Antibiotics and Opportunities of Their Alternatives in Pig Production: Mechanisms Through Modulating Intestinal Microbiota on Intestinal Health and Growth
Abstract
:1. Introduction
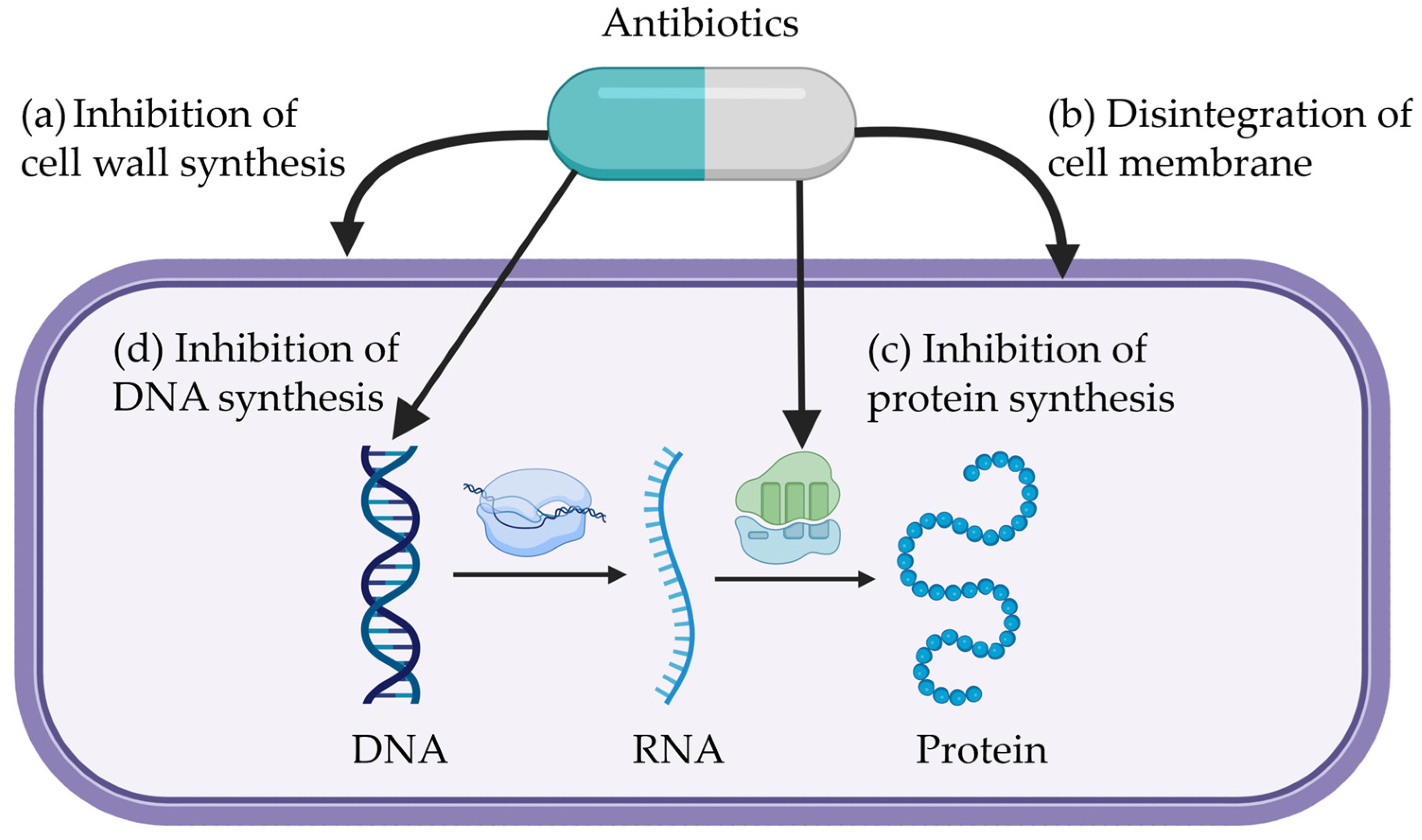
2. Mechanisms of Antibiotics
2.1. Inhibition of Cell Wall Synthesis
2.2. Disintegration of Cell Membrane
2.3. Inhibition of Protein Synthesis
2.4. Inhibition of DNA Synthesis
2.5. Antimicrobial Resistance
3. Mechanisms of Alternatives to Antimicrobial Growth Promoters
3.1. Organic Acid
3.2. Phytobiotics
3.3. Probiotics
3.4. Other Potentials: Postbiotics, Functional Fatty Acid, and Bacteriophage
4. Impact of Antimicrobial Growth Promoters on Intestinal Microbiota, Intestinal Health, and Growth of Pigs
| Initial BW, kg (Age, d) | Feeding Duration, d | Inclusion Rate, mg/kg | Sample Type | Alpha-/Beta- Diversity and Relative Abundance | Intestinal Health | Growth Performance | Reference |
|---|---|---|---|---|---|---|---|
| 9.3 kg (d 28) | 28 | 15 | Cecal digesta | Beta diversity: p < 0.05 (Unweighted and Weighted UniFrac distance) Prevotellaceae_NK3B31 ↓ Lachnospiraceae_unclassified ↓ Streptococcus ↓ | Villus heigh to crypt depth in the ileum, 100% ↑ | - | Lin and Yu [197] |
| 9.0 kg (d 21) | 42 | 15 | Feces | Beta diversity: p < 0.05 (Weighted principal coordinate analysis) Streptococcus ↓ Treponema 2 ↑ Lachnospiraceae_unclassified ↑ | - | ADG, 7% ↑ ADFI, 6% ↑ | Lin and Yu [198] |
| 6.6 kg (d 21) * | 28 | 30 | Jejunal mucosa | Chao 1 ↑ Simpson ↑ Shannon ↑ Acinetobacter ↑ Bifidobacterium ↑ Pseudomonas ↑ | Villus height in the jejunum, 22% ↑ Villus heigh to crypt depth in the jejunum, 30% ↑ | ADG, 39% ↑ ADFI, 23% ↑ G:F, 10% ↑ | Xu et al. [25] |
| 6.3 kg (d 21) * | 28 | 30 | Feces | - | Protein carbonyl in the jejunum, 29% ↓ | ADG, 22% ↑ ADFI, 12% ↑ G:F, 11% ↑ | Duarte et al. [80] |
| Jejunal mucosa | - | ||||||
| 6.6 kg (d 21) | 30 | 150 | Jejunum | Chao 1 ↓ Shannon ↓ Beta diversity: p < 0.05 (Bray-Curtis) Clostridium_sensu_stricto ↓ Butyrivibrio ↑ | mRNA expression of aminopeptidase, maltase-glucoamylase, and sucrase-isomaltase in the jejunum ↑ | ADG, 6% ↑ ADFI, 3% ↑ G:F, 3% ↑ | Ángel-Isaza et al. [199] |
| 7.9 kg (d 21) * | 28 | 30 | Jejunal mucosa | Simpson ↑ Shannon ↑ Sphingomonadaceae ↑ Propionibacteriaceae ↑ | TNF-α in the jejunum, 22% ↓ Malondialdehyde in the jejunum, 46% ↓ mRNA expression of interferon-γ ↑ mRNA expression of TLR4 and NOD1 ↓ | ADG, 12% ↑ ADFI, 6% ↑ G:F, 3% ↑ | Duarte et al. [26] |
| 9.9 kg (d 28) | 28 | 30 | Feces | Shannon ↓ Beta diversity: p < 0.05 (Unweighted and Weighted UniFrac distance) [Ruminococcus] gauvreauii ↓ Ruminococcus UCG-005 ↓ | - | ADG, 3% ↑ ADFI, 6% ↑ G:F, 2% ↓ | Hung et al. [168] |
| Initial BW, kg (Age, d) | Feeding Duration, d | Inclusion Rate, mg/kg | Sample Type | Alpha-/Beta- Diversity and Relative Abundance | Intestinal Health | Growth Performance | Reference |
|---|---|---|---|---|---|---|---|
| (d 21) | 28 | 55 (first 14 d) 27.5 (last 14 d) | Feces | Chao 1 ↓ Shannon ↓ Faith’s phylogenetic diversity ↓ Slackia ↓ Peptococcus ↓ Catenibacterium ↓ | - | - | Lourenco et al. [200] |
| 5.8 kg (d 19) | 33 | 55 | Feces | Veillonellaceae ↑ Streptococcus ↓ | - | - | Muurinen et al. [201] |
| 6.9 kg * | 12 | 0.5 | Colonic digesta | Lactobacillaceae ↓ | - | ADG, 17% ↓ ADFI, 7% ↓ G:F, 11% ↓ | Kim et al. [202,203] |
| 12 | 50 | Colonic digesta | Lactobacillaceae ↑ | Villus heigh to crypt depth in the jejunum, 49% ↑ | ADG, 17% ↑ ADFI, 5% ↑ G:F, 23% ↑ | ||
| 18 | 0.5 | Colonic digesta | - | - | ADG, 6% ↓ ADFI, 5% ↓ G:F, 1% ↓ | ||
| 18 | 50 | Colonic digesta | Beta diversity: p < 0.05 (Bray-Curtis) Prevotellaceae ↓ Lactobacillaceae ↓ Clostridiaceae ↑ | Villus heigh to crypt depth in the jejunum, 35% ↑ Villus height in the jejunum, 20% ↑ mRNA expression of ZO-1 and occludin in the ileum ↑ mRNA expression of IL-1β, IL-6, and TNF-α in the ileum ↓ | ADG, 14% ↑ G:F, 15% ↑ | ||
| 6.2 kg (d 21) * | 28 | 50 | Jejunal digesta | Streptococcus ↑ Bifidobacterium ↓ | Villus height in the jejunum, 25% ↑ mRNA expression of IL-6 in the ileum ↓ | ADG, 53% ↑ ADFI, 35% ↑ G:F, 15% ↑ | Jinno et al. [204] He et al. [177] |
| 28 | 50 | Ileal digesta | Shannon ↑ Lactobacillaceae ↓ Clostridium sensu stricto 1 ↑ | ||||
| 28 | 50 | Colonic digesta | Lactobacillaceae ↓ Streptococcus ↓ Clostridium sensu stricto 1 ↑ | ||||
| 7.2 kg (d 21 to 24) * | 12 | 50 | Colonic digesta | Streptococcaceae ↓ | mRNA expression of ZO-1 and occludin in the jejunum ↑ mRNA expression of IL-1β and IL-6 in the ileum ↓ | ADG, 26% ↑ ADFI, 5% ↓ G:F, 33% ↑ | Kim et al. [205,206] |
| 18 | 50 | Colonic digesta | Clostridiaceae ↑ Lactobacillaceae ↓ | mRNA expression of occludin in the jejunum ↑ | ADG, 19% ↑ ADFI, 2% ↓ G:F, 16% ↑ | ||
| 7.4 kg (d 21) * | 7 | 50 | Feces | Blautia ↑ Escherichia-Shigella ↓ | - | ADG, 15% ↑ ADFI, 30% ↑ G:F, 12% ↓ | Jinno et al. [207] |
| 14 | 50 | Feces | Agathobacter ↓ | - | ADG, 26% ↑ ADFI, 6% ↑ G:F, 19% ↑ | ||
| 21 | 50 | Feces | Dorea ↓ Streptococcus ↓ | - | ADG, 15% ↑ ADFI, 8% ↑ G:F, 6% ↑ | ||
| 28 | 50 | Feces | Blautia ↓ Dorea ↓ Lactobacillus ↓ | - | ADG, 16% ↑ ADFI, 15% ↑ G:F, 1% ↑ | ||
| 28 | 50 | Ileal digesta | Clostridium sensu stricto 1 ↑ Megasphaera ↓ | - | ADG, 16% ↑ ADFI, 15% ↑ G:F, 1% ↑ |
5. Impact of Antimicrobial Growth Promoter Alternatives on Intestinal Microbiota, Intestinal Health, and Growth of Pigs
5.1. Organic Acid
5.2. Phytobiotics
5.3. Probiotics
| Type | Initial BW, kg | Feeding Duration, d | Inclusion Rate, % | Sample Type | Alpha-/Beta- Diversity and Relative Abundance | Intestinal Health | Growth Performance | Reference |
|---|---|---|---|---|---|---|---|---|
| Protected sodium butyrate | 6.5 | 39 | 0.3, 0.2, 0.1 in phase 1, 2, 3, respectively | Feces | Escherichia coli ↓ Total coliforms ↓ | Lipid peroxidation in the jejunum, 19% ↓ Glutathione peroxidase in the jejunum, 58% ↑ Superoxide dismutase, 58% ↑ | ADG, 2% ↓ ADFI, 7% ↓ G:F, 3% ↑ | Marchiori et al. [222] |
| Tributyrin | 6.5 | 39 | 0.3, 0.2, 0.1 in phase 1, 2, 3, respectively | Feces | Escherichia coli ↓ Total coliforms ↓ | Lipid peroxidation in the jejunum, 38% ↓ Superoxide dismutase, 46% ↑ | ADG, 7% ↑ ADFI, 3% ↓ G:F, 10% ↑ | |
| Lauric acid, butyrate, medium-chain fatty acids | 7.4 | 42 | 0.2 | Feces | Spirochaetes ↓ | mRNA expression of superoxide dismutase 1, glutathione peroxidase 1, and ZO-1 in the jejunum ↑ Villus height to crypt depth ratio in the jejunum, 31% ↑ | ADG, 13% ↑ ADFI, 10% ↑ G:F, 4% ↑ | Cai et al. [218] |
| Sodium butyrate | 5.9 | 14 | 0.1 | Colonic digesta | Lactobacillus ↑ Enterobacteriaceae ↓ Escherichia coli ↓ | Goblet cells in the ileum, 26% ↑ | ADG, 3% ↓ ADFI, 9% ↓ G:F, 11% ↑ | Sadurni et al. [221] |
| Gluconic acid | 8.2 | 42 | 1.8 | Distal small intestinal/Colonic digesta | Chao1 ↓ Simpson ↓ Lactobacillus amylovorus ↑ Faecalibacterium prausnitzii ↑ Megasphaera elsdenii ↑ | Butyrate concentration in the cecum and colon ↑ mRNA expression of MUC2 and IFN-γ in the ileum ↑ | ADG, 7% ↑ ADFI, 11% ↑ G:F, 1% ↓ | Michiels et al. [219] |
| Encapsulated sodium butyrate | 4.7 | 49 | 0.20, 0.15, 0.10 in phase 1, 2, 3, respectively | Cecal digesta | Streptococcaceae ↓ | - | ADG, 5% ↑ ADFI, 1% ↓ G:F, 6% ↑ | da Silva et al. [299] |
| Formic acid, ammonium formate, acetic acid | 5.3 | 49 | 0.2 | Cecal digesta | Beta-diversity: p < 0.05 (Jaccard distances) Coprococcus ↑ Blautia ↑ | - | ADG, 4% ↑ ADFI, 3% ↑ G:F, 1% ↑ | Xiang et al. [220] |
| Sodium butyrate, benzoic acid | 6.9 | 35 | 0.105 sodium butyrate, 0.5 benzoic acid | Feces | Shannon ↑ Beta-diversity: p < 0.05 (Bray-Curtis) Veillonella ↓ Sarcina ↓ Turicibacter ↑ | - | ADG, 3% ↓ ADFI, 11% ↓ G:F, 8% ↑ | Wei et al. [300] |
| Sorbic acid, medium chain fatty acid, formic acid, short chain fatty acid | 6.7 | 20 | 0.2 Presan FX and 0.3 Fysal MP | Feces | Ruminococcaceae ↑ Lachnospiraceae ↑ Lactobacillaceae ↑ | - | ADG, 10% ↑ ADFI, 1% ↑ G:F, 5% ↑ | Pluske et al. [226] |
| Formic acid, ammonium formate, propionic acid, acetic acid, citric acid | 7.8 | 28 | 0.3 | Cecal digesta | Lachnospiraceae ↓ Escherichia-Shigella ↓ | mRNA expression of claudin-1 and ZO-1 ↑ Acetic acid concentration in the cecum, 29% ↑ | ADG, 9% ↑ ADFI, 5% ↓ G:F, 14% ↑ | Ma et al. [301] |
| Short chain fatty acid | 8.7 | 42 | 0.2 | Feces | Clostridium sensu stricto 1 ↑ Streptococcus ↓ | - | ADG, 4% ↑ ADFI, 2% ↑ G:F, 2% ↑ | Lingbeek et al. [302] |
| Type | Initial BW, kg | Feeding Duration, d | Inclusion Rate, % | Sample Type | Alpha-/Beta- Diversity and Relative Abundance | Intestinal Health | Growth Performance | Reference |
|---|---|---|---|---|---|---|---|---|
| Mixture of castor oil and cashew nutshell liquid | 7.0 | 34 | 0.50, 0.75, 1.00, or 1.50 | Jejunal mucosa | Helicobacteraceae ↓ Lactobacillus kitasatonis ↑ | Protein carbonyl in the jejunal mucosa ↓ Villus height in the jejunum ↑ | - | Moita et al. [303] |
| Herb | 6.4 | 28 | 1 | Jejunal mucosa | Chao1 ↓ Shannon ↓ Simpson ↓ | Protein carbonyl in the jejunum, 44% ↓ Villus height to crypt depth ratio in the jejunum, 35% ↑ Ki-67+ in the jejunum, 28% ↓ | ADG, 13% ↓ ADFI, 13% ↓ | Garavito-Duarte et al. [78] |
| Essential oil | 6.4 | 28 | 1 | Jejunal mucosa | Syntrophococcus ↓ Corynebacterium ↓ | Villus height to crypt depth ratio in the jejunum, 29% ↑ Ki-67+ in the jejunum, 21% ↓ | ADG, 2% ↑ ADFI, 2% ↓ | |
| Essential oil | 6.3 | 28 | 0.05 | Cecal digesta | - | Glutathione peroxidase in the jejunum, 13% ↑ mRNA expression of TLR4 (460% ↑), TLR8 (1455% ↑), TNF-α (161% ↑), and IL-1β (366% ↑) in the ileum | ADFI, 13% ↓ G:F, 4% ↓ | Mo et al. [261] |
| Microencapsulated essential oil | 6.3 | 28 | 0.05 | Cecal digesta | 8 potential pathogenic bacteria ↓ | Glutathione peroxidase in the jejunum, 30% ↑ | ADG, 17% ↑ ADFI, 4% ↓ G:F, 2% ↑ | |
| Essential oil | 7.6 | 28 | 0.04 | Colonic digesta | Holdemanella ↑ phascolarctobacterium ↑ | Villus height in the ileum ↑ Expression of TLR4 and NF-κB in the ileum ↓ | ADG, 27% ↑ ADFI, 25% ↑ | Shao et al. [304] |
| Herbal plant extract | 8.7 | 33 | 0.5 | Feces | E. coli ↓ Lactobacillus ↑ Bifidobacterium ↑ | Villus height in the ileum, 80% ↑ | - | Shuo et al. [305] |
| Tannin | 8.6 | 21 | 0.15 | Colonic digesta | Clostridium_sp_Culture_27 ↓ Lactococcus ↑ | The activity of maltase and sucrase in the jejunum ↑ | ADG, 22% ↑ ADFI, 10% ↑ G:F, 11% ↑ | Xu et al. [306] |
| Tannic acid | 7.7 | 28 | 0.1 | Colonic digesta | E. coli ↓ | Butyrate concentration in cecal digesta, 98% ↑ Villus height to crypt depth ratio in the ileum, 20% ↑ | - | Song et al. [307] |
| Type | Initial BW, kg | Feeding Duration, d | Inclusion Rate (%), Daily Oral Administration (CFU/d), or Concentration of Probiotics in Diet (CFU/kg) | Sample Type | Alpha-/Beta- Diversity and Relative Abundance | Intestinal Health | Growth Performance | Reference |
|---|---|---|---|---|---|---|---|---|
| Lactobacillus | 6.1 | 47 | 0.1% | Feces | C. Incertae Sedis XIII ↑ | Villus height to crypt depth ratio in the jejunum, 5% ↑ | ADG, 1% ↓ ADFI, 1% ↓ G:F, 2% ↓ | Zuniga et al. [276] |
| Bifidobacterium | 6.1 | 47 | 0.1% | Feces | Streptococcaceae ↓ | Villus height to crypt depth ratio in the jejunum, 19% ↑ | ADFI, 3% ↓ G:F, 1% ↓ | |
| Enterococcus hirae | 6.4 | 21 | 1.0 × 1010 CFU/d | Colonic digesta | Chao1 ↑ Simpson ↑ Channon ↑ Beta-diversity: p < 0.05 Prevotellaceae ↑ Lactobacillaceae ↓ Bacteroidaceae ↑ | Acetic acid concentration in the colon ↑ mRNA expression of proliferating cell nuclear antigen and villus height in the jejunum ↑ | ADG, 14% ↑ G:F, 11% ↓ | Zhang et al. [270] |
| Lactiplantibacillus argentoratensis | 5.9 | 24 | 1.0 × 108 CFU/d | Feces | Beta-diversity: p < 0.05 (UniFrac distances) Streptococcus ↑ Clostridium ↑ Campylobacter ↓ | - | ADG, 36 folds ↑ | Yoon et al. [271] |
| Bacillus | 8.1 | 31 | 0.04% | Feces | Streptococcus ↓ Lactobacillus ↑ | Goblet cells in the ileum, 71% ↑ mRNA expression of TNF-α (81% ↓) and occludin (147% ↑) in the ileum | ADG, 5% ↑ ADFI, 2% ↑ G:F, 2% ↑ | Xue et al. [278] |
| Bacillus licheniformis | 8.2 | 10 | 2.5 × 109 CFU/kg | Ileal digesta Colonic digesta | Bacteroidetes ↓ | mRNA expression of ZO-1 (133% ↑), occludin (156% ↑), SGLT1 (202% ↑), and aminopeptidase N (98% ↑) in the ileum | ADG, 27% ↑ ADFI, 3% ↓ G:F, 26% ↑ | Xu et al. [308] |
| Lactobacillus plantarum, lactobacillus reuteri, bifidobacterium longum | 7.1 | 14 | 1.0 × 109 CFU/kg | Feces | Beta-diversity: p < 0.05 (Bray–Curtis) Faecalibacterium ↑ Parabacteroides ↑ Clostridium ↑ | mRNA expression of IL-1β, IL-6, TNF-α, and NF-κB in the duodenum ↓ | ADG, 15% ↑ | Tang et al. [272] |
| Enterococcus faecium, bacillus subtilis, and saccharomyces cerevisiae | 13.1 | 42 | 0.5% | Feces | Shannon ↑ Beta-diversity: p < 0.05 (Bray–Curtis) Ruminococcaceae ↑ Prevotella ↑ Eubaterium coprostanoligenes ↑ | - | ADG, 19% ↑ | Park et al. [273] |
| Clostridium butyricum | 8.2 | 28 | 1.5 × 109 CFU/d | Feces | Beta-diversity: p < 0.05 (unifrac distance) Faecalibacterium ↑ Rikenellaceae ↓ | - | ADG, 25% ↑ ADFI, 7% ↑ G:F, 19% ↑ | Liu et al. [274] |
| Lactiplantibacillus plantarum, Bacillus subtilis | 8.9 | 50 | 1.0 × 109 CFU/kg | Feces | Shannon ↑ Beta-diversity: p < 0.05 (NMDS) Lactobacillus ↑ Streptococcus ↓ Clostridium ↓ | - | ADG, 15% ↑ ADFI, 9% ↑ G:F, 4% ↑ | Chen et al. [275] |
| Bacillus licheniformis | 6.5 | 14 | 0.1% | Cecal digesta | Lactobacillus ↑ Clostridium ↓ | Lipase activity, occludin, ZO-1, and villus height to crypt depth ratio in the jejunum ↑ Lactic acid concentration in the cecum, 212% ↑ | ADG, 25% ↑ ADFI, 19% ↑ G:F, 7% ↑ | Sun et al. [277] |
| Bacillus subtilis | 6.4 | 28 | 2.0 × 109 CFU/d | Feces | Escherichia coli ↓ Total coliforms ↓ Bacillus spp. ↑ | mRNA expression of occludin and proliferating cell nuclear antigen in the ileum ↑ mRNA expression of TLR-4 in the ileum ↓ | ADFI, 9% ↓ G:F, 13% ↑ | Sudan et al. [309] |
5.4. Other Potentials: Postbiotics, Functional Fatty Acid, and Bacteriophage
6. Comparison of Antimicrobial Growth Promoters and Their Alternatives and Future Research
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castanon, J.I.R. History of the use of antibiotic as growth promoters in European poultry feeds. Poult. Sci. 2007, 86, 2466–2471. [Google Scholar] [CrossRef]
- Cromwell, G.L. Why and how antibiotics are used in swine production. Anim. Biotechnol. 2002, 13, 7–27. [Google Scholar] [CrossRef]
- Zimmerman, D.R. Role of subtherapeutic levels of antimicrobials in pig production. J. Anim. Sci. 1986, 62, 6–16. [Google Scholar] [CrossRef]
- Dritz, S.S.; Tokach, M.D.; Goodband, R.D.; Nelssen, J.L. Effects of administration of antimicrobials in feed on growth rate and feed efficiency of pigs in multisite production systems. J. Am. Vet. Med. Assoc. 2002, 220, 1690–1695. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-G.; Johnson, T.A.; Su, J.-Q.; Qiao, M.; Guo, G.-X.; Stedtfeld, R.D.; Hashsham, S.A.; Tiedje, J.M. Diverse and abundant antibiotic resistance genes in Chinese swine farms. Proc. Natl. Acad. Sci. USA 2013, 110, 3435–3440. [Google Scholar] [CrossRef] [PubMed]
- Fournier, P.-E.; Vallenet, D.; Barbe, V.; Audic, S.; Ogata, H.; Poirel, L.; Richet, H.; Robert, C.; Mangenot, S.; Abergel, C. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet. 2006, 2, e7. [Google Scholar] [CrossRef]
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Mohamed, M.G. Antimicrobial resistance: Impacts, challenges, and future prospects. J. Med. Surg. Public Health 2024, 2, 100081. [Google Scholar] [CrossRef]
- Rigueira, L.L.; Perecmanis, S. Concerns about the use of antimicrobials in swine herds and alternative trends. Transl. Anim. Sci. 2024, 8, txae039. [Google Scholar] [CrossRef]
- Littmann, J.; Viens, A.M. The Ethical Significance of Antimicrobial Resistance; Oxford University Press: Oxford, UK, 2015; Volume 8, pp. 209–224. [Google Scholar]
- Patel, S.J.; Wellington, M.; Shah, R.M.; Ferreira, M.J. Antibiotic stewardship in food-producing animals: Challenges, progress, and opportunities. Clin. Ther. 2020, 42, 1649–1658. [Google Scholar] [CrossRef]
- FDA. Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals; U.S. Department of Health and Human Services: Rockville, MD, USA, 2023. Available online: https://www.fda.gov/animal-veterinary/antimicrobial-resistance/2023-summary-report-antimicrobials-sold-or-distributed-use-food-producing-animals (accessed on 12 April 2024).
- European Medicines Agency. Annual Report 2023; European Medicines Agency: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Regulation (EU) 2019/6 on Veterinary Medicines and Regulation. 2020. Available online: https://eur-lex.europa.eu/eli/reg/2019/6/oj (accessed on 10 March 2025).
- Zhao, Q.; Jiang, Z.; Li, T.; Cheng, M.; Sun, H.; Cui, M.; Zhang, C.; Xu, S.; Wang, H.; Wu, C. Current status and trends in antimicrobial use in food animals in China, 2018–2020. One Health Adv. 2023, 1, 29. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, L. China’s national plan to combat antimicrobial resistance. Lancet Infect. Dis. 2016, 16, 1216–1218. [Google Scholar] [CrossRef] [PubMed]
- Dutra, M.C.; Moreno, L.Z.; Dias, R.A.; Moreno, A.M. Antimicrobial use in Brazilian swine herds: Assessment of use and reduction examples. Microorganisms 2021, 9, 881. [Google Scholar] [CrossRef]
- Tiseo, K.; Huber, L.; Gilbert, M.; Robinson, T.P.; Van Boeckel, T.P. Global trends in antimicrobial use in food animals from 2017 to 2030. Antibiotics 2020, 9, 918. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.R.; Innes, G.K.; Johnson, K.A.; Lhermie, G.; Ivanek, R.; Greiner Safi, A.; Lansing, D. Consumer perceptions of antimicrobial use in animal husbandry: A scoping review. PLoS ONE 2021, 16, e0261010. [Google Scholar] [CrossRef]
- Albernaz-Gonçalves, R.; Olmos Antillón, G.; Hötzel, M.J. Linking animal welfare and antibiotic use in pig farming—A review. Animals 2022, 12, 216. [Google Scholar] [CrossRef]
- Sun, Y.; Duarte, M.E.; Kim, S.W. Dietary inclusion of multispecies probiotics to reduce the severity of post-weaning diarrhea caused by Escherichia coli F18+ in pigs. Anim. Nutr. 2021, 7, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.E.; Tyus, J.; Kim, S.W. Synbiotic effects of enzyme and probiotics on intestinal health and growth of newly weaned pigs challenged with enterotoxigenic F18+ Escherichia coli. Front. Vet. Sci. 2020, 7, 573. [Google Scholar] [CrossRef]
- Li, P.; Niu, Q.; Wei, Q.; Zhang, Y.; Ma, X.; Kim, S.W.; Lin, M.; Huang, R. Microbial shifts in the porcine distal gut in response to diets supplemented with Enterococcus faecalis as alternatives to antibiotics. Sci. Rep. 2017, 7, 41395. [Google Scholar] [CrossRef]
- Kim, K.; Ehrlich, A.; Perng, V.; Chase, J.A.; Raybould, H.; Li, X.; Atwill, E.R.; Whelan, R.; Sokale, A.; Liu, Y. Algae-derived β-glucan enhanced gut health and immune responses of weaned pigs experimentally infected with a pathogenic E. coli. Anim. Feed Sci. Technol. 2019, 248, 114–125. [Google Scholar] [CrossRef]
- Li, Y.S.; San Andres, J.V.; Trenhaile-Grannemann, M.D.; van Sambeek, D.M.; Moore, K.C.; Winkel, S.M.; Fernando, S.C.; Burkey, T.E.; Miller, P.S. Effects of mannan oligosaccharides and Lactobacillus mucosae on growth performance, immune response, and gut health of weanling pigs challenged with Escherichia coli lipopolysaccharides. J. Anim. Sci. 2021, 99, skab286. [Google Scholar] [CrossRef]
- Xu, X.; Duarte, M.E.; Kim, S.W. Postbiotic effects of Lactobacillus fermentate on intestinal health, mucosa-associated microbiota, and growth efficiency of nursery pigs challenged with F18+ Escherichia coli. J. Anim. Sci. 2022, 100, skac210. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.E.; Deng, Z.; Kim, S.W. Effects of dietary Lactobacillus postbiotics and bacitracin on the modulation of mucosa-associated microbiota and pattern recognition receptors affecting immunocompetence of jejunal mucosa in pigs challenged with enterotoxigenic F18+ Escherichia coli. J. Anim. Sci. Biotechnol. 2024, 15, 139. [Google Scholar] [CrossRef]
- Kim, S.W.; Duarte, M.E. Saccharomyces yeast postbiotics supplemented in feeds for sows and growing pigs for its impact on growth performance of offspring and growing pigs in commercial farm environments. Anim. Biosci. 2024, 37, 1463. [Google Scholar] [CrossRef]
- Duarte, M.E.; Kim, S.W. Efficacy of Saccharomyces yeast postbiotics on cell turnover, immune responses, and oxidative stress in the jejunal mucosa of young pigs. Sci. Rep. 2024, 14, 19235. [Google Scholar] [CrossRef]
- Gormley, A.R.; Duarte, M.E.; Deng, Z.; Kim, S.W. Saccharomyces yeast postbiotics mitigate mucosal damages from F18+ Escherichia coli challenges by positively balancing the mucosal microbiota in the jejunum of young pigs. Anim. Microbiome 2024, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Holanda, D.M.; Kim, Y.I.; Parnsen, W.; Kim, S.W. Phytobiotics with adsorbent to mitigate toxicity of multiple mycotoxins on health and growth of pigs. Toxins 2021, 13, 442. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.E.; Kim, S.W. Phytobiotics from oregano extracts enhance the intestinal health and growth performance of pigs. Antioxidants 2022, 11, 2066. [Google Scholar] [CrossRef] [PubMed]
- Kommera, S.K.; Mateo, R.D.; Neher, F.J.; Kim, S.W. Phytobiotics and organic acids as potential alternatives to the use of antibiotics in nursery pig diets. Asian-Australas. J. Anim. Sci. 2006, 19, 1784–1789. [Google Scholar] [CrossRef]
- Park, I.; Lee, J.K.; Wang, J.; Kim, S.W. 0948 Effects of dietary supplementation of phytobiotics on intestinal health and growth performance of nursery pigs. J. Anim. Sci. 2016, 94, 456. [Google Scholar] [CrossRef]
- Garavito-Duarte, Y.; Bonetti, A.; Tugnoli, B.; Choi, H.; Piva, A.; Grilli, E.; Kim, S.W. Investigation of the nutritional and functional roles of a microencapsulated blend of botanicals on intestinal health and growth of nursery pigs challenged with F18+ Escherichia coli. J. Anim. Sci. 2025, 103, skaf047. [Google Scholar] [CrossRef]
- Choi, H.; Chen, Y.; Longo, F.; Kim, S.W. Comparative effects of benzoic acid and sodium benzoate in diets for nursery pigs on growth performance and acidification of digesta and urine. J. Anim. Sci. 2023, 101, skad116. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.B.; Song, Y.S.; Seo, S.; Kim, B.G. Effects of Supplemental Benzoic Acid, Bromelain, Adipic Acid, and Humic Substances on Nitrogen Utilization, Urine pH, Slurry pH, and Manure Odorous Compounds in Pigs. Animals 2023, 14, 82. [Google Scholar] [CrossRef]
- Kim, J.S.; Hosseindoust, A.; Lee, S.H.; Choi, Y.H.; Kim, M.J.; Lee, J.H.; Kwon, I.K.; Chae, B.J. Bacteriophage cocktail and multi-strain probiotics in the feed for weanling pigs: Effects on intestine morphology and targeted intestinal coliforms and Clostridium. Animal 2017, 11, 45–53. [Google Scholar] [CrossRef]
- Choi, Y.; Hosseindoust, A.; Ha, S.H.; Kim, J.; Min, Y.; Jeong, Y.; Mun, J.; Sa, S.; Kim, J. Effects of dietary supplementation of bacteriophage cocktail on health status of weanling pigs in a non-sanitary environment. J. Anim. Sci. Biotechnol. 2023, 14, 64. [Google Scholar] [CrossRef]
- Gardiner, G.E.; Metzler-Zebeli, B.U.; Lawlor, P.G. Impact of intestinal microbiota on growth and feed efficiency in pigs: A review. Microorganisms 2020, 8, 1886. [Google Scholar] [CrossRef]
- Duarte, M.E.; Kim, S.W. Intestinal microbiota and its interaction to intestinal health in nursery pigs. Anim. Nutr. 2022, 8, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Espinosa, C.D.; Abelilla, J.J.; Casas, G.A.; Lagos, L.V.; Lee, S.A.; Kwon, W.B.; Mathai, J.K.; Navarro, D.M.; Jaworski, N.W. Non-antibiotic feed additives in diets for pigs: A review. Anim. Nutr. 2018, 4, 113–125. [Google Scholar] [CrossRef]
- Meroueh, S.O.; Bencze, K.Z.; Hesek, D.; Lee, M.; Fisher, J.F.; Stemmler, T.L.; Mobashery, S. Three-dimensional structure of the bacterial cell wall peptidoglycan. Proc. Natl. Acad. Sci. USA 2006, 103, 4404–4409. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Yarlagadda, V.; Ghosh, C.; Haldar, J. A review on cell wall synthesis inhibitors with an emphasis on glycopeptide antibiotics. Medchemcomm 2017, 8, 516–533. [Google Scholar] [CrossRef]
- Shockman, G.D.; Daneo-Moore, L.; Kariyama, R.; Massidda, O. Bacterial walls, peptidoglycan hydrolases, autolysins, and autolysis. Microb. Drug Resist. 1996, 2, 95–98. [Google Scholar] [CrossRef]
- Hashemian, S.M.R.; Farhadi, Z.; Farhadi, T. Fosfomycin: The characteristics, activity, and use in critical care. Ther. Clin. Risk Manag. 2019, 15, 525–530. [Google Scholar] [CrossRef]
- E l Zoeiby, A.; Sanschagrin, F.; Levesque, R.C. Structure and function of the Mur enzymes: Development of novel inhibitors. Mol. Microbiol. 2003, 47, 1–12. [Google Scholar] [CrossRef]
- Brandish, P.E.; Kimura, K.-i.; Inukai, M.; Southgate, R.; Lonsdale, J.T.; Bugg, T.D. Modes of action of tunicamycin, liposidomycin B, and mureidomycin A: Inhibition of phospho-N-acetylmuramyl-pentapeptide translocase from Escherichia coli. Antimicrob. Agents Chemother. 1996, 40, 1640–1644. [Google Scholar] [CrossRef] [PubMed]
- Butaye, P.; Devriese, L.A.; Haesebrouck, F. Antimicrobial growth promoters used in animal feed: Effects of less well known antibiotics on gram-positive bacteria. Clin. Microbiol. Rev. 2003, 16, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.; Chen, L.; Hu, Y.; Rew, Y.; Shin, D.; Boger, D.L. Chemistry and biology of ramoplanin: A lipoglycodepsipeptide with potent antibiotic activity. Chem. Rev. 2005, 105, 449–476. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Bradford, P.A. β-Lactams and β-lactamase inhibitors: An overview. Cold Spring Harb. Perspect. Med. 2016, 6, a025247. [Google Scholar] [CrossRef]
- Epand, R.M.; Walker, C.; Epand, R.F.; Magarvey, N.A. Molecular mechanisms of membrane targeting antibiotics. Biochim. Biophys. Acta Biomembr. 2016, 1858, 980–987. [Google Scholar] [CrossRef]
- Taylor, S.D.; Palmer, M. The action mechanism of daptomycin. Bioorg. Med. Chem. 2016, 24, 6253–6268. [Google Scholar] [CrossRef]
- Russell, J.B.; Strobel, H. Effect of ionophores on ruminal fermentation. Appl. Environ. Microbiol. 1989, 55, 1–6. [Google Scholar] [CrossRef]
- Beutler, B. LPS in microbial pathogenesis: Promise and fulfilment. J. Endotoxin Res. 2002, 8, 329–335. [Google Scholar] [CrossRef]
- Wiedemann, I.; Breukink, E.; Van Kraaij, C.; Kuipers, O.P.; Bierbaum, G.; De Kruijff, B.; Sahl, H.-G. Specific binding of nisin to the peptidoglycan precursor lipid II combines pore formation and inhibition of cell wall biosynthesis for potent antibiotic activity. J. Biol. Chem. 2001, 276, 1772–1779. [Google Scholar] [CrossRef]
- Shajani, Z.; Sykes, M.T.; Williamson, J.R. Assembly of bacterial ribosomes. Annu. Rev. Biochem. 2011, 80, 501–526. [Google Scholar] [CrossRef] [PubMed]
- McCoy, L.S.; Xie, Y.; Tor, Y. Antibiotics that target protein synthesis. Wiley Interdiscip. Rev. RNA 2011, 2, 209–232. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.L.; Moore, P.B.; Steitz, T.A. Structures of five antibiotics bound at the peptidyl transferase center of the large ribosomal subunit. J. Mol. Biol. 2003, 330, 1061–1075. [Google Scholar] [CrossRef]
- Zhang, W.; Dunkle, J.A.; Cate, J.H.D. Structures of the ribosome in intermediate states of ratcheting. Science 2009, 325, 1014–1017. [Google Scholar] [CrossRef]
- Mukhtar, T.A.; Wright, G.D. Streptogramins, oxazolidinones, and other inhibitors of bacterial protein synthesis. Chem. Rev. 2005, 105, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.L.; Ippolito, J.A.; Ban, N.; Nissen, P.; Moore, P.B.; Steitz, T.A. The structures of four macrolide antibiotics bound to the large ribosomal subunit. Mol. Cell 2002, 10, 117–128. [Google Scholar] [CrossRef]
- Ebright, R.H. RNA polymerase: Structural similarities between bacterial RNA polymerase and eukaryotic RNA polymerase II. J. Mol. Biol. 2000, 304, 687–698. [Google Scholar] [CrossRef]
- Floss, H.G.; Yu, T.-W. Rifamycin mode of action, resistance, and biosynthesis. Chem. Rev. 2005, 105, 621–632. [Google Scholar] [CrossRef]
- Roca, J.; Berger, J.M.; Harrison, S.C.; Wang, J.C. DNA transport by a type II topoisomerase: Direct evidence for a two-gate mechanism. Proc. Natl. Acad. Sci. USA 1996, 93, 4057–4062. [Google Scholar] [CrossRef]
- Abbass, E.M.; Khalil, A.K.; Mohamed, M.M.; Eissa, I.H.; El-Naggar, A.M. Design, efficient synthesis, docking studies, and anticancer evaluation of new quinoxalines as potential intercalative Topo II inhibitors and apoptosis inducers. Bioorg. Chem. 2020, 104, 104255. [Google Scholar] [CrossRef] [PubMed]
- Zhanteng, S.; Xia, F.; Jingrong, Z.; Zhiming, X.; Yang, L.; Shi, W.; Decheng, S. Multi-residue determination of five quinoxalines and three their metabolites in poultry feathers and its application for depletions of olaquindox and quincetone in chickens. J. Pharm. Biomed. Anal. 2023, 236, 115684. [Google Scholar] [CrossRef] [PubMed]
- Gaskins, H.R.; Collier, C.T.; Anderson, D.B. Antibiotics as growth promotants: Mode of action. Anim. Biotechnol. 2002, 13, 29–42. [Google Scholar] [CrossRef]
- Broom, L.J. The sub-inhibitory theory for antibiotic growth promoters. Poult. Sci. 2017, 96, 3104–3108. [Google Scholar] [CrossRef] [PubMed]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Emran, T.B.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef]
- Tenover, F.C. Mechanisms of antimicrobial resistance in bacteria. Am. J. Med. 2006, 119, S3–S10. [Google Scholar] [CrossRef]
- Holmes, A.H.; Moore, L.S.P.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J.V. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef]
- McManus, M.C. Mechanisms of bacterial resistance to antimicrobial agents. Am. J. Health-Syst. Pharm. 1997, 54, 1420–1433. [Google Scholar] [CrossRef]
- Parsley, L.C.; Consuegra, E.J.; Kakirde, K.S.; Land, A.M.; Harper, W.F., Jr.; Liles, M.R. Identification of diverse antimicrobial resistance determinants carried on bacterial, plasmid, or viral metagenomes from an activated sludge microbial assemblage. Appl. Environ. Microbiol. 2010, 76, 3753–3757. [Google Scholar] [CrossRef]
- Leistikow, K.R.; Beattie, R.E.; Hristova, K.R. Probiotics beyond the farm: Benefits, costs, and considerations of using antibiotic alternatives in livestock. Front. Antibiot. 2022, 1, 1003912. [Google Scholar] [CrossRef]
- Scicutella, F.; Mannelli, F.; Daghio, M.; Viti, C.; Buccioni, A. Polyphenols and organic acids as alternatives to antimicrobials in poultry rearing: A review. Antibiotics 2021, 10, 1010. [Google Scholar] [CrossRef]
- Suiryanrayna, M.V.A.N.; Ramana, J.V. A review of the effects of dietary organic acids fed to swine. J. Anim. Sci. Biotechnol. 2015, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Garavito-Duarte, Y.R.; Deng, Z.; Kim, S.W. Literature review: Opportunities with phytobiotics for health and growth of pigs. Ann. Anim. Sci. 2024. ahead of print. [Google Scholar] [CrossRef]
- Garavito-Duarte, Y.R.; Duarte, M.E.; Kim, S.W. Efficacy of ground herb-based and essential oil-based phytobiotics on the intestinal health and performance of nursery pigs challenged with F18+ Escherichia coli. J. Anim. Sci. 2025, 103, skaf018. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Gormley, A.; Jang, K.B.; Duarte, M.E. Current status of global pig production: An overview and research trends. Anim. Biosci. 2024, 37, 719–729. [Google Scholar] [CrossRef]
- Duarte, M.E.; Stahl, C.H.; Kim, S.W. Intestinal damages by F18+ Escherichia coli and its amelioration with an antibacterial bacitracin fed to nursery pigs. Antioxidants 2023, 12, 1040. [Google Scholar] [CrossRef]
- Ferreira, J.L.; Watanabe, P.H.; Mendonça, I.B.; Nogueira, B.D.; Ferreira, A.C.S.; Nepomuceno, R.C.; Pascoal, L.A.F.; Almeida, J.M.S.; Guerra, R.R.; Trevisan, M.T.S. Calcium anacardate and citric acid as growth promoters for weaned piglets. Livest. Sci. 2020, 238, 104084. [Google Scholar] [CrossRef]
- Risley, C.R.; Kornegay, E.T.; Lindemann, M.D.; Weakland, S.M. Effects of organic acids with and without a microbial culture on performance and gastrointestinal tract measurements of weanling pigs. Anim. Feed Sci. Technol. 1991, 35, 259–270. [Google Scholar] [CrossRef]
- Blank, R.; Sauer, W.C.; Mosenthin, R.; Zentek, J.; Huang, S.; Roth, S. Effect of fumaric acid supplementation and dietary buffering capacity on the concentration of microbial metabolites in ileal digesta of young pigs. Can. J. Anim. Sci. 2001, 81, 345–353. [Google Scholar] [CrossRef]
- Luise, D.; Motta, V.; Salvarani, C.; Chiappelli, M.; Fusco, L.; Bertocchi, M.; Mazzoni, M.; Maiorano, G.; Costa, L.N.; Van Milgen, J. Long-term administration of formic acid to weaners: Influence on intestinal microbiota, immunity parameters and growth performance. Anim. Feed Sci. Technol. 2017, 232, 160–168. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, D.; Yu, B.; He, J.; Yu, J.; Mao, X.; Huang, Z.; Luo, Y.; Luo, J.; Zheng, P. Lactic Acid and Glutamine Have Positive Synergistic Effects on Growth Performance, Intestinal Function, and Microflora of Weaning Piglets. Animals 2024, 14, 3532. [Google Scholar] [CrossRef] [PubMed]
- Ming, D.; Xu, X.; Jiang, X.; Li, Y.; Sun, W.; Xiang, J.; Huang, M.; Pi, Y.; Li, X. Indole-3-propionic acid enhances growth performance and reduces diarrhea via modulating redox status and intestinal inflammation in weaned piglets. Anim. Nutr. 2024, 19, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Huyghebaert, G.; Ducatelle, R.; Van Immerseel, F. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 2011, 187, 182–188. [Google Scholar] [CrossRef]
- Perrin, D.D.; Dempsey, B.; Serjeant, E.P. pKa Prediction for Organic Acids and Bases; Springer: Berlin/Heidelberg, Germany; Chapman and Hall: London, UK, 1981; Volume 1. [Google Scholar]
- Theobald, P. Principles of Using Organic Acids in Animal Nutrition. 2015. Available online: https://pdfs.semanticscholar.org/3529/208446f1fd200efad0050191b0e3effd420c.pdf (accessed on 12 April 2024).
- Holyoak, C.; Stratford, M.; McMullin, Z.; Cole, M.; Crimmins, K.; Brown, A.; Coote, P. Activity of the plasma membrane H (+)-ATPase and optimal glycolytic flux are required for rapid adaptation and growth of Saccharomyces cerevisiae in the presence of the weak-acid preservative sorbic acid. Appl. Environ. Microbiol. 1996, 62, 3158–3164. [Google Scholar] [CrossRef] [PubMed]
- Cetin-Karaca, H. Evaluation of Natural Antimicrobial Phenolic Compounds Against Foodborne Pathogens. Master’s Thesis, University of Kentucky Libraries, Lexington, KY, USA, 2011. [Google Scholar]
- Lambert, R.; Stratford, M. Weak-acid preservatives: Modelling microbial inhibition and response. J. Appl. Microbiol. 1999, 86, 157–164. [Google Scholar] [CrossRef]
- Choi, H.; Kim, S.W. Dietary intervention of benzoic acid for intestinal health and growth of nursery pigs. Animals 2024, 14, 2394. [Google Scholar] [CrossRef]
- Hirshfield, I.N.; Terzulli, S.; O’Byrne, C. Weak organic acids: A panoply of effects on bacteria. Sci. Prog. 2003, 86, 245–270. [Google Scholar] [CrossRef]
- Ng, W.K.; Koh, C.B. The utilization and mode of action of organic acids in the feeds of cultured aquatic animals. Rev. Aquac. 2017, 9, 342–368. [Google Scholar] [CrossRef]
- Temiz, U.; Öztürk, E. Encapsulation methods and use in animal nutrition. Selcuk J. Agric. Food Sci. 2018, 32, 624–631. [Google Scholar] [CrossRef]
- Piva, A.; Pizzamiglio, V.; Morlacchini, M.; Tedeschi, M.; Piva, G. Lipid microencapsulation allows slow release of organic acids and natural identical flavors along the swine intestine. J. Anim. Sci. 2007, 85, 486–493. [Google Scholar] [CrossRef]
- Windisch, W.; Schedle, K.; Plitzner, C.h.; Kroismayr, A. Use of phytogenic products as feed additives for swine and poultry. J. Anim. Sci. 2008, 86, E140–E148. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Kim, E.S.; Cho, J.H.; Song, M.; Doo, H.; Kim, S.; Keum, G.B.; Kwak, J.; Ryu, S.; Choi, Y. Cutting-edge knowledge on the roles of phytobiotics and their proposed modes of action in swine. Front. Vet. Sci. 2023, 10, 1265689. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Romero, J.C.; González-Ríos, H.; Borges, A.; Simões, M. Antibacterial effects and mode of action of selected essential oils components against Escherichia coli and Staphylococcus aureus. J. Evid. Based Complement. Altern. Med. 2015, 2015, 795435. [Google Scholar] [CrossRef]
- Kim, J.; Marshall, M.R.; Wei, C.-i. Antibacterial activity of some essential oil components against five foodborne pathogens. J. Agric. Food Chem. 1995, 43, 2839–2845. [Google Scholar] [CrossRef]
- Han, G.; Lee, D.G. Antibacterial mode of action of β-Amyrin promotes apoptosis-like death in Escherichia coli by producing reactive oxygen species. J. Microbiol. Biotechnol. 2022, 32, 1547. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.H.; Cha, H.-J.; Lee, H.; Hong, S.-H.; Park, C.; Park, S.-H.; Kim, G.-Y.; Kim, S.; Kim, H.-S.; Hwang, H.-J. Protective effect of glutathione against oxidative stress-induced cytotoxicity in RAW 264.7 macrophages through activating the nuclear factor erythroid 2-related factor-2/heme oxygenase-1 pathway. Antioxidants 2019, 8, 82. [Google Scholar] [CrossRef]
- Gessner, D.K.; Fiesel, A.; Most, E.; Dinges, J.; Wen, G.; Ringseis, R.; Eder, K. Supplementation of a grape seed and grape marc meal extract decreases activities of the oxidative stress-responsive transcription factors NF-κB and Nrf2 in the duodenal mucosa of pigs. Acta Vet. Scand. 2013, 55, 18. [Google Scholar] [CrossRef]
- Girennavar, B.; Cepeda, M.L.; Soni, K.A.; Vikram, A.; Jesudhasan, P.; Jayaprakasha, G.; Pillai, S.D.; Patil, B.S. Grapefruit juice and its furocoumarins inhibits autoinducer signaling and biofilm formation in bacteria. Int. J. Food Microbiol. 2008, 125, 204–208. [Google Scholar] [CrossRef]
- Vattem, D.A.; Mihalik, K.; Crixell, S.H.; McLean, R.J. Dietary phytochemicals as quorum sensing inhibitors. Fitoterapia 2007, 78, 302–310. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Jensen, P.Ø.; Rasmussen, T.B.; Christophersen, L.; Calum, H.; Hentzer, M.; Hougen, H.-P.; Rygaard, J.; Moser, C.; Eberl, L. Garlic blocks quorum sensing and promotes rapid clearing of pulmonary Pseudomonas aeruginosa infections. Microbiology 2005, 151, 3873–3880. [Google Scholar] [CrossRef]
- Vendeville, A.; Winzer, K.; Heurlier, K.; Tang, C.M.; Hardie, K.R. Making ‘sense’of metabolism: Autoinducer-2, LuxS and pathogenic bacteria. Nat. Rev. Microbiol. 2005, 3, 383–396. [Google Scholar] [CrossRef] [PubMed]
- LaSarre, B.; Federle, M.J. Exploiting quorum sensing to confuse bacterial pathogens. Microbiol. Mol. Biol. 2013, 77, 73–111. [Google Scholar] [CrossRef] [PubMed]
- Van Noten, N.m.; Van Liefferinge, E.; Degroote, J.; De Smet, S.; Desmet, T.; Michiels, J. Fate of thymol and its monoglucosides in the gastrointestinal tract of piglets. ACS Omega 2020, 5, 5241–5248. [Google Scholar] [CrossRef]
- Fuller, R. Probiotics in man and animals. J. Appl. Bacteriol. 1989, 66, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Chang, S.Y.; Bogere, P.; Won, K.; Choi, J.-Y.; Choi, Y.-J.; Lee, H.K.; Hur, J.; Park, B.-Y.; Kim, Y. Beneficial roles of probiotics on the modulation of gut microbiota and immune response in pigs. PLoS ONE 2019, 14, e0220843. [Google Scholar] [CrossRef]
- Oelschlaeger, T.A. Mechanisms of probiotic actions–a review. Int. J. Med. Microbiol. 2010, 300, 57–62. [Google Scholar] [CrossRef]
- Braat, H.; van den Brande, J.; van Tol, E.; Hommes, D.; Peppelenbosch, M.; van Deventer, S. Lactobacillus rhamnosus induces peripheral hyporesponsiveness in stimulated CD4+ T cells via modulation of dendritic cell function. Am. J. Clin. Nutr. 2004, 80, 1618–1625. [Google Scholar] [CrossRef]
- Kamada, N.; Maeda, K.; Inoue, N.; Hisamatsu, T.; Okamoto, S.; Hong, K.S.; Yamada, T.; Watanabe, N.; Tsuchimoto, K.; Ogata, H. Nonpathogenic Escherichia coli strain Nissle 1917 inhibits signal transduction in intestinal epithelial cells. Infect. Immun. 2008, 76, 214–220. [Google Scholar] [CrossRef]
- Yan, F.; Cao, H.; Cover, T.L.; Whitehead, R.; Washington, M.K.; Polk, D.B. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 2007, 132, 562–575. [Google Scholar] [CrossRef]
- Yousefi, B.; Eslami, M.; Ghasemian, A.; Kokhaei, P.; Salek Farrokhi, A.; Darabi, N. Probiotics importance and their immunomodulatory properties. J. Cell. Physiol. 2019, 234, 8008–8018. [Google Scholar] [CrossRef]
- Dongarrà, M.L.; Rizzello, V.; Muccio, L.; Fries, W.; Cascio, A.; Bonaccorsi, I.; Ferlazzo, G. Mucosal immunology and probiotics. Curr. Allergy Asthma Rep. 2013, 13, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Fuller, R.; Gibson, G.R. Modification of the intestinal microflora using probiotics and prebiotics. Scand. J. Gastroenterol. 1997, 32, 28–31. [Google Scholar] [CrossRef]
- Mack, D.R.; Michail, S.; Wei, S.; McDougall, L.; Hollingsworth, M.A. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am. J. Physiol. Gastrointest. Liver Physiol. 1999, 276, G941–G950. [Google Scholar] [CrossRef]
- Mykhal’chyshyn, H.P.; Bodnar, P.M.; Kobyliak, N.M. Effect of probiotics on proinflammatory cytokines level in patients with type 2 diabetes and nonalcoholic fatty liver disease. Likars’ Ka Sprav. 2013, 2, 56–62. [Google Scholar]
- Plaza-Diaz, J.; Gomez-Llorente, C.; Fontana, L.; Gil, A. Modulation of immunity and inflammatory gene expression in the gut, in inflammatory diseases of the gut and in the liver by probiotics. World J. Gastroenterol. 2014, 20, 15632. [Google Scholar] [CrossRef] [PubMed]
- Brown, M. Modes of action of probiotics: Recent developments. J. Vet. Adv. 2011, 14, 1895–1900. [Google Scholar] [CrossRef]
- Gilliland, S.; Speck, M. Deconjugation of bile acids by intestinal lactobacilli. Appl. Environ. Microbiol. 1977, 33, 15–18. [Google Scholar] [CrossRef]
- Maqueda, M.; Sanchez-Hidalgo, M.; Fernández, M.; Montalban-Lopez, M.; Valdivia, E.; Martínez-Bueno, M. Genetic features of circular bacteriocins produced by Gram-positive bacteria. FEMS Microbiol. Rev. 2008, 32, 2–22. [Google Scholar] [CrossRef]
- Dunne, C. Adaptation of bacteria to the intestinal niche: Probiotics and gut disorder. Inflamm. Bowel Dis. 2001, 7, 136–145. [Google Scholar] [CrossRef]
- Ohashi, Y.; Ushida, K. Health-beneficial effects of probiotics: Its mode of action. Anim. Sci. J. 2009, 80, 361–371. [Google Scholar] [CrossRef]
- Takahashi, S.; Egawa, Y.; Simojo, N.; Tsukahara, T.; Ushida, K. Oral administration of Lactobacillus plantarum strain Lq80 to weaning piglets stimulates the growth of indigenous lactobacilli to modify the lactobacillal population. J. Gen. Appl. Microbiol. 2007, 53, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Inoue, R.; Tsukahara, T.; Nakanishi, N.; Ushida, K. Development of the intestinal microbiota in the piglet. J. Gen. Appl. Microbiol. 2005, 51, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.E.; Garavito-Duarte, Y.; Kim, S.W. Impacts of F18+ Escherichia coli on intestinal health of nursery pigs and dietary interventions. Animals 2023, 13, 2791. [Google Scholar] [CrossRef]
- Mantziari, A.; Salminen, S.; Szajewska, H.; Malagón-Rojas, J.N. Postbiotics against pathogens commonly involved in pediatric infectious diseases. Microorganisms 2020, 8, 1510. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.F.J.; Wootton, M.; Howe, R.A. Antimicrobial susceptibility testing breakpoints and methods from BSAC to EUCAST. J. Antimicrob. Chemother. 2016, 71, 3–5. [Google Scholar] [CrossRef]
- Sun, Z.; Harris, H.M.; McCann, A.; Guo, C.; Argimón, S.; Zhang, W.; Yang, X.; Jeffery, I.B.; Cooney, J.C.; Kagawa, T.F. Expanding the biotechnology potential of lactobacilli through comparative genomics of 213 strains and associated genera. Nat. Commun. 2015, 6, 8322. [Google Scholar] [CrossRef]
- Corr, S.C.; Li, Y.; Riedel, C.U.; O’Toole, P.W.; Hill, C.; Gahan, C.G. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc. Natl. Acad. Sci. USA 2007, 104, 7617–7621. [Google Scholar] [CrossRef]
- Duarte, M.E.; Parnsen, W.; Zhang, S.; Abreu, M.L.; Kim, S.W. Low crude protein formulation with supplemental amino acids for its impacts on intestinal health and growth performance of growing-finishing pigs. J. Anim. Sci. Biotechnol. 2024, 15, 55. [Google Scholar] [CrossRef]
- Wu, Z.; Pan, D.-D.; Guo, Y.; Zeng, X. Structure and anti-inflammatory capacity of peptidoglycan from Lactobacillus acidophilus in RAW-264.7 cells. Carbohydr. Polym. 2013, 96, 466–473. [Google Scholar] [CrossRef]
- Kolling, Y.; Salva, S.; Villena, J.; Marranzino, G.; Alvarez, S. Non-viable immunobiotic Lactobacillus rhamnosus CRL1505 and its peptidoglycan improve systemic and respiratory innate immune response during recovery of immunocompromised-malnourished mice. Int. Immunopharmacol. 2015, 25, 474–484. [Google Scholar] [CrossRef]
- Jackman, J.A.; Boyd, R.D.; Elrod, C.C. Medium-chain fatty acids and monoglycerides as feed additives for pig production: Towards gut health improvement and feed pathogen mitigation. J. Anim. Sci. Biotechnol. 2020, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Rocha, G.C.; Kim, S.W. Effects of dietary supplementation of myristic acid on jejunal mucosa-associated microbiota, mucosal immunity, and growth performance of nursery pigs. Anim. Sci. J. 2025, 96, e70027. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Fatty acids, inflammation and intestinal health in pigs. J. Anim. Sci. Biotechnol. 2015, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.K.; Jackman, J.A.; Valle-González, E.R.; Cho, N.-J. Antibacterial free fatty acids and monoglycerides: Biological activities, experimental testing, and therapeutic applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef]
- Kundukad, B.; Schussman, M.; Yang, K.; Seviour, T.; Yang, L.; Rice, S.A.; Kjelleberg, S.; Doyle, P.S. Mechanistic action of weak acid drugs on biofilms. Sci. Rep. 2017, 7, 4783. [Google Scholar] [CrossRef]
- Diether, N.E.; Hulshof, T.G.; Willing, B.P.; van Kempen, T.A.T.G. A blend of medium-chain fatty acids, butyrate, organic acids, and a phenolic compound accelerates microbial maturation in newly weaned piglets. PLoS ONE 2023, 18, e0289214. [Google Scholar] [CrossRef]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef]
- Liu, C.-H.; Huang, H.-Y. Antimicrobial activity of curcumin-loaded myristic acid microemulsions against Staphylococcus epidermidis. Chem. Pharm. Bull. 2012, 60, 1118–1124. [Google Scholar] [CrossRef]
- Stulnig, T.M.; Huber, J.; Leitinger, N.; Imre, E.-M.; Angelisová, P.; Nowotny, P.; Waldhäusl, W. Polyunsaturated eicosapentaenoic acid displaces proteins from membrane rafts by altering raft lipid composition. J. Biol. Chem. 2001, 276, 37335–37340. [Google Scholar] [CrossRef]
- Boyaval, P.; Corre, C.; Dupuis, C.; Roussel, E. Effects of free fatty acids on propionic acid bacteria. Le Lait 1995, 75, 17–29. [Google Scholar] [CrossRef]
- Shin, S.Y.; Bajpai, V.K.; Kim, H.R.; Kang, S.C. Antibacterial activity of eicosapentaenoic acid (EPA) against foodborne and food spoilage microorganisms. LWT-Food Sci. Technol. 2007, 40, 1515–1519. [Google Scholar] [CrossRef]
- Sado-Kamdem, S.L.; Vannini, L.; Guerzoni, M.E. Effect of α-linolenic, capric and lauric acid on the fatty acid biosynthesis in Staphylococcus aureus. Int. J. Food Microbiol. 2009, 129, 288–294. [Google Scholar] [CrossRef]
- Galbraith, H.; Miller, T.B. Effect of long chain fatty acids on bacterial respiration and amino acid uptake. J. Appl. Microbiol. 1973, 36, 659–675. [Google Scholar] [CrossRef] [PubMed]
- Jassim, S.A.A.; Limoges, R.G. Natural solution to antibiotic resistance: Bacteriophages ‘The Living Drugs’. World J. Microbiol. Biotechnol. 2014, 30, 2153–2170. [Google Scholar] [CrossRef]
- Huang, C.; Shi, J.; Ma, W.; Li, Z.; Wang, J.; Li, J.; Wang, X. Isolation, characterization, and application of a novel specific Salmonella bacteriophage in different food matrices. Food Res. Int. 2018, 111, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Hosseindoust, A.R.; Lee, S.H.; Kim, J.S.; Choi, Y.H.; Kwon, I.K.; Chae, B.J. Productive performance of weanling piglets was improved by administration of a mixture of bacteriophages, targeted to control Coliforms and Clostridium spp. shedding in a challenging environment. J. Anim. Physiol. Anim. Nutr. 2017, 101, e98–e107. [Google Scholar] [CrossRef]
- Young, R.Y. Bacteriophage lysis: Mechanism and regulation. Microbiol. Rev. 1992, 56, 430–481. [Google Scholar] [CrossRef]
- Young, R. Phage lysis: Three steps, three choices, one outcome. J. Microbiol. 2014, 52, 243–258. [Google Scholar] [CrossRef]
- Wright, E.E.; Elliman, J.R.; Owens, L. Induction and characterization of lysogenic bacteriophages from Streptococcus iniae. J. Appl. Microbiol. 2013, 114, 1616–1624. [Google Scholar] [CrossRef]
- Keen, E.C.; Bliskovsky, V.V.; Malagon, F.; Baker, J.D.; Prince, J.S.; Klaus, J.S.; Adhya, S.L. Novel “superspreader” bacteriophages promote horizontal gene transfer by transformation. MBio 2017, 8, e02115-16. [Google Scholar] [CrossRef]
- Visek, W.J. The mode of growth promotion by antibiotics. J. Anim. Sci. 1978, 46, 1447–1469. [Google Scholar] [CrossRef]
- Forbes, M.; Park, J.T. Growth of germ-free and conventional chicks: Effect of diet, dietary penicillin and bacterial environment. J. Nutr. 1959, 67, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Foster, D.N.; Shurson, G.C. Effects of feeding diets containing bacitracin methylene disalicylate to heat-stressed finishing pigs. J. Anim. Sci. 2011, 89, 1830–1843. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.-T.; Hu, Q.; Faris, R.J.; Guo, J.; Urriola, P.E.; Shurson, G.C.; Chen, C.; Saqui-Salces, M. Analysis of gastrointestinal responses revealed both shared and specific targets of zinc oxide and carbadox in weaned pigs. Antibiotics 2020, 9, 463. [Google Scholar] [CrossRef]
- Lekagul, A.; Tangcharoensathien, V.; Yeung, S. Patterns of antibiotic use in global pig production: A systematic review. Vet. Anim. Sci. 2019, 7, 100058. [Google Scholar] [CrossRef]
- Davies, P.R.; Singer, R.S. Antimicrobial use in wean to market pigs in the United States assessed via voluntary sharing of proprietary data. Zoonoses Public Health 2020, 67, 6–21. [Google Scholar] [CrossRef]
- USDA. Antimicrobial Use and Stewardship on U.S. Swine Operations, 2017. 2019. Available online: https://www.aphis.usda.gov/sites/default/files/amu-swine-operations.pdf (accessed on 10 March 2025).
- Holman, D.B.; Chénier, M.R. Antimicrobial use in swine production and its effect on the swine gut microbiota and antimicrobial resistance. Can. J. Microbiol. 2015, 61, 785–798. [Google Scholar] [CrossRef]
- Bergeland, M.E.; Henry, S.C. Infectious diarrheas of young pigs. Vet. Clin. N. Am. 1982, 4, 389–399. [Google Scholar] [CrossRef]
- Hu, C.; Niu, X.; Chen, S.; Wen, J.; Bao, M.; Mohyuddin, S.G.; Yong, Y.; Liu, X.; Wu, L.; Yu, Z. A comprehensive analysis of the colonic flora diversity, short chain fatty acid metabolism, transcripts, and biochemical indexes in heat-stressed pigs. Front. Immunol. 2021, 12, 717723. [Google Scholar] [CrossRef]
- Hung, D.-Y.; Cheng, Y.-H.; Chen, W.-J.; Hua, K.-F.; Pietruszka, A.; Dybus, A.; Lin, C.-S.; Yu, Y.-H. Bacillus licheniformis-fermented products reduce diarrhea incidence and alter the fecal microbiota community in weaning piglets. Animals 2019, 9, 1145. [Google Scholar] [CrossRef]
- Mateos, I.; Combes, S.; Pascal, G.; Cauquil, L.; Barilly, C.; Cossalter, A.-M.; Laffitte, J.; Botti, S.; Pinton, P.; Oswald, I.P. Fumonisin-exposure impairs age-related ecological succession of bacterial species in weaned pig gut microbiota. Toxins 2018, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Sun, Y.; Ma, L.; Chen, Q.; Hu, C.; Yang, H.; Hong, Q.; Xiao, Y. Comparative analysis of fecal microbiota between diarrhea and non-diarrhea piglets reveals biomarkers of gut microbiota associated with diarrhea. Anim. Nutr. 2024, 19, 401–410. [Google Scholar] [CrossRef]
- Shin, J.-H.; Sim, M.; Lee, J.-Y.; Shin, D.-M. Lifestyle and geographic insights into the distinct gut microbiota in elderly women from two different geographic locations. J. Physiol. Anthropol. 2016, 35, 31. [Google Scholar] [CrossRef] [PubMed]
- Rajilić–Stojanović, M.; Biagi, E.; Heilig, H.G.; Kajander, K.; Kekkonen, R.A.; Tims, S.; de Vos, W.M. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology 2011, 141, 1792–1801. [Google Scholar] [CrossRef]
- Modesto, M.; D’Aimmo, M.R.; Stefanini, I.; Trevisi, P.; De Filippi, S.; Casini, L.; Mazzoni, M.; Bosi, P.; Biavati, B. A novel strategy to select Bifidobacterium strains and prebiotics as natural growth promoters in newly weaned pigs. Livest. Sci. 2009, 122, 248–258. [Google Scholar] [CrossRef]
- Martínez, E.A.; Babot, J.D.; Lorenzo-Pisarello, M.J.; Apella, M.C.; Chaia, A.P. Feed supplementation with avian Propionibacterium acidipropionici contributes to mucosa development in early stages of rearing broiler chickens. Benef. Microbes 2016, 7, 687–698. [Google Scholar] [CrossRef]
- Liu, X.; Mao, B.; Gu, J.; Wu, J.; Cui, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Blautia—A new functional genus with potential probiotic properties? Gut Microbes 2021, 13, 1875796. [Google Scholar] [CrossRef]
- Henryon, M.; Berg, P.; Jensen, J.; Andersen, S. Genetic variation for resistance to clinical and subclinical diseases exists in growing pigs. Anim. Sci. 2001, 73, 375–387. [Google Scholar] [CrossRef]
- He, Y.; Kim, K.; Kovanda, L.; Jinno, C.; Song, M.; Chase, J.; Li, X.; Tan, B.; Liu, Y. Bacillus subtilis: A potential growth promoter in weaned pigs in comparison to carbadox. J. Anim. Sci. 2020, 98, skaa290. [Google Scholar] [CrossRef]
- Rahman, M.d.R.T.; Fliss, I.; Biron, E. Insights in the development and uses of alternatives to antibiotic growth promoters in poultry and swine production. Antibiotics 2022, 11, 766. [Google Scholar] [CrossRef]
- Niewold, T.A. The nonantibiotic anti-inflammatory effect of antimicrobial growth promoters, the real mode of action? A hypothesis. Poult. Sci. 2007, 86, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.T.; Tabori, C.; Miller, E.R.; Hogberg, M.G. The effects of antibiotics in the weanling pig diet on growth and the excretion of volatile phenolic and aromatic bacterial metabolites. Am. J. Clin. Nutr. 1982, 35, 1417–1424. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, M.; Yang, Y.; Mu, C.; Su, Y.; Zhu, W. Effect of early antibiotic administration on cecal bacterial communities and their metabolic profiles in pigs fed diets with different protein levels. Anaerobe 2016, 42, 188–196. [Google Scholar] [CrossRef]
- Abt, M.C.; Pamer, E.G. Commensal bacteria mediated defenses against pathogens. Curr. Opin. Immunol. 2014, 29, 16–22. [Google Scholar] [CrossRef]
- Vervaeke, I.J.; Decuypere, J.A.; Dierick, N.A.; Henderickx, H.K. Quantitative in vitro evaluation of the energy metabolism influenced by virginiamycin and spiramycin used as growth promoters in pig nutrition. J. Anim. Sci. 1979, 49, 846–856. [Google Scholar] [CrossRef]
- Elvers, K.T.; Wilson, V.J.; Hammond, A.; Duncan, L.; Huntley, A.L.; Hay, A.D.; Van Der Werf, E.T. Antibiotic-induced changes in the human gut microbiota for the most commonly prescribed antibiotics in primary care in the UK: A systematic review. BMJ Open 2020, 10, e035677. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Feng, S.; Huo, F.; Liu, H. Effects of four antibiotics on the diversity of the intestinal microbiota. Microbiol. Spectr. 2022, 10, e01904–e01921. [Google Scholar] [CrossRef]
- Holman, D.B.; Chénier, M.R. Temporal changes and the effect of subtherapeutic concentrations of antibiotics in the gut microbiota of swine. FEMS Microbiol. Ecol. 2014, 90, 599–608. [Google Scholar] [CrossRef]
- Saladrigas-García, M.; Durán, M.; D’Angelo, M.; Coma, J.; Pérez, J.F.; Martín-Orúe, S.M. An insight into the commercial piglet’s microbial gut colonization: From birth towards weaning. Anim. Microbiome 2022, 4, 68. [Google Scholar] [CrossRef]
- Sutton, T.A.; O’Neill, H.V.M.; Bedford, M.R.; McDermott, K.; Miller, H.M. Effect of xylanase and xylo-oligosaccharide supplementation on growth performance and faecal bacterial community composition in growing pigs. Anim. Feed Sci. Technol. 2021, 274, 114822. [Google Scholar] [CrossRef]
- Maltecca, C.; Dunn, R.; He, Y.; McNulty, N.P.; Schillebeeckx, C.; Schwab, C.; Shull, C.; Fix, J.; Tiezzi, F. Microbial composition differs between production systems and is associated with growth performance and carcass quality in pigs. Anim. Microbiome 2021, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Piazuelo, D.; Lawlor, P.G.; Ranjitkar, S.; Cormican, P.; Villodre, C.; Bouwhuis, M.A.; Marsh, A.; Crispie, F.; Rattigan, R.; Gardiner, G.E. Intestinal microbiota modulation and improved growth in pigs with post-weaning antibiotic and ZnO supplementation but only subtle microbiota effects with Bacillus altitudinis. Sci. Rep. 2021, 11, 23304. [Google Scholar] [CrossRef] [PubMed]
- Jeon, B.S.; Kim, S.; Sang, B.-I. Megasphaera hexanoica sp. nov., a medium-chain carboxylic acid-producing bacterium isolated from a cow rumen. Int. J. Syst. Evol. Microbiol. 2017, 67, 2114–2120. [Google Scholar] [CrossRef]
- Sung, J.Y.; Johnson, T.A.; Ragland, D.; Adeola, O. Impact of ileal indigestible protein on fecal nitrogen excretion and fecal microbiota may be greater compared with total protein concentration of diets in growing pigs. J. Anim. Sci. 2023, 101, skac409. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, S.; Zhong, R.; Su, D.; Xia, B.; Liu, L.; Chen, L.; Zhang, H. Time-course alterations of gut microbiota and short-chain fatty acids after short-term lincomycin exposure in young swine. Appl. Microbiol. Biotechnol. 2021, 105, 8441–8456. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Bai, Y.; Zhang, G.; Liu, L.; Lai, C. Relationship between dietary fiber fermentation and volatile fatty acids’ concentration in growing pigs. Animals 2020, 10, 263. [Google Scholar] [CrossRef]
- Hăbeanu, M.; Lefter, N.A.; Toma, S.M.; Dumitru, M.; Cismileanu, A.; Surdu, I.; Gheorghe, A.; Dragomir, C.; Untea, A. Changes in ileum and cecum volatile fatty acids and their relationship with microflora and enteric methane in pigs fed different fiber levels. Agriculture 2022, 12, 451. [Google Scholar] [CrossRef]
- Duncan, S.H.; Louis, P.; Thomson, J.M.; Flint, H.J. The role of pH in determining the species composition of the human colonic microbiota. Environ. Microbiol. 2009, 11, 2112–2122. [Google Scholar] [CrossRef]
- Lin, K.-H.; Yu, Y.-H. Evaluation of Bacillus licheniformis-fermented feed additive as an antibiotic substitute: Effect on the growth performance, diarrhea incidence, and cecal microbiota in weaning piglets. Animals 2020, 10, 1649. [Google Scholar] [CrossRef]
- Lin, K.H.; Yu, Y.H. A field study of Bacillus licheniformis-fermented products on growth performance and faecal microbiota of weaning piglets. S. Afr. J. Anim. Sci. 2022, 52, 718–730. [Google Scholar] [CrossRef]
- Ángel-Isaza, J.A.; Herrera Franco, V.; López-Herrera, A.; Parra-Suescun, J.E. Nutraceutical Additives Modulate Microbiota and Gut Health in Post-Weaned Piglets. Vet. Sci. 2024, 11, 332. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, J.M.; Hampton, R.S.; Johnson, H.M.; Callaway, T.R.; Rothrock, M.J., Jr.; Azain, M.J. The effects of feeding antibiotic on the intestinal microbiota of weanling pigs. Front. Vet. Sci. 2021, 8, 601394. [Google Scholar] [CrossRef] [PubMed]
- Muurinen, J.; Richert, J.; Wickware, C.L.; Richert, B.; Johnson, T.A. Swine growth promotion with antibiotics or alternatives can increase antibiotic resistance gene mobility potential. Sci. Rep. 2021, 11, 5485. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Jinno, C.; Ji, P.; Liu, Y. Trace amounts of antibiotic altered metabolomic and microbial profiles of weaned pigs infected with a pathogenic E. coli. J. Anim. Sci. Biotechnol. 2022, 13, 59. [Google Scholar] [CrossRef]
- Kim, K.; He, Y.; Jinno, C.; Kovanda, L.; Li, X.; Song, M.; Liu, Y. Trace amounts of antibiotic exacerbated diarrhea and systemic inflammation of weaned pigs infected with a pathogenic Escherichia coli. J. Anim. Sci. 2021, 99, skab073. [Google Scholar] [CrossRef]
- Jinno, C.; Li, X.; Liu, Y. Dietary supplementation of Bacillus subtilis or antibiotics modified intestinal microbiome of weaned pigs under enterotoxigenic Escherichia coli infection. Front. Microbiol. 2022, 13, 1064328. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Jinno, C.; Li, X.; Bravo, D.; Cox, E.; Ji, P.; Liu, Y. Impact of an oligosaccharide-based polymer on the metabolic profiles and microbial ecology of weanling pigs experimentally infected with a pathogenic E. coli. J. Anim. Sci. Biotechnol. 2024, 15, 1. [Google Scholar] [CrossRef]
- Kim, K.; He, Y.; Jinno, C.; Kovanda, L.; Li, X.; Bravo, D.; Cox, E.; Liu, Y. Supplementation of oligosaccharide-based polymer enhanced growth and disease resistance of weaned pigs by modulating intestinal integrity and systemic immunity. J. Anim. Sci. Biotechnol. 2022, 13, 10. [Google Scholar] [CrossRef]
- Jinno, C.; Wong, B.; Klünemann, M.; Htoo, J.; Li, X.; Liu, Y. Effects of supplementation of Bacillus amyloliquefaciens on performance, systemic immunity, and intestinal microbiota of weaned pigs experimentally infected with a pathogenic enterotoxigenic E. coli F18. Front. Microbiol. 2023, 14, 1101457. [Google Scholar] [CrossRef]
- Franco, V.H.H.; Carrasco, S.C.P.; Suescún, J.E.P. Antimicrobials added to the feed of weaned piglets at two ages improves the molecular expression of intestinal barrier proteins. Anim. Prod. Sci. 2022, 62, 511–520. [Google Scholar] [CrossRef]
- Han, Y.; Zhan, T.; Tang, C.; Zhao, Q.; Dansou, D.M.; Yu, Y.; Barbosa, F.F.; Zhang, J. Effect of replacing in-feed antibiotic growth promoters with a combination of egg immunoglobulins and phytomolecules on the performance, serum immunity, and intestinal health of weaned pigs challenged with Escherichia coli K88. Animals 2021, 11, 1292. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Piao, X. Essential oil blend could decrease diarrhea prevalence by improving antioxidative capability for weaned pigs. Animals 2019, 9, 847. [Google Scholar] [CrossRef] [PubMed]
- Dahmer, P.L.; Jones, C.K. Evaluating dietary acidifiers as alternatives for conventional feed-based antibiotics in nursery pig diets. Transl. Anim. Sci. 2021, 5, txab040. [Google Scholar] [CrossRef]
- Dahmer, P.L.; Leubcke, G.E.; Lerner, A.B.; Jones, C.K. Effects of medium-chain fatty acids as alternatives to ZnO or antibiotics in nursery pig diets. Transl. Anim. Sci. 2020, 4, txaa151. [Google Scholar] [CrossRef]
- Outlaw, A.; Gachman, A.; Kim, H.; Xu, X.; Tan, Z.; Qin, Z.; Peng, X.; Rudar, M. Evaluation of protected benzoic acid on growth performance, nutrient digestibility, and gut health indices in starter pigs. Transl. Anim. Sci. 2023, 7, txad111. [Google Scholar] [CrossRef]
- Wilt, H.D.; Carlson, M.S. Effect of supplementing zinc oxide and biotin with or without carbadox on nursery pig performance. J. Anim. Sci. 2009, 87, 3253–3258. [Google Scholar] [CrossRef]
- Bischoff, S.C. ‘Gut health’: A new objective in medicine? BMC Med. 2011, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, J.; Ren, E.; Su, Y.; Zhu, W. Co-occurrence of early gut colonization in neonatal piglets with microbiota in the maternal and surrounding delivery environments. Anaerobe 2018, 49, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Huang, S.; Jiang, L.; Wang, W.; Li, T.; Zuo, B.; Li, Z.; Wang, J. Differences in the gut microbiota establishment and metabolome characteristics between low-and normal-birth-weight piglets during early-life. Front. Microbiol. 2018, 9, 1798. [Google Scholar] [CrossRef]
- Cai, L.; Zhao, Y.; Chen, W.N.; Li, Y.P.; Han, Y.M.; Zhang, B.; Pineda, L.; Li, X.L.; Jiang, X.R. Effect of an organic acid blend as an antibiotic alternative on growth performance, antioxidant capacity, intestinal barrier function, and fecal microbiota in weaned piglets. J. Anim. Sci. 2024, 102, skae149. [Google Scholar] [CrossRef]
- Michiels, J.; Truffin, D.; Majdeddin, M.; Van Poucke, M.; Van Liefferinge, E.; Van Noten, N.; Vandaele, M.; Van Kerschaver, C.; Degroote, J.; Peelman, L.; et al. Gluconic acid improves performance of newly weaned piglets associated with alterations in gut microbiome and fermentation. Porc. Health Manag. 2023, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.D.; Deng, Z.C.; Wang, Y.W.; Sun, H.; Wang, L.; Han, Y.M.; Wu, Y.Y.; Liu, J.G.; Sun, L.H. Organic Acids Improve Growth Performance with Potential Regulation of Redox Homeostasis, Immunity, and Microflora in Intestines of Weaned Piglets. Antioxidants 2021, 10, 1665. [Google Scholar] [CrossRef] [PubMed]
- Sadurni, M.; Barroeta, A.C.; Sol, C.; Puyalto, M.; Castillejos, L. Effects of dietary crude protein level and sodium butyrate protected by medium-chain fatty acid salts on performance and gut health in weaned piglets. J. Anim. Sci. 2023, 101, skad090. [Google Scholar] [CrossRef]
- Marchiori, M.S.; Paiano, D.; Zatti, E.; Tarasconi, L.; Ficagna, C.; Amaral, M.A.F.D.; Milarch, C.F.; Horn, V.W.; Mendes, R.E.; Galli, G.M.; et al. Butyric acid glycerides as substitutes for antibiotics as growth enhancers in the diet of nursery piglets. Res. Vet. Sci. 2024, 167, 105110. [Google Scholar] [CrossRef]
- Cui, C.; Wei, Y.L.; Wang, Y.B.; Ma, W.; Zheng, X.Y.; Wang, J.; Ma, Z.W.; Wu, C.C.; Chu, L.C.; Zhang, S.H.; et al. Dietary supplementation of benzoic acid and essential oils combination enhances intestinal resilience against LPS stimulation in weaned piglets. J. Anim. Sci. Biotechnol. 2024, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- López-Colom, P.; Castillejos, L.; Rodríguez-Sorrento, A.; Puyalto, M.; Mallo, J.J.; Martín-Orúe, S.M. Impact of in-feed sodium butyrate or sodium heptanoate protected with medium-chain fatty acids on gut health in weaned piglets challenged with Escherichia coli F4+. Arch. Anim. Nutr. 2020, 74, 271–295. [Google Scholar] [CrossRef]
- Long, S.; Xu, Y.; Pan, L.; Wang, Q.; Wang, C.; Wu, J.; Wu, Y.; Han, Y.; Yun, C.; Piao, X. Mixed organic acids as antibiotic substitutes improve performance, serum immunity, intestinal morphology and microbiota for weaned piglets. Anim. Feed Sci. Technol. 2018, 235, 23–32. [Google Scholar] [CrossRef]
- Pluske, J.R.; Turpin, D.L.; Sahibzada, S.; Pineda, L.; Han, Y.M.; Collins, A. Impacts of feeding organic acid-based feed additives on diarrhea, performance, and fecal microbiome characteristics of pigs after weaning challenged with an enterotoxigenic strain of Escherichia coli. Transl. Anim. Sci. 2021, 5, txab212. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Ma, N.; Guo, P.; Zhang, J.; He, T.; Kim, S.W.; Zhang, G.; Ma, X. Nutrients mediate intestinal bacteria–mucosal immune crosstalk. Front. Immunol. 2018, 9, 5. [Google Scholar] [CrossRef]
- Papadopoulos, G.A.; Poutahidis, T.; Tallarico, N.; Hardas, A.; Teliousis, K.; Arsenos, G.; Fortomaris, P.D. Dietary supplementation of encapsulated organic acids enhances performance and modulates immune regulation and morphology of jejunal mucosa in piglets. Res. Vet. Sci. 2017, 115, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Grilli, E.; Tugnoli, B.; Passey, J.L.; Stahl, C.H.; Piva, A.; Moeser, A.J. Impact of dietary organic acids and botanicals on intestinal integrity and inflammation in weaned pigs. BMC Vet. Res. 2015, 11, 96. [Google Scholar] [CrossRef]
- Lallès, J.P.; Bosi, P.; Janczyk, P.; Koopmans, S.; Torrallardona, D. Impact of bioactive substances on the gastrointestinal tract and performance of weaned piglets: A review. Animal 2009, 3, 1625–1643. [Google Scholar] [CrossRef] [PubMed]
- Tugnoli, B.; Giovagnoni, G.; Piva, A.; Grilli, E. From acidifiers to intestinal health enhancers: How organic acids can improve growth efficiency of pigs. Animals 2020, 10, 134. [Google Scholar] [CrossRef]
- Kil, D.Y.; Kwon, W.B.; Kim, B.G. Dietary acidifiers in weanling pig diets: A review. Rev. Colomb. Cienc. Pec. 2011, 24, 231–247. [Google Scholar]
- Gottlob, R.O.; Groesbeck, C.N.; DeRuochey, J.M.; Neill, C.R.; Tokach, M.D.; Goodband, R.D.; Nelssen, J.L.; Dritz, S.S. Effects of water-based citric acid on growth performance and water disappearance of weanling pigs. Kans. Agric. Exp. Stn. Res. Rep. 2005, 964, 60–63. [Google Scholar] [CrossRef]
- Blank, R.; Mosenthin, R.; Sauer, W.C.; Huang, S. Effect of fumaric acid and dietary buffering capacity on ileal and fecal amino acid digestibilities in early-weaned pigs. J. Anim. Sci. 1999, 77, 2974–2984. [Google Scholar] [CrossRef]
- Manzanilla, E.G.; Perez, J.F.; Martin, M.; Kamel, C.; Baucells, F.; Gasa, J. Effect of plant extracts and formic acid on the intestinal equilibrium of early-weaned pigs. J. Anim. Sci. 2004, 82, 3210–3218. [Google Scholar] [CrossRef]
- Partanen, K.H.; Mroz, Z. Organic acids for performance enhancement in pig diets. Nutr. Res. Rev. 1999, 12, 117–145. [Google Scholar] [CrossRef]
- Eisemann, J.H.; Van Heugten, E. Response of pigs to dietary inclusion of formic acid and ammonium formate. J. Anim. Sci. 2007, 85, 1530–1539. [Google Scholar] [CrossRef]
- Canibe, N.; Højberg, O.; Højsgaard, S.; Jensen, B.B. Feed physical form and formic acid addition to the feed affect the gastrointestinal ecology and growth performance of growing pigs. J. Anim. Sci. 2005, 83, 1287–1302. [Google Scholar] [CrossRef]
- Eckel, B.; Kirchgessner, M.; Roth, F.X. Zum Einfluß von Ameisensäure auf tägliche Zunahmen, Futteraufnahme, Futterverwertung und Verdaulichkeit: 1. Mitteilung. Untersuchungen zur nutritiven Wirksamkeit von organischen Säuren in der Ferkelaufzucht. J. Anim. Physiol. Anim. Nutr. 1992, 67, 93–100. [Google Scholar] [CrossRef]
- Taube, V.A.; Neu, M.E.; Hassan, Y.; Verspohl, J.; Beyerbach, M.; Kamphues, J. Effects of dietary additives (potassium diformate/organic acids) as well as influences of grinding intensity (coarse/fine) of diets for weaned piglets experimentally infected with Salmonella Derby or Escherichia coli. J. Anim. Physiol. Anim. Nutr. 2009, 93, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Upadhaya, S.D.; Lee, K.Y.; Kim, I.H. Protected organic acid blends as an alternative to antibiotics in finishing pigs. Asian-Australas. J. Anim. Sci. 2014, 27, 1600. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.J.; Park, J.W.; Baek, D.H.; Kim, J.K.; Kim, I.H. Feeding the blend of organic acids and medium chain fatty acids reduces the diarrhea in piglets orally challenged with enterotoxigenic Escherichia coli K88. Anim. Feed Sci. Technol. 2017, 224, 46–51. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Lee, K.Y.; Kim, I.H. Effect of protected organic acid blends on growth performance, nutrient digestibility and faecal micro flora in growing pigs. J. Appl. Anim. Res. 2016, 44, 238–242. [Google Scholar] [CrossRef]
- Creus, E.; Pérez, J.; Peralta, B.; Baucells, F.; Mateu, E. Effect of acidified feed on the prevalence of Salmonella in market-age pigs. Zoonoses Public Health 2007, 54, 314–319. [Google Scholar] [CrossRef]
- Tsao, R.; Deng, Z. Separation procedures for naturally occurring antioxidant phytochemicals. J. Chromatogr. B 2004, 812, 85–99. [Google Scholar] [CrossRef]
- Yang, Y.-F.; Zhao, L.-L.; Shao, Y.-X.; Liao, X.-D.; Zhang, L.-Y.; Lin, L.; Luo, X.-G. Effects of dietary graded levels of cinnamon essential oil and its combination with bamboo leaf flavonoid on immune function, antioxidative ability and intestinal microbiota of broilers. J. Integr. Agric. 2019, 18, 2123–2132. [Google Scholar] [CrossRef]
- Krauze, M.; Cendrowska-Pinkosz, M.; Matuseviĉius, P.; Stępniowska, A.; Jurczak, P.; Ognik, K. The effect of administration of a phytobiotic containing cinnamon oil and citric acid on the metabolism, immunity, and growth performance of broiler chickens. Animals 2021, 11, 399. [Google Scholar] [CrossRef]
- Singh, I. Antimicrobials in higher plants: Classification, mode of action and bioactivities. Chem. Biol. Lett. 2017, 4, 48–62. [Google Scholar]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential oils of oregano: Biological activity beyond their antimicrobial properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, Y.F.; Zou, Y.; Hu, X.M.; Zheng, L.F.; Wei, H.; Giannenas, I.; Jin, L.Z.; Peng, J.; Jiang, S.W. Effects of dietary oregano essential oil supplementation on the stress response, antioxidative capacity, and HSPs mRNA expression of transported pigs. Livest. Sci. 2015, 180, 143–149. [Google Scholar] [CrossRef]
- Zou, Y.; Xiang, Q.; Wang, J.; Wei, H.; Peng, J. Effects of oregano essential oil or quercetin supplementation on body weight loss, carcass characteristics, meat quality and antioxidant status in finishing pigs under transport stress. Livest. Sci. 2016, 192, 33–38. [Google Scholar] [CrossRef]
- Ariza-Nieto, C.; Bandrick, M.; Baidoo, S.K.; Anil, L.; Molitor, T.W.; Hathaway, M. Effect of dietary supplementation of oregano essential oils to sows on colostrum and milk composition, growth pattern and immune status of suckling pigs. J. Anim. Sci. 2011, 89, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Janz, J.A.M.; Morel, P.C.H.; Wilkinson, B.H.P.; Purchas, R.W. Preliminary investigation of the effects of low-level dietary inclusion of fragrant essential oils and oleoresins on pig performance and pork quality. Meat Sci. 2007, 75, 350–355. [Google Scholar] [CrossRef]
- Dauqan, E.M.A.; Abdullah, A. Medicinal and functional values of thyme (Thymus vulgaris L.) herb. J. Appl. Biol. 2017, 5, 17–22. [Google Scholar] [CrossRef]
- El-Guendouz, S.; Aazza, S.; Anahi Dandlen, S.; Majdoub, N.; Lyoussi, B.; Raposo, S.; Dulce Antunes, M.; Gomes, V.; Graça Miguel, M. Antioxidant activity of thyme waste extract in O/W emulsions. Antioxidants 2019, 8, 243. [Google Scholar] [CrossRef]
- Czech, A.; Klimiuk, K.; Sembratowicz, I. The effect of thyme herb in diets for fattening pigs on their growth performance and health. PLoS ONE 2023, 18, e0291054. [Google Scholar] [CrossRef]
- Klimiuk, K.; Sembratowicz, I.; Tutaj, K.; Czech, A. Effect of thyme (Thymus vulgaris L.) used in diets with extruded flaxseed on the antioxidant and lipid profile of the blood and tissues of fattening pigs. Antioxidants 2023, 12, 1045. [Google Scholar] [CrossRef]
- Lei, X.J.; Yun, H.M.; Kim, I.H. Effects of dietary supplementation of natural and fermented herbs on growth performance, nutrient digestibility, blood parameters, meat quality and fatty acid composition in growing-finishing pigs. Ital. J. Anim. Sci. 2018, 17, 984–993. [Google Scholar] [CrossRef]
- Yan, L.; Meng, Q.W.; Kim, I.H. The effects of dietary Houttuynia cordata and Taraxacum officinale extract powder on growth performance, nutrient digestibility, blood characteristics and meat quality in finishing pigs. Livest. Sci. 2011, 141, 188–193. [Google Scholar] [CrossRef]
- Mo, K.; Li, J.; Liu, F.; Xu, Y.; Huang, X.; Ni, H. Superiority of microencapsulated essential oils compared with common essential oils and antibiotics: Effects on the intestinal health and gut microbiota of weaning piglet. Front. Nutr. 2022, 8, 808106. [Google Scholar] [CrossRef] [PubMed]
- Baldissera, M.D.; Souza, C.F.; De Matos, A.F.I.M.; Baldisserotto, B.; Stefani, L.M.; da Silva, A.S. Purinergic system as a potential target for inflammation and toxicity induced by thymol in immune cells and tissues. Mol. Cell. Biochem. 2019, 452, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Hashemipour, H.; Khaksar, V.; Rubio, L.; Veldkamp, T.; Van Krimpen, M.M. Effect of feed supplementation with a thymol plus carvacrol mixture, in combination or not with an NSP-degrading enzyme, on productive and physiological parameters of broilers fed on wheat-based diets. Anim. Feed Sci. Technol. 2016, 211, 117–131. [Google Scholar] [CrossRef]
- Timmerman, H.M.; Koning, C.J.; Mulder, L.; Rombouts, F.M.; Beynen, A.C. Monostrain, multistrain and multispecies probiotics—A comparison of functionality and efficacy. Int. J. Food Microbiol. 2004, 96, 219–233. [Google Scholar] [CrossRef]
- Bosi, P.; Trevisi, P. New topics and limits related to the use of beneficial microbes in pig feeding. Benef. Microbes 2010, 1, 447–454. [Google Scholar] [CrossRef]
- Gaggìa, F.; Mattarelli, P.; Biavati, B. Probiotics and prebiotics in animal feeding for safe food production. Int. J. Food Microbiol. 2010, 141, S15–S28. [Google Scholar] [CrossRef]
- Musa, H.H.; Wu, S.; Zhu, C.; Seri, H.; Zhu, G. The potential benefits of probiotics in animal production and health. J. Anim. Vet. Adv. 2009, 8, 313–321. [Google Scholar]
- Chen, W.; Mi, J.; Lv, N.; Gao, J.; Cheng, J.; Wu, R.; Ma, J.; Lan, T.; Liao, X. Lactation stage-dependency of the sow milk microbiota. Front. Microbiol. 2018, 9, 945. [Google Scholar] [CrossRef]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut microbiota dysbiosis in postweaning piglets: Understanding the keys to health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, Z.; Li, J.Y.; Li, H.; Liu, Z.; Wang, J.; Tan, B. Ningxiang pig-derived HNAU0516 ameliorates postweaning diarrhoea by promoting intestinal health and modulating the gut microbiota in piglets. Animal 2024, 18, 101220. [Google Scholar] [CrossRef]
- Yoon, K.N.; Lee, H.G.; Yeom, S.J.; Kim, S.S.; Park, J.H.; Song, B.S.; Yi, S.W.; Do, Y.J.; Oh, B.; Oh, S.I.; et al. AGMB00912 alleviates salmonellosis and modulates gut microbiota in weaned piglets: A pilot study. Sci. Rep. 2024, 14, 15466. [Google Scholar] [CrossRef]
- Tang, J.L.; Zhao, M.C.; Yang, W.Y.; Chen, H.; Dong, Y.H.; He, Q.; Miao, X.; Zhang, J.T. Effect of Composite Probiotics on Antioxidant Capacity, Gut Barrier Functions, and Fecal Microbiome of Weaned Piglets and Sows. Animals 2024, 14, 1359. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, Y.H.; Kim, S.J.; Han, J.H. Impact of Long-Term Supplementation with Probiotics on Gut Microbiota and Growth Performance in Post-Weaned Piglets. Animals 2024, 14, 1652. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qiu, X.Y.; Yang, Y.; Wang, J.; Wang, Q.; Liu, J.B.; Huang, J.X.; Yang, F.Y.; Liu, Z.H.; Qi, R.L. Uncovering the mechanism of CBX 2021 to improve pig health based on and studies. Front. Microbiol. 2024, 15, 1394332. [Google Scholar] [CrossRef]
- Chen, J.; Mou, L.Y.; Wang, L.; Wu, G.F.; Dai, X.M.; Chen, Q.F.; Zhang, J.B.; Luo, X.; Xu, F.F.; Zhang, M.; et al. Mixed and fermented feed improves gut microbiota and immunity of Bamei piglet. Front. Microbiol. 2024, 15, 1442373. [Google Scholar] [CrossRef]
- Zuniga, J.C.; Rueda, A.M.F.; Samuel, R.S.; St-Pierre, B.; Levesque, C.L. Impact of Lactobacillus- and Bifidobacterium-Based Direct-Fed Microbials on the Performance, Intestinal Morphology, and Fecal Bacterial Populations of Nursery Pigs. Microorganisms 2024, 12, 1786. [Google Scholar] [CrossRef]
- Sun, W.J.; Chen, W.N.; Meng, K.; Cai, L.; Li, G.G.; Li, X.L.; Jiang, X.R. Dietary Supplementation with Probiotic Improves Intestinal Integrity via Modulating Intestinal Barrier Function and Microbial Diversity in Weaned Piglets. Biology 2023, 12, 238. [Google Scholar] [CrossRef]
- Xue, L.; Long, S.F.; Cheng, B.; Song, Q.; Zhang, C.; Hansen, L.H.B.; Sheng, Y.S.; Zang, J.J.; Piao, X.S. Dietary Triple-Strain-Based Probiotic Supplementation Improves Performance, Immune Function, Intestinal Morphology, and Microbial Community in Weaned Pigs. Microorganisms 2024, 12, 1536. [Google Scholar] [CrossRef]
- Dowarah, R.; Verma, A.K.; Agarwal, N.; Singh, P.; Singh, B.R. Selection and characterization of probiotic lactic acid bacteria and its impact on growth, nutrient digestibility, health and antioxidant status in weaned piglets. PLoS ONE 2018, 13, e0192978. [Google Scholar] [CrossRef] [PubMed]
- Ryan, K.M.; Ernst, M.K.; Rice, N.R.; Vousden, K.H. Role of NF-κB in p53-mediated programmed cell death. Nature 2000, 404, 892–897. [Google Scholar] [CrossRef]
- Willing, B.P.; Van Kessel, A.G. Enterocyte proliferation and apoptosis in the caudal small intestine is influenced by the composition of colonizing commensal bacteria in the neonatal gnotobiotic pig. J. Anim. Sci. 2007, 85, 3256–3266. [Google Scholar] [CrossRef]
- Garrett, W.S.; Gordon, J.I.; Glimcher, L.H. Homeostasis and inflammation in the intestine. Cell 2010, 140, 859–870. [Google Scholar] [CrossRef]
- Burrough, E.R.; Arruda, B.L.; Plummer, P.J. Comparison of the luminal and mucosa-associated microbiota in the colon of pigs with and without swine dysentery. Front. Vet. Sci. 2017, 4, 139. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, S.; Park, I.; Kim, S.W. Impacts of energy feeds and supplemental protease on growth performance, nutrient digestibility, and gut health of pigs from 18 to 45 kg body weight. Anim. Nutr. 2017, 3, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Mazur-Kuśnirek, M.; Lipiński, K.; Jørgensen, J.N.; Hansen, L.H.B.; Antoszkiewicz, Z.; Zabielski, R.; Konieczka, P. The effect of a Bacillus-based probiotic on sow and piglet performance in two production cycles. Animals 2023, 13, 3163. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Hou, C.; Zeng, X.; Qiao, S. The use of lactic acid bacteria as a probiotic in swine diets. Pathogens 2015, 4, 34–45. [Google Scholar] [CrossRef]
- Niu, Q.; Li, P.; Hao, S.; Zhang, Y.; Kim, S.W.; Li, H.; Ma, X.; Gao, S.; He, L.; Wu, W. Dynamic distribution of the gut microbiota and the relationship with apparent crude fiber digestibility and growth stages in pigs. Sci. Rep. 2015, 5, 9938. [Google Scholar] [CrossRef]
- Yang, H.; Huang, X.; Fang, S.; Xin, W.; Huang, L.; Chen, C. Uncovering the composition of microbial community structure and metagenomics among three gut locations in pigs with distinct fatness. Sci. Rep. 2016, 6, 27427. [Google Scholar] [CrossRef]
- Conlon, M.A.; Bird, A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2014, 7, 17–44. [Google Scholar] [CrossRef] [PubMed]
- Haenen, D.; Zhang, J.; da Silva, C.S.; Bosch, G.; van der Meer, I.M.; van Arkel, J.; van den Borne, J.J.; Gutiérrez, O.P.; Smidt, H.; Kemp, B. A diet high in resistant starch modulates microbiota composition, SCFA concentrations, and gene expression in pig intestine. J. Nutr. 2013, 143, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Haffner, F.B.; Diab, R.; Pasc, A. Encapsulation of probiotics: Insights into academic and industrial approaches. AIMS Mater. Sci. 2016, 3, 114–136. [Google Scholar] [CrossRef]
- Chapman, C.; Gibson, G.R.; Rowland, I. Health benefits of probiotics: Are mixtures more effective than single strains? Eur. J. Nutr. 2011, 50, 1–17. [Google Scholar] [CrossRef]
- Ahmed, S.T.; Hoon, J.; Mun, H.-S.; Yang, C.-J. Evaluation of Lactobacillus and Bacillus-based probiotics as alternatives to antibiotics in enteric microbial challenged weaned piglets. Afr. J. Microbiol. Res. 2014, 8, 96–104. [Google Scholar] [CrossRef]
- Cai, L.; Indrakumar, S.; Kiarie, E.; Kim, I.H. Effects of a multi-strain Bacillus species–based direct-fed microbial on growth performance, nutrient digestibility, blood profile, and gut health in nursery pigs fed corn–soybean meal–based diets. J. Anim. Sci. 2015, 93, 4336–4342. [Google Scholar] [CrossRef]
- Jaworski, N.W.; Owusu-Asiedu, A.; Walsh, M.C.; McCann, J.C.; Loor, J.J.; Stein, H.H. Effects of a 3 strain Bacillus-based direct-fed microbial and dietary fiber concentration on growth performance and expression of genes related to absorption and metabolism of volatile fatty acids in weanling pigs. J. Anim. Sci. 2017, 95, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Kim, J.S.; Ingale, S.L.; Kim, K.H.; Shinde, P.L.; Kwon, I.K.; Chae, B.J. Effect of potential multimicrobe probiotic product processed by high drying temperature and antibiotic on performance of weanling pigs. J. Anim. Sci. 2011, 89, 1795–1804. [Google Scholar] [CrossRef]
- Choi, Y.; Goel, A.; Hosseindoust, A.; Lee, S.; Kim, K.; Jeon, S.; Noh, H.; Kyong Kwon, I.; Chae, B. Effects of dietary supplementation of Ecklonia cava with or without probiotics on the growth performance, nutrient digestibility, immunity and intestinal health in weanling pigs. Ital. J. Anim. Sci. 2016, 15, 62–68. [Google Scholar] [CrossRef]
- Boyle, R.J.; Robins-Browne, R.M.; Tang, M.L. Probiotic use in clinical practice: What are the risks? Am. J. Clin. Nutr. 2006, 83, 1256–1264. [Google Scholar] [CrossRef]
- da Silva, C.A.; Dias, C.P.; Callegari, M.A.; Romano, G.D.; de Souza, K.L.; Jacob, D.V.; Ulbrich, A.J.; Goossens, T. Phytogenics and encapsulated sodium butyrate can replace antibiotics as growth promoters for lightly weaned piglets. PLoS ONE 2022, 17, e0279197. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.Y.; Bottoms, K.A.; Stein, H.H.; Blavi, L.; Bradley, C.L.; Bergstrom, J.; Knapp, J.; Story, R.; Maxwell, C.; Tsai, T.C.; et al. Dietary Organic Acids Modulate Gut Microbiota and Improve Growth Performance of Nursery Pigs. Microorganisms 2021, 9, 110. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.Y.; Piao, X.S.; Shang, Q.H.; Long, S.F.; Liu, S.J.; Mahfuz, S. Mixed organic acids as an alternative to antibiotics improve serum biochemical parameters and intestinal health of weaned piglets. Anim. Nutr. 2021, 7, 737–749. [Google Scholar] [CrossRef]
- Lingbeek, M.M.; Borewicz, K.; Febery, E.; Han, Y.M.; Doelman, J.; van Kuijk, S.J.A. Short-chain fatty acid administration via water acidifier improves feed efficiency and modulates fecal microbiota in weaned piglets. J. Anim. Sci. 2021, 99, skab307. [Google Scholar] [CrossRef]
- Moita, V.H.C.; Duarte, M.E.; da Silva, S.N.; Kim, S.W. Supplemental effects of functional oils on the modulation of mucosa-associated microbiota, intestinal health, and growth performance of nursery pigs. Animals 2021, 11, 1591. [Google Scholar] [CrossRef]
- Shao, Y.; Peng, Q.; Wu, Y.; Peng, C.; Wang, S.; Zou, L.; Qi, M.; Peng, C.; Liu, H.; Li, R. The effect of an essential oil blend on growth performance, intestinal health, and microbiota in early-weaned piglets. Nutrients 2023, 15, 450. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sui, M.; Wu, F.; Chen, X.; Chen, B.; Yao, L. Effects of Chinese herbal plant extracts on diarrhea rate, intestinal morphology, nutrient digestibility, and immunity of weaned piglets. Can. J. Anim. Sci. 2023, 103, 388–393. [Google Scholar] [CrossRef]
- Xu, T.; Ma, X.; Zhou, X.; Qian, M.; Yang, Z.; Cao, P.; Han, X. Coated tannin supplementation improves growth performance, nutrients digestibility, and intestinal function in weaned piglets. J. Anim. Sci. 2022, 100, skac088. [Google Scholar] [CrossRef]
- Song, Y.; Luo, Y.; Yu, B.; He, J.; Zheng, P.; Mao, X.; Huang, Z.; Luo, J.; Luo, Y.; Yan, H. Tannic acid extracted from gallnut prevents post-weaning diarrhea and improves intestinal health of weaned piglets. Anim. Nutr. 2021, 7, 1078–1086. [Google Scholar] [CrossRef]
- Xu, H.X.; Gong, J.S.; Lu, P.; Azevedo, P.; Li, L.Y.; Yu, H.; Yang, C.B. Functional evaluation of Bacillus licheniformis PF9 for its potential in controlling enterotoxigenic Escherichia coli in weaned piglets. Transl. Anim. Sci. 2024, 8, txae050. [Google Scholar] [CrossRef]
- Sudan, S.; Fletcher, L.; Zhan, X.S.; Dingle, S.; Patterson, R.; Huber, L.A.; Friendship, R.; Kiarie, E.G.; Li, J.L. Comparative efficacy of a novel-based probiotic and pharmacological zinc oxide on growth performance and gut responses in nursery pigs. Sci. Rep. 2023, 13, 4659. [Google Scholar] [CrossRef] [PubMed]
- Oltval, Z.N.; Milliman, C.L.; Korsmeyer, S.J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programed cell death. Cell 1993, 74, 609–619. [Google Scholar] [CrossRef]
- Cheng, Y.-C.; Kim, S.W. Use of microorganisms as nutritional and functional feedstuffs for nursery pigs and broilers. Animals 2022, 12, 3141. [Google Scholar] [CrossRef] [PubMed]
- Qi, N.; Liu, S.; Yan, F.; Chen, B.; Wu, S.; Lin, X.; Yan, Z.; Zhou, Q.; Liao, S.; Li, J. Study of microencapsulated fatty acid antimicrobial activity in vitro and its prevention ability of Clostridium perfringens induced necrotic enteritis in broiler chicken. Gut Pathog. 2023, 15, 1. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.-H.; Kim, Y.-G.; Hu, L.; Lee, J. Fatty acids as aminoglycoside antibiotic adjuvants against Staphylococcus aureus. Front. Microbiol. 2022, 13, 876932. [Google Scholar] [CrossRef] [PubMed]
- Kelsey, J.A.; Bayles, K.W.; Shafii, B.; McGuire, M.A. Fatty acids and monoacylglycerols inhibit growth of Staphylococcus aureus. Lipids 2006, 41, 951–961. [Google Scholar] [CrossRef]
- Marounek, M.; Skrivanova, E.; Skrivanova, V. A note on the effect of caprylic acid and triacylglycerols of caprylic and capric acid on growth rate and shedding of coccidia oocysts in weaned piglets. J. Anim. Feed Sci. 2004, 13, 269–274. [Google Scholar] [CrossRef]
- Hanczakowska, E.; Szewczyk, A.; Okoń, K. Effects of dietary caprylic and capric acids on piglet performance and mucosal epithelium structure of the ileum. J. Anim. Feed Sci 2011, 20, 556–565. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Lee, K.Y.; Tran, H.N.; Kim, I.H. Effect of a protected blend of organic acids and medium-chain fatty acids on growth performance, nutrient digestibility, blood profiles, meat quality, faecal microflora, and faecal gas emission in finishing pigs. Can. J. Anim. Sci. 2018, 99, 448–455. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, F.; Odle, J.; Lin, X.; Jacobi, S.K.; Zhu, H.; Wu, Z.; Hou, Y. Fish Oil Enhances Intestinal Integrity and Inhibits TLR4 and NOD2 Signaling Pathways in Weaned Pigs after LPS Challenge, 3. J. Nutr. 2012, 142, 2017–2024. [Google Scholar] [CrossRef]
- Patterson, R.; Connor, M.L.; Krause, D.O.; Nyachoti, C.M. Response of piglets weaned from sows fed diets supplemented with conjugated linoleic acid (CLA) to an Escherichia coli K88+ oral challenge. Animal 2008, 2, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Zentek, J.; Buchheit-Renko, S.; Ferrara, F.; Vahjen, W.; Van Kessel, A.; Pieper, R. Nutritional and physiological role of medium-chain triglycerides and medium-chain fatty acids in piglets. Anim. Health Res. Rev. 2011, 12, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Pluske, J.R.; Black, J.L.; Kim, J.C.; Dunshea, F.R. Suppressing feed intake of finisher pigs: A preliminary study. Anim. Prod. Sci. 2015, 55, 1546. [Google Scholar] [CrossRef]
- Pluske, J.; Turpin, D.; Abraham, S.; Collins, A.; Dunshea, F. 2C-125: Lauric Acid, a Potentially New Feed Additive for the Australian Pork Industry; Pork CRC: Des Moines, IA, USA, 2018. [Google Scholar]
- Yoon, B.K.; Jackman, J.A.; Kim, M.C.; Sut, T.N.; Cho, N.-J. Correlating membrane morphological responses with micellar aggregation behavior of capric acid and monocaprin. Langmuir 2017, 33, 2750–2759. [Google Scholar] [CrossRef]
- Dierick, N.A.; Decuypere, J.A. Endogenous lipolysis in feedstuffs and compound feeds for pigs: Effects of storage time and conditions and lipase and/or emulsifier addition. Anim. Feed Sci. Technol. 2002, 102, 53–70. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, Z.; Zou, T.; Chen, J.; Li, G.; Zheng, L.; Li, S.; You, J. Bacteriophage as an alternative to antibiotics promotes growth performance by regulating intestinal inflammation, intestinal barrier function and gut microbiota in weaned piglets. Front. Vet. Sci. 2021, 8, 623899. [Google Scholar] [CrossRef]
- Hosseindoust, A.R.; Lee, S.H.; Kim, J.S.; Choi, Y.H.; Noh, H.S.; Lee, J.H.; Jha, P.K.; Kwon, I.K.; Chae, B.J. Dietary bacteriophages as an alternative for zinc oxide or organic acids to control diarrhoea and improve the performance of weanling piglets. Vet. Med. 2017, 62, 53–61. [Google Scholar] [CrossRef]
- Kingkan, P.; Supcharoenkul, T.; Rakangthong, C.; Bunchasak, C.; Surachat, K.; Loongyai, W. Effect of bacteriophages on intestinal colonization of Escherichia coli, cecal microbiota composition, intestinal morphology, and growth performance in nursery pigs from commercial pig farms. Adv. Anim. Vet. Sci 2023, 11, 960–967. [Google Scholar] [CrossRef]
- Oliver, W.T.; Wells, J.E. Lysozyme as an alternative to growth promoting antibiotics in swine production. J. Anim. Sci. Biotechnol. 2015, 6, 35. [Google Scholar] [CrossRef]
- Oliver, W.T.; Wells, J.E.; Maxwell, C.V. Lysozyme as an alternative to antibiotics improves performance in nursery pigs during an indirect immune challenge. J. Anim. Sci. 2014, 92, 4927–4934. [Google Scholar] [CrossRef]
- Nyachoti, C.M.; Kiarie, E.; Bhandari, S.K.; Zhang, G.; Krause, D.O. Weaned pig responses to Escherichia coli K88 oral challenge when receiving a lysozyme supplement. J. Anim. Sci. 2012, 90, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.T.; Duarte, M.E.; Holanda, D.M.; Kim, S.W. Friend or foe? Impacts of dietary xylans, xylooligosaccharides, and xylanases on intestinal health and growth performance of monogastric animals. Animals 2021, 11, 609. [Google Scholar] [CrossRef]
- Baker, J.T.; Deng, Z.; Sokale, A.; Frederick, B.; Kim, S.W. Nutritional and functional roles of β-mannanase on intestinal health and growth of newly weaned pigs fed two different types of feeds. J. Anim. Sci. 2024, 102, skae206. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, S.; Kim, S.W. Effects of supplemental xylanase on health of the small intestine in nursery pigs fed diets with corn distillers’ dried grains with solubles. J. Anim. Sci. 2020, 98, skaa185. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.T.; Duarte, M.E.; Kim, S.W. Effects of dietary xylanase supplementation on growth performance, intestinal health, and immune response of nursery pigs fed diets with reduced metabolizable energy. J. Anim. Sci. 2024, 102, skae026. [Google Scholar] [CrossRef]
- Jang, K.B.; Kim, Y.I.; Duarte, M.E.; Kim, S.W. Effects of β-mannanase supplementation on intestinal health and growth of nursery pigs. J. Anim. Sci. 2024, 102, skae052. [Google Scholar] [CrossRef]
- Jang, K.B.; Zhao, Y.; Kim, Y.I.; Pasquetti, T.; Kim, S.W. Effects of bacterial β-mannanase on apparent total tract digestibility of nutrients in various feedstuffs fed to growing pigs. Anim. Biosci. 2023, 36, 1700. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Duarte, Y.G.; Pasquali, G.A.; Kim, S.W. Investigation of the nutritional and functional roles of a combinational use of xylanase and β-glucanase on intestinal health and growth of nursery pigs. J. Anim. Sci. Biotechnol. 2024, 15, 63. [Google Scholar] [CrossRef]
- Moita, V.H.C.; Kim, S.W. Nutritional and functional roles of phytase and xylanase enhancing the intestinal health and growth of nursery pigs and broiler chickens. Animals 2022, 12, 3322. [Google Scholar] [CrossRef]
- Tiwari, U.P.; Chen, H.; Kim, S.W.; Jha, R. Supplemental effect of xylanase and mannanase on nutrient digestibility and gut health of nursery pigs studied using both in vivo and in vitro models. Anim. Feed Sci. Technol. 2018, 245, 77–90. [Google Scholar] [CrossRef]
- Xu, Y.; Lahaye, L.; He, Z.; Zhang, J.; Yang, C.; Piao, X. Micro-encapsulated essential oils and organic acids combination improves intestinal barrier function, inflammatory responses and microbiota of weaned piglets challenged with enterotoxigenic Escherichia coli F4 (K88+). Anim. Nutr. 2020, 6, 269–277. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Lee, J.M.; Kim, I.H. Effects of Enterococcus faecium DSM 7134 on weanling pigs were influenced by dietary energy and crude protein density. Livest. Sci. 2014, 169, 106–111. [Google Scholar] [CrossRef]
- Devi, S.M.; Kim, I.H. Effect of medium chain fatty acids (MCFA) and probiotic (Enterococcus faecium) supplementation on the growth performance, digestibility and blood profiles in weanling pigs. Vet. Med. 2014, 59, 527–535. [Google Scholar] [CrossRef]
- Lei, X.J.; Lee, S.I.; Lee, K.Y.; Nguyen, D.H.; Kim, I.H. Effects of a blend of organic acids and medium-chain fatty acids with and without Enterococcus faecium on growth performance, nutrient digestibility, blood parameters, and meat quality in finishing pigs. Can. J. Anim. Sci. 2018, 98, 852–859. [Google Scholar] [CrossRef]
- Foster, J.W. Low pH adaptation and the acid tolerance response of Salmonella typhimurium. Crit. Rev. Microbiol. 1995, 21, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Mani-López, E.; García, H.; López-Malo, A. Organic acids as antimicrobials to control Salmonella in meat and poultry products. Food Res. Int. 2012, 45, 713–721. [Google Scholar] [CrossRef]
- Li, T.; Teng, D.; Mao, R.; Hao, Y.; Wang, X.; Wang, J. A critical review of antibiotic resistance in probiotic bacteria. Food Res. Int. 2020, 136, 109571. [Google Scholar] [CrossRef]
- Mater, D.D.G.; Langella, P.; Corthier, G.; Flores, M.-J. A probiotic Lactobacillus strain can acquire vancomycin resistance during digestive transit in mice. J. Mol. Microbiol. Biotechnol. 2008, 14, 123–127. [Google Scholar] [CrossRef]
- Flórez, A.-B.; Ladero, V.; Álvarez-Martín, P.; Ammor, M.-S.; Álvarez, M.-Á.; Mayo, B. Acquired macrolide resistance in the human intestinal strain Lactobacillus rhamnosus E41 associated with a transition mutation in 23S rRNA genes. Int. J. Antimicrob. Agents 2007, 30, 341–344. [Google Scholar] [CrossRef]
- Gueimonde, M.; Sánchez, B.; de los Reyes-Gavilán, C.G.; Margolles, A. Antibiotic resistance in probiotic bacteria. Front. Microbiol. 2013, 4, 202. [Google Scholar] [CrossRef]
- Serafini, F.; Bottacini, F.; Viappiani, A.; Baruffini, E.; Turroni, F.; Foroni, E.; Lodi, T.; van Sinderen, D.; Ventura, M. Insights into physiological and genetic mupirocin susceptibility in bifidobacteria. Appl. Environ. Microbiol. 2011, 77, 3141–3146. [Google Scholar] [CrossRef] [PubMed]
- Mayrhofer, S.; Mair, C.; Kneifel, W.; Domig, K.J. Susceptibility of bifidobacteria of animal origin to selected antimicrobial agents. Chemother. Res. Pract. 2011, 2011, 989520. [Google Scholar] [CrossRef] [PubMed]
- Sheu, C.W.; Freese, E. Lipopolysaccharide layer protection of gram-negative bacteria against inhibition by long-chain fatty acids. J. Bacteriol. 1973, 115, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Nunn, W.D. Two-carbon compounds and fatty acids as carbon sources. Escherichia coli and Salmonella typhimurium: Cellular and molecular biology. In Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology; Neidhardt, F.C., Curtiss, R., III, Ingraham, J.L., Lin, E.C.C., Low, K.B., Magasanik, B., Reznikoff, W.S., Riley, M., Schaechter, M., Umbarger, H.E., Eds.; American Society for Microbiology: Washington, DC, USA, 1987; Volume 1, pp. 285–299. [Google Scholar]
- Sulakvelidze, A.; Alavidze, Z.; Morris, J.G., Jr. Bacteriophage therapy. Antimicrob. Agents Chemother. 2001, 45, 649–659. [Google Scholar] [CrossRef]
- Yoichi, M.; Morita, M.; Mizoguchi, K.; Fischer, C.R.; Unno, H.; Tanji, Y. The criterion for selecting effective phage for Escherichia coli O157: H7 control. Biochem. Eng. J. 2004, 19, 221–227. [Google Scholar] [CrossRef]

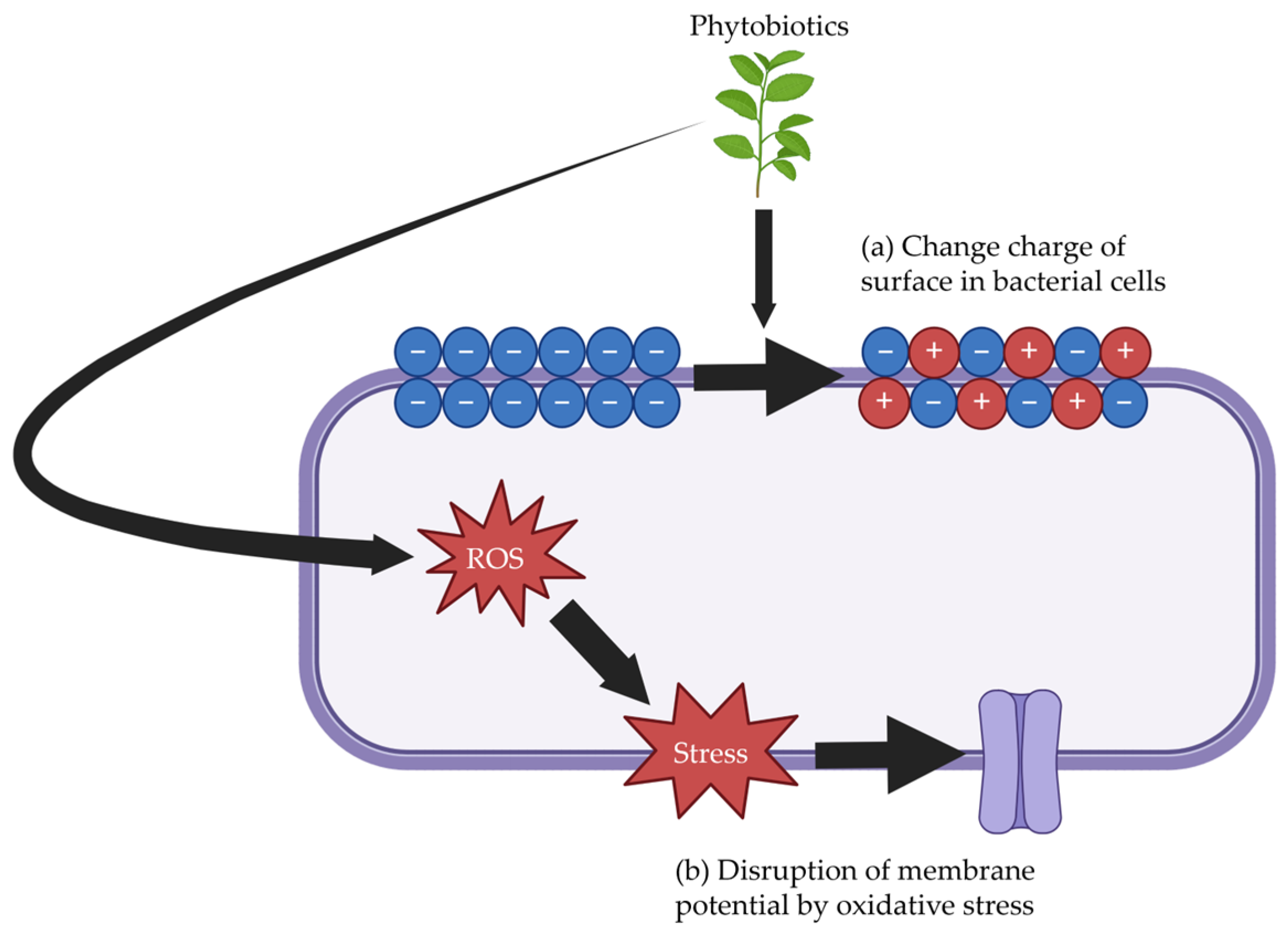

| Main Mechanism | Specific Mechanism | Example |
|---|---|---|
| Inhibition of cell wall synthesis | Inhibition of the cytoplasmic stage | Mur enzymes, alanine racemase, D-alanyl-D-alanine ligase, fosfomycin, and seromycin |
| Inhibition of the membrane-associated stage | Tunicamycin, liposidomycin, mureidomycin, mannopeptimycin, lantibiotic, defensin, and glycopeptide antibiotics | |
| Inhibition of the extra-cytoplasmic stage | Transpeptidase, endopeptidase, carboxypeptidase, transglycosylase, penicillin, cephalosporin, and cephamycin | |
| Disintegration of cell membrane | Formation of pores that disrupt the integrity of the cell membrane | Monensin, salinomycin, narasin, and nisin |
| Inhibition of protein synthesis | Interference with the aminoacyl site on the 30S subunit | Neomycin, kanamycin, puromycin, and tetracycline |
| Interference with peptidyl transferase center on the 50S subunit | Chloramphenicol, clindamycin, sparsomycin, streptogramin, and oxazolidinone | |
| Interference with the exit site on the 50S subunit | Macrolide antibiotics | |
| Inhibition of DNA synthesis | Inhibition of RNA transcription | Rifamycin |
| Inhibition of Type II topoisomerases | Olaquindox, mequindox, quincetone, cyadox, and carbadox |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, J.Y.; Deng, Z.; Kim, S.W. Antibiotics and Opportunities of Their Alternatives in Pig Production: Mechanisms Through Modulating Intestinal Microbiota on Intestinal Health and Growth. Antibiotics 2025, 14, 301. https://doi.org/10.3390/antibiotics14030301
Sung JY, Deng Z, Kim SW. Antibiotics and Opportunities of Their Alternatives in Pig Production: Mechanisms Through Modulating Intestinal Microbiota on Intestinal Health and Growth. Antibiotics. 2025; 14(3):301. https://doi.org/10.3390/antibiotics14030301
Chicago/Turabian StyleSung, Jung Yeol, Zixiao Deng, and Sung Woo Kim. 2025. "Antibiotics and Opportunities of Their Alternatives in Pig Production: Mechanisms Through Modulating Intestinal Microbiota on Intestinal Health and Growth" Antibiotics 14, no. 3: 301. https://doi.org/10.3390/antibiotics14030301
APA StyleSung, J. Y., Deng, Z., & Kim, S. W. (2025). Antibiotics and Opportunities of Their Alternatives in Pig Production: Mechanisms Through Modulating Intestinal Microbiota on Intestinal Health and Growth. Antibiotics, 14(3), 301. https://doi.org/10.3390/antibiotics14030301






