A Simple In Vitro Method to Determine Bactericidal Activity Against Mycobacterium abscessus Under Hypoxic Conditions
Abstract
1. Introduction
2. Results and Discussion
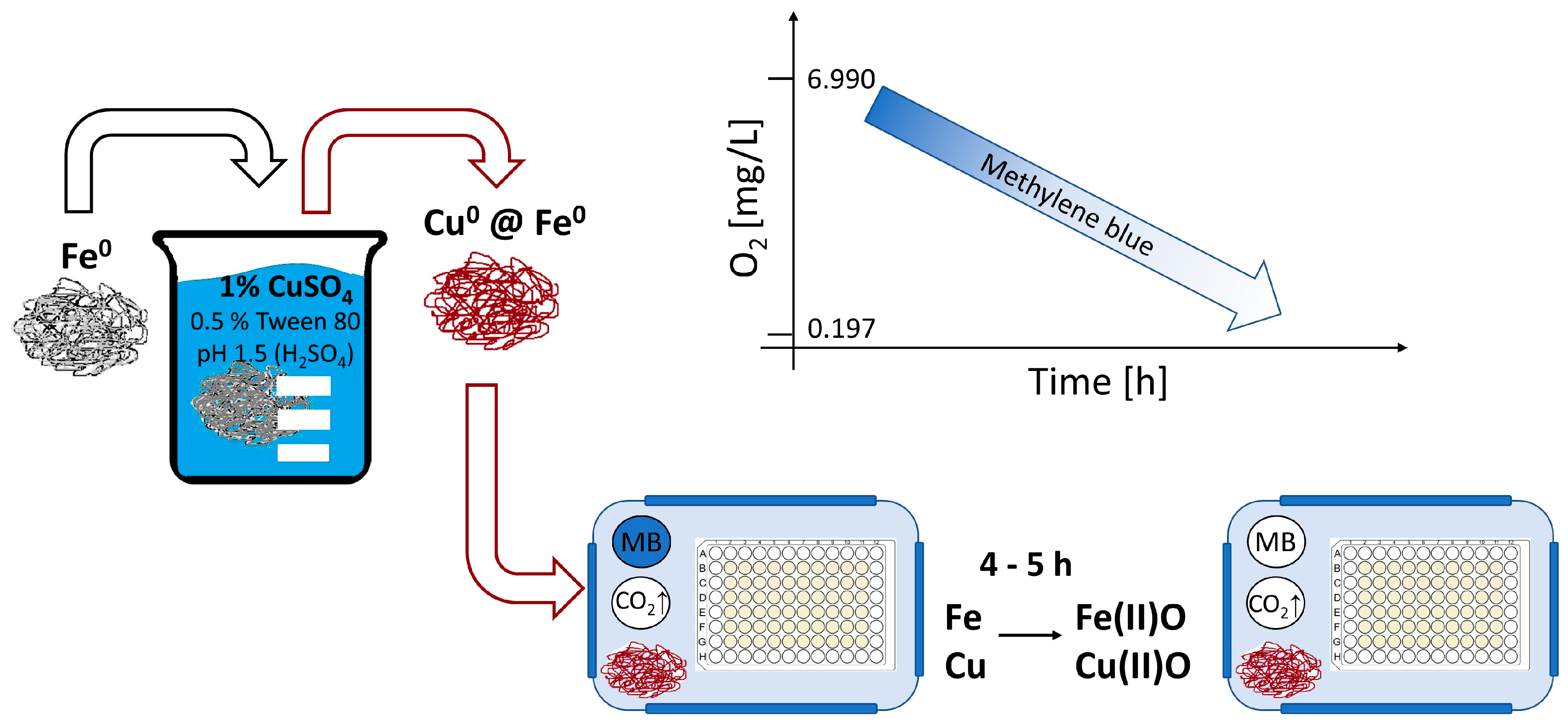
2.1. Generation of a Hypoxic Environment by Activated Iron Wool
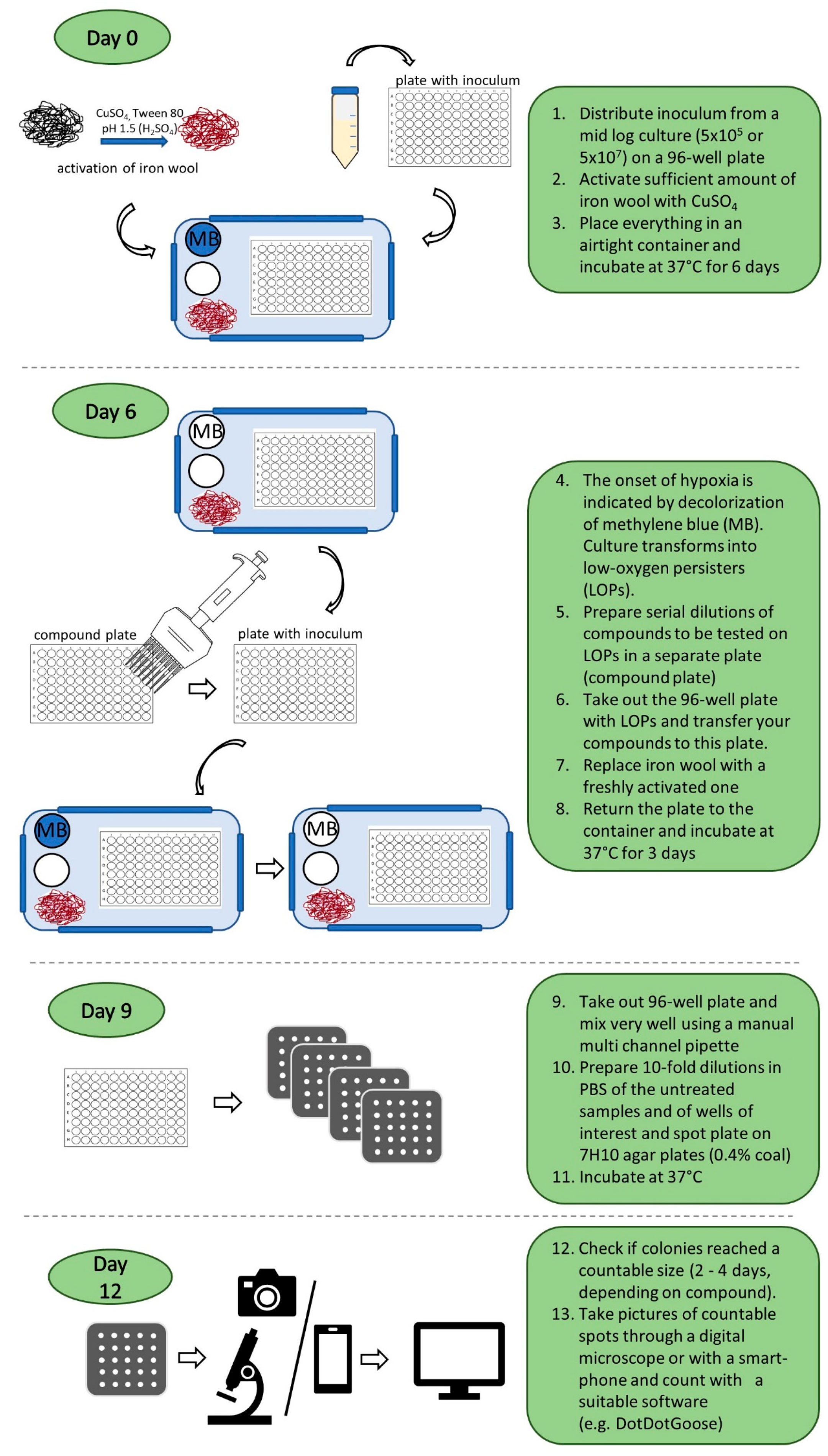
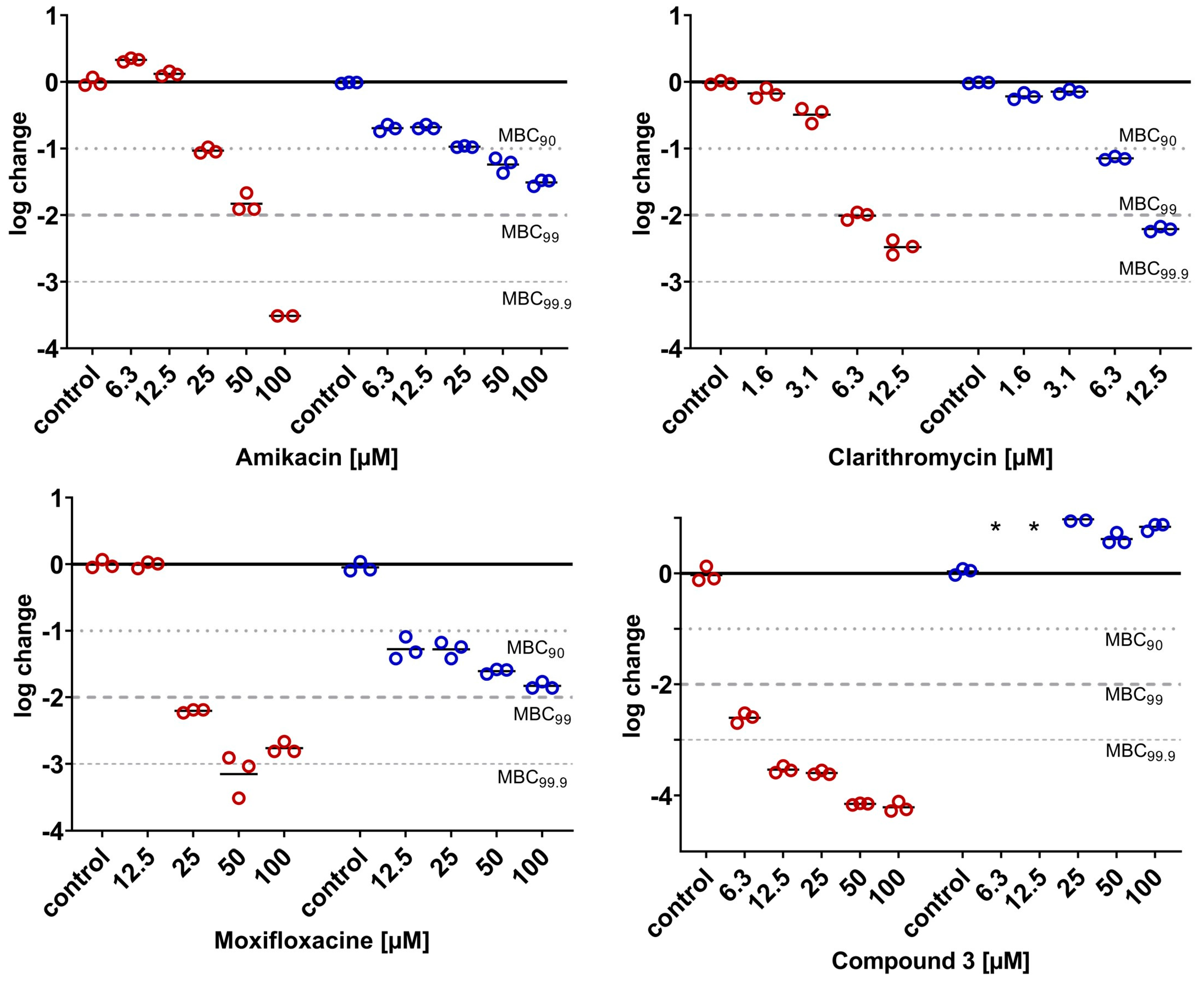
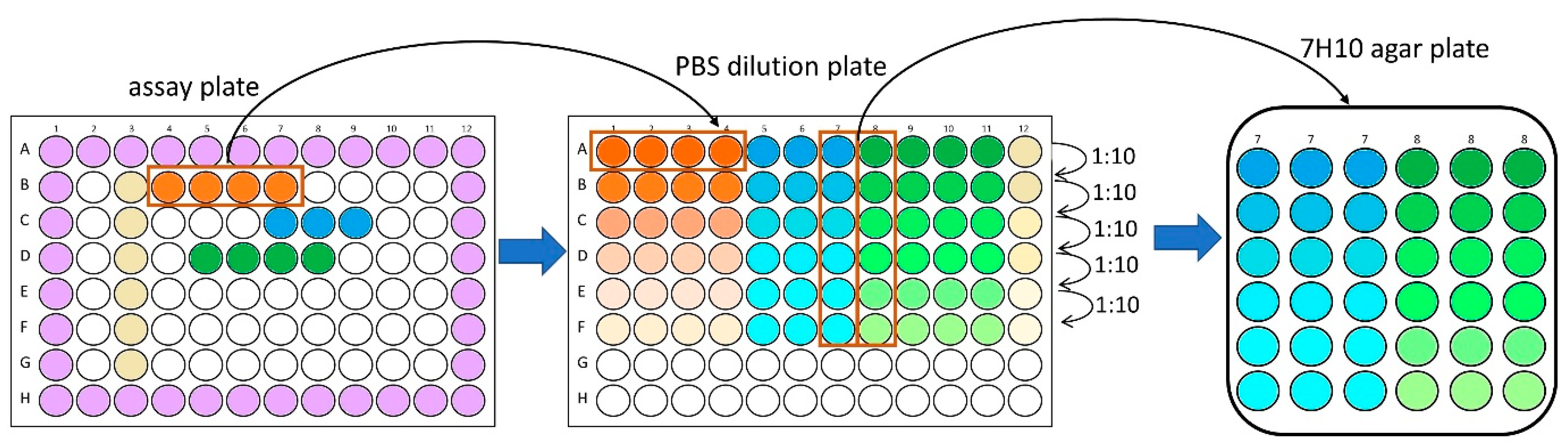
2.2. M. abscessus LOP Assay for Determination of Drug Activity In Vitro
2.3. Evidence of M. abscessus Transitioning to the LOP State
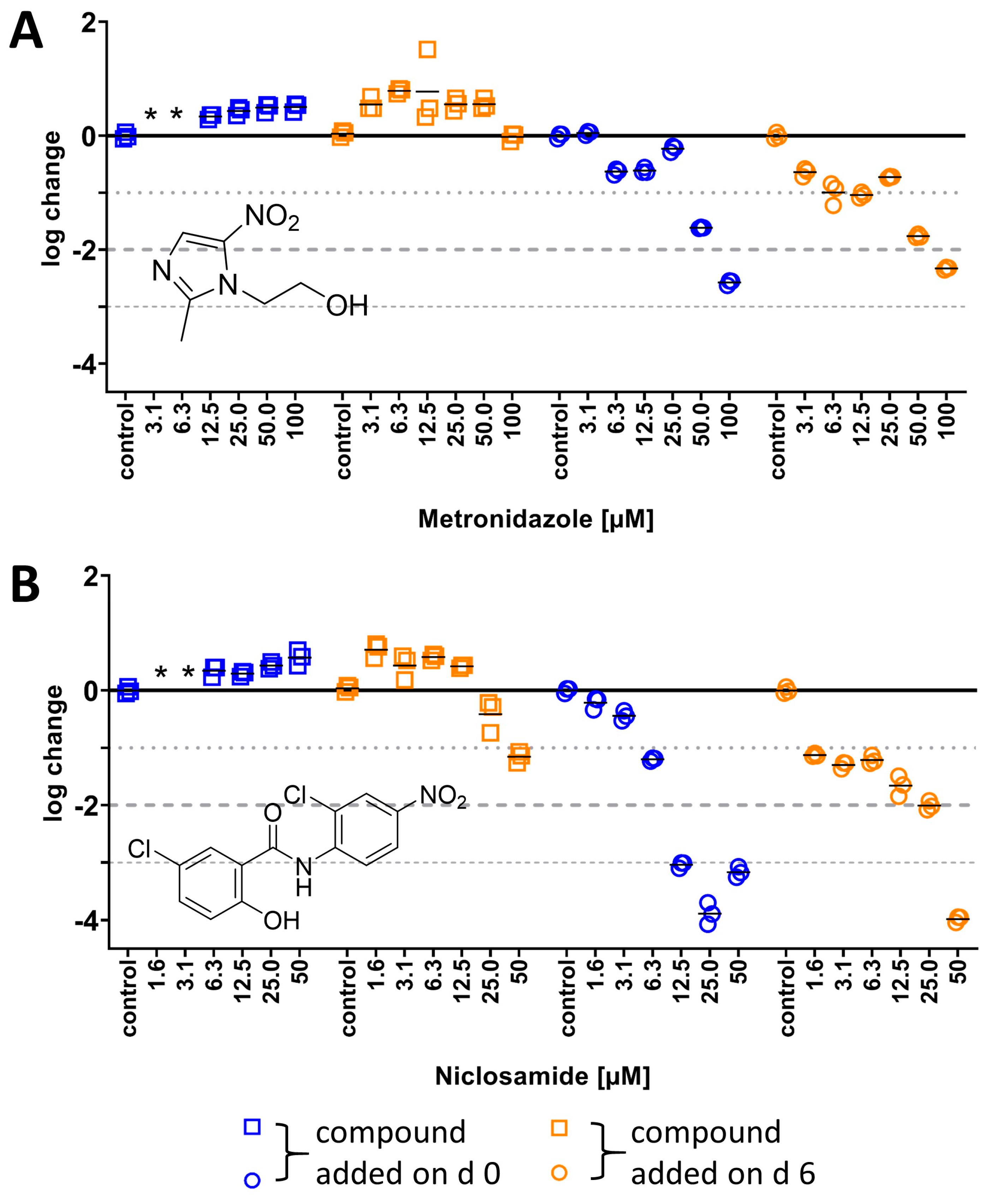
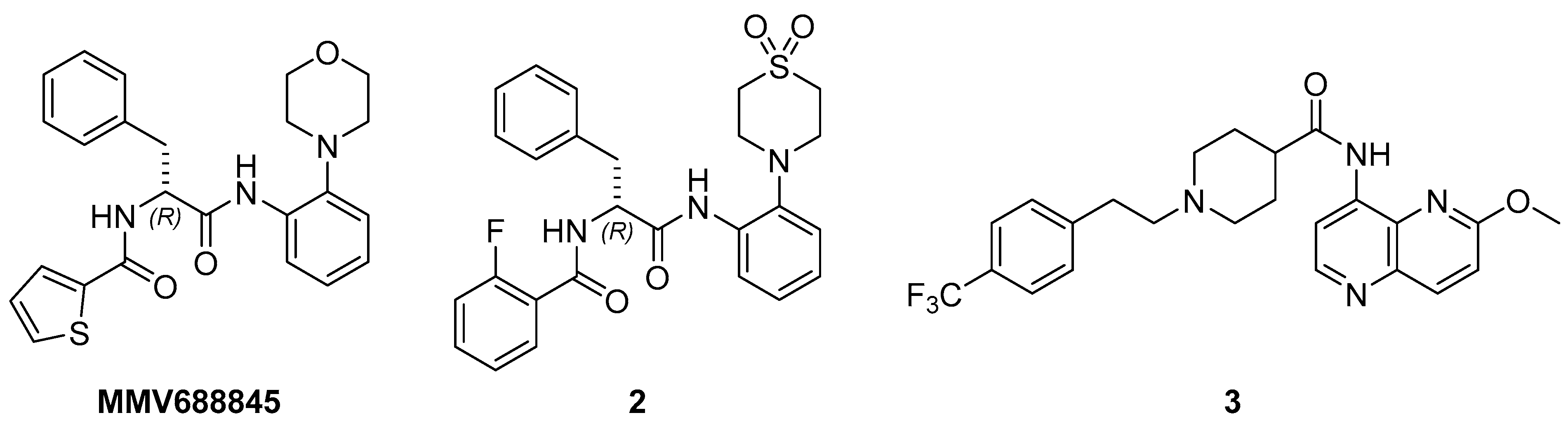
2.3.1. Activity of Nitroaromatics Against M. abscessus LOPs
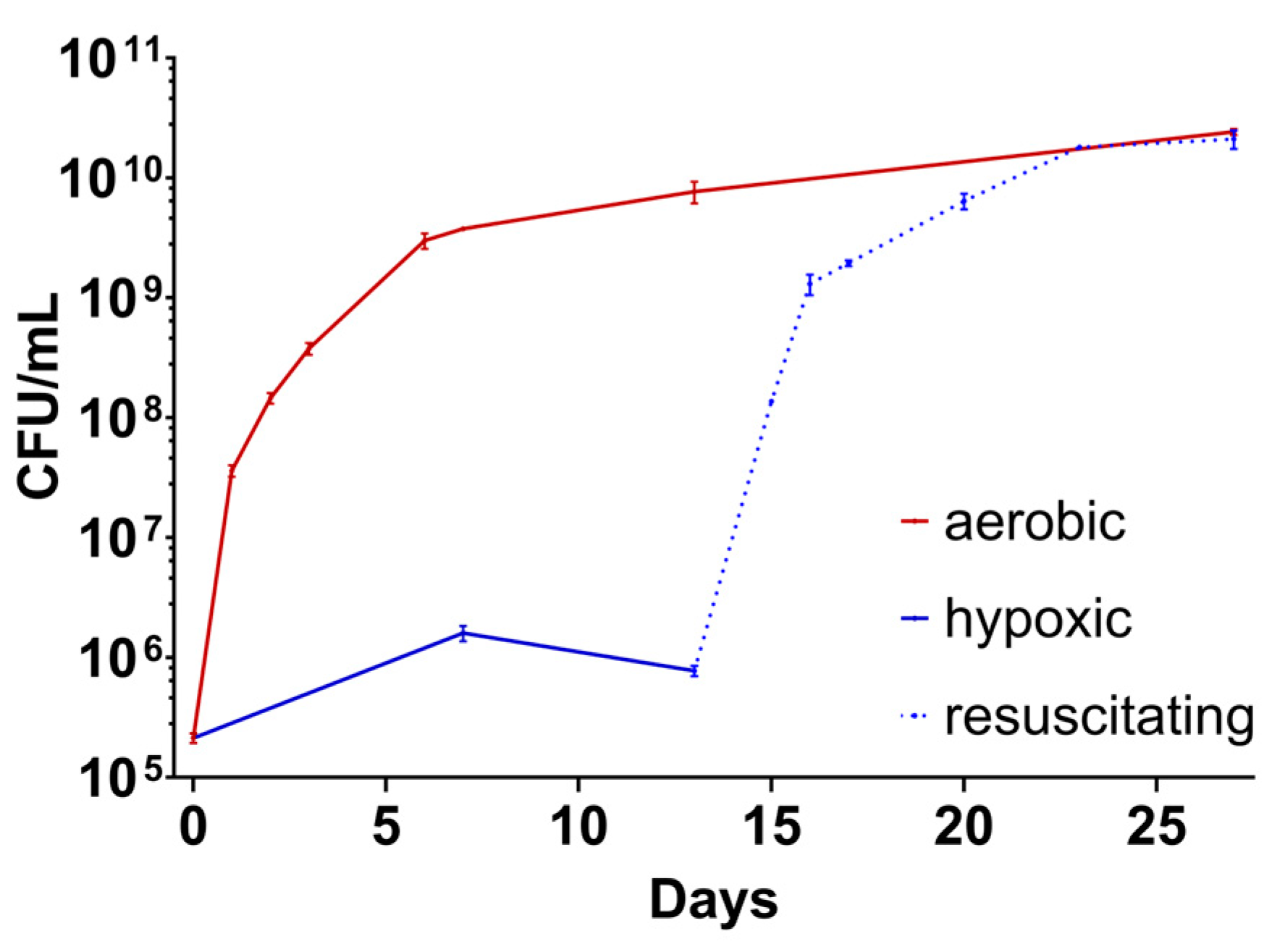
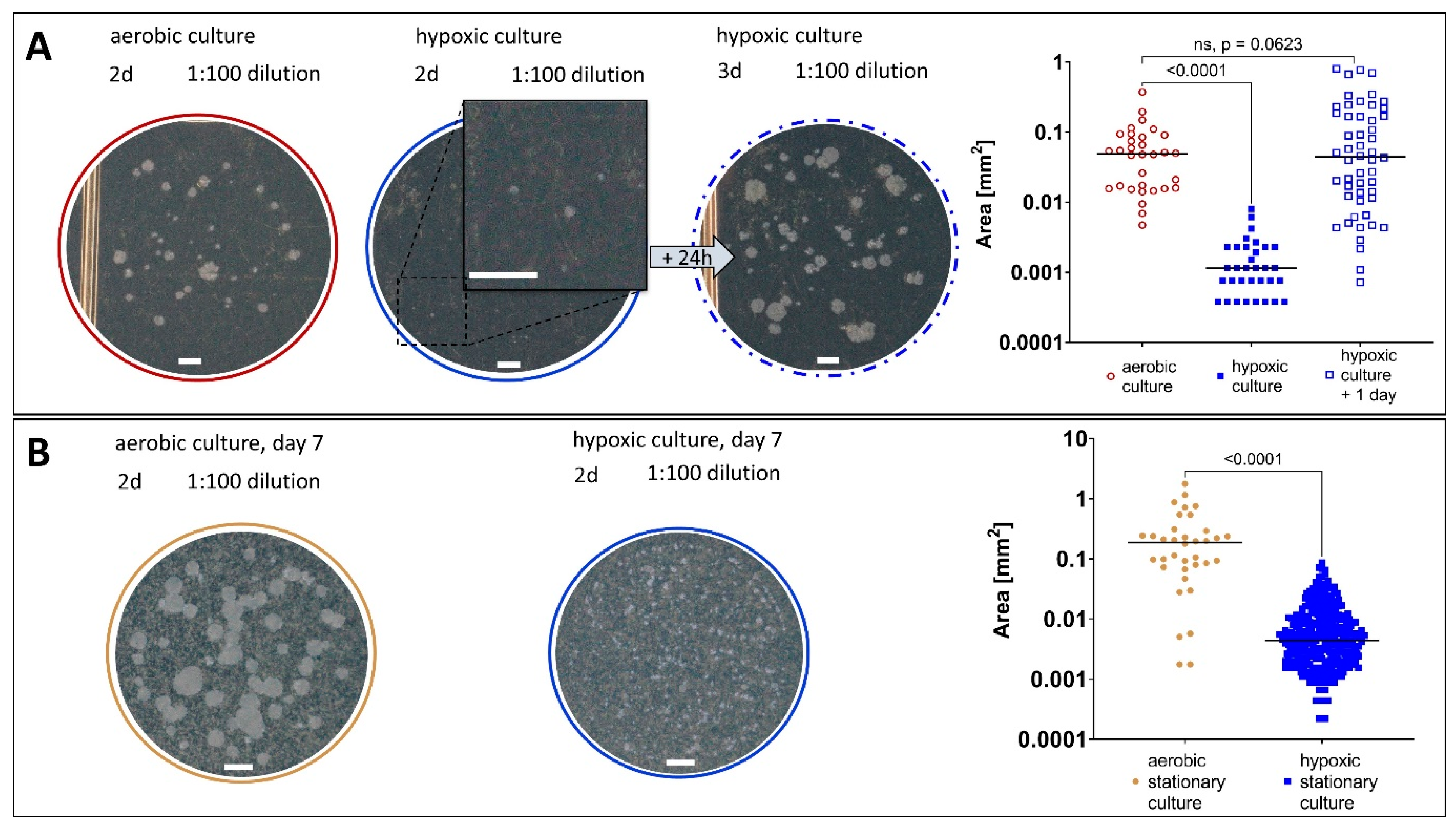
2.3.2. Growth Behaviour of M. abscessus LOPs
2.3.3. Resuscitation of M. abscessus LOPs on Solid and in Liquid Medium
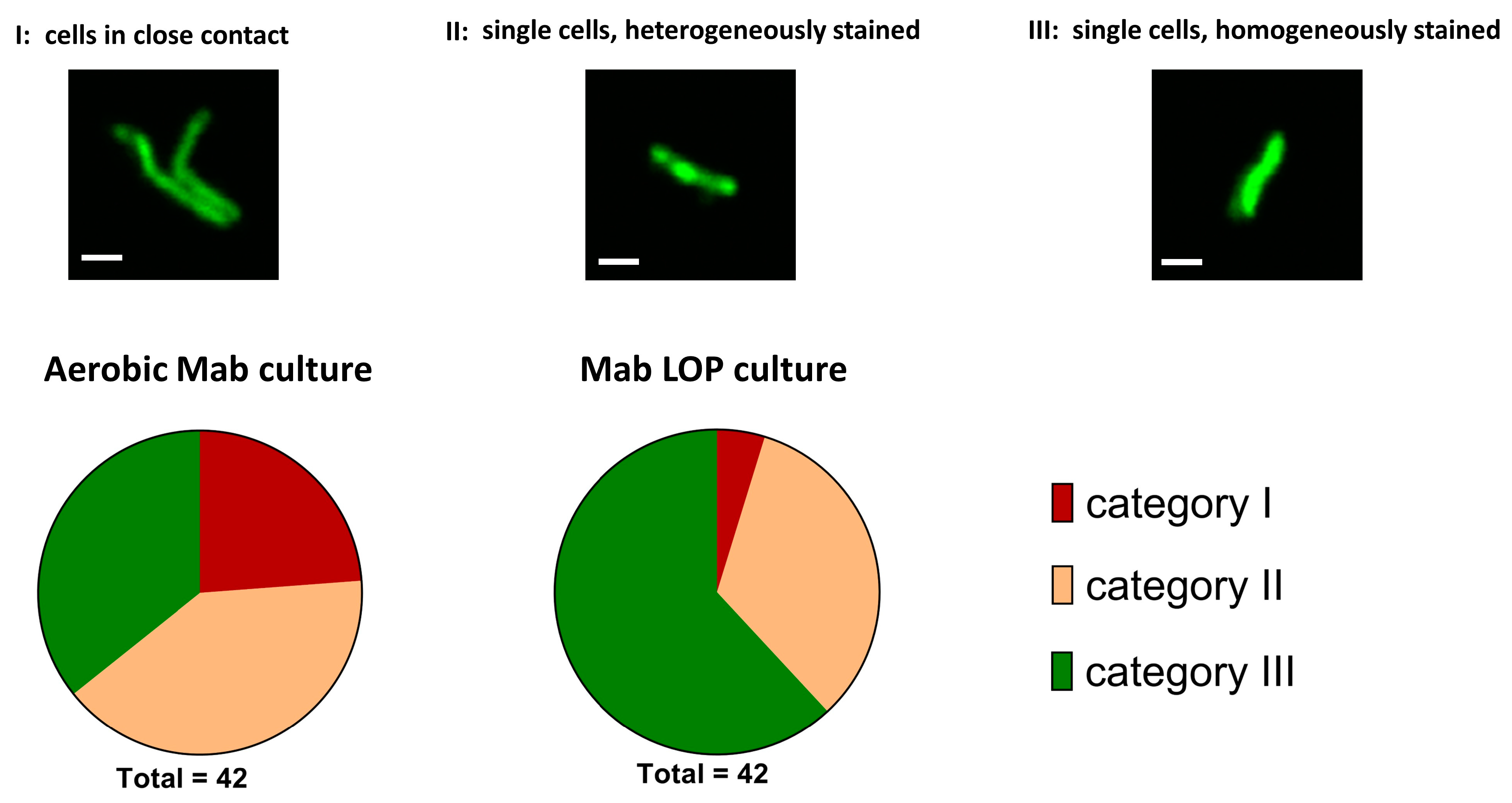
2.3.4. Morphology of M. abscessus Single Cells
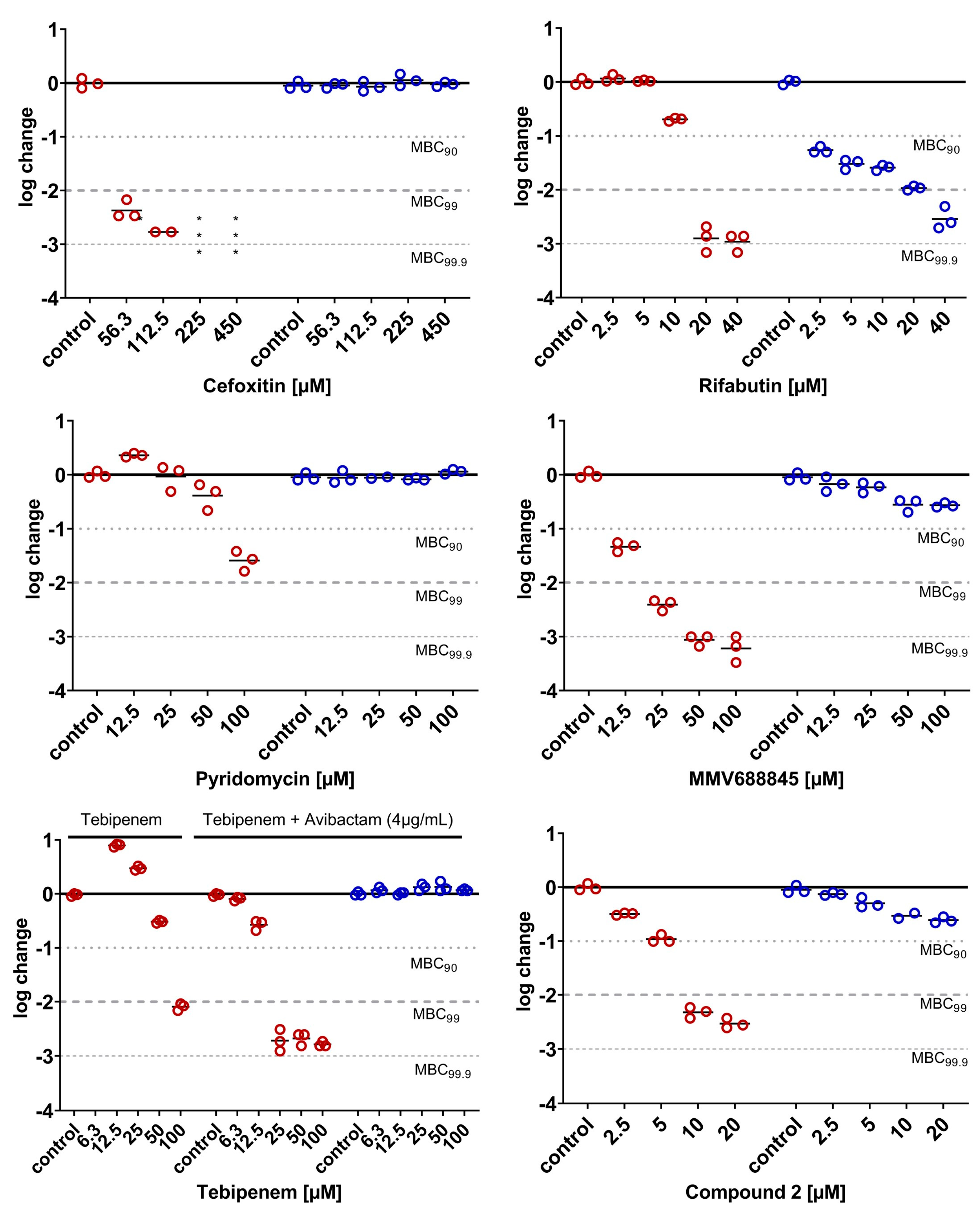
2.3.5. Phenotypical Resistance of LOPs to Antimycobacterial Compounds
| Literature Data | |||||||
|---|---|---|---|---|---|---|---|
| Drug | MIC90 (µM) Aer | MBC (µM) Aer | MBC (µM) LOPs | MIC90 (µM) Aer | MBC (µM) Aer | MBC (µM) Wayne | Ref. |
| Pyridomycin | 12.5 | MBC90 100 | MBC90 n.a. | 11.6 | - | - | [44] |
| MBC99 n.a. | MBC99 n.a. | ||||||
| Cefoxitin | 28 | MBC90 56 | MBC90 n.a. | 16 | MBC90 25 a | MBC90 > 100 | [10] |
| Tebipenem (+Avibactam 4 µg/mL) | 6.3 | MBC90 25 | MBC90 n.a. | 3–4 | MBC90 8 | - | [52] |
| MBC99 25 | MBC99 n.a. | MBC99 16 | - | [52] | |||
| MBC99.9 24 | - | [52] | |||||
| Rifabutin | 1.3 | MBC90 20 | MBC90 2.5 | 2.3 | MBC90 > 3.5 b | MBC90 3.5 b | [22] |
| Clarithromycin | 0.8 | MBC90 6 | MBC90 6.3 | 0.2 | MBC90 > 100 a | MBC90 > 100 a | [10] |
| MBC99 6 | MBC99 12.5 | 0.3 | MBC99 2.7 b | MBC90 2.7 b | [22] | ||
| Amikacin | 6.3 | MBC90 25 | MBC90 25 | 2 | MBC90 12.5 a | MBC90 > 100 a | [10] |
| 13.6 | MBC90 > 13.6 b | MBC90 > 13.6 b | [22] | ||||
| Moxifloxacin | 6.3 | MBC90 25 | MBC90 12.5 | 2 | MBC90 3 a | MBC90 > 100 a | [10] |
| MBC99 25 | MBC99 n.a. | 10 | MBC99.9 10 b | MBC90 > 10 b | [22] | ||
3. Materials and Methods
3.1. Source of the Materials
3.2. Bacterial Strain and Culture Medium (Liquid and Solid Medium)
3.3. Minimum Inhibitory Concentration (MIC) Determination in 7H9 by OD600 Measurement
3.4. Calculation of MIC90
3.5. LOPs Assay Under Hypoxic Conditions
3.6. MBC Determination on Agar
3.7. Calculation of Log Change
3.8. Fluorescent Labeling of M. abscessus Cells and Preparation of Samples for Microscopy
3.9. Confocal Laser Scanning Microscopy
3.10. Automated Object Counting Using Fluorescence Microscopy
3.11. Measuring of CFU Characteristics (Area, Perimeter and Length) on Pictures
3.12. Oxygen Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LOP | Low-oxygen persister |
| MB | Methylene blue |
| MBC | Minimum bactericidal concentration |
| MIC | Minimum inhibitory concentration |
| NRP | Non-replicating persister |
| OD | Optical density |
References
- Johansen, M.D.; Herrmann, J.L.; Kremer, L. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat. Rev. Microbiol. 2020, 18, 392–407. [Google Scholar] [CrossRef] [PubMed]
- Dartois, V.; Dick, T. Therapeutic developments for tuberculosis and nontuberculous mycobacterial lung disease. Nat. Rev. Drug Discov. 2024, 23, 381–403. [Google Scholar] [CrossRef]
- Pasipanodya, J.G.; Ogbonna, D.; Ferro, B.E.; Magombedze, G.; Srivastava, S.; Deshpande, D.; Gumbo, T. Systematic Review and Meta-analyses of the Effect of Chemotherapy on Pulmonary Mycobacterium abscessus Outcomes and Disease Recurrence. Antimicrob. Agents Chemother. 2017, 61, e01206-17. [Google Scholar] [CrossRef] [PubMed]
- Boudehen, Y.M.; Kremer, L. Mycobacterium abscessus. Trends Microbiol. 2021, 29, 951–952. [Google Scholar] [CrossRef]
- Griffith, D.E.; Daley, C.L. Treatment of Mycobacterium abscessus Pulmonary Disease. Chest 2022, 161, 64–75. [Google Scholar] [CrossRef]
- Ganapathy, U.S.; Dick, T. Why Matter Matters: Fast-Tracking Mycobacterium abscessus Drug Discovery. Molecules 2022, 27, 6948. [Google Scholar] [CrossRef] [PubMed]
- Datta, M.; Via, L.E.; Kamoun, W.S.; Liu, C.; Chen, W.; Seano, G.; Weiner, D.M.; Schimel, D.; England, K.; Martin, J.D.; et al. Anti-vascular endothelial growth factor treatment normalizes tuberculosis granuloma vasculature and improves small molecule delivery. Proc. Natl. Acad. Sci. USA 2015, 112, 1827–1832. [Google Scholar] [CrossRef] [PubMed]
- Via, L.E.; Lin, P.L.; Ray, S.M.; Carrillo, J.; Allen, S.S.; Eum, S.Y.; Taylor, K.; Klein, E.; Manjunatha, U.; Gonzales, J.; et al. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect. Immun. 2008, 76, 2333–2340. [Google Scholar] [CrossRef]
- Worlitzsch, D.; Tarran, R.; Ulrich, M.; Schwab, U.; Cekici, A.; Meyer, K.C.; Birrer, P.; Bellon, G.; Berger, J.; Weiss, T.; et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Investig. 2002, 109, 317–325. [Google Scholar]
- Yam, Y.K.; Alvarez, N.; Go, M.L.; Dick, T. Extreme Drug Tolerance of Mycobacterium abscessus “Persisters”. Front. Microbiol. 2020, 11, 359. [Google Scholar] [CrossRef]
- Early, J.V.; Mullen, S.; Parish, T. A rapid, low pH, nutrient stress, assay to determine the bactericidal activity of compounds against non-replicating Mycobacterium tuberculosis. PLoS ONE 2019, 14, e0222970. [Google Scholar] [CrossRef]
- Gold, B.; Warrier, T.; Nathan, C. A multi-stress model for high throughput screening against non-replicating Mycobacterium tuberculosis. Methods Mol. Biol. 2015, 1285, 293–315. [Google Scholar]
- Loebel, R.O.; Shorr, E.; Richardson, H.B. The Influence of Foodstuffs upon the Respiratory Metabolism and Growth of Human Tubercle Bacilli. J. Bacteriol. 1933, 26, 139–166. [Google Scholar] [CrossRef]
- Martins, O.; Lee, J.; Kaushik, A.; Ammerman, N.C.; Dooley, K.E.; Nuermberger, E.L. In Vitro Activity of Bedaquiline and Imipenem against Actively Growing, Nutrient-Starved, and Intracellular Mycobacterium abscessus. Antimicrob. Agents Chemother. 2021, 65, e0154521. [Google Scholar] [CrossRef]
- Berube, B.J.; Castro, L.; Russell, D.; Ovechkina, Y.; Parish, T. Novel Screen to Assess Bactericidal Activity of Compounds Against Non-replicating Mycobacterium abscessus. Front. Microbiol. 2018, 9, 2417. [Google Scholar] [CrossRef]
- Voskuil, M.I.; Schnappinger, D.; Visconti, K.C.; Harrell, M.I.; Dolganov, G.M.; Sherman, D.R.; Schoolnik, G.K. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J. Exp. Med. 2003, 198, 705–713. [Google Scholar] [CrossRef]
- Mulyukin, A.L.; Recchia, D.; Kostrikina, N.A.; Artyukhina, M.V.; Martini, B.A.; Stamilla, A.; Degiacomi, G.; Salina, E.G. Distinct Effects of Moxifloxacin and Bedaquiline on Growing and ‘Non-Culturable’ Mycobacterium abscessus. Microorganisms 2023, 11, 2690. [Google Scholar] [CrossRef]
- Salina, E.G.; Waddell, S.J.; Hoffmann, N.; Rosenkrands, I.; Butcher, P.D.; Kaprelyants, A.S. Potassium availability triggers Mycobacterium tuberculosis transition to, and resuscitation from, non-culturable (dormant) states. Open Biol. 2014, 4, 140106. [Google Scholar] [CrossRef]
- Wayne, L.G.; Hayes, L.G. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect. Immun. 1996, 64, 2062–2069. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Warit, S.; Wan, B.; Hwang, C.H.; Pauli, G.F.; Franzblau, S.G. Low-oxygen-recovery assay for high-throughput screening of compounds against nonreplicating Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2007, 51, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Belardinelli, J.M.; Verma, D.; Li, W.; Avanzi, C.; Wiersma, C.J.; Williams, J.T.; Johnson, B.K.; Zimmerman, M.; Whittel, N.; Angala, B.; et al. Therapeutic efficacy of antimalarial drugs targeting DosRS signaling in Mycobacterium abscessus. Sci. Transl. Med. 2022, 14, eabj3860. [Google Scholar] [CrossRef]
- Lanni, A.; Borroni, E.; Iacobino, A.; Russo, C.; Gentile, L.; Fattorini, L.; Giannoni, F. Activity of Drug Combinations against Mycobacterium abscessus Grown in Aerobic and Hypoxic Conditions. Microorganisms 2022, 10, 1421. [Google Scholar] [CrossRef]
- Simcox, B.S.; Tomlinson, B.R.; Shaw, L.N.; Rohde, K.H. Mycobacterium abscessus DosRS two-component system controls a species-specific regulon required for adaptation to hypoxia. Front. Cell Infect. Microbiol. 2023, 13, 1144210. [Google Scholar] [CrossRef]
- Batyrshina, Y.R.; Schwartz, Y.S. Modeling of Mycobacterium tuberculosis dormancy in bacterial cultures. Tuberculosis 2019, 117, 7–17. [Google Scholar] [CrossRef]
- Parker, C.A. Anaerobiosis with iron wool. Aust. J. Exp. Biol. Med. Sci. 1955, 33, 33–37. [Google Scholar] [CrossRef]
- Wayne, L.G.; Lin, K.Y. Glyoxylate metabolism and adaptation of Mycobacterium tuberculosis to survival under anaerobic conditions. Infect. Immun. 1982, 37, 1042–1049. [Google Scholar] [CrossRef]
- Richter, A.; Strauch, A.; Chao, J.; Ko, M.; Av-Gay, Y. Screening of Preselected Libraries Targeting Mycobacterium abscessus for Drug Discovery. Antimicrob. Agents Chemother. 2018, 62, e00828-18. [Google Scholar] [CrossRef]
- Mukherjee, T.; Boshoff, H.; Barry, C.E., 3rd. Comment on: Identification of antimicrobial activity among FDA-approved drugs for combating Mycobacterium abscessus and Mycobacterium chelonae. J. Antimicrob. Chemother. 2012, 67, 252–253. [Google Scholar] [CrossRef]
- Yang, J.J.; Goff, A.; Wild, D.J.; Ding, Y.; Annis, A.; Kerber, R.; Foote, B.; Passi, A.; Duerksen, J.L.; London, S.; et al. Computational drug repositioning identifies niclosamide and tribromsalan as inhibitors of Mycobacterium tuberculosis and Mycobacterium abscessus. Tuberculosis 2024, 146, 102500. [Google Scholar] [CrossRef]
- Burock, S.; Daum, S.; Tröger, H.; Kim, T.D.; Krüger, S.; Rieke, D.T.; Ochsenreither, S.; Welter, K.; Herrmann; Sleegers, A. Niclosamide a new chemotherapy agent? Pharmacokinetics of the potential anticancer drug in a patient cohort of the NIKOLO trial. J. Clin. Oncol. 2018, 36, e14536. [Google Scholar]
- Khan, A.; Sarkar, D. A simple whole cell based high throughput screening protocol using Mycobacterium bovis BCG for inhibitors against dormant and active tubercle bacilli. J. Microbiol. Methods 2008, 73, 62–68. [Google Scholar] [CrossRef]
- Franzblau, S.G.; DeGroote, M.A.; Cho, S.H.; Andries, K.; Nuermberger, E.; Orme, I.M.; Mdluli, K.; Angulo-Barturen, I.; Dick, T.; Dartois, V.; et al. Comprehensive analysis of methods used for the evaluation of compounds against Mycobacterium tuberculosis. Tuberculosis 2012, 92, 453–488. [Google Scholar] [CrossRef]
- Ayrapetyan, M.; Williams, T.; Oliver, J.D. Relationship between the Viable but Nonculturable State and Antibiotic Persister Cells. J. Bacteriol. 2018, 200, e00249-18. [Google Scholar] [CrossRef]
- Voskuil, M.I.; Visconti, K.C.; Schoolnik, G.K. Mycobacterium tuberculosis gene expression during adaptation to stationary phase and low-oxygen dormancy. Tuberculosis 2004, 84, 218–227. [Google Scholar] [CrossRef]
- Van, N.; Degefu, Y.N.; Leus, P.A.; Larkins-Ford, J.; Klickstein, J.; Maurer, F.P.; Stone, D.; Poonawala, H.; Thorpe, C.M.; Smith, T.C.; et al. Novel Synergies and Isolate Specificities in the Drug Interaction Landscape of Mycobacterium abscessus. Antimicrob. Agents Chemother. 2023, 67, e0009023. [Google Scholar] [CrossRef]
- North, R.J.; Izzo, A.A. Mycobacterial virulence. Virulent strains of Mycobacteria tuberculosis have faster in vivo doubling times and are better equipped to resist growth-inhibiting functions of macrophages in the presence and absence of specific immunity. J. Exp. Med. 1993, 177, 1723–1733. [Google Scholar]
- Mann, L.; Siersleben, F.; Lang, M.; Richter, A. Determination of bactericidal activity against 3HC-2-Tre-labelled Mycobacterium abscessus (Mycobacteroides abscessus) by automated fluorescence microscopy. J. Microbiol. Methods 2024, 224, 107002. [Google Scholar] [CrossRef]
- Richter, A.; Goddard, R.; Mann, L.; Siersleben, F.; Seidel, R.W. Structural characterization of a 3-hydroxychromone dye trehalose conjugate for fluorescent labelling of mycobacteria. J. Mol. Struct. 2024, 1298, 137010. [Google Scholar] [CrossRef]
- Du, P.; Sohaskey, C.D.; Shi, L. Transcriptional and Physiological Changes during Mycobacterium tuberculosis Reactivation from Non-replicating Persistence. Front. Microbiol. 2016, 7, 1346. [Google Scholar] [CrossRef]
- Sarathy, J.P.; Via, L.E.; Weiner, D.; Blanc, L.; Boshoff, H.; Eugenin, E.A.; Barry, C.E., 3rd; Dartois, V.A. Extreme Drug Tolerance of Mycobacterium tuberculosis in Caseum. Antimicrob. Agents Chemother. 2018, 62, e02266-17. [Google Scholar] [PubMed]
- Wallis, R.S.; Patil, S.; Cheon, S.H.; Edmonds, K.; Phillips, M.; Perkins, M.D.; Joloba, M.; Namale, A.; Johnson, J.L.; Teixeira, L.; et al. Drug tolerance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 1999, 43, 2600–2606. [Google Scholar] [CrossRef]
- Xie, Z.; Siddiqi, N.; Rubin, E.J. Differential antibiotic susceptibilities of starved Mycobacterium tuberculosis isolates. Antimicrob. Agents Chemother. 2005, 49, 4778–4780. [Google Scholar] [CrossRef]
- Holt, M.R.; Baird, T. Treatment Approaches to Mycobacterium abscessus Pulmonary Disease. Clin. Chest Med. 2023, 44, 785–798. [Google Scholar] [CrossRef]
- Hartkoorn, R.C.; Sala, C.; Neres, J.; Pojer, F.; Magnet, S.; Mukherjee, R.; Uplekar, S.; Boy-Rottger, S.; Altmann, K.H.; Cole, S.T. Towards a new tuberculosis drug: Pyridomycin–nature’s isoniazid. EMBO Mol. Med. 2012, 4, 1032–1042. [Google Scholar] [CrossRef]
- Seidel, R.W.; Goddard, R.; Lang, M.; Richter, A. Nalpha-Aroyl-N-Aryl-Phenylalanine Amides: A Promising Class of Antimycobacterial Agents Targeting the RNA Polymerase. Chem. Biodivers. 2024, 21, e202400267. [Google Scholar] [CrossRef]
- Lang, M.; Ganapathy, U.S.; Mann, L.; Abdelaziz, R.; Seidel, R.W.; Goddard, R.; Sequenzia, I.; Hoenke, S.; Schulze, P.; Aragaw, W.W.; et al. Synthesis and Characterization of Phenylalanine Amides Active against Mycobacterium abscessus and Other Mycobacteria. J. Med. Chem. 2023, 66, 5079–5098. [Google Scholar] [CrossRef]
- Beuchel, A.; Robaa, D.; Negatu, D.A.; Madani, A.; Alvarez, N.; Zimmerman, M.D.; Richter, A.; Mann, L.; Hoenke, S.; Csuk, R.; et al. Structure-Activity Relationship of Anti-Mycobacterium abscessus Piperidine-4-carboxamides, a New Class of NBTI DNA Gyrase Inhibitors. ACS Med. Chem. Lett. 2022, 13, 417–427. [Google Scholar] [CrossRef]
- Miranda-CasoLuengo, A.A.; Staunton, P.M.; Dinan, A.M.; Lohan, A.J.; Loftus, B.J. Functional characterization of the Mycobacterium abscessus genome coupled with condition specific transcriptomics reveals conserved molecular strategies for host adaptation and persistence. BMC Genom. 2016, 17, 553. [Google Scholar] [CrossRef]
- Jakkala, K.; Ajitkumar, P. Hypoxic Non-replicating Persistent Mycobacterium tuberculosis Develops Thickened Outer Layer That Helps in Restricting Rifampicin Entry. Front. Microbiol. 2019, 10, 2339. [Google Scholar] [CrossRef]
- Lin, W.; Mandal, S.; Degen, D.; Liu, Y.; Ebright, Y.W.; Li, S.; Feng, Y.; Zhang, Y.; Mandal, S.; Jiang, Y.; et al. Structural Basis of Mycobacterium tuberculosis Transcription and Transcription Inhibition. Mol. Cell 2017, 66, 169–179.e8. [Google Scholar] [CrossRef]
- Greendyke, R.; Byrd, T.F. Differential antibiotic susceptibility of Mycobacterium abscessus variants in biofilms and macrophages compared to that of planktonic bacteria. Antimicrob. Agents Chemother. 2008, 52, 2019–2026. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Ganapathy, U.S.; Lan, T.; Osiecki; Sarathy, J.P.; Dartois, V.; Aldrich, C.C.; Dick, T. ADP-ribosylation-resistant rifabutin analogs show improved bactericidal activity against drug-tolerant M. abscessus in caseum surrogate. Antimicrob. Agents Chemother. 2023, 67, e0038123. [Google Scholar] [CrossRef] [PubMed]
- Kolpen, M.; Jensen, P.Ø.; Qvist, T.; Kragh, K.N.; Ravnholt, C.; Fritz, B.G.; Johansen, U.R.; Bjarnsholt, T.; Høiby, N. Biofilms of Mycobacterium abscessus complex can be sensitized to antibiotics by disaggregation and oxygenation. Antimicrob. Agents Chemother. 2020, 64, e01212-19. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.; Goddard, R.; Siersleben, F.; Mann, L.; Seidel, R.W. Structural Elucidation of 2-(6-(Diethylamino) benzofuran-2-yl)-3-hydroxy-4 H-chromen-4-one and Labelling of Mycobacterium aurum Cells. Molbank 2023, 2023, M1647. [Google Scholar] [CrossRef]
- Ersts, P.J. DotDotGoose (1.7.0). American Museum of Natural History, Center for Biodiversity and Conservation. Available online: https://biodiversityinformatics.amnh.org/open_source/dotdotgoose/ (accessed on 1 September 2023).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feilcke, R.; Eckenstaler, R.; Lang, M.; Richter, A.; Imming, P. A Simple In Vitro Method to Determine Bactericidal Activity Against Mycobacterium abscessus Under Hypoxic Conditions. Antibiotics 2025, 14, 299. https://doi.org/10.3390/antibiotics14030299
Feilcke R, Eckenstaler R, Lang M, Richter A, Imming P. A Simple In Vitro Method to Determine Bactericidal Activity Against Mycobacterium abscessus Under Hypoxic Conditions. Antibiotics. 2025; 14(3):299. https://doi.org/10.3390/antibiotics14030299
Chicago/Turabian StyleFeilcke, Ruth, Robert Eckenstaler, Markus Lang, Adrian Richter, and Peter Imming. 2025. "A Simple In Vitro Method to Determine Bactericidal Activity Against Mycobacterium abscessus Under Hypoxic Conditions" Antibiotics 14, no. 3: 299. https://doi.org/10.3390/antibiotics14030299
APA StyleFeilcke, R., Eckenstaler, R., Lang, M., Richter, A., & Imming, P. (2025). A Simple In Vitro Method to Determine Bactericidal Activity Against Mycobacterium abscessus Under Hypoxic Conditions. Antibiotics, 14(3), 299. https://doi.org/10.3390/antibiotics14030299






