Recent Advances in Biofilm Control Technologies for the Food Industry
Abstract
1. Introduction
2. Physical Strategies for Controlling Bacterial Biofilms in the Food Industry
2.1. Thermal Processing for Biofilm Removal in Food Processing Environments
2.2. Bioelectric Effects for Enhanced Biofilm Control in Food Processing Applications
2.3. Applications of Ultrasound for Biofilm Disruption in Food Industry
2.4. Promising Surface Modification Strategies for Biofilm Control in Food Equipment
3. Chemical Treatments in Food Processing and Biofilm Control
3.1. Chemical Agents for Biofilm Control in Food Processing
3.2. Acidulants for Biofilm Control in Food Processing
3.3. Enzyme-Based Approaches for Biofilm Control in Food Processing
4. Alternative Methods for Biofilm Elimination in Food Industry
4.1. Challenges and Potential of Bacteriophages in Food Biofilm Management
4.2. Plant-Based and Peptide Solutions for Biofilm Control in Food Safety
4.3. Probiotics for Biofilm Control in Food Systems
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Chen, D.; Peters, B.M.; Li, L.; Li, B.; Xu, Z.; Shirliff, M.E. Staphylococcal chromosomal cassettes mec (SCCmec): A mobile genetic element in methicillin-resistant Staphylococcus aureus. Microb. Pathogen. 2016, 101, 56–67. [Google Scholar] [CrossRef]
- Redmond, E.C.; Griffith, C.J. Consumer food handling in the home: A review of food safety studies. J. Food Prot. 2003, 66, 130–161. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Lin, C.H.; Aljuffali, I.A.; Fang, J.Y. Current pathogenic Escherichia coli foodborne outbreak cases and therapy development. Arch. Microbiol. 2017, 199, 811–825. [Google Scholar] [CrossRef]
- Nahar, S.; Mizan, M.F.R.; Ha, A.J.; Ha, S.D. Advances and future prospects of enzyme-based biofilm prevention approaches in the food industry. Comprehen. Rev. Food Sci. Food Saf. 2018, 17, 1484–1502. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, M.; Warner, R.D.; Fang, Z. Incorporating nisin and grape seed extract in chitosan-gelatine edible coating and its effect on cold storage of fresh pork. Food Cont. 2020, 110, 107018. [Google Scholar] [CrossRef]
- El-Saber Batiha, G.; Hussein, D.E.; Algammal, A.M.; George, T.T.; Jeandet, P.; Al-Snafi, A.E.; Tiwari, A.; Pagnossa, J.P.; Lima, C.M.; Thorat, N.D.; et al. Application of natural antimicrobials in food preservation: Recent views. Food Cont. 2021, 126, 108066. [Google Scholar] [CrossRef]
- Aladhadh, M. A review of modern methods for the detection of foodborne pathogens. Microorganisms 2023, 11, 1111. [Google Scholar] [CrossRef]
- Negash, A.W.; Tsehai, B.A. Current applications of bacteriocin. Int. J. Microbiol. 2020, 2020, 4374891. [Google Scholar] [CrossRef] [PubMed]
- Alonso, V.P.P.; Kabuki, D.Y. Formation and dispersal of biofilms in dairy substrates. Int. J. Dairy Technol. 2019, 72, 472–478. [Google Scholar] [CrossRef]
- Amrutha, B.; Sundar, K.; Shetty, P.H. Effect of organic acids on biofilm formation and quorum signaling of pathogens from fresh fruits and vegetables. Microb. Pathogen. 2017, 111, 156–162. [Google Scholar] [CrossRef]
- Chmielewski, R.A.N.; Frank, J.F. Biofilm formation and control in food processing facilities. Comprehen. Rev. Food Sci. Food Saf. 2003, 2, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Galie, S.; Garcia-Gutierrez, C.; Miguelez, E.M.; Villar, C.J.; Lombo, F. Biofilms in the food industry: Health aspects and control methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef]
- Valderrama, W.B.; Cutter, C.N. An ecological perspective of Listeria monocytogenes biofilms in food processing facilities. Crit. Rev. Food Sci. Nutr. 2013, 53, 801–817. [Google Scholar] [CrossRef]
- Zhu, T.; Yang, C.; Bao, X.; Chen, F.; Guo, X. Strategies for controlling biofilm formation in food industry. Grain Oil Sci. Technol. 2022, 5, 179–186. [Google Scholar] [CrossRef]
- Simões, M.; Simões, L.C.; Vieira, M.J. A review of current and emergent biofilm control strategies. LWT-Food Sci. Technol. 2010, 43, 573–583. [Google Scholar] [CrossRef]
- de Souza, E.L.; Meira, Q.G.; de Medeiros Barbosa, I.; Athayde, A.J.; da Conceição, M.L.; de Siqueira Júnior, J.P. Biofilm formation by Staphylococcus aureus from food contact surfaces in a meat-based broth and sensitivity to sanitizers. Braz. J. Microbiol. 2014, 45, 67–75. [Google Scholar] [CrossRef]
- Yang, Y.; Hoe, Y.W.; Zheng, Q.; Chung, H.J.; Yuk, H.G. Biofilm formation by Salmonella Enteritidis in a simulated liquid egg processing environment and its sensitivity to chlorine and hot water treatment. Food Cont. 2017, 73, 595–600. [Google Scholar] [CrossRef]
- Hoseinzadeh, E.; Wei, C.; Farzadkia, M.; Rezaee, A. Effects of low frequency-low voltage alternating electric current on apoptosis progression in bioelectrical reactor biofilm. Front. Bioeng. Biotechnol. 2020, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- van der Borden, A.J.; van der Mei, H.C.; Busscher, H.J. Electric block current induced detachment from surgical stainless steel and decreased viability of Staphylococcus epidermidis. Biomaterials 2005, 26, 6731–6735. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Malan, S.M.; Karau, M.J.; Cede, J.; Greenwood-Quaintance, K.E.; Brinkman, C.L.; Mandrekar, J.N.; Patel, R. Antibiofilm activity of low-amperage continuous and intermittent direct electrical current. Antimicrob. Agents Chemother. 2015, 59, 4610–4615. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Abdallah, M.; Campistron, P.; Moulin, E.; Callens, D.; Khelissa, S.O.; Debreyne, P.; Chihib, N.-E.; Delaplace, G. Detection of biofilm formation by ultrasonic Coda Wave Interferometry. J. Food Eng. 2021, 290, 110219. [Google Scholar] [CrossRef]
- Sun, J.; Wang, D.; Sun, Z.; Liu, F.; Du, L.; Wang, D. The combination of ultrasound and chlorogenic acid to inactivate Staphylococcus aureus under planktonic, biofilm, and food systems. Ultrason. Sonochem. 2021, 80, 105801. [Google Scholar] [CrossRef]
- Unal Turhan, E.; Polat, S.; Erginkaya, Z.; Konuray, G. Investigation of synergistic antibacterial effect of organic acids and ultrasound against pathogen biofilms on lettuce. Food Biosci. 2022, 47, 101643. [Google Scholar] [CrossRef]
- Shao, L.; Dong, Y.; Chen, X.; Xu, X.; Wang, H. Modeling the elimination of mature biofilms formed by Staphylococcus aureus and Salmonella spp. Using combined ultrasound and disinfectants. Ultrason. Sonochem. 2020, 69, 105269. [Google Scholar] [CrossRef] [PubMed]
- Awad, T.S.; Asker, D.; Hatton, B.D. Food-safe modification of stainless steel food-processing surfaces to reduce bacterial biofilms. ACS Appl. Mater. Interfaces 2018, 10, 22902–22912. [Google Scholar] [CrossRef]
- Fontecha-Umaña, F.; Ríos-Castillo, A.G.; Ripolles-Avila, C.; Rodríguez-Jerez, J.J. Antimicrobial activity and bacterial biofilm prevention on polyester surfaces with silver and zinc oxide nanoparticles at varying concentrations. Foods 2020, 9, 442. [Google Scholar] [CrossRef]
- Pontin, K.P.; Borges, K.A.; Furian, T.Q.; Carvalho, D.; Wilsmann, D.E.; Cardoso, H.R.P.; Alves, A.K.; Chitolina, G.Z.; Salle, C.T.P.; Moraes, H.L.d.S.; et al. Antimicrobial activity of copper surfaces against biofilm formation by Salmonella Enteritidis and its potential application in the poultry industry. Food Microbiol. 2021, 94, 103645. [Google Scholar] [CrossRef] [PubMed]
- Pijls, B.G.; Sanders, I.; Kujiper, E.J.; Nelissen, R. Induction heating for eradicating Staphylococcus epidermidis from biofilm. Bone Jt. Res. 2020, 9, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Scher, K.; Romling, U.; Yaron, S. Effect of heat, acidification, and chlorination on Salmonella enterica serovar typhimurium cells in a biofilm formed at the air-liquid interface. Appl. Environ. Microbiol. 2005, 71, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Hu, X.; Ren, L. Biofilm control strategies in food industry: Inhibition and utilization. Trends Food Sci. Technol. 2022, 123, 103–113. [Google Scholar] [CrossRef]
- Ravikumar, K.; Basu, B.; Dubey, A.K. Analysis of electrical analogue of a biological cell and its response to external electric field. Regen. Eng. Transl. Med. 2019, 5, 10–21. [Google Scholar] [CrossRef]
- Barki, K.G.; Das, A.; Dixith, S.; Ghatak, P.D.; Mathew-Steiner, S.; Schwab, E.; Khanna, S.; Wozniak, D.J.; Roy, S.; Sen, C.K. Electric field based dressing disrupts mixed-species bacterial biofilm infection and restores functional wound healing. Ann. Surg. 2019, 269, 756–766. [Google Scholar] [CrossRef]
- Ruiz-Ruigomez, M.; Badiola, J.; Schmidt-Malan, S.M.; Greenwood-Quaintance, K.; Karau, M.J.; Brinkman, C.L.; Mandrekar, J.N.; Patel, R. Direct electrical current reduces bacterial and yeast biofilm formation. Int. J. Bacteriol. 2016, 2016, 9727810. [Google Scholar] [CrossRef]
- del Pozo, J.L.; Rouse, M.S.; Mandrekar, J.N.; Steckelberg, J.M.; Patel, R. The electricidal effect: Reduction of Staphylococcus and Pseudomonas biofilms by prolonged exposure to low-intensity electrical current. Antimicrob. Agents Chemother. 2009, 53, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Liu, Y.; Li, L.; Guo, Y.; Xie, Y.; Cheng, Y.; Yao, W. Ultrasound-involved emerging strategies for controlling foodborne microbial biofilms. Trends Food Sci. Technol. 2020, 96, 91–101. [Google Scholar] [CrossRef]
- Vyas, N.; Manmi, K.; Wang, Q.; Jadhav, A.J.; Barigou, M.; Sammons, R.L.; Kuehne, S.A.; Walmsley, A.D. Which parameters affect biofilm removal with acoustic cavitation? A review. Ultrasound Med. Biol. 2019, 45, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Erriu, M.; Blus, C.; Szmukler-Moncler, S.; Buogo, S.; Levi, R.; Barbato, G.; Madonnaripa, D.; Denotti, G.; Piras, V.; Orru, G. Microbial biofilm modulation by ultrasound: Current concepts and controversies. Ultrason. Sonochem. 2014, 21, 15–22. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- LuTheryn, G.; Glynne-Jones, P.; Webb, J.S.; Carugo, D. Ultrasound-mediated therapies for the treatment of biofilms in chronic wounds: A review of present knowledge. Microb. Biotechnol. 2020, 13, 613–628. [Google Scholar] [CrossRef]
- Oulahal, N.; Martial-Gros, A.; Bonneau, M.; Blum, L.J. Removal of meat biofilms from surfaces by ultrasounds combined with enzymes and/or a chelating agent. Innov. Food Sci. Emerg. Technol. 2007, 8, 192–196. [Google Scholar] [CrossRef]
- Sultana, S.T.; Babauta, J.T.; Beyenal, H. Electrochemical biofilm control: A review. Biofouling 2015, 31, 745–758. [Google Scholar] [CrossRef]
- Gomes, I.B.; Simões, M.; Simões, L.C. Copper surfaces in biofilm control. Nanomaterials 2020, 10, 2491. [Google Scholar] [CrossRef] [PubMed]
- Damrongsaktrakul, P.; Ruengvisesh, S.; Rahothan, A.; Sukhumrat, N.; Tuitemwong, P.; Phung-On, I. Removal of Salmonella Typhimurium biofilm from food contact surfaces using quercus infectoria gall extract in combination with a surfactant. J. Microbiol. Biotechnol. 2021, 31, 439–446. [Google Scholar] [CrossRef]
- Byun, K.-H.; Han, S.H.; Yoon, J.-w.; Park, S.H.; Ha, S.-D. Efficacy of chlorine-based disinfectants (sodium hypochlorite and chlorine dioxide) on Salmonella Enteritidis planktonic cells, biofilms on food contact surfaces and chicken skin. Food Cont. 2021, 123, 107838. [Google Scholar] [CrossRef]
- Bonneville, L.; Maia, V.; Barroso, I.; Martínez-Suárez, J.V.; Brito, L. Lactobacillus plantarum in dual-species biofilms with Listeria monocytogenes enhanced the anti-Listeria activity of a commercial disinfectant based on hydrogen peroxide and peracetic acid. Front. Microbiol. 2021, 12, 631627. [Google Scholar] [CrossRef]
- Kim, H.; Moon, M.J.; Kim, C.Y.; Ryu, K. Efficacy of chemical sanitizers against Bacillus cereus on food contact surfaces with scratch and biofilm. Food Sci. Biotechnol. 2019, 28, 581–590. [Google Scholar] [CrossRef]
- Akbas, M.Y.; Cag, S. Use of organic acids for prevention and removal of Bacillus subtilis biofilms on food contact surfaces. Food Sci. Technol. Int. 2016, 22, 587–597. [Google Scholar] [CrossRef]
- Cisneros, L.; Cattelan, N.; Villalba, M.I.; Rodriguez, C.; Serra, D.O.; Yantorno, O.; Fadda, S. Lactic acid bacteria biofilms and their ability to mitigate Escherichia coli O157:H7 surface colonization. Lett. Appl. Microbiol. 2021, 73, 247–256. [Google Scholar] [CrossRef]
- Toushik, S.H.; Kim, K.; Ashrafudoulla, M.; Mizan, M.F.R.; Roy, P.K.; Nahar, S.; Kim, Y.; Ha, S.-D. Korean kimchi-derived lactic acid bacteria inhibit foodborne pathogenic biofilm growth on seafood and food processing surface materials. Food Cont. 2021, 129, 108276. [Google Scholar] [CrossRef]
- Li, Y.; Dong, R.; Ma, L.; Qian, Y.; Liu, Z. Combined anti-biofilm enzymes strengthen the eradicate effect of Vibrio parahaemolyticus biofilm: Mechanism on cpsA-J expression and application on different carriers. Foods 2022, 11, 1305. [Google Scholar] [CrossRef]
- Ge, L.; Zhao, Y.-S.; Mo, T.; Li, J.-R.; Li, P. Immobilization of glucose oxidase in electrospun nanofibrous membranes for food preservation. Food Cont. 2012, 26, 188–193. [Google Scholar] [CrossRef]
- Mnif, S.; Jardak, M.; Yaich, A.; Aifa, S. Enzyme-based strategy to eradicate monospecies Macrococcus caseolyticus biofilm contamination in dairy industries. Int. Dairy J. 2020, 100, 104560. [Google Scholar] [CrossRef]
- Korany, A.M.; Hua, Z.; Green, T.; Hanrahan, I.; El-Shinawy, S.H.; El-Kholy, A.; Hassan, G.; Zhu, M.J. Efficacy of ozonated water, chlorine, chlorine dioxide, quaternary ammonium compounds and peroxyacetic acid against Listeria monocytogenes biofilm on polystyrene surfaces. Front. Microbiol. 2018, 9, 2296. [Google Scholar] [CrossRef]
- Lee, S.H.I.; Cappato, L.P.; Corassin, C.H.; Cruz, A.G.; Oliveira, C.A.F. Effect of peracetic acid on biofilms formed by Staphylococcus aureus and Listeria monocytogenes isolated from dairy plants. J. Dairy Sci. 2016, 99, 2384–2390. [Google Scholar] [CrossRef]
- Scaramuzza, N.; Mutti, P.; Cigarini, M.; Berni, E. Effect of peracetic acid on ascospore-forming molds and test microorganisms used for bio-validations of sanitizing processes in food plants. Int. J. Food Microbiol. 2020, 332, 108772. [Google Scholar] [CrossRef] [PubMed]
- Møretrø, T.; Fanebust, H.; Fagerlund, A.; Langsrud, S. Whole room disinfection with hydrogen peroxide mist to control Listeria monocytogenes in food industry related environments. Int. J. Food Microbiol. 2019, 292, 118–125. [Google Scholar] [CrossRef]
- Stearns, R.; Freshour, A.; Shen, C. Literature review for applying peroxyacetic acid and/or hydrogen peroxide to control foodborne pathogens on food products. J. Agric. Food Res. 2022, 10, 100442. [Google Scholar] [CrossRef]
- De Roos, J.; De Vuyst, L. Acetic acid bacteria in fermented foods and beverages. Curr. Opin. Biotechnol. 2018, 49, 115–119. [Google Scholar] [CrossRef]
- Secchi, E.; Savorana, G.; Vitale, A.; Eberl, L.; Stocker, R.; Rusconi, R. The structural role of bacterial eDNA in the formation of biofilm streamers. Proc. Natl. Acad. Sci. USA 2022, 119, e2113723119. [Google Scholar] [CrossRef] [PubMed]
- Beck, C.I.; Scott, D.O.N. Enzymes in foods-for better or worse. In Food Related Enzymes; Advances in Chemistry; American Chemical Society: Washington, DC, USA, 1974; Volume 136, pp. 1–30. [Google Scholar]
- Kim, H.S.; Ashrafudoulla, M.; Kim, B.R.; Mizan, M.F.R.; Jung, S.J.; Sadekuzzaman, M.; Park, S.H.; Ha, S.D. The application of bacteriophage to control Cronobacter sakazakii planktonic and biofilm growth in infant formula milk. Biofouling 2021, 37, 606–614. [Google Scholar] [CrossRef]
- Byun, K.-H.; Han, S.H.; Choi, M.W.; Kim, B.-H.; Park, S.H.; Ha, S.-D. Biofilm eradication ability of phage cocktail against Listeria monocytogenes biofilms formed on food contact materials and effect on virulence-related genes and biofilm structure. Food Res. Int. 2022, 157, 111367. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Yang, X.; Euler, C.W.; Han, X.; Liu, J.; Hossen, M.I.; Zhou, Y.; Li, J. Application of a novel phage ZPAH7 for controlling multidrug-resistant Aeromonas hydrophila on lettuce and reducing biofilms. Food Cont. 2021, 122, 107785. [Google Scholar] [CrossRef]
- Marchese, A.; Arciola, C.R.; Coppo, E.; Barbieri, R.; Barreca, D.; Chebaibi, S.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M.; Daglia, M. The natural plant compound carvacrol as an antimicrobial and anti-biofilm agent: Mechanisms, synergies and bio-inspired anti-infective materials. Biofouling 2018, 34, 630–656. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, P.; Zhu, L.; Zhang, Y.; Luo, X. Effect of four kinds of natural antimicrobial compounds on the biofilm formation ability of Listeria monocytogenes isolated from beef processing plants in China. LWT-Food Sci. Technol. 2020, 133, 110020. [Google Scholar] [CrossRef]
- Pleva, P.; Bartošová, L.; Máčalová, D.; Zálešáková, L.; Sedlaříková, J.; Janalíková, M. Biofilm formation reduction by eugenol and thymol on biodegradable food packaging material. Foods 2021, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Lee, N.K.; Paik, H.D. Antimicrobial and anti-biofilm effects of probiotic Lactobacillus plantarum KU200656 isolated from kimchi. Food Sci. Biotechnol. 2021, 30, 97–106. [Google Scholar] [CrossRef]
- Tazehabadi, M.H.; Algburi, A.; Popov, I.V.; Ermakov, A.M.; Chistyakov, V.A.; Prazdnova, E.V.; Weeks, R.; Chikindas, M.L. Probiotic Bacilli inhibit Salmonella biofilm formation without killing planktonic cells. Front. Microbiol. 2021, 12, 615328. [Google Scholar] [CrossRef]
- Rezaei, Z.; Salari, A.; Khanzadi, S.; Rhim, J.W.; Shamloo, E. Preparation of milk-based probiotic lactic acid bacteria biofilms: A new generation of probiotics. Food Sci. Nutr. 2023, 11, 2915–2924. [Google Scholar] [CrossRef]
- Mgomi, F.C.; Yuan, L.; Chen, C.w.; Zhang, Y.s.; Yang, Z.q. Bacteriophages: A weapon against mixed-species biofilms in the food processing environment. J. Appl. Microbiol. 2022, 133, 2107–2121. [Google Scholar] [CrossRef]
- Gutierrez, D.; Rodriguez-Rubio, L.; Martinez, B.; Rodriguez, A.; Garcia, P. Bacteriophages as weapons against bacterial biofilms in the food industry. Front. Microbiol. 2016, 7, 825. [Google Scholar] [CrossRef]
- Jaroni, D.; Litt, P.K.; Bule, P.; Rumbaugh, K. Effectiveness of bacteriophages against biofilm-forming shiga-toxigenic Escherichia coli in vitro and on food-contact surfaces. Foods 2023, 12, 2787. [Google Scholar] [CrossRef]
- Wang, C.; Hang, H.; Zhou, S.; Niu, Y.D.; Du, H.; Stanford, K.; McAllister, T.A. Bacteriophage biocontrol of Shiga toxigenic Escherichia coli (STEC) O145 biofilms on stainless steel reduces the contamination of beef. Food Microbiol. 2020, 92, 103572. [Google Scholar] [CrossRef]
- Radford, D.; Guild, B.; Strange, P.; Ahmed, R.; Lim, L.T.; Balamurugan, S. Characterization of antimicrobial properties of Salmonella phage Felix O1 and Listeria phage A511 embedded in xanthan coatings on Poly(lactic acid) films. Food Microbiol. 2017, 66, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Zhou, Y.; Liang, L.; Nime, I.; Liu, K.; Yan, T.; Wang, X.; Li, J. Application of a phage cocktail for control of Salmonella in foods and reducing biofilms. Viruses 2019, 11, 841. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.P.; Melo, L.D.R.; Azeredo, J. Understanding the complex phage-host interactions in biofilm communities. Annu. Rev. Virol. 2021, 8, 73–94. [Google Scholar] [CrossRef]
- Visnapuu, A.; Van der Gucht, M.; Wagemans, J.; Lavigne, R. Deconstructing the phage-bacterial biofilm interaction as a basis to establish new antibiofilm strategies. Viruses 2022, 14, 1057. [Google Scholar] [CrossRef]
- Rossi, C.; Chaves-López, C.; Serio, A.; Casaccia, M.; Maggio, F.; Paparella, A. Effectiveness and mechanisms of essential oils for biofilm control on food-contact surfaces: An updated review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2172–2191. [Google Scholar] [CrossRef]
- Sadekuzzaman, M.; Mizan, M.F.R.; Kim, H.-S.; Yang, S.; Ha, S.-D. Activity of thyme and tea tree essential oils against selected foodborne pathogens in biofilms on abiotic surfaces. LWT-Food Sci. Technol. 2018, 89, 134–139. [Google Scholar] [CrossRef]
- dos Santos Rodrigues, J.B.; de Carvalho, R.J.; de Souza, N.T.; de Sousa Oliveira, K.; Franco, O.L.; Schaffner, D.; de Souza, E.L.; Magnani, M. Effects of oregano essential oil and carvacrol on biofilms of Staphylococcus aureus from food-contact surfaces. Food Cont. 2017, 73, 1237–1246. [Google Scholar] [CrossRef]
- Greene, A.C.; Acharya, A.P.; Lee, S.B.; Gottardi, R.; Zaleski, E.; Little, S.R. Cranberry extract-based formulations for preventing bacterial biofilms. Drug Deliv. Transl. Res. 2021, 11, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Santos, R.R.; Fink-Gremmels, J. Analyzing the antibacterial effects of food ingredients: Model experiments with allicin and garlic extracts on biofilm formation and viability of Staphylococcus epidermidis. Food Sci. Nutr. 2015, 3, 158–168. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, X.; Zhang, F.; Feng, L.; Li, J. Inhibition of quorum sensing, biofilm, and spoilage potential in Shewanella baltica by green tea polyphenols. J. Microbiol. 2015, 53, 829–836. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Yang, K.; Guo, R.; Zhu, X.; Shi, Y.; Huang, A. Antibiofilm mechanism of a novel milk-derived antimicrobial peptide against Staphylococcus aureus by downregulating agr quorum sensing system. J. Appl. Microbiol. 2022, 133, 2198–2209. [Google Scholar] [CrossRef]
- Palmieri, G.; Balestrieri, M.; Capuano, F.; Proroga, Y.T.R.; Pomilio, F.; Centorame, P.; Riccio, A.; Marrone, R.; Anastasio, A. Bactericidal and antibiofilm activity of bactenecin-derivative peptides against the food-pathogen Listeria monocytogenes: New perspectives for food processing industry. Int. J. Food Microbiol. 2018, 279, 33–42. [Google Scholar] [CrossRef]
- Tomé, A.R.; Carvalho, F.M.; Teixeira-Santos, R.; Burmølle, M.; Mergulhão, F.J.M.; Gomes, L.C. Use of probiotics to control biofilm formation in food industries. Antibiotics 2023, 12, 754. [Google Scholar] [CrossRef]
- Salas-Jara, M.J.; Ilabaca, A.; Vega, M.; García, A. Biofilm forming Lactobacillus: New challenges for the development of probiotics. Microorganisms 2016, 4, 35. [Google Scholar] [CrossRef] [PubMed]
- Vuotto, C.; Longo, F.; Donelli, G. Probiotics to counteract biofilm-associated infections: Promising and conflicting data. Int. J. Oral Sci. 2014, 6, 189–194. [Google Scholar] [CrossRef]
- Wasfi, R.; Abd El-Rahman, O.A.; Zafer, M.M.; Ashour, H.M. Probiotic Lactobacillus sp. inhibit growth, biofilm formation and gene expression of caries-inducing Streptococcus mutans. J. Cell. Mol. Med. 2018, 22, 1972–1983. [Google Scholar] [CrossRef]
- Carvalho, F.M.; Mergulhão, F.J.M.; Gomes, L.C. Using Lactobacilli to fight Escherichia coli and Staphylococcus aureus biofilms on urinary tract devices. Antibiotics 2021, 10, 1525. [Google Scholar] [CrossRef] [PubMed]
- Sarikaya, H.; Aslim, B.; Yuksekdag, Z. Assessment of anti-biofilm activity and bifidogenic growth stimulator (BGS) effect of lyophilized exopolysaccharides (l-EPSs) from Lactobacilli strains. Int. J. Food Propert. 2017, 20, 362–371. [Google Scholar] [CrossRef]
- Krishnamoorthi, R.; Srinivash, M.; Mahalingam, P.U.; Malaikozhundan, B.; Suganya, P.; Gurushankar, K. Antimicrobial, anti-biofilm, antioxidant and cytotoxic effects of bacteriocin by Lactococcus lactis strain CH3 isolated from fermented dairy products-An in vitro and in silico approach. Int. J. Biol. Macromol. 2022, 220, 291–306. [Google Scholar] [CrossRef] [PubMed]
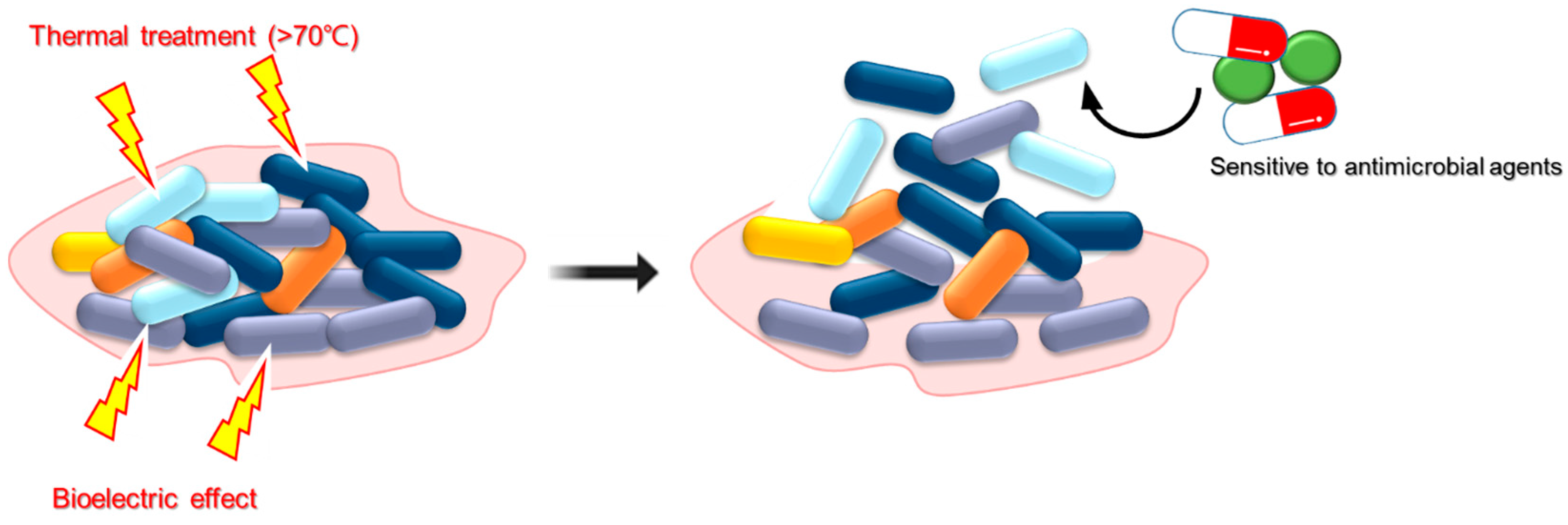

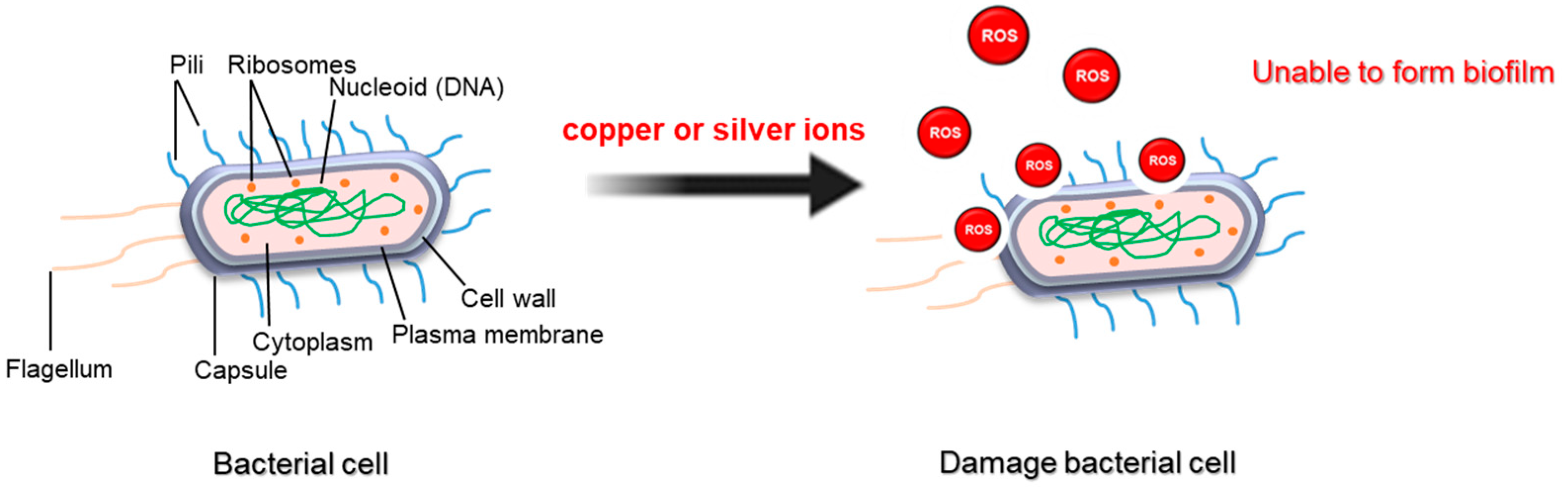
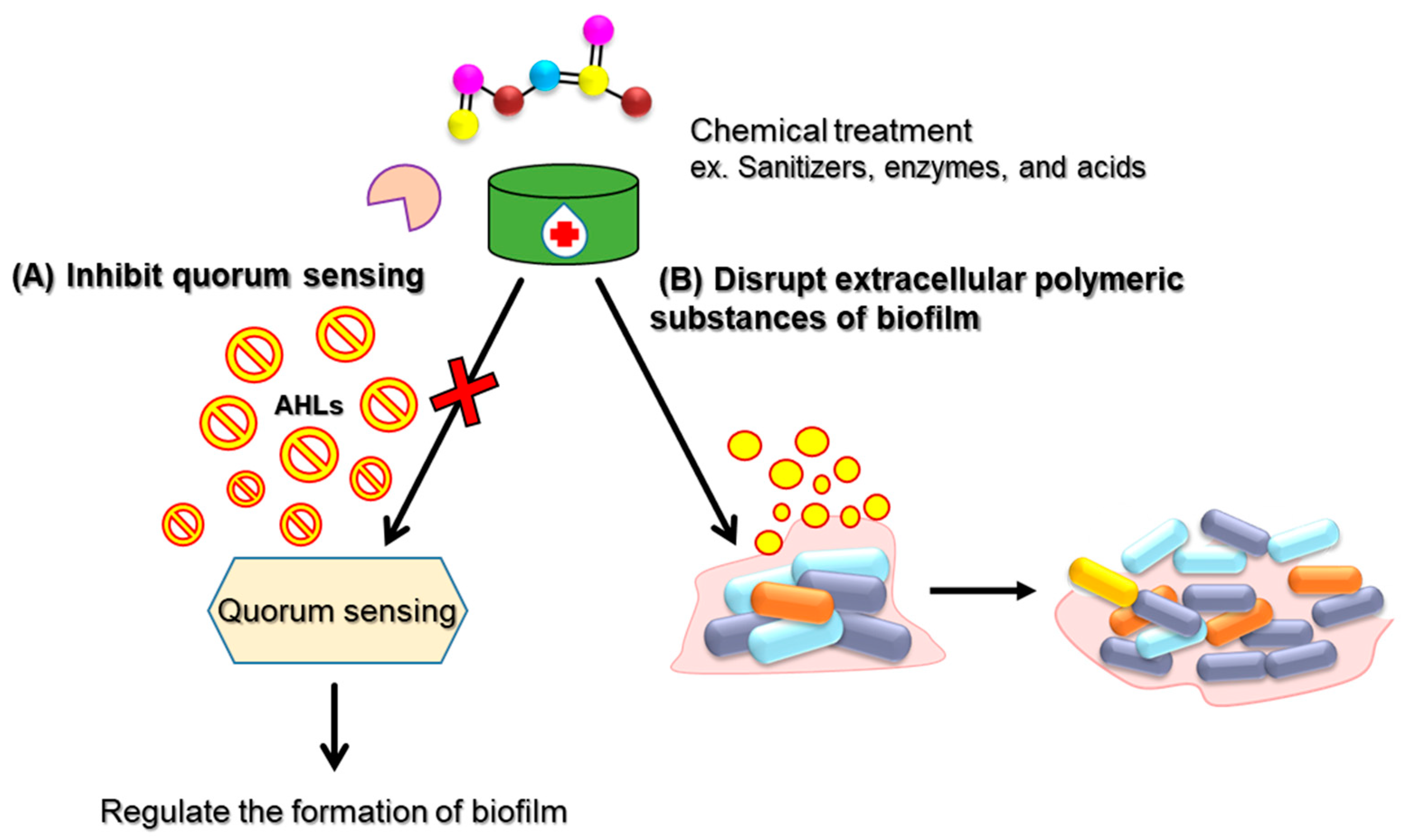

| Physical Treatment | Target Strain | Anti-Biofilm Activity | Reference |
|---|---|---|---|
| Thermal | Staphylococcus aureus | Superheated steam (SHS) treatment (150 °C, 15 s) can effectively eradicate mature biofilm of S. aureus formed on food contact surfaces | [17] |
| Staphylococcus epidermidis | S. epidermidis biofilm cells that formed in liquid egg processing environment were sensitive to hot-water treatment (71 °C). | [18] | |
| Electrical field | Sludge containing mixed-bacteria strains from wastewater treatment plant | Low-frequency and low-voltage range of 8 vpp can stimulate the attachment of the bacteria to solid surfaces and therefore diminish bacterial biofilm-forming ability. | [19] |
| S. epidermidis | 100 μA electric block currents were demonstrated to enable 76% detachment of S. epidermidis from stainless steel. | [20] | |
| Pseudomonas aeruginosa, S. epidermidis, and S. aureus | The biofilm of multiple bacterial species was inhibited by great electricidal effects using low-amperage currents and intermittent strategies. | [21] | |
| Ultrasonic | S. aureus | Ultrasonic coda wave interferometry was able to detect the early stage of biofilm formation of on stainless steel. | [22] |
| S. aureus | The treatment of ultrasonic plus 1% of chlorogenic acid for 60 min exhibited synergistic antibacterial and antibiofilm effects by cause damage to cell morphology and decreasing the exopolysaccharide contents in S. aureus. | [23] | |
| E. coli and L. monocytogenes | The combination of ultrasound and different organic acids (acetic acid, malic acid, citric acid and lactic acid) can detach bacteria on the surface of lettuce. | [24] | |
| Salmonella spp. | Combination of ultrasound (40 kHz) and acidic electrolyzed water produced a synergistic effect on the reduction of Salmonella spp. biofilm formed on stainless steel surfaces. | [25] | |
| Surface modification | E. coli and P. aeruginosa | Oil-based slippery coatings significantly inhibited bacterial adhesion and reduced biofilm formation on stainless steel surface. | [26] |
| E. coli and S. aureus | Silver and zinc oxide nanoparticle-containing polyester surfaces prevented biofilm formation in both Gram-positive and Gram-negative bacteria | [27] | |
| S. enteritidis | Compared to uncoated surfaces, a reduction in biofilm formation was observed on copper-coated surfaces (3–4 log CFU reduction) | [28] |
| Chemical Treatment | Target Strain | Anti-Biofilm Activity | Reference |
|---|---|---|---|
| Sanitizers and disinfectants | S. Enteritidis | Chlorine dioxide showed biofilm removal ability on food contact surfaces (stainless steel, silicone rubber, and plastic) and chicken skin. | [45] |
| L. monocytogenes | Peracetic acid-based commercial disinfectant showed biofilm formation-inhibitory effect on stainless steel surface at low temperature. | [46] | |
| B. cereus. | Chemical sanitizers containing quaternary ammonium compound (QAC) dramatically reduced the presence of a biofilm on food contact surfaces (glass, polyethylene, polypropylene, and wood). | [47] | |
| Acidulants | B. subtilis | 2% citric acid showed biofilm removal ability on stainless steel surface. | [48] |
| E. coli O157:H7 | Lactic acid bacteria inhibited growth and surface colonization of E. coli O157:H7 at 10 °C. | [49] | |
| V. parahaemolyticus and P. aeruginosa | Lactic acid bacteria isolated from Korean fermented vegetable (kimchi) showed biofilm removal ability on seafood model and food contact surfaces (rubber and high-density polyethylene plastic). | [50] | |
| V. parahaemolyticus | Enzymatic cocktail of lipase, cellulase, and proteinase K showed biofilm removal ability by disrupted EPS of bacterial biofilm. | [51] | |
| Enzymes | E. coli and S. aureus | A novel food packaging material was obtained by immobilizing glucose oxidase in polyvinyl alcohol/chitosan/tea extract electrospun nanofibrous membrane. | [52] |
| Macrococcus caseolyticus | Enzymatic cocktail of protease, lipase, cellulase, a-amylase, and DNase showed biofilm removal ability in dairy industries by disrupted EPS of bacterial biofilm. | [53] |
| Biological Treatment | Target Strain | Anti-Biofilm Activity | References |
|---|---|---|---|
| Bacteriophages | Cronobacter sakazakii | Bacteriophages isolated from sewage showed biofilm removal ability in infant formula milk industry by targeting biofilm matrix. | [62] |
| Listeria monocytogenes | Listeria-specific bacteriophage cocktail effectively eradicate matured biofilm formed on food contact materials including polyethylene, polypropylene, and stainless steel. | [63] | |
| Aeromonas hydrophila | Bacteriophages isolated from the sediment of a fish farm inhibited biofilm formation and degraded and killed bacteria in matured biofilms on lettuce. | [64] | |
| Natural compounds | Staphylococcus aureus and Enterococcus faecalis | Carvacrol isolated from the leaves of wild bergamot showed antibacterial and antibiofilm activities by inhibiting bacterial motility and interfering with bacterial adhesion. | [65] |
| L. monocytogenes | Cinnamaldehyde, eugenol, resveratrol, and thymoquinone isolated from plants inhibited biofilm formation in beef processing plants by interfering with quorum sensing systems. | [66] | |
| Escherichia coli, S. aureus, and Bacillus pumilus | Thymol and eugenol can be used to prepare novel active food packaging for the dairy industry to prevent biofilm formation. | [67] | |
| Probiotics | S. aureus, L. monocytogenes, and S. Typhimurium | Lactobacillus plantarum isolated from Korean fermented kimchi showed antibiofilm formation by inhibiting bacterial adhesion to surface. | [68] |
| S. Enteritidis | Bacteriocins produced from Bacillus subtilis and B. amyloliquefaciens inhibited biofilm formation in poultry products by interfering with the quorum sensing system. | [69] | |
| S. aureus | Probiotic biofilms of Lactiplantibacillus plantarum and Lacticaseibacillus rhamnosus prevented biofilm formation of bacteria in milk and yogurt during processing and storage conditions. | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dawan, J.; Zhang, S.; Ahn, J. Recent Advances in Biofilm Control Technologies for the Food Industry. Antibiotics 2025, 14, 254. https://doi.org/10.3390/antibiotics14030254
Dawan J, Zhang S, Ahn J. Recent Advances in Biofilm Control Technologies for the Food Industry. Antibiotics. 2025; 14(3):254. https://doi.org/10.3390/antibiotics14030254
Chicago/Turabian StyleDawan, Jirapat, Song Zhang, and Juhee Ahn. 2025. "Recent Advances in Biofilm Control Technologies for the Food Industry" Antibiotics 14, no. 3: 254. https://doi.org/10.3390/antibiotics14030254
APA StyleDawan, J., Zhang, S., & Ahn, J. (2025). Recent Advances in Biofilm Control Technologies for the Food Industry. Antibiotics, 14(3), 254. https://doi.org/10.3390/antibiotics14030254







