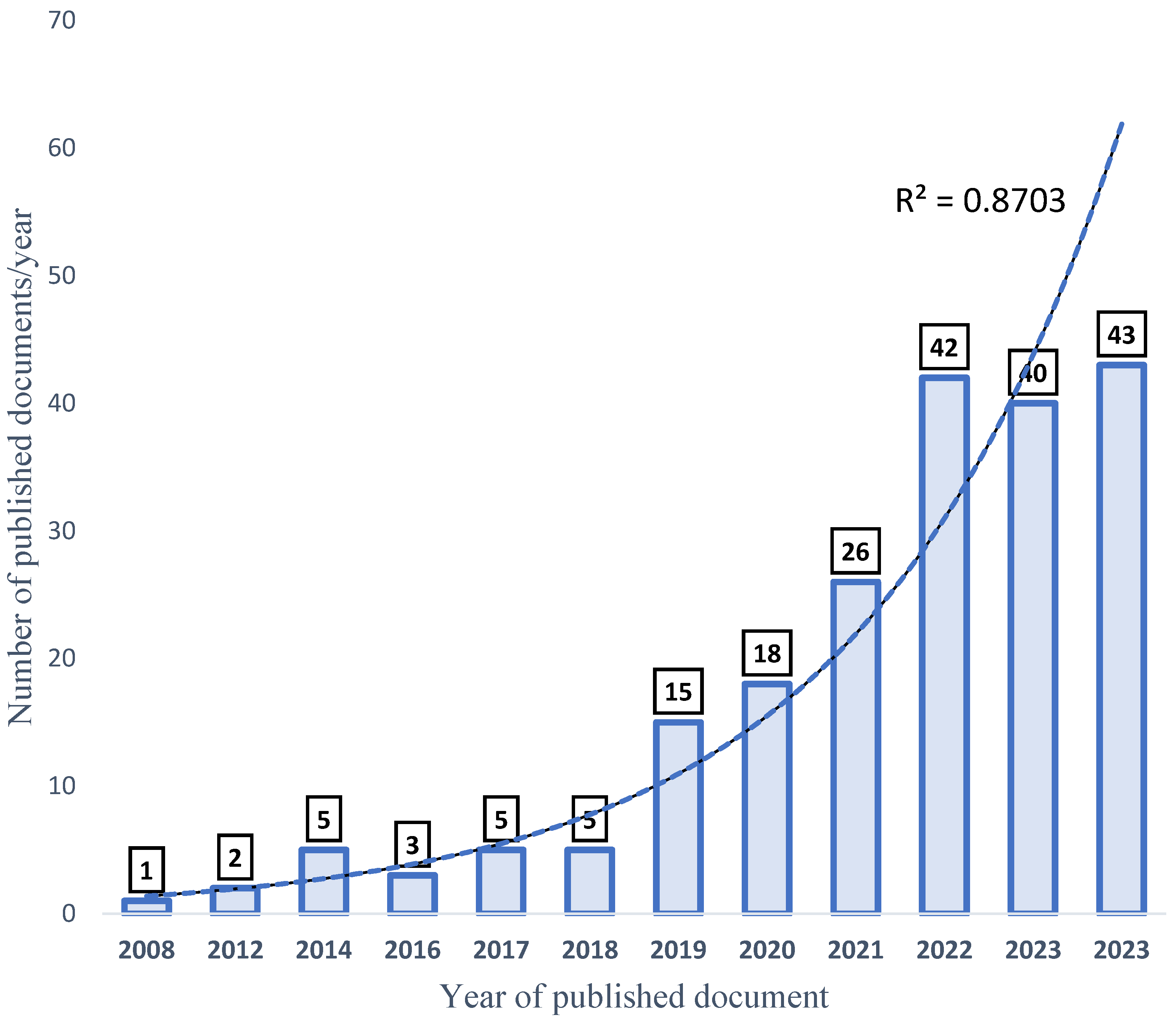
The total amount of documents published about the selected keywords (aquaculture, probiotics, growth performance, and disease resistance (APGD)) in the date range from 2008 to 2024 was 175. However, in one decade (between 2008 and 2018), only 19 documents (1–5 documents/year) were published about APGD, representing 10.98% of the total published documents. Later, a high increase in the publication rate (%/year) occurred, and the progress was exponential, which is justified by the high R2 value (0.9) (Figure 3). In 2019, the number of published documents was 13 (7.5%), while 15 (8.7%), 24 (13.9%), 37 (21.4%), 30 (17.3%), and 37 (21.4%) documents were published in the years 2019, 2020, 2021, 2022, 2023, and 2024, respectively. This rapid increase in the publication of scientific papers signifies a rapid response to address the importance of aquaculture, the rapidly growing human population, and food security-related issues. Projections indicate that, by the year 2050, the global population will be more than 9 billion, with a projected 85% increase in food production [29].
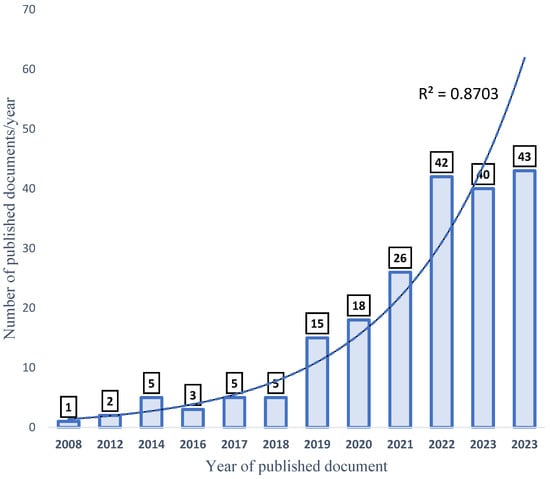
Figure 3.
Progress of publication of APGD documents, based on Web of Science (WoS) database, between 2008 and 2024. APGD: aquaculture, probiotics, growth performance, and disease resistance.
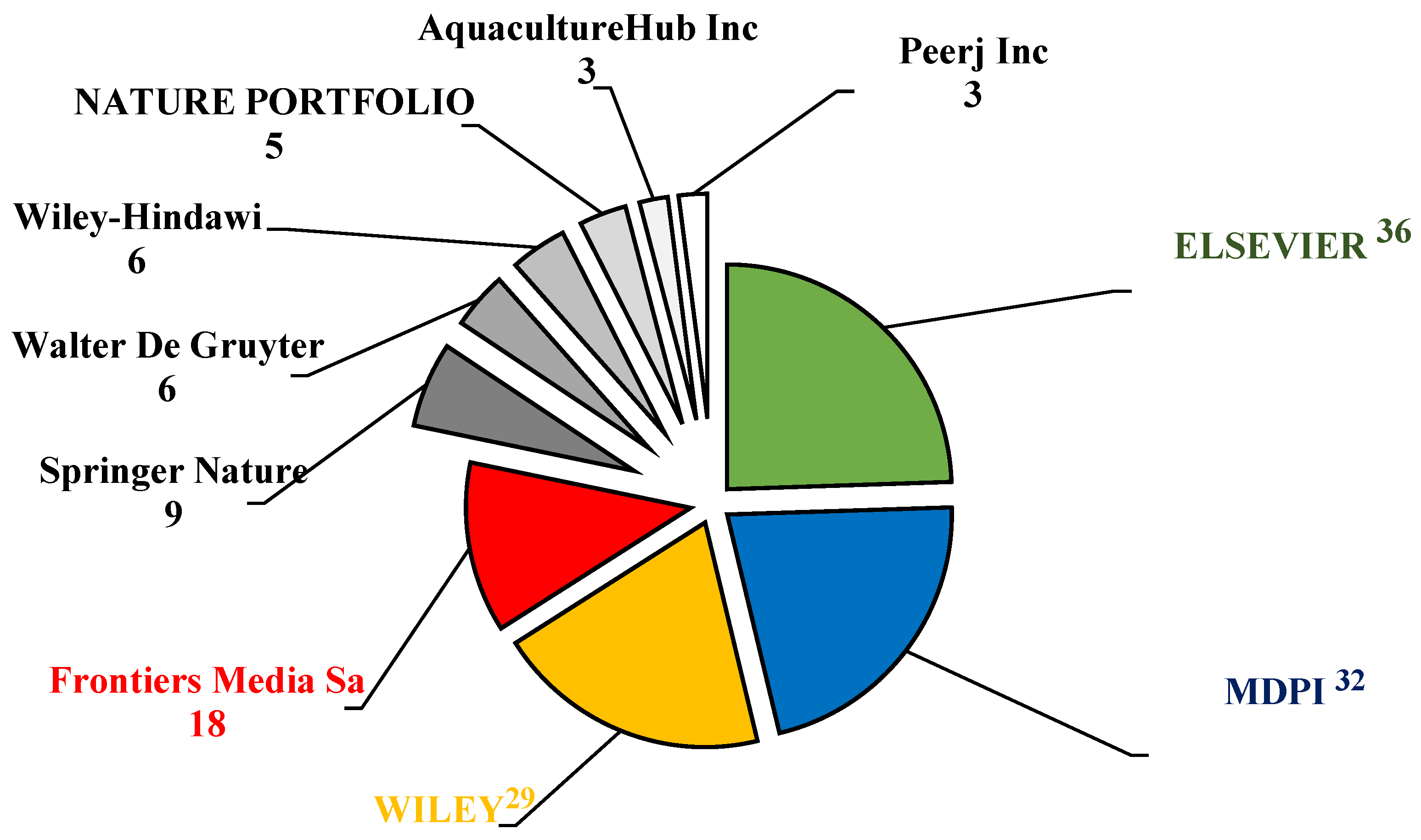
Elsevier and the Multidisciplinary Digital Publishing Institute (MDPI) were the most prominent academic publishers in aquaculture-related sciences. In this investigation, the top three publishers of APGD documents were Elsevier (36 documents), MDPI (32 documents), and Wily (30 documents) (Figure 4). These publishers played a vital role by publishing more than 58% of the total APGD documents published between 2008 and 2024. The dramatic increase in the research output by China has positioned it as a leading player in global academic publishing, including the publishing of studies related to aquaculture. China now exceeds the United States and any other nation in terms of its number of researchers and scientific publications, with a significant rise in the quality of these publications also being seen, as evidenced by their increasing number of citations in top-ranked journals [30]. MDPI has emerged as a major player in the publication of APGD documents and is particularly noted for its open access model, which allows for the rapid dissemination of research results. This is crucial in a field like aquaculture, where timely information can significantly impact practices and policies. For instance, a recent analysis highlighted a gradual increase in sustainable aquaculture publications, with a notable peak in 2023, indicating a growing interest and the need for accessible research in this area [31]. The open access nature of the MDPI journals ensures that research is readily available to practitioners and policymakers, facilitating the application of new findings in real-world settings.
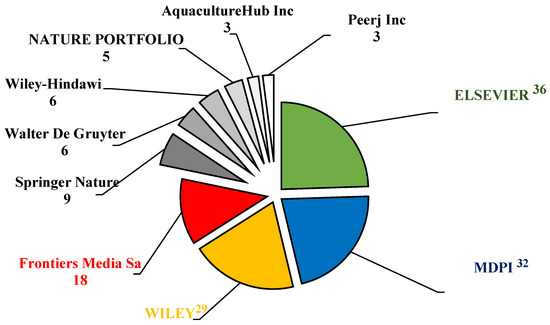
Figure 4.
Leading publishers of the APGD documents based on Web of Science (WoS) database, 2008–2024. APGD: aquaculture, probiotics, growth performance, and disease resistance.
3.2. Volume of Research on APGD Based on WoS Database
3.2.1. Co-Occurrence of All Keywords
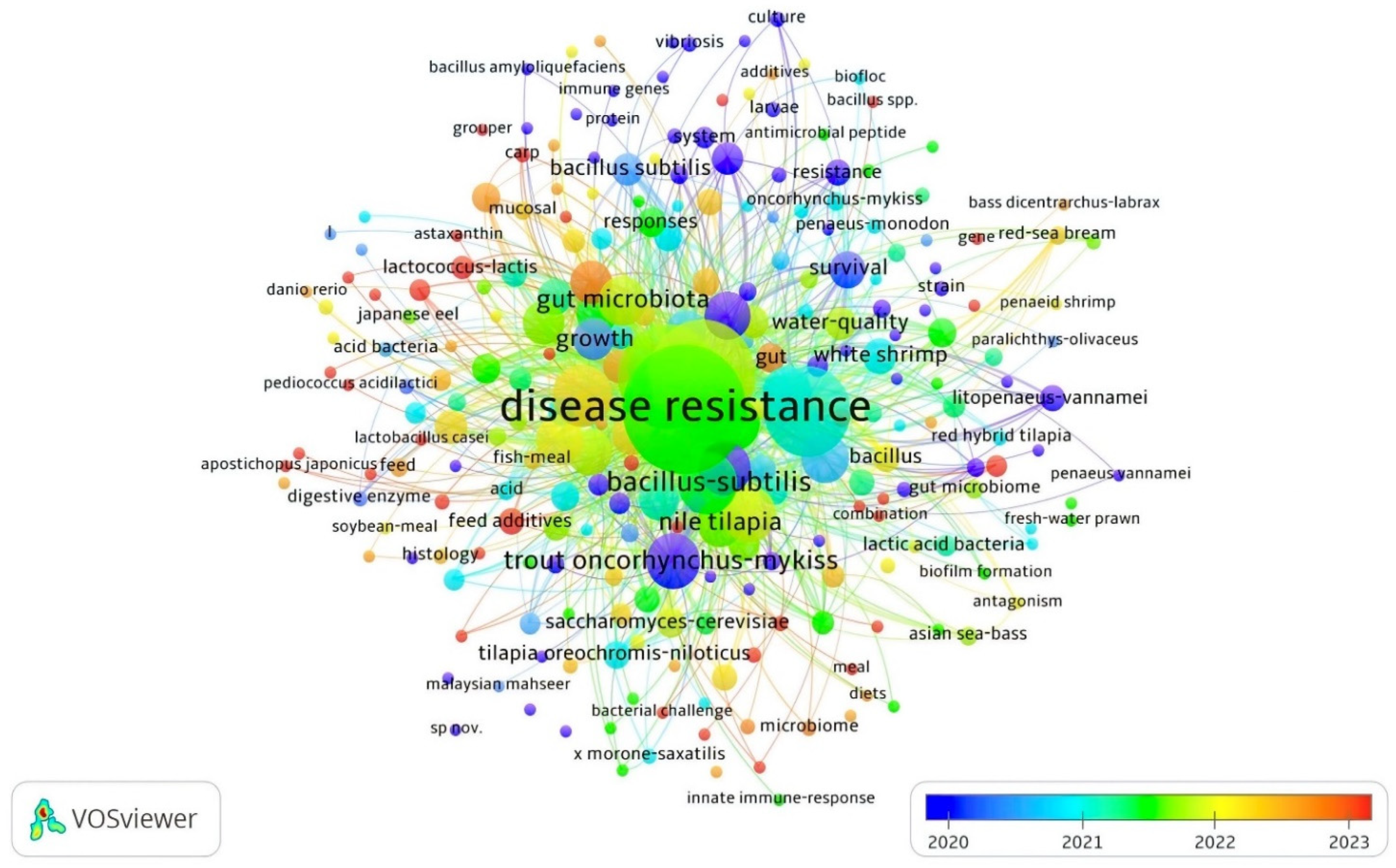
According to an analysis of co-occurrence of all keywords in the APGD documents from between the years 2008 and 2024, the total number of instances of the co-occurrence of all keywords was 939. However, the most significant keywords appearing in the APGD documents and their frequency of appearance (per time) were disease resistance (130), probiotics (99), aquaculture (88), growth-performance (67), Bacillus subtilis (35), and fish (36), with the total length strength recorded being 1793, 1348, 1169, 913, 491, and 482 for disease resistance, probiotics, aquaculture, growth performance, Bacillus subtilis, and fish, respectively (Table 2, Figure 5). This means that these keywords, within the past few years, have had central importance in aquaculture probiotics research. In fact, a higher total link strength of a keyword indicates a higher degree of connectivity of that word with other keywords and a higher likelihood that it appears with other keywords in the same document. The other significant keywords were performance, immunity, trout (Oncorhynchus mykiss), dietary supplementation, rainbow trout, immune response, lactic acid bacteria, bacteria, gut microbiota, innate immunity, growth performance, Nile tilapia, and probiotic, respectively (Table 2).

Table 2.
Co-occurrence and total link strength of top keywords in the APGD documents based on WoS, 2008–2024.
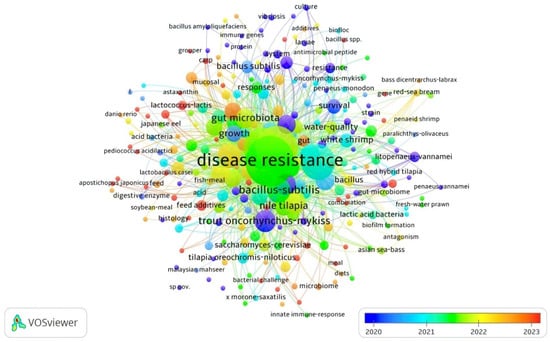
Figure 5.
Network visualization of all keywords in APGD documents based on Web of Science (WoS) database, 2008–2024.
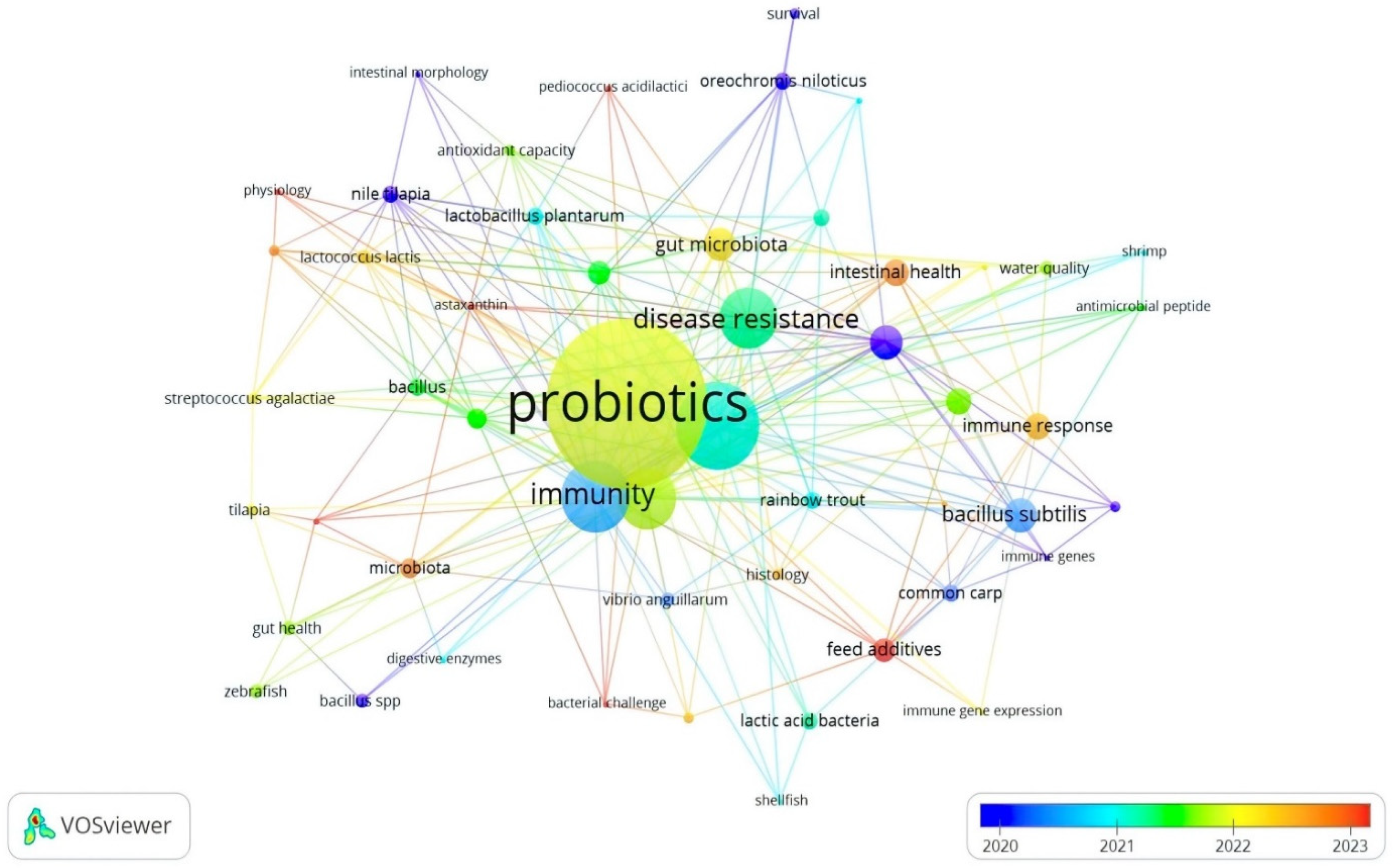
3.2.2. Co-Occurrence of Author Keywords
Similarly, the most significant author keywords were probiotics (62), aquaculture (30), immunity (24), probiotic (22), and growth performance (21). The total length strength of these keywords was 291, 154, 122, 109, and 108 for probiotics, aquaculture, immunity, probiotic, and growth performance, respectively (Table 3). The other keywords were disease resistance, growth, bacillus subtilis, Litopenaeus vannamei, gut microbiota, immune response, intestinal microbiota, intestinal health, gene expression, feed additives, Aeromonas hydrophila, and microbiota (Table 3, Figure 6). Based on the obtained results (Table 2), the keywords disease resistance (1793), probiotics (1348), aquaculture (1169), growth-performance (913), Bacillus subtilis (491), fish (482), and performance (456) yielded the highest length strengths, which can indicate that, between 2008 and 2024, the APGD documents focused on improving growth and disease resistance through the implementation of feed-based probiotics. Numerous studies have shown that overcrowding in intensive aquaculture systems can lead to stress in fish, making them more susceptible to infections and allowing pathogens to be more aggressive [32,33,34]. The stress induced by high stocking densities can compromise the immune systems of fish, increasing their vulnerability to diseases such as streptococcosis and viral infections [35]. However, probiotics are implemented for sustainable aquaculture purposes and to enhance growth parameters [20,21,22,36,37] or decrease the likelihood of disease outbreaks [22,25,38].

Table 3.
Co-occurrence and total link strength of the most powerful author keywords in APGD documents based on WoS literature searches, 2008–2024.
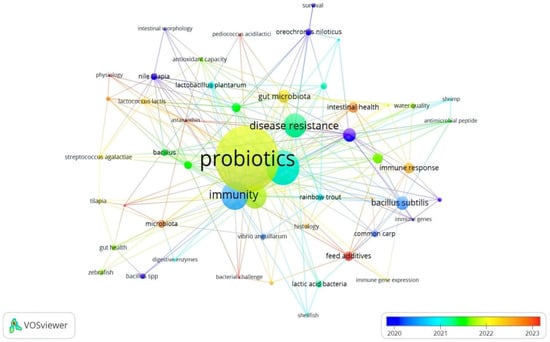
Figure 6.
Network visualization of co-occurrence of author keywords in APGD documents based on Web of Science (WoS) database, 2008–2024. APGD: aquaculture, probiotics, growth performance, and disease resistance.
3.3. Impact of Probiotics on Growth Parameters
3.3.1. Growth Indices
The parameters that are commonly used to evaluate animal growth performance after treatment with probiotics-based feed include the final body weight (FBW, g), survival rate (%), feed conversion ratio (FCR, g/g), and specific growth rate (SGR, %). The FCR value is calculated using the formula FCR = F/ (Wf − Wi), where F represents the feed intake, Wf is the final body weight, and Wi is the initial wet body weight. The SGR is determined by the formula SGR = (lnWf − lnWi)/t × 100, where Wf is the final body weight, Wi is the initial wet body weight (g), and t is the time (days).
Enhanced growth-related parameters are reflected in faster harvesting times and improved production efficiency, which is crucial for meeting the rising global demand for seafood [39]. Numerous studies have discussed the positive correlation between probiotics and the growth performance of different aquatic animals. For instance, a report from China stated that the growth performance of sea cucumber (Apostichopus japonicus) can significantly be improved when the animal receives a diet supplemented with the probiotic Sporosarcina aquimarina MS4 at 107 cfu/g after 60 days [40]. In addition, the FCR, FBW, SGR (%/day), and weight gain (%) of common carp (Cyprinus carpio) can be significantly improved when the fish receive a diet supplemented with probiotic Pediococcus pentosaceus for 45 days [41]. Similarly, a report from China indicated that the probiotics Limosilactobacillus reuteri FS3052, Lacticaseibacillus rhamnosus FS3051, and Bacillus subtilis natto NTU-18 significantly improved the growth indices of Grey mullet (Mugil cephalus) when the fish (1.07 ± 0.07 g) were fed a diet supplemented with probiotics for 28 days [42].
In the last few years, extensive research studies have been performed on a wide range of aquaculture species, especially fish and shrimp, to evaluate the impact of probiotic-based diets on their growth parameters and feed efficiency (Table 4). Many research studies have confirmed the positive correlation between the use of probiotic and fish growth indices; however, the exact mechanisms by which probiotics improve growth parameters are still not fully understood.
3.3.2. Mechanism of Action
According to Eliopoulos et al. [43] and Yang et al. [44], probiotics play an important role in improving feed palatability through the fermentation process. The improvement of feed palatability by probiotics can be attributed to their ability to modify the sensory characteristics of feed. For instance, the fermentation process carried out by certain probiotic strains can produce metabolites that enhance the aroma of feed, which plays an important role in sensory perception, making it more attractive to animals. Yang et al. [44] demonstrated that the palatability of traditional Chinese herbs could be effectively enhanced by probiotic fermentation, enhancing their flavor and taste. Furthermore, a recent study showed that the flavor and palatability of plants could be altered after their fermentation by probiotics, which results in the subsequent stimulation of the animals’ appetite by the formation of aroma and flavor compounds [45].
Lactic acid bacteria, particularly Lactobacillus lactis, have been shown to play a critical function in the digestion of the macro-nutrients of feedstuffs fed to fish, which has been associated with enhanced hydrolysis and increased nutrient utilization in several fish species [46]. Furthermore, probiotic-supplemented diets have been shown to have the capability to improve fish performance through enhancing the activity of digestive enzymes and improving food absorbability [17,47,48]. For example, Zhang et al. [49] noticed that the digestive enzymes (lipase, amylase, and trypsin) of farmed tilapia (Oreochromis niloticus) were efficiently improved after 45 days of the fish receiving diets supplemented with the probiotic Clostridium butyricum at concentrations of 1.5 × 1011 CFU/kg. In addition, the dietary inclusion of Lactobacillus spp. and L. pentosus at concentrations of 107 and 5 × 108 CFU per g improved several types of digestive enzymes of white shrimp (Litopenaeus vannamei) [50,51]. Furthermore, Tang et al. [52] noticed that the activity of protease and amylases enzymes of Siniperca chuatsi significantly improved when fish received a diet supplemented with the probiotic Bacillus velezensis GY65.

Table 4.
Specific effects of probiotics on fish growth performance and feed utilization based on recent studies.
Table 4.
Specific effects of probiotics on fish growth performance and feed utilization based on recent studies.
| Country | Host × Probiotic | Experimental Condition | Main Outcomes | Reference |
|---|---|---|---|---|
| China |
|
|
| [42] |
| Mexico |
|
|
| [53] |
| China |
|
|
| [54] |
| China |
|
|
| [52] |
| Egypt |
|
|
| [55] |
| China |
|
|
| [26] |
| Indonesia |
|
|
| [56] |
| Spain |
|
|
| [57] |
| Pakistan |
|
|
| [58] |
| China |
|
|
| [59] |
| Iran |
|
|
| [60] |
| China |
|
|
| [61] |
| Egypt |
|
|
| [62] |
AGR: average growth rate, FBW: final body weight, FCR: feed conversion ratio, SGR: significant growth rate, LG: body length gains, FL: final body length, FER: feed efficiency ratio, LGR: length gain rate, WGR: weight gain rate, PER: protein efficiency ratio. ↑: increase, ↓: decrease. Different letters mean significant differences. ns: non-significant.
3.4. Impact of Probiotic on Disease Resistance, Immunity and Water Quality
In addition to enhancing growth performance, the integration of probiotics in aquaculture has gained considerable attention for its ability to enhance disease resistance, modulate the immune system, improve gut health, and enhance microbial diversity within the guts of diverse fish and shrimp species. Thus, probiotics foster overall health in aquatic animals and contribute to the sustainability of aquaculture. In a previous study, Zokaeifar et al. [63] examined the impact of the probiotic Bacillus subtilis strains on juvenile white shrimp (Litopenaeus vannamei). They noticed that the cumulative mortality rate (%) of the shrimp in the control group following a challenge with Vibrio harveyi was 63.3%, while the probiotic groups exhibited significantly lower mortality rates, ranging from 20.0% to 33.3%.
A pivotal mechanism through which probiotics impart their salutary effects is by means of enhancing the expression of immune-related genes. For instance, the supplementation of Bacillus cereus in pengze crucian carp (Carassius auratus) has been shown to activate both specific and non-specific immune responses, thereby increasing their resistance to pathogens [64]. Furthermore, the administration of Lactobacillus plantarum has been associated with an enhancement in resistance against Aeromonas sobria in Nile tilapia (Oreochromis niloticus) [62] and A. hydrophila in Caspian whitefish (Rutilus frisii kutum) [65], suggesting a clear correlation between probiotic supplementation and enhanced disease resistance. A similar result has been recorded between the probiotic Bacillus clausii and increased lysozyme activity and complement C3 in grouper (Epinephelus coioides) following a 60-day period of feeding on a supplemented diet [66]. In recent years, numerous studies have examined the positive correlation between the administration of probiotics in aquatic animals and a reduction in pathogens, particularly Staphylococcus, Mycoplasma, and Weissella [67], Mycoplasma and Rhodobacter [42], and Vibrio harveyi [26] (Table 5).
Probiotic bacteria have been shown to have considerable beneficial effects on aquatic environments [68,69,70]. The concentrations of NO3⁻ (nitrate), NO2⁻ (nitrite), and ammonia (NH4+) are the main water quality parameters which effect fish performance and should be monitored regularly. These compounds are toxic to fish and can accumulate in aquatic ecosystems due to the decomposition of organic matter. Probiotics play a key role in reducing the concentrations of harmful substances in aquaculture water. Probiotic bacteria, particularly strains that belong to the genus Bacillus, have demonstrated the ability to assimilate nitrogen compounds and break down organic matter, thereby lowering the ammonia levels in water. It is believed that probiotic bacteria such as Clostridium butyricum can increase the number of denitrifying bacteria that can degrade NH4+ and NO3− in water, resulting in a large amount of NH4-N and NO3-N being degraded into substances that are harmless to fish [71]. This process not only improves water quality but also creates a healthier environment for fish growth and development. Recently, Liu et al. [72] noticed that a mixture of probiotics (Lactobacillus plantarum and Pediococcus pentosaceus) significantly reduced the concentrations of ammonia and nitrite in the aquatic environment of Tilapia (Oreochromis niloticus) when the fish received a diet supplemented with C. butyricum at 3.0 × 1010, 1.5 × 1011, and 3.0 × 1011 CFU/kg [49].

Table 5.
Impact of probiotics on disease resistance and immune-related parameters of some aquatic species.
Table 5.
Impact of probiotics on disease resistance and immune-related parameters of some aquatic species.
| Country | Host × Probiotic | Experimental Condition | Main Outcomes | Reference |
|---|---|---|---|---|
| China |
|
|
| [42] |
| Iran |
|
|
| [65] |
| China |
|
|
| [73] |
| China |
|
|
| [26] |
| China |
|
|
| [67] |
| China |
|
|
| [74] |
| Bangladesh |
|
|
| [75] |
| China |
|
|
| [76] |
| China |
|
|
| [77] |
| China |
|
|
| [78] |
| México |
|
|
| [79] |
| China |
|
|
| [49] |
| China |
|
|
| [72] |
ALT: serum alanine aminotransferase, AST: aspartate aminotransferase, IL-8, IL-1β, TNF-α, IFN-γ: immune-related genes. ↑: increase, ↓: decrease.
3.5. Multi-Strain Probiotics and Synbiotics in Aquaculture
3.5.1. Effect of Multi-Strain Probiotics
The utilization of multi-strain probiotics in aquaculture has garnered considerable attention due to their demonstrated capacity to enhance growth performance, fortify immune responses, and augment disease resistance in diverse aquatic species (Table 6). Multi-strain probiotics, which consist of a combination of different probiotic strains, can provide a synergistic effect that enhances the overall health and resilience of fish and shrimp against pathogens. For instance, the inclusion of multi-species probiotics has been shown to enhance the innate immune responses of whiteleg shrimp (Litopenaeus vannamei), leading to improved survival rates when they are challenged with pathogens such as Vibrio parahaemolyticus [80]. In a similar manner, dietary supplementation with a combination of Bacillus subtilis and Lactobacillus plantarum strains has been demonstrated to considerably enhance the immune parameters and disease resistance of Nile tilapia (O. niloticus) against Aeromonas hydrophila [62,81]. Furthermore, multi-strain probiotics have been associated with enhanced growth parameters in aquaculture species. Studies have shown that the use of multi-strain probiotic formulations can lead to better feed conversion ratios and increased weight gain in fish and shrimp [82]. For instance, a combination of Bacillus subtilis and Lactobacillus casei has been demonstrated to enhance growth performance in catfish (Clarias gariepinus) [83]. The synergistic effects of multiple strains have been demonstrated to optimize nutrient utilization and improve gut health, both of which are critical to growth and overall health in aquaculture [84].
3.5.2. Synergistic Effect of Probiotics and Prebiotics
The combination of probiotics and prebiotics in synbiotics has been shown to have a synergistic effect [85]. Such a combination could improve the survival of probiotic organisms because, in the absence of its food source, a probiotic would face significant challenges in surviving in the digestive system. This is due to their inability to tolerate oxygen and low-pH and low-temperature conditions [86]. Synbiotics are therefore used to enhance growth and stimulate immune responses in the host [87].
A study by Saba et al. [88] reported that synbiotics exhibited superior performance compared to single-strain and multi-strain probiotics. The improved efficacy of synbiotics over single-strain and multi-strain probiotics can be attributed to the synergistic effect that occurs when the combined action of probiotics and prebiotics produces health benefits greater than the sum of their individual effects [89]. This synergistic effect has the potential to enhance digestive enzymes, health, and immune function [90]. Synbiotics have been shown to modulate the gut microbial ecosystem more efficiently than probiotics or prebiotics used in separate forms [91]. This enhanced efficacy stems from the distinct mechanisms of action of probiotics and prebiotics, which can modulate the gut microbiota in varied ways. The combination of these two approaches has been shown to promote a more balanced and diverse gut microbiota composition [91]. Consequently, there is a significant need for further investigation, through empirical research, to explore the potential benefits of multi-strain probiotics and synbiotics. Such studies will facilitate more profound analyses, offering enhanced insights into the mechanisms underlying these effects.

Table 6.
Impact of the interaction between different strains of probiotics and synbiotics in aquaculture.
Table 6.
Impact of the interaction between different strains of probiotics and synbiotics in aquaculture.
| Country | Host × Probiotics | Experimental Condition | Main Outcomes | Reference |
|---|---|---|---|---|
| Bangladesh |
|
|
| [92] |
| Kazakhstan |
|
|
| [93] |
| Australia |
|
|
| [94] |
| China |
|
|
| [95] |
| Egypt |
|
|
| [96] |
| Republic of Korea |
|
|
| [97] |
| Indonesia |
|
|
| [83] |
| Republic of Korea |
|
|
| [74] |
| Pakistan |
|
|
| [98] |
| Pakistan |
|
|
| [99] |
↑: increase, ↓: decrease, Different letters mean significant differences. ns: non-significant differences (p < 0.05)
4. Key Limitations of Aquaculture Probiotics
The utilization of probiotics in aquaculture has received considerable attention as an alternative solution to the use of antibiotics to enhance fish performance. However, the use of probiotics in aquaculture has limitations despite its potential benefits. One of the main limitations of the use of probiotics in aquaculture is their specificity and variability across species and environmental conditions. Probiotics must be adapted to specific host species to achieve optimal results, as a bacterium that is beneficial to one species may not provide the same benefits to other hosts [100]. In addition, the bacteria that are beneficial to certain fish species may have a pathogenic effect on another fish species [100]. This species specification requires the extensive testing and characterization of probiotic strains before they can be effectively used in aquaculture [101]. In addition, the environmental conditions in which probiotics are applied, such as the water temperature, salinity, and pH, can significantly affect their survival, effectiveness, and stability [39,102]. For example, probiotics must be able to withstand the harsh conditions of the fish gastrointestinal tract, including exposure to bile salts and low pH [103]. In addition, some aquaculture probiotics have been associated with alterations in the dynamics of nutrient cycling. Although probiotics are frequently implemented to enhance nutrient utilization and promote water quality improvement, their presence can also result in unforeseen consequences. For instance, the introduction of particular probiotic strains has been observed to suppress the growth of cyanobacteria by increasing the relative abundance of other microbial groups, such as Verrucomicrobiota (from 2.61% to 6.35%) [104]. This alteration, while it seems beneficial, has the potential to disrupt the natural balance of primary producers in aquatic ecosystems, which may result in declines in biodiversity in aquatic ecosystems [104]. These factors may limit the number of probiotic strains that can be effectively used in aquaculture.
5. Conclusions
Probiotics have demonstrated efficacy in improving the growth performance, feed utilization, disease resistance, and water quality management in various aquaculture systems. The mechanisms underlying these effects, which include improving gut health, modulating immune responses, and reducing environmental stressors, position probiotics as an essential tool in promoting sustainable aquaculture. The present bibliometric study highlights the importance of probiotics as a viable solution within the context of aquaculture. A detailed analysis of global research trends reveals a substantial increase in the publication of APGD documents from 2019 to 2024, signifying a notable rise in scientific and practical interest in probiotics as eco-friendly alternatives to antibiotic use in aquaculture. Asia, particularly China, has emerged as a leader in research on the use of probiotics in aquaculture, driven by its substantial aquaculture industry. A bibliometric analysis using VOSviewer software also revealed a significant co-occurrence of keywords such as “disease resistance”, “growth performance”, and “gut microbiota”, underscoring their prominence in recent research efforts. This may indicate that the majority of APGD documents are focused on the relationship between disease resistance and probiotics.
According to the recently reviewed literature, probiotics such as Pediococcus spp., Lactobacillus casei, Lactobacillus planta, Clostridium butyricum, Bacillus subtilis, Saccharomyces cereviciae, and Halobacterium salinarum have shown remarkable improvements in a wide range of aquaculture species through many ways such as improving their growth parameters, feed utilization, and immune-related parameters. In addition, the synergistic effects of multi-strain probiotics and synbiotics have demonstrated promising results in optimizing health benefits, highlighting the need for further experimental investigation.
A well-conducted bibliometric review can play a powerful role in advancing the discipline of aquaculture probiotics by providing a comprehensive perspective, identifying the research gaps, and establishing guidelines for future research studies. Further research is needed to explore strain-specific effects, optimal dosages, timing, combinations of probiotics with prebiotics, and the impacts on aquatic ecosystems for the purpose of sustainable aquaculture. By incorporating probiotics into aquaculture practices, the industry can meet the growing demand for aquatic products while aligning with global sustainability goals.
Author Contributions
Conceptualization, E.A.H.M.; methodology, E.A.H.M.; software, E.A.H.M.; formal analysis, E.A.H.M.; investigation, E.A.H.M.; resources, K.P. and B.K.; data curation, E.A.H.M. and A.E.M.A.; writing—original draft preparation, E.A.H.M.; writing—review and editing, K.P., B.K. and A.E.M.A., visualization, E.A.H.M.; supervision, K.P.; project administration, K.P. and E.A.H.M.; funding acquisition, B.K. All authors have read and agreed to the published version of the manuscript.
Funding
Project No. TKP2021-NKTA-32 was implemented with support from the National Research, Development, and Innovation Fund of Hungary, financed under the TKP2021-NKTA funding scheme, and supported by the University of Debrecen Program for Scientific Publication. The current study is also supported by the Stipendium Hungaricum Scholarship (SHS) program administered by the Tempus Public Foundation (TPF), Hungary. In addition, we would like to express our deep thanks to the University of Debrecen, Hungary, for facilitating unrestricted open access publishing in MDPI.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting this study’s findings are available from the corresponding author upon request.
Conflicts of Interest
The author, Elshafia Ali Hamid Mohammed, was employed by the Agricultural Research Corporation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- FAO. The State of World Fisheries and Aquaculture; Food and Agriculture Organization: Rome, Italy, 2016. [Google Scholar]
- FAO. Yearbook. Fishery and Aquaculture Statistics 2017; Food and Agriculture Organization of the United Nation: Rome, Italy, 2019. [Google Scholar]
- Lewbart, G.A. Bacteria and Ornamental Fish. Semin. Avian Exot. Pet Med. 2001, 10, 48–56. [Google Scholar] [CrossRef]
- Abarike, E.D.; Jian, J.; Tang, J.; Cai, J.; Yu, H.; Lihua, C.; Jun, L. Influence of Traditional Chinese Medicine and Bacillus Species (TCMBS) on Growth, Immune Response and Disease Resistance in Nile Tilapia, Oreochromis Niloticus. Aquac. Res. 2018, 49, 2366–2375. [Google Scholar] [CrossRef]
- Hasan, K.N.; Banerjee, G. Recent Studies on Probiotics as Beneficial Mediator in Aquaculture: A Review. J. Basic Appl. Zool. 2020, 81, 53. [Google Scholar] [CrossRef]
- Gao, P.; Mao, D.; Luo, Y.; Wang, L.; Xu, B.; Xu, L. Occurrence of Sulfonamide and Tetracycline-Resistant Bacteria and Resistance Genes in Aquaculture Environment. Water Res. 2012, 46, 2355–2364. [Google Scholar] [CrossRef] [PubMed]
- Watts, J.; Schreier, H.; Lanska, L.; Hale, M. The Rising Tide of Antimicrobial Resistance in Aquaculture: Sources, Sinks and Solutions. Mar. Drugs 2017, 15, 158. [Google Scholar] [CrossRef]
- Elsabagh, M.; Mohamed, R.; Moustafa, E.M.; Hamza, A.; Farrag, F.; Decamp, O.; Dawood, M.A.O.; Eltholth, M. Assessing the Impact of Bacillus Strains Mixture Probiotic on Water Quality, Growth Performance, Blood Profile and Intestinal Morphology of Nile Tilapia, Oreochromis niloticus. Aquac. Nutr. 2018, 24, 1613–1622. [Google Scholar] [CrossRef]
- Won, S.; Hamidoghli, A.; Choi, W.; Bae, J.; Jang, W.J.; Lee, S.; Bai, S.C. Evaluation of Potential Probiotics Bacillus subtilis WB60, Pediococcus pentosaceus, and Lactococcus lactis on Growth Performance, Immune Response, Gut Histology and Immune-Related Genes in Whiteleg Shrimp, Litopenaeus vannamei. Microorganisms 2020, 8, 281. [Google Scholar] [CrossRef] [PubMed]
- Zibiene, G.; Zibas, A. Impact of Commercial Probiotics on Growth Parameters of European Catfish (Silurus glanis) and Water Quality in Recirculating Aquaculture Systems. Aquac. Int. 2019, 27, 1751–1766. [Google Scholar] [CrossRef]
- Gismondo, M.R.; Drago, L.; Lombardi, A. Review of Probiotics Available to Modify Gastrointestinal Flora. Int. J. Antimicrob. Agents 1999, 12, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Parker, R. Probiotics, the Other Half of the Antibiotic Story. Anim. Nutr. Health 1974, 4–6. [Google Scholar]
- FAO/WHO. Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Liver Lactic Acid Bacteria; Food and Agriculture Organization and World Health Organization Joint Report; FAO/WHO: Geneva, Switzerland, 2001. [Google Scholar]
- Mujeeb Rahiman, K.M.; Jesmi, Y.; Thomas, A.P.; Mohamed Hatha, A.A. Probiotic Effect of Bacillus NL110 and Vibrio NE17 on the Survival, Growth Performance and Immune Response of Macrobrachium Rosenbergii (de Man): Probiotic Effect of Bacteria on M. rosenbergii. Aquac. Res. 2010, 41, e120–e134. [Google Scholar] [CrossRef]
- Vijayan, K.K.; Bright Singh, I.S.; Jayaprakash, N.S.; Alavandi, S.V.; Somnath Pai, S.; Preetha, R.; Rajan, J.J.S.; Santiago, T.C. A Brackishwater Isolate of Pseudomonas PS-102, a Potential Antagonistic Bacterium against Pathogenic Vibrios in Penaeid and Non-Penaeid Rearing Systems. Aquaculture 2006, 251, 192–200. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Salminen, S.; Isolauri, E. Probiotics: An Overview of Beneficial Effects. Antonie Leeuwenhoek 2002, 82, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Yanbo, W.; Zirong, X. Effect of Probiotics for Common Carp (Cyprinus carpio) Based on Growth Performance and Digestive Enzyme Activities. Anim. Feed Sci. Technol. 2006, 127, 283–292. [Google Scholar] [CrossRef]
- Tovar-Ramírez, D.; Zambonino Infante, J.; Cahu, C.; Gatesoupe, F.J.; Vázquez-Juárez, R. Influence of Dietary Live Yeast on European Sea Bass (Dicentrarchus labrax) Larval Development. Aquaculture 2004, 234, 415–427. [Google Scholar] [CrossRef]
- Kari, Z.A.; Kabir, M.A.; Dawood, M.A.O.; Razab, M.K.A.A.; Ariff, N.S.N.A.; Sarkar, T.; Pati, S.; Edinur, H.A.; Mat, K.; Ismail, T.A.; et al. Effect of Fish Meal Substitution with Fermented Soy Pulp on Growth Performance, Digestive Enzyme, Amino Acid Profile, and Immune-Related Gene Expression of African Catfish (Clarias gariepinus). Aquaculture 2022, 546, 737418. [Google Scholar] [CrossRef]
- Boonthai, T.; Vuthiphandchai, V.; Nimrat, S. Probiotic Bacteria Effects on Growth and Bacterial Composition of Black Tiger Shrimp (Penaeus monodon): Probiotic Bacteria Effects on Black Tiger Shrimp. Aquac. Nutr. 2011, 17, 634–644. [Google Scholar] [CrossRef]
- Kumar, R.; Mukherjee, S.C.; Prasad, K.P.; Pal, A.K. Evaluation of Bacillus subtilis as a Probiotic to Indian Major Carp Labeo rohita (Ham.). Aquac. Res. 2006, 37, 1215–1221. [Google Scholar] [CrossRef]
- Silva, E.F.; Soares, M.A.; Calazans, N.F.; Vogeley, J.L.; Do Valle, B.C.; Soares, R.; Peixoto, S. Effect of Probiotic (Bacillus spp.) Addition during Larvae and Postlarvae Culture of the White Shrimp Litopenaeus vannamei. Aquac. Res. 2012, 44, 13–21. [Google Scholar] [CrossRef]
- Wu, Y.-S.; Chu, Y.-T.; Chen, Y.-Y.; Chang, C.-S.; Lee, B.-H.; Nan, F.-H. Effects of Dietary Lactobacillus reuteri and Pediococcus acidilactici on the Cultured Water Qualities, the Growth and Non-Specific Immune Responses of Penaeus vannamei. Fish Shellfish Immunol. 2022, 127, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Ahmadifar, M.; Esfahani, D.E.; Ahmadifar, E.; Sheikhzadeh, N.; Mood, S.M.; Moradi, S.Z. Combined Effects of Spirulina Platensis and Pediococcus acidilactici on the Growth Performance, Digestive Enzyme Activity, Antioxidative Status, and Immune Genes in Zebrafish. Ann. Anim. Sci. 2023, 23, 1159–1167. [Google Scholar] [CrossRef]
- Newaj-Fyzul, A.; Adesiyun, A.A.; Mutani, A.; Ramsubhag, A.; Brunt, J.; Austin, B. Bacillus Subtilis AB1 Controls Aeromonas Infection in Rainbow Trout (Oncorhynchus mykiss, Walbaum): Control of Aeromonas Infection in Rainbow Trout. J. Appl. Microbiol. 2007, 103, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Song, S.; Cui, C.; Cai, Y.; Zhou, Y.; Wang, J.; Bei, W.; Zhang, D.; Guo, W.; Wang, S. Strain-Specific Benefits of Bacillus Probiotics in Hybrid Grouper: Growth Enhancement, Metabolic Health, Immune Modulation, and Vibrio Harveyi Resistance. Animals 2024, 14, 1062. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. Software Survey: VOSviewer, a Computer Program for Bibliometric Mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gao, S. Current Status of Industrialized Aquaculture in China: A Review. Environ. Sci. Pollut. Res. 2023, 30, 32278–32287. [Google Scholar] [CrossRef]
- Choruma, D.J.; Balkovic, J.; Odume, O.N. Calibration and Validation of the EPIC Model for Maize Production in the Eastern Cape, South Africa. Agronomy 2019, 9, 494. [Google Scholar] [CrossRef]
- Hyland, K. Enter the Dragon: China and Global Academic Publishing. Learn. Publ. 2023, 36, 394–403. [Google Scholar] [CrossRef]
- Tucciarone, I.; Secci, G.; Contiero, B.; Parisi, G. Sustainable Aquaculture over the Last 30 Years: An Analysis of the Scientific Literature by the Text Mining Approach. Rev. Aquac. 2024, 16, 2064–2076. [Google Scholar] [CrossRef]
- Subasinghe, R.P.; Delamare-Deboutteville, J.; Mohan, C.V.; Phillips, M.J. Vulnerabilities in Aquatic Animal Production: -EN- -FR- Les Vulnérabilités de La Production d’animaux Aquatiques -ES- Factores de Vulnerabilidad de La Producción de Animales Acuáticos. Rev. Sci. Tech. OIE 2019, 38, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Mugimba, K.K.; Chengula, A.A.; Wamala, S.; Mwega, E.D.; Kasanga, C.J.; Byarugaba, D.K.; Mdegela, R.H.; Tal, S.; Bornstein, B.; Dishon, A.; et al. Detection of Tilapia Lake Virus (Ti LV) Infection by PCR in Farmed and Wild Nile Tilapia (Oreochromis niloticus) from Lake Victoria. J. Fish Dis. 2018, 41, 1181–1189. [Google Scholar] [CrossRef]
- Le, N.T.T.; Armstrong, C.W.; Brækkan, E.H.; Eide, A. Climatic Events and Disease Occurrence in Intensive Litopenaeus vannamei Shrimp Farming in the Mekong Area of Vietnam. Aquaculture 2024, 587, 740867. [Google Scholar] [CrossRef]
- Rebl, A.; Korytář, T.; Borchel, A.; Bochert, R.; Strzelczyk, J.E.; Goldammer, T.; Verleih, M. The Synergistic Interaction of Thermal Stress Coupled with Overstocking Strongly Modulates the Transcriptomic Activity and Immune Capacity of Rainbow Trout (Oncorhynchus mykiss). Sci. Rep. 2020, 10, 14913. [Google Scholar] [CrossRef] [PubMed]
- Eissa, E.-S.H.; Baghdady, E.S.; Gaafar, A.Y.; El-Badawi, A.A.; Bazina, W.K.; Abd Al-Kareem, O.M.; Abd El-Hamed, N.N.B. Assessing the Influence of Dietary Pediococcus acidilactici Probiotic Supplementation in the Feed of European Sea Bass (Dicentrarchus labrax L.) (Linnaeus, 1758) on Farm Water Quality, Growth, Feed Utilization, Survival Rate, Body Composition, Blood Biochemical Parameters, and Intestinal Histology. Aquac. Nutr. 2022, 2022, 5841220. [Google Scholar] [CrossRef]
- Eissa, M.E.H.; Alaryani, F.S.; Elbahnaswy, S.; Khattab, M.S.; Elfeky, A.; AbouelFadl, K.Y.; Eissa, E.-S.H.; Ahmed, R.A.; Van Doan, H.; El-Haroun, E. Dietary Inclusion of Pediococcus acidilactici Probiotic Promoted the Growth Indices, Hemato-Biochemical Indices, Enzymatic Profile, Intestinal and Liver Histomorphology, and Resistance of Nile Tilapia against Aspergillus flavus. Anim. Feed Sci. Technol. 2023, 306, 115814. [Google Scholar] [CrossRef]
- Huang, J.-B.; Wu, Y.-C.; Chi, S.-C. Dietary Supplementation of Pediococcus pentosaceus Enhances Innate Immunity, Physiological Health and Resistance to Vibrio anguillarum in Orange-Spotted Grouper (Epinephelus coioides). Fish Shellfish Immunol. 2014, 39, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Amenyogbe, E. Application of Probiotics for Sustainable and Environment-Friendly Aquaculture Management—A Review. Cogent Food Agric. 2023, 9, 2226425. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, B.; Li, Y.; Zhan, H.; Liu, Y.; Xiao, S.; Zhao, Q.; Wang, J. Dietary Supplementation with Sporosarcina Aquimarina MS4 Enhances Juvenile Sea Cucumber (Apostichopus japonicus) Growth, Immunity and Disease Resistance against Vibrio splendidus Infection at Low Temperature. Aquac. Nutr. 2021, 27, 918–926. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Sadegh, T.H.; Dawood, M.A.O.; Dadar, M.; Sheikhzadeh, N. The Effects of Dietary Pediococcus pentosaceus on Growth Performance, Hemato-Immunological Parameters and Digestive Enzyme Activities of Common Carp (Cyprinus carpio). Aquaculture 2020, 516, 734656. [Google Scholar] [CrossRef]
- Chan, C.-H.; Chen, L.-H.; Chen, K.-Y.; Chen, I.-H.; Lee, K.-T.; Lai, L.-C.; Tsai, M.-H.; Chuang, E.Y.; Lin, M.-T.; Yan, T.-R. Single-Strain Probiotics Enhance Growth, Anti-Pathogen Immunity, and Resistance to Nocardia seriolae in Grey Mullet (Mugil cephalus) via Gut Microbiota Modulation. Anim. Microbiome 2024, 6, 67. [Google Scholar] [CrossRef]
- Eliopoulos, C.; Markou, G.; Chorianopoulos, N.; Haroutounian, S.A.; Arapoglou, D. Transformation of Mixtures of Olive Mill Stone Waste and Oat Bran or Lathyrus clymenum Pericarps into High Added Value Products Using Solid State Fermentation. Waste Manag. 2022, 149, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, Y.; Zheng, L.; Peng, M.; Mai, Y.; Wang, X. Comparative Analysis of the Effect of Dietary Supplementation with Fermented and Water-Extracted Leaf Extracts of Eucommia ulmoides on Egg Production and Egg Nutrition. Foods 2024, 13, 1521. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Chitrakar, B.; Regenstein, J.M.; Sang, Y.; Zhou, P. Microbiology, Flavor Formation, and Bioactivity of Fermented Soybean Curd (Furu): A Review. Food Res. Int. 2023, 163, 112183. [Google Scholar] [CrossRef] [PubMed]
- Yeganeh, S.; Adel, M.; Nosratimovafagh, A.; Dawood, M.A.O. The Effect of Lactococcus lactis subsp. Lactis PTCC 1403 on the Growth Performance, Digestive Enzymes Activity, Antioxidative Status, Immune Response, and Disease Resistance of Rainbow Trout (Oncorhynchus mykiss). Probiotics Antimicrob. Proteins 2021, 13, 1723–1733. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, X.-W.; Zhu, J.-Y.; Liu, L.-L.; Liu, Y.-C.; Zhu, H. Dietary Sanguinarine Affected Immune Response, Digestive Enzyme Activity and Intestinal Microbiota of Koi Carp (Cryprinus carpiod). Aquaculture 2019, 502, 72–79. [Google Scholar] [CrossRef]
- Cao, H.; Yu, R.; Zhang, Y.; Hu, B.; Jian, S.; Wen, C.; Kajbaf, K.; Kumar, V.; Yang, G. Effects of Dietary Supplementation with β-Glucan and Bacillus subtilis on Growth, Fillet Quality, Immune Capacity, and Antioxidant Status of Pengze Crucian Carp (Carassius auratus Var. Pengze). Aquaculture 2019, 508, 106–112. [Google Scholar] [CrossRef]
- Zhang, M.; Dong, B.; Lai, X.; Chen, Z.; Hou, L.; Shu, R.; Huang, Y.; Shu, H. Effects of Clostridium Butyricum on Growth, Digestive Enzyme Activity, Antioxidant Capacity and Gut Microbiota in Farmed Tilapia (Oreochromis niloticus). Aquac. Res. 2021, 52, 1573–1584. [Google Scholar] [CrossRef]
- Zuo, Z.; Shang, B.; Shao, Y.; Li, W.; Sun, J. Screening of Intestinal Probiotics and the Effects of Feeding Probiotics on the Growth, Immune, Digestive Enzyme Activity and Intestinal Flora of Litopenaeus vannamei. Fish Shellfish Immunol. 2019, 86, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wang, B.; Jiang, K.; Wang, M.; Zhou, S.; Liu, M.; Wang, L. Exploring the Influence of the Surface Proteins on Probiotic Effects Performed by Lactobacillus pentosus HC-2 Using Transcriptome Analysis in Litopenaeus vannamei Midgut. Fish Shellfish Immunol. 2019, 87, 853–870. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.-Y.; Zhu, X.-Z.; Chen, J.-C.; Wang, Y.; Zhang, D.-F.; Liu, L.-Y.; Wang, Q.; Shi, C.-B.; Wang, Y.-Y.; Wang, Y.-J. In Vitro Antiviral Effect of Compound Chinese Herbal Medicine and Probiotic Fermentation Effect on Siniperca chuatsi. Aquac. Res. 2024, 2024, 9976156. [Google Scholar] [CrossRef]
- Pérez-Jiménez, G.M.; Alvarez-Villagomez, C.S.; Martínez-Porchas, M.; Garibay-Valdez, E.; Sepúlveda-Quiroz, C.A.; Méndez-Marín, O.; Martínez-García, R.; Jesús-Contreras, R.; Alvarez-González, C.A.; De La Rosa-García, S.D.C. The Indigenous Probiotic Lactococcus lactis PH3-05 Enhances the Growth, Digestive Physiology, and Gut Microbiota of the Tropical Gar (Atractosteus tropicus) Larvae. Animals 2024, 14, 2663. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Z.; He, H.; He, W.; Zhang, Z.; Sun, S.; Wang, W. Multi-Omics Analysis Reveals the Regulatory Mechanism of Different Probiotics on Growth Performance and Intestinal Health of Salmo trutta (S. trutta). Microorganisms 2024, 12, 1410. [Google Scholar] [CrossRef] [PubMed]
- Redhwan, A.; Eissa, E.-S.H.; Ezzo, O.H.; Abdelgeliel, A.S.; Munir, M.B.; Chowdhury, A.J.K.; Kari, Z.A.; Syafaat, M.N.; Suryani, A.E.; Eissa, M.E.H.; et al. Effects of Water Additive Mixed Probiotics on Water Quality, Growth Performance, Feed Utilization, Biochemical Analyses and Disease Resistance against Aeromonas sobria of Nile Tilapia. Desalination Water Treat. 2024, 319, 100480. [Google Scholar] [CrossRef]
- Mawardi, M.; Indrawati, A.; Lusiastuti, A.M.; Wibawan, I.W.T. Antibiotic Resistance Gene-Free Probiont Administration to Tilapia for Growth Performance and Streptococcus agalactiae Resistance. Vet. World 2023, 16, 2504–2514. [Google Scholar] [CrossRef]
- Messina, C.M.; Madia, M.; Manuguerra, S.; Espinosa-Ruiz, C.; Esteban, M.A.; Santulli, A. Dietary Inclusion of Halobacterium Salinarum Modulates Growth Performances and Immune Responses in Farmed Gilthead Seabream (Sparus aurata L.). Animals 2023, 13, 2743. [Google Scholar] [CrossRef]
- Noshair, I.; Kanwal, Z.; Jabeen, G.; Arshad, M.; Yunus, F.-U.-N.; Hafeez, R.; Mairaj, R.; Haider, I.; Ahmad, N.; Alomar, S.Y. Assessment of Dietary Supplementation of Lactobacillus rhamnosus Probiotic on Growth Performance and Disease Resistance in Oreochromis niloticus. Microorganisms 2023, 11, 1423. [Google Scholar] [CrossRef]
- Li, W.; Huang, X.; Lu, X.; Jiang, B.; Liu, C.; Huang, Y.; Su, Y. Effects of Dietary Lactobacillus Reuteri on Growth Performance, Nutrient Retention, Gut Health and Microbiota of the Nile Tilapia (Oreochromis niloticus). Aquac. Rep. 2022, 26, 101275. [Google Scholar] [CrossRef]
- Safari, R.; Imanpour, M.R.; Hoseinifar, S.H.; Faheem, M.; Dadar, M.; Van Doan, H. Effects of Dietary Lactobacillus casei on the Immune, Growth, Antioxidant, and Reproductive Performances in Male Zebrafish (Danio rerio). Aquac. Rep. 2022, 25, 101176. [Google Scholar] [CrossRef]
- Xia, R.; Hao, Q.; Xie, Y.; Zhang, Q.; Ran, C.; Yang, Y.; Zhou, W.; Chu, F.; Zhang, X.; Wang, Y.; et al. Effects of Dietary Saccharomyces cerevisiae on Growth, Intestinal and Liver Health, Intestinal Microbiota and Disease Resistance of Channel Catfish (Ictalurus punctatus). Aquac. Rep. 2022, 24, 101157. [Google Scholar] [CrossRef]
- Abou-El-Atta, M.E.; Abdel-Tawwab, M.; Abdel-Razek, N.; Abdelhakim, T.M.N. Effects of Dietary Probiotic Lactobacillus plantarum and Whey Protein Concentrate on the Productive Parameters, Immunity Response and Susceptibility of Nile Tilapia, Oreochromis niloticus (L.), to Aeromonas Sobria Infection. Aquac. Nutr. 2019, 25, 1367–1377. [Google Scholar] [CrossRef]
- Zokaeifar, H.; Balcázar, J.L.; Saad, C.R.; Kamarudin, M.S.; Sijam, K.; Arshad, A.; Nejat, N. Effects of Bacillus Subtilis on the Growth Performance, Digestive Enzymes, Immune Gene Expression and Disease Resistance of White Shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2012, 33, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Cao, H.; Jiang, W.; Hu, B.; Jian, S.; Wen, C.; Kajbaf, K.; Kumar, V.; Tao, Z.; Peng, M. Dietary Supplementation of Bacillus cereus as Probiotics in Pengze Crucian Carp (Carassius auratus Var. Pengze): Effects on Growth Performance, Fillet Quality, Serum Biochemical Parameters and Intestinal Histology. Aquac. Res. 2019, 50, 2207–2217. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Hoseini, S.M.; Taheri Mirghaed, A.; Ghelichpour, M.; Shirzad-Aski, H.; Van Doan, H.; El-Haroun, E.; Safari, R.; Khanzadeh, M. Comparison of the Effects of Host-Associated (Autochthonous) and Commercial Probiotics on Immune Responses, Growth Parameters and Intestinal Microbiota of Caspian Whitefish (Rutilus frisii kutum) Fry. Front. Mar. Sci. 2024, 11, 1446927. [Google Scholar] [CrossRef]
- Wang, J.; Yang, H.-L.; Xia, H.-Q.; Ye, J.-D.; Lu, K.-L.; Hu, X.; Feng, Y.; Ruan, L.; Sun, Y.-Z. Supplementation of Heat-Inactivated Bacillus clausii DE5 in Diets for Grouper, Epinephelus coioides, Improves Feed Utilization, Intestinal and Systemic Immune Responses and Not Growth Performance. Aquac. Nutr. 2018, 24, 821–831. [Google Scholar] [CrossRef]
- Yu, G.; Zhao, S.; Ou, W.; Ai, Q.; Zhang, W.; Mai, K.; Zhang, Y. Host-Associated Bacillus Velezensis T20 Improved Disease Resistance and Intestinal Health of Juvenile Turbot (Scophthalmus maximus). Aquac. Rep. 2024, 35, 101927. [Google Scholar] [CrossRef]
- Nathanailides, C.; Kolygas, M.; Choremi, K.; Mavraganis, T.; Gouva, E.; Vidalis, K.; Athanassopoulou, F. Probiotics Have the Potential to Significantly Mitigate the Environmental Impact of Freshwater Fish Farms. Fishes 2021, 6, 76. [Google Scholar] [CrossRef]
- Hancz, C. Application of Probiotics for Environmentally Friendly and Sustainable Aquaculture: A Review. Sustainability 2022, 14, 15479. [Google Scholar] [CrossRef]
- Iribarren, D.; Dagá, P.; Moreira, M.T.; Feijoo, G. Potential environmental effects of probiotics used in aquaculture. Aquac. Int. 2012, 20, 779–789. [Google Scholar] [CrossRef]
- Randall, D.J.; Tsui, T.K.N. Ammonia Toxicity in Fish. Mar. Pollut. Bull. 2002, 45, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wu, W.; Xu, X.; Wang, Y.; Yu, T.; Wang, J.; Zheng, Q.; Changru, X.; Wu, P. Rhodopseudomonas palustris in Effluent Enhances the Disease Resistance, TOR and NF-κB Signalling Pathway, Intestinal Microbiota and Aquaculture Water Quality of Pelteobagrus vachelli. Aquac. Res. 2020, 51, 3959–3971. [Google Scholar] [CrossRef]
- Hu, X.; Xu, W.; Li, H.; Lu, B.; Du, Y.; Chen, J. Investigation of the Protective Effect of Probiotic Lactobacillus plantarum Ep-M17 on the Hepatopancreas of Penaeus vannamei. Aquac. Nutr. 2024, 2024, 8216782. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.; Chang, C.-C.; Lin, Y.-H.; Lin, Y.-H. Effect of Fermented Lemon Peel as a Functional Feed Additive on Growth, Non-Specific Immune Responses, and Vibrio alginolyticus Resistance in Whiteleg Shrimp, Litopenaeus vannamei. Aquac. Rep. 2024, 34, 101918. [Google Scholar] [CrossRef]
- Ferdous, Z.; Hossain, M.K.; Hadiuzzaman, M.; Rafiquzzaman, S.M.; Halim, K.A.; Rahman, T.; Faruk, M.A.R.; Kari, Z.A.; Shahjahan, M. Multi-Species Probiotics Enhance Survival, Growth, Intestinal Microbiota and Disease Resistance of Rohu (Labeo rohita) Larvae. Water Biol. Secur. 2024, 3, 100234. [Google Scholar] [CrossRef]
- Ji, Z.; Zhu, C.; Zhu, X.; Ban, S.; Yu, L.; Tian, J.; Dong, L.; Wen, H.; Lu, X.; Jiang, M. Dietary Host-Associated Bacillus subtilis Supplementation Improves Intestinal Microbiota, Health and Disease Resistance in Chinese Perch (Siniperca chuatsi). Anim. Nutr. 2023, 13, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-H.; Chen, S.-W.; Wen, Z.-H.; Hu, S.-Y. Administration of the Potential Probiotic Paenibacillus ehimensis NPUST1 Enhances Expression of Indicator Genes Associated with Nutrient Metabolism, Growth and Innate Immunity against Aeromonas hydrophila and Streptococcus indie Infections in Zebrafish (Danio rerio). Fishes 2022, 7, 386. [Google Scholar] [CrossRef]
- Xue, M.; Wu, Y.; Hong, Y.; Meng, Y.; Xu, C.; Jiang, N.; Li, Y.; Liu, W.; Fan, Y.; Zhou, Y. Effects of Dietary Bacillus amyloliquefaciens on the Growth, Immune Responses, Intestinal Microbiota Composition and Disease Resistance of Yellow Catfish, Pelteobagrus fulvidraco. Front. Cell. Infect. Microbiol. 2022, 12, 1047351. [Google Scholar] [CrossRef]
- Becerril-Cortés, D.; Hamdan-Partida, A.; Mata-Sotres, J.A.; Coelho Emerenciano, M.G.; Monroy-Dosta, M.D.C. Yeast Rhodoturula Glutinis as a Modulator of Innate Immune and Oxidative Stress-Related Genes in Oreochromis Niloticus Cultured in a Biofloc System. Lat. Am. J. Aquat. Res. 2022, 50, 739–752. [Google Scholar] [CrossRef]
- Kesselring, J.C.; Gruber, C.; Standen, B.; Wein, S. Continuous and pulse-feeding application of multispecies probiotic bacteria in whiteleg shrimp, Litopenaeus vannamei. J. World Aquac. Soc. 2019, 50, 1123–1132. [Google Scholar] [CrossRef]
- Giri, S.S.; Sukumaran, V.; Sen, S.S.; Jena, P.K. Effects of Dietary Supplementation of Potential Probiotic Bacillus subtilis VSG1 Singularly or in Combination with Lactobacillus plantarum VSG3 or/and Pseudomonas aeruginosa VSG2 on the Growth, Immunity and Disease Resistance of Labeo rohita. Aquac. Nutr. 2014, 20, 163–171. [Google Scholar] [CrossRef]
- Puvanasundram, P.; Chong, C.M.; Sabri, S.; Yusoff, M.S.; Karim, M. Multi-Strain Probiotics: Functions, Effectiveness and Formulations for Aquaculture Applications. Aquac. Rep. 2021, 21, 100905. [Google Scholar] [CrossRef]
- Aini, N.; Putri, D.S.Y.R.; Achhlam, D.H.; Fatimah, F.; Andriyono, S.; Hariani, D.; Do, H.D.K.; Wahyuningsih, S.P.A. Supplementation of Bacillus subtilis and Lactobacillus casei to Increase Growth Performance and Immune System of Catfish (Clarias gariepinus) Due to Aeromonas hydrophila Infection. Vet. World 2024, 17, 602–611. [Google Scholar] [CrossRef]
- Zabidi, A.; Yusoff, F.M.; Amin, N.; Yaminudin, N.J.M.; Puvanasundram, P.; Karim, M.M.A. Effects of Probiotics on Growth, Survival, Water Quality and Disease Resistance of Red Hybrid Tilapia (Oreochromis spp.) Fingerlings in a Biofloc System. Animals 2021, 11, 3514. [Google Scholar] [CrossRef]
- Roberfroid, M.B. Prebiotics and Synbiotics: Concepts and Nutritional Properties. Br. J. Nutr. 1998, 80, 197–202. [Google Scholar] [CrossRef]
- Collins, M.D.; Gibson, G.R. Probiotics, Prebiotics, and Synbiotics: Approaches for Modulating the Microbial Ecology of the Gut. Am. J. Clin. Nutr. 1999, 69, 1052–1057. [Google Scholar] [CrossRef]
- Nayak, S. Probiotics and Immunity: A Fish Perspective. Fish Shellfish Immunol. 2010, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Saba, A.O.; Yasin, I.S.M.; Azmai, M.N.A. Meta-Analyses Indicate That Dietary Probiotics Significantly Improve Growth, Immune Response, and Disease Resistance in Tilapia. Aquac. Int. 2024, 32, 4841–4867. [Google Scholar] [CrossRef]
- Butt, U.D.; Lin, N.; Akhter, N.; Siddiqui, T.; Li, S.; Wu, B. Overview of the Latest Developments in the Role of Probiotics, Prebiotics and Synbiotics in Shrimp Aquaculture. Fish Shellfish Immunol. 2021, 114, 263–281. [Google Scholar] [CrossRef]
- Yousefi, M.; Naderi Farsani, M.; Ghafarifarsani, H.; Raeeszadeh, M. Dietary Lactobacillus helveticus and Gum Arabic Improves Growth Indices, Digestive Enzyme Activities, Intestinal Microbiota, Innate Immunological Parameters, Antioxidant Capacity, and Disease Resistance in Common Carp. Fish Shellfish Immunol. 2023, 135, 108652. [Google Scholar] [CrossRef] [PubMed]
- Hijová, E. Synbiotic Supplements in the Prevention of Obesity and Obesity-Related Diseases. Metabolites 2022, 12, 313. [Google Scholar] [CrossRef] [PubMed]
- Shahriar, F.; Ibnasina, A.H.E.A.; Uddin, N.; Munny, F.J.; Hussain, M.A.; Jang, W.J.; Lee, J.M.; Lee, E.-W.; Hasan, M.T.; Kawsar, M.A. Effects of Commercial Probiotics on the Growth, Hematology, Immunity, and Resistance to Aeromonas hydrophila Challenge in Climbing Perch, Anabas testudineus. Aquac. Res. 2024, 2024, 3901035. [Google Scholar] [CrossRef]
- Paritova, A.; Nurgaliyev, A.; Nurgaliyeva, G.; Abekeshev, N.; Abuova, A.; Zakirova, F.; Zwierzchowski, G.; Kuanchaleyev, Z.; Issabekova, S.; Kizatova, M.; et al. The Dietary Effects of Two Strain Probiotics (Leuconostoc mesenteroides, Lactococcus lactis) on Growth Performance, Immune Response and Gut Microbiota in Nile Tilapia (Oreochromis niloticus). PLoS ONE 2024, 19, e0312580. [Google Scholar] [CrossRef] [PubMed]
- Siddik, M.A.B.; Francis, D.S.; Islam, S.M.M.; Salini, M.J.; Fotedar, R. Fermentation and Fortification of Sargassum Linearifolium with Multi-Strain Probiotics Improves Mucosal Barrier Status, Inflammatory Response and Resistance to Vibrio Harveyi Infection in Barramundi Lates Calcarifer. Aquaculture 2025, 595, 741502. [Google Scholar] [CrossRef]
- Xie, G.; Chen, X.; Feng, Y.; Yu, Z.; Lu, Q.; Li, M.; Ye, Z.; Lin, H.; Yu, W.; Shu, H. Effects of Dietary Multi-Strain Probiotics on Growth Performance, Antioxidant Status, Immune Response, and Intestinal Microbiota of Hybrid Groupers (Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂). Microorganisms 2024, 12, 1358. [Google Scholar] [CrossRef]
- Shehata, A.I.; Soliman, A.A.; Ahmed, H.A.; Gewaily, M.S.; Amer, A.A.; Shukry, M.; Abdel-Latif, H.M.R. Evaluation of Different Probiotics on Growth, Body Composition, Antioxidant Capacity, and Histoarchitecture of Mugil capito. Sci. Rep. 2024, 14, 7379. [Google Scholar] [CrossRef]
- Cadangin, J.; Lee, J.-H.; Jeon, C.-Y.; Lee, E.-S.; Moon, J.-S.; Park, S.-J.; Hur, S.-W.; Jang, W.-J.; Choi, Y.-H. Effects of Dietary Supplementation of Bacillus, β-Glucooligosaccharide and Their Synbiotic on the Growth, Digestion, Immunity, and Gut Microbiota Profile of Abalone, Haliotis discus hannai. Aquac. Rep. 2024, 35, 102027. [Google Scholar] [CrossRef]
- Muhammad, Z.; Anjum, M.Z.; Akhter, S.; Irfan, M.; Amin, S.; Jamal, Y.; Khalid, S.; Ghazanfar, S. Effect of Lactobacillus plantarum and Pediococcus pentosaceus on the Growth Performance and Morphometry of the Genetically Improved Farmed Tilapia (Oreochromis niloticus). Pak. J. Zool. 2023, 56, 1–8. [Google Scholar] [CrossRef]
- Ullah, S.; Shao, Q.-J.; Ullah, I.; Zuberi, A.; Khattak, M.N.K.; Dawar, F.U.; Imran, M.; Ullah, A.; Shah, A.B. Commercially Available Probiotic Enhanced Growth, Digestion and Immune Response of Rohu (Labeo rohita) Reared in Earthen Pond. Isr. J. Aquac. Bamidgeh 2020, 72, 1–10. [Google Scholar] [CrossRef]
- Hai, N.V. The Use of Probiotics in Aquaculture. J. Appl. Microbiol. 2015, 119, 917–935. [Google Scholar] [CrossRef]
- Vulla, K.E.; Mmanda, F.P.; Nyangoko, B.P.; Makule, E.E. Unlocking Potential Benefits on Applications of Probiotics in Inland Aquaculture Industry: A Review. Aquac. Fish Fish. 2024, 4, e70027. [Google Scholar] [CrossRef]
- Shefat, S.H.T. Probiotic strains used in aquaculture. Int. Res. J. Microbiol. 2018, 7, 43–55. [Google Scholar] [CrossRef]
- Jaafar, R.S.; Al-Knany, F.N.; Mahdi, B.A.; Al-Taee, A.M.R. Study the Probiotic Properties of Pediococcus pentosaceus Isolated from Fish Ponds in Basra City, South of Iraq. J. Pure Appl. Microbiol. 2019, 13, 2343–2351. [Google Scholar] [CrossRef]
- Mang, Q.; Gao, J.; Li, Q.; Sun, Y.; Xu, G.; Xu, P. Metagenomic Insight into the Effect of Probiotics on Nitrogen Cycle in the Coilia nasus Aquaculture Pond Water. Microorganisms 2024, 12, 627. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).