Kinetic Patterns of Antibiotic Consumption in German Acute Care Hospitals from 2017 to 2023
Abstract
1. Introduction
2. Results
2.1. Hospitals
2.2. Total Antibiotics and AWaRe-Categories
2.3. Selected Antibiotic Classes
2.3.1. Betalactams
2.3.2. Macrolides
2.3.3. Fluoroquinolones
2.3.4. Glycopeptides and Selected Reserve-Antibiotics
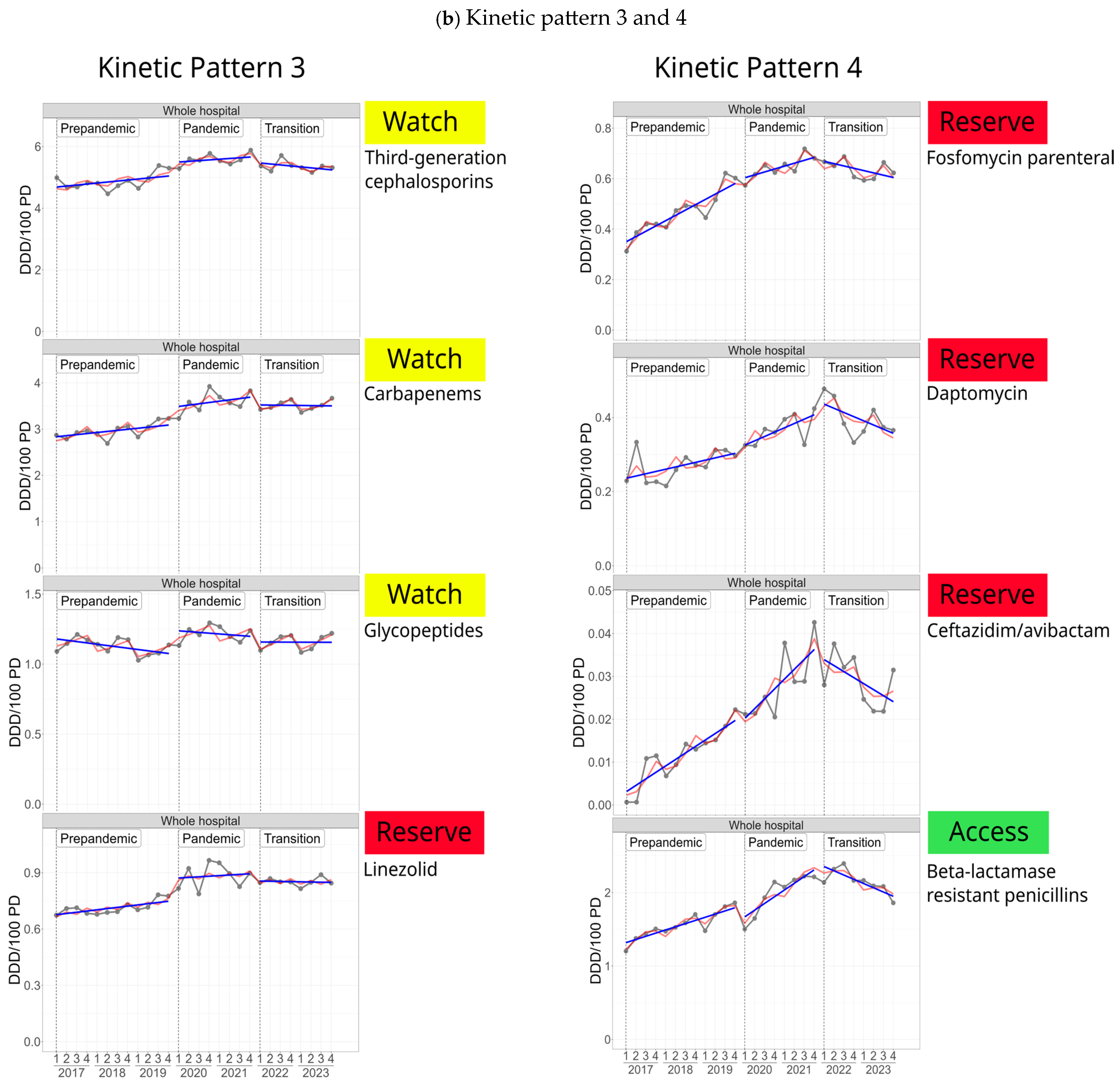
2.3.5. Common Kinetic Patterns of Selected Antibiotic Classes/Substances
3. Discussion
4. Materials and Methods
Statistical Analysis
- Investigation of the presence of a linear increasing or decreasing trend or no linear trend in AMC over the entire time period (2017–2023)
- Investigation of differences in phase-specific mean consumption levels
- Investigation of the presence and change in intra-phasic trends according to the three phases
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| TLA | Three letter acronym |
| LD | Linear dichroism |
References
- Moynihan, R.; Sanders, S.; Michaleff, Z.A.; Scott, A.M.; Clark, J.; To, E.J.; Jones, M.; Kitchener, E.; Fox, M.; Johansson, M.; et al. Impact of COVID-19 pandemic on utilisation of healthcare services: A systematic review. BMJ Open 2021, 11, e045343. [Google Scholar] [CrossRef] [PubMed]
- Kapsner, L.A.; Kampf, M.O.; Seuchter, S.A.; Gruendner, J.; Gulden, C.; Mate, S.; Mang, J.M.; Schüttler, C.; Deppenwiese, N.; Krause, L.; et al. Reduced Rate of Inpatient Hospital Admissions in 18 German University Hospitals During the COVID-19 Lockdown. Front. Public Health 2021, 8, 594117. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Clinical Management of COVID-19: Living Guideline. 18 August 2023. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2023.2 (accessed on 28 January 2025).
- Khan, S.; Hasan, S.S.; Bond, S.E.; Conway, B.R.; Aldeyab, M.A. Antimicrobial consumption in patients with COVID-19: A systematic review and meta-analysis. Expert Rev. Anti Infect. Ther. 2022, 20, 749–772. [Google Scholar] [CrossRef]
- Cong, W.; Poudel, A.N.; Alhusein, N.; Wang, H.; Yao, G.; Lambert, H. Antimicrobial Use in COVID-19 Patients in the First Phase of the SARS-CoV-2 Pandemic: A Scoping Review. Antibiotics 2021, 10, 745. [Google Scholar] [CrossRef]
- Abu-Rub, L.I.; Abdelrahman, H.A.; Johar, A.A.; Alhussain, H.A.; Hadi, H.A.; Eltai, N.O. Antibiotics Prescribing in Intensive Care Settings during the COVID-19 Era: A Systematic Review. Antibiotics 2021, 10, 935. [Google Scholar] [CrossRef] [PubMed]
- United Kingdom (UK) Health Security Agency. English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR) Report 2023 to 2024. Available online: https://assets.publishing.service.gov.uk/media/6734e208b613efc3f1823095/ESPAUR-report-2023-2024.pdf (accessed on 28 January 2025).
- European Surveillance of Antimicrobial Consumption (ESAC)-Net. European Centre for Disease Prevention and Control Antimicrobial Consumption Database (ESAC-Net). 2023. Available online: https://www.ecdc.europa.eu/en/antimicrobial-consumption/surveillance-and-disease-data/database (accessed on 28 January 2025).
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef]
- Cong, W.; Stuart, B.; AIhusein, N.; Liu, B.; Tang, Y.; Wang, H.; Wang, Y.; Manchundiya, A.; Lambert, H. Antibiotic Use and Bacterial Infection in COVID-19 Patients in the Second Phase of the SARS-CoV-2 Pandemic: A Scoping Review. Antibiotics 2022, 11, 991. [Google Scholar] [CrossRef]
- Suleiman, A.S.; Islam, M.A.; Akter, M.S.; Amin, M.R.; Werkneh, A.A.; Bhattacharya, P. A meta-meta-analysis of co-infection, secondary infections, and antimicrobial resistance in COVID-19 patients. J. Infect. Public Health 2023, 16, 1562–1590. [Google Scholar] [CrossRef]
- Granata, G.; Cicalini, S. The Evolving Challenge of Appropriate Antibiotics Use in Hospitalized COVID-19 Patients: A Systematic Literature Review. Antibiotics 2024, 13, 545. [Google Scholar] [CrossRef]
- Sili, U.; Tekin, A.; Bilgin, H.; Khan, S.A.; Domecq, J.P.; Vadgaonkar, G.; Segu, S.S.; Rijhwani, P.; Raju, U.; Surapaneni, K.M.; et al. Early empiric antibiotic use in COVID-19 patients: Results from the international VIRUS registry. Int. J. Infect. Dis. 2024, 140, 39–48. [Google Scholar] [CrossRef]
- Durà-Miralles, X.; Abelenda-Alonso, G.; Bergas, A.; Laporte-Amargós, J.; Sastre-Escolà, E.; Padullés, A.; Carratalà, J.; Gudiol, C. An Ocean between the Waves: Trends in Antimicrobial Consumption in Hospitalized Patients with COVID-19. Antibiotics 2024, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Friedli, O.; Gasser, M.; Cusini, A.; Fulchini, R.; Vuichard-Gysin, D.; Halder Tobler, R.; Wassilew, N.; Plüss-Suard, C.; Kronenberg, A. Impact of the COVID-19 Pandemic on Inpatient Antibiotic Consumption in Switzerland. Antibiotics 2022, 11, 792. [Google Scholar] [CrossRef]
- Aldeyab, M.A.; Crowe, W.; Karasneh, R.A.; Patterson, L.; Sartaj, M.; Ewing, J.; Lattyak, W.J.; Al-Azzam, S.; Araydah, M.; Darwish Elhajji, F.; et al. The impact of the COVID-19 pandemic on antibiotic consumption and prevalence of pathogens in primary and secondary healthcare settings in Northern Ireland. Br. J. Clin. Pharmacol. 2023, 89, 2851–2866. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Wolford, H.; Baggs, J.; Reddy, S.; Hicks, L.A.; Neuhauser, M.M.; Kabbani, S. Antibiotic Use Among Hospitalized Patients With COVID-19 in the United States, March 2020–June 2022. Open Forum Infect. Dis. 2023, 10, ofad503. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, E.N.; Neuhauser, M.M.; Srinivasan, A.; Dubendris, H.; Webb, A.K.; Soe, M.M.; Hicks, L.A.; Wu, H.; Kabbani, S.; Edwards, J.R. Impact of the COVID-19 Pandemic on Inpatient Antibiotic Use in the United States, January 2019 Through July 2022. Clin. Infect. Dis. 2024, 78, 24–26. [Google Scholar] [CrossRef]
- Marks, K.M.; Gulick, R.M. COVID-19. Ann. Intern. Med. 2023, 176, ITC145–ITC160. [Google Scholar] [CrossRef]
- Ghosh, S.; Bornman, C.; Zafer, M.M. Antimicrobial Resistance Threats in the emerging COVID-19 pandemic: Where do we stand? J. Infect. Public Health 2021, 14, 555–560. [Google Scholar] [CrossRef]
- Seneghini, M.; Rüfenacht, S.; Babouee-Flury, B.; Flury, D.; Schlegel, M.; Kuster, S.P.; Kohler, P.P. It is complicated: Potential short- and long-term impact of coronavirus disease 2019 (COVID-19) on antimicrobial resistance-An expert review. Antimicrob. Steward. Healthc. Epidemiol. 2022, 2, e27. [Google Scholar] [CrossRef]
- Yang, X.; Li, X.; Qiu, S.; Liu, C.; Chen, S.; Xia, H.; Zeng, Y.; Shi, L.; Chen, J.; Zheng, J.; et al. Global antimicrobial resistance and antibiotic use in COVID-19 patients within health facilities: A systematic review and meta-analysis of aggregated participant data. J. Infect. 2024, 89, 106183. [Google Scholar] [CrossRef]
- Kern, W.V.; Steib-Bauert, M.; Baumann, J.; Kramme, E.; Först, G.; de With, K. Impact of the COVID-19 Pandemic on Inpatient Antibiotic and Antifungal Drug Prescribing Volumes in Germany. Antibiotics 2024, 13, 837. [Google Scholar] [CrossRef] [PubMed]
- Robert Koch Institute. Surveillance-System for Antimicrobial Consumption. Available online: https://amr.rki.de/Content/AVS/Main.aspx (accessed on 28 January 2025).
- Andrews, A.; Budd, E.L.; Hendrick, A.; Ashiru-Oredope, D.; Beech, E.; Hopkins, S.; Gerver, S.; Muller-Pebody, B.; The Amu Covid-Stakeholder Group. Surveillance of Antibacterial Usage during the COVID-19 Pandemic in England, 2020. Antibiotics 2021, 10, 841. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Bond, S.E.; Lee-Milner, J.; Conway, B.R.; Lattyak, W.J.; Aldeyab, M.A. Antimicrobial consumption in an acute NHS Trust during the COVID-19 pandemic: Intervention time series analysis. JAC Antimicrob. Resist. 2024, 6, dlae013. [Google Scholar] [CrossRef]
- Meschiari, M.; Onorato, L.; Bacca, E.; Orlando, G.; Menozzi, M.; Franceschini, E.; Bedini, A.; Cervo, A.; Santoro, A.; Sarti, M.; et al. Long-Term Impact of the COVID-19 Pandemic on In-Hospital Antibiotic Consumption and Antibiotic Resistance: A Time Series Analysis (2015–2021). Antibiotics 2022, 11, 826. [Google Scholar] [CrossRef]
- Rothe, K.; Spinner, C.D.; Panning, M.; Pletz, M.W.; Rohde, G.; Rupp, J.; Witzenrath, M.; Erber, J.; Eberhardt, F.; Essig, A.; et al. Evaluation of a multiplex PCR screening approach to identify community-acquired bacterial co-infections in COVID-19: A multicenter prospective cohort study of the German competence network of community-acquired pneumonia (CAPNETZ). Infection 2021, 49, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften e.V. (AWMF). S3-Leitlinie Empfehlungen zur Therapie von Patienten mit COVID-19—Living Guideline, AWMF-Register-Nr. 113/001. 2024. Available online: https://register.awmf.org/de/leitlinien/detail/113-001 (accessed on 28 January 2025).
- RECOVERY Collaborative Group. Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2021, 397, 605–612. [Google Scholar]
- Sievers, C.; Zacher, B.; Ullrich, A.; Huska, M.; Fuchs, S.; Buda, S.; Haas, W.; Diercke, M.; An der Heiden, M.; Kröger, S. SARS-CoV-2 Omicron variants BA.1 and BA.2 both show similarly reduced disease severity of COVID-19 compared to Delta, Germany, 2021 to 2022. Euro Surveill. 2022, 27, 2200396. [Google Scholar] [CrossRef]
- Roger, P.M.; Lesselingue, D.; Gérard, A.; Roghi, J.; Quint, P.; Un, S.; Chincholle, A.; Assi, A.; Bouchard, O.; Javaudin, V.; et al. Antibiotic Consumption 2017–2022 in 30 Private Hospitals in France: Impact of Antimicrobial Stewardship Tools and COVID-19 Pandemic. Antibiotics 2024, 13, 180. [Google Scholar] [CrossRef]
- European Medicines Agency. Fluoroquinolone and Quinolone Antibiotics: PRAC Recommends Restrictions on Use. New Restrictions Follow Review of Disabling and Potentially Long-Lasting Side Effects. 5 October 2018. Available online: https://www.ema.europa.eu/en/documents/referral/quinolone-fluoroquinolone-article-31-referral-prac-recommends-restrictions-use_en.pdf (accessed on 28 January 2025).
- Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften e.V. (AWMF). S3-Leitlinie Strategien zur Sicherung Rationaler Antibiotikaanwendung im Krankenhaus. Available online: https://register.awmf.org/de/leitlinien/detail/092-001 (accessed on 28 January 2025).
- Aghdassi, S.J.S.; Hansen, S.; Peña Diaz, L.A.; Gropmann, A.; Saydan, S.; Geffers, C.; Gastmeier, P.; Piening, B.; Behnke, M. Healthcare-Associated Infections and the Use of Antibiotics in German Hospitals. Dtsch. Arztebl. Int. 2024, 121, 277–283. [Google Scholar] [CrossRef]
- Omoush, S.A.; Alzyoud, J.A.M. The Prevalence and Impact of Coinfection and Superinfection on the Severity and Outcome of COVID-19 Infection: An Updated Literature Review. Pathogens 2022, 11, 445. [Google Scholar] [CrossRef]
- Ruzsa, R.; Benkő, R.; Hambalek, H.; Papfalvi, E.; Csupor, D.; Nacsa, R.; Csatordai, M.; Soós, G.; Hajdú, E.; Matuz, M. Hospital Antibiotic Consumption before and during the COVID-19 Pandemic in Hungary. Antibiotics 2024, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- Rubinić, I.; Leung, V.H.; Högberg, L.D.; Monnet, D.L.; Vlahović-Palčevski, V.; ESAC-Net study group. Measuring hospital antibiotic consumption in EU/EEA countries: Comparison of different metrics, 2017 to 2021. Euro Surveill. 2024, 29, 2400317. [Google Scholar] [CrossRef]
- Brueggemann, A.B.; Jansen van Rensburg, M.J.; Shaw, D.; McCarthy, N.D.; Jolley, K.A.; Maiden, M.C.J.; van der Linden, M.P.G.; Amin-Chowdhury, Z.; Bennett, D.E.; Borrow, R.; et al. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID-19 pandemic in 26 countries and territories in the Invasive Respiratory Infection Surveillance Initiative: A prospective analysis of surveillance data. Lancet Digit. Health 2021, 3, e360–e370. [Google Scholar] [CrossRef]
- Buchholz, U.; Lehfeld, A.S.; Tolksdorf, K.; Cai, W.; Reiche, J.; Biere, B.; Dürrwald, R.; Buda, S. Respiratory infections in children and adolescents in Germany during the COVID-19 pandemic. J. Health Monit. 2023, 8, 20–38. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Intensified Circulation of Respiratory Syncytial Virus (RSV) and Associated Hospital Burden in the EU/EEA. Available online: https://www.ecdc.europa.eu/en/publications-data/intensified-circulation-respiratory-syncytial-virus-rsv-and-associated-hospital (accessed on 28 January 2025).
- Singer, R.; Abu Sin, M.; Tenenbaum, T.; Toepfner, N.; Berner, R.; Buda, S.; Schlaberg, J.; Schönfeld, V.; Reinacher, U.; van der Linden, M.; et al. The Increase in Invasive Bacterial Infections with Respiratory Transmission in Germany, 2022/2023. Dtsch. Arztebl. Int. 2024, 121, 114–120. [Google Scholar] [CrossRef]
- WHO. Disease Outbreak News; Increased Incidence of Scarlet Fever and Invasive Group a Streptococcus Infection—Multi-country. Available online: www.who.int/emergencies/disease-outbreak-news/item/2022-DON429 (accessed on 28 January 2025).
- Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM). Arzneimittelinformationen. Available online: https://www.bfarm.de/DE/Arzneimittel/Arzneimittelinformationen/Lieferengpaesse/Antibiotika.html (accessed on 28 January 2025).
- Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften e.V. (AWMF). S3-Leitlinie Behandlung von Erwachsenen Patienten mit Ambulant Erworbener Pneumonie. 2021. Available online: https://register.awmf.org/de/leitlinien/detail/020-020 (accessed on 28 January 2025).
- Sandfort, M.; Hans, J.B.; Fischer, M.A.; Reichert, F.; Cremanns, M.; Eisfeld, J.; Pfeifer, Y.; Heck, A.; Eckmanns, T.; Werner, G.; et al. Increase in NDM-1 and NDM-1/OXA-48-producing Klebsiella pneumoniae in Germany associated with the war in Ukraine, 2022. Euro Surveill. 2022, 27, 2200926. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control (ECDC). Antimicrobial Resistance in the EU/EEA (EARS-Net). Surveillance and Disease Data for Antimicrobial Resistance. Available online: https://www.ecdc.europa.eu/en/antimicrobial-resistance/surveillance-and-disease-data (accessed on 8 March 2025).
- Musuuza, J.S.; Watson, L.; Parmasad, V.; Putman-Buehler, N.; Christensen, L.; Safdar, N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251170. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Leung, V.; Raybardhan, S.; Lo, J.; Kan, T.; Leung, F.; Westwood, D.; Daneman, N.; MacFadden, D.R.; et al. Predictors and microbiology of respiratory and bloodstream bacterial infection in patients with COVID-19: Living rapid review update and meta-regression. Clin. Microbiol. Infect. 2022, 28, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Mathé, P.; Göpel, S.; Hornuss, D.; Tobys, D.; Käding, N.; Eisenbeis, S.; Kohlmorgen, B.; Trauth, J.; Gölz, H.; Walker, S.V.; et al. Increasing numbers and complexity of Staphylococcus aureus bloodstream infection-14 years of prospective evaluation at a German tertiary care centre with multi-centre validation of findings. Clin. Microbiol. Infect. 2023, 29, 1197.e9–1197.e15. [Google Scholar] [CrossRef]
- Gagliotti, C.; Högberg, L.D.; Billström, H.; Eckmanns, T.; Giske, C.G.; Heuer, O.E.; Jarlier, V.; Kahlmeter, G.; Lo Fo Wong, D.; Monen, J.; et al. Staphylococcus aureus bloodstream infections: Diverging trends of meticillin-resistant and meticillin-susceptible isolates, EU/EEA, 2005 to 2018. EARS-Net study group participants. Euro Surveill. 2021, 26, 2002094. [Google Scholar] [CrossRef]
- Allel, K.; Peters, A.; Conejeros, J.; Martínez, J.R.W.; Spencer-Sandino, M.; Riquelme-Neira, R.; Rivas, L.; Rojas, P.; Orellana Chea, C.; García, P.; et al. Antibiotic Consumption During the Coronavirus Disease 2019 Pandemic and Emergence of Carbapenemase-Producing Klebsiella pneumoniae Lineages Among Inpatients in a Chilean Hospital: A Time-Series Study and Phylogenomic Analysis. Clin. Infect. Dis. 2023, 77 (Suppl. S1), S20–S28. [Google Scholar] [CrossRef] [PubMed]
- Wick, M.; Poshtiban, A.; Kramer, R.; Bangert, M.; Lange, M.; Wetzke, M.; Damm, O. Inpatient burden of respiratory syncytial virus in children 2 years of age in Germany: A retrospective analysis of nationwide hospitalization data, 2019–2022. Influenza Other Respir Viruses 2023, 17, e13211. [Google Scholar] [CrossRef] [PubMed]
- Statistisches Bundesamt (DESTATIS). Einrichtungen, Betten und Patientenbewegung. Available online: https://www.destatis.de/DE/Themen/Gesellschaft-Umwelt/Gesundheit/Krankenhaeuser/Tabellen/gd-krankenhaeuser-jahre.html (accessed on 28 January 2025).
- Klauber, J.; Jürgen Wasem, J.; Andreas Beivers, A.; Carina Mostert, C.; Scheller-Kreinsen, D. Hrsg. Krankenhaus-Report 2024; Springer: Berlin/Heidelberg, Germany, 2024; ISBN 978-3-662-68791-8. [Google Scholar] [CrossRef]
- Yusuf, E.; Zeitlinger, M.; Meylan, S. A narrative review of the intermediate category of the antimicrobial susceptibility test: Relation with dosing and possible impact on antimicrobial stewardship. J. Antimicrob. Chemother. 2023, 78, 338–345. [Google Scholar] [CrossRef]
- Munting, A.; Regina, J.; Damas, J.; Lhopitallier, L.; Kritikos, A.; Guery, B.; Senn, L.; Viala, B. Impact of 2020 EUCAST criteria on meropenem prescription for the treatment of Pseudomonas aeruginosa infections: An observational study in a university hospital. Clin. Microbiol. Infect. 2022, 28, 558–563. [Google Scholar] [CrossRef]
- Wantia, N.; Gatermann, S.G.; Rothe, K.; Laufenberg, R. New EUCAST definitions of, S.; I and R from 2019—German physicians are largely not aware of the changes. Infection 2020, 48, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Hentschker, C.; Mostert, C.; Klauber, J.; Malzahn, J.; Scheller-Kreinsen, D.; Schillinger, G.; Karagiannidis, C.; Busse, R. Structure of hospital care for COVID-19 patients up to July 2020 in Germany. Med. Klin. Intensivmed. Notfmed. 2021, 116, 431–439. [Google Scholar] [CrossRef]
- Shin, J.; Park, J.Y.; Chae, J.; Kim, H.S.; Moon, S.M.; Heo, E.; Park, S.Y.; Seo, D.M.; Chun, H.J.; Kim, Y.C.; et al. Difference in Baseline Antimicrobial Prescription Patterns of Hospitals According to Participation in the National Antimicrobial Monitoring and Feedback System in Korea. J. Korean Med. Sci. 2024, 39, e216. [Google Scholar] [CrossRef]
- Schweickert, B.; Feig, M.; Schneider, M.; Willrich, N.; Behnke, M.; Peña Diaz, L.A.; Gastmeier, P.; Richter, D.; Blank, H.P.; Eckmanns, T.; et al. Antibiotic consumption in Germany: First data of a newly implemented web-based tool for local and national surveillance. J. Antimicrob. Chemother. 2018, 73, 3505–3515. [Google Scholar] [CrossRef]
- Moja, L.; Zanichelli, V.; Mertz, D.; Gandra, S.; Cappello, B.; Cooke, G.S.; Chuki, P.; Harbarth, S.; Pulcini, C.; Mendelson, M.; et al. WHO’s essential medicines and AWaRe: Recommendations on first- and second-choice antibiotics for empiric treatment of clinical infections. Clin. Microbiol. Infect. 2024, 30 (Suppl. S2), S1–S51. [Google Scholar] [CrossRef]



| Pre-Pandemic | Pandemic | Transition | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | Difference 2017–2023 | Change (%) | Trend a (95%CI) | Trend p-Value | ||
| Whole hospital | PD b | 57.1 | 54.2 | 51.8 | 53.1 | 52.3 | 52.9 | 53.0 | −4.1 | −7.2 | −0.13 (−0.22; −0.04) | 0.006 |
| AD b | 277.1 | 263.7 | 246.1 | 247.5 | 242.2 | 246.0 | 244.7 | −32.4 | −11.7 | −1.21 (−1.71; −0.72) | <0.001 | |
| ICU c | PD | 107.3 | 105.7 | 103.7 | 104.5 | 104.0 | 104.6 | 105.0 | −2.4 | −2.2 | −0.08 (−0.25; 0.09) | 0.344 |
| AD | 467.9 | 472.9 | 441.1 | 472.2 | 490.8 | 454.6 | 430.4 | −37.5 | −8.0 | −0.87 (−2.12; 0.38) | 0.162 | |
| General Ward | PD | 53.3 | 50.4 | 47.9 | 48.7 | 47.8 | 48.7 | 49.0 | −4.2 | −8.0 | −1.30 (−1.85; −0.75) | <0.001 |
| AD | 264.1 | 249.8 | 232.6 | 230.4 | 223.7 | 230.5 | 231.2 | −32.9 | −12.5 | −0.14 (−0.23; −0.05) | 0.003 | |
| Pre-Pandemic Phase | Pandemic Phase | Transition Phase | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2017–2019 | 2020–2021 | 2022–2023 | |||||||||||
| Diff a 17–19 | Change (%) | Trend | p-Value | Diff 19−21 | Change (%) | Change of Trend b | p-Value | Diff 21–23 | Change (%) | Change of Trend c | p-Value | ||
| Whole hospital | PD d | −5.3 | −9.2 | −0.69 (−0.88; −0.50) | <0.001 | 0.5 | 1.0 | 0.56 (1.03; 0.09) | 0.019 | 0.7 | 1.3 | 0.21 (0.80; −0.37) | 0.630 |
| AD d | −31 | −11.2 | −4.05 (−5.16; −2.94) | <0.001 | −3.9 | −1.6 | 3.02 (5.80; 0.23) | 0.032 | 2.6 | 1.1 | 1.19 (4.62; −2.23) | 0.656 | |
| ICU e | PD | −3.6 | −3.4 | −0.50 (−1.13; 0.14) | 0.119 | 0.2 | 0.2 | 0.66 (2.25; −0.93) | 0.550 | 1.0 | 1.0 | −0.18 (1.77; −2.14) | 0.970 |
| AD | −26.8 | −5.7 | −3.54 (−6.71; −0.37) | 0.031 | 49.7 | 11.3 | 8.51 (16.46; 0.57) | 0.035 | −60.4 | −12.3 | −10.68 (−0.90; −20.47) | 0.031 | |
| General Ward | PD | −31.4 | −11.9 | −0.70 (−0.88; −0.53) | <0.001 | −8.9 | −3.8 | 0.54 (0.98; 0.10) | 0.014 | 7.4 | 3.3 | 0.30 (0.84; −0.25) | 0.368 |
| AD | −5.4 | −10.1 | −4.11 (−5.25; −2.97) | <0.001 | −0.1 | −0.2 | 2.72 (5.57; −0.13) | 0.062 | 1.2 | 2.5 | 2.05 (5.55; −1.46) | 0.321 | |
| Pre-Pandemic Phase | Pandemic Phase | Transition Phase | Difference | Difference | ||||
|---|---|---|---|---|---|---|---|---|
| 2017–2019 | 2020–2021 | 2022–2023 | Pre-Pandemic—Pandemic | Pandemic—Transition | ||||
| Mean Value | Mean Value | Mean Value | Difference | Difference | ||||
| (95%CI) | (95%CI) | (95%CI) | (95%CI) | p-Value | (95%CI) | p-Value | ||
| Whole hospital | PD a | 54.3 (53.7; 55.0) | 52.7 (51.9; 53.5) | 52.9 (52.2; 53.7) | −1.6 (−0.62; −2.63) | 0.003 | 0.2 (1.32; −0.87) | 0.673 |
| AD a | 262.2 (258.5; 265.9) | 244.8 (240.2; 249.4) | 245.4 (240.8; 250.0) | −17.42 (−11.52; −23.32) | <0.001 | 0.60 (7.07; −5.86) | 0.847 | |
| ICU b | PD | 105.5 (103.4; 107.7) | 104.2 (101.6; 106.8) | 104.8 (102.2; 107.4) | −1.34 (2.03; −4.71) | 0.415 | 0.59 (4.28; −3.10) | 0.742 |
| AD | 460.5 (449.9; 471.2) | 482.4 (469.3; 495.4) | 442.6 (429.5; 455.6) | 21.83 (38.69; 4.97) | 0.014 | −39.77 (−21.30; −58.24) | <0.001 | |
| General Ward | PD | 50.5 (49.9; 51.1) | 48.3 (47.5; 49.0) | 48.9 (48.2; 49.6) | −2.23 (−1.29; −3.16) | <0.001 | 0.62 (1.64; −0.41) | 0.222 |
| AD | 248.7 (244.9; 252.6) | 227.0 (222.3; 231.6) | 230.9 (226.2; 235.5) | −21.78 (−15.74; −27.82) | <0.001 | 3.90 (10.51; −2.71) | 0.202 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schweickert, B.; Willrich, N.; Feig, M.; Schneider, M.; Behnke, M.; Peña Diaz, L.A.; Geffers, C.; Wieters, I.; Gröschner, K.; Richter, D.; et al. Kinetic Patterns of Antibiotic Consumption in German Acute Care Hospitals from 2017 to 2023. Antibiotics 2025, 14, 316. https://doi.org/10.3390/antibiotics14030316
Schweickert B, Willrich N, Feig M, Schneider M, Behnke M, Peña Diaz LA, Geffers C, Wieters I, Gröschner K, Richter D, et al. Kinetic Patterns of Antibiotic Consumption in German Acute Care Hospitals from 2017 to 2023. Antibiotics. 2025; 14(3):316. https://doi.org/10.3390/antibiotics14030316
Chicago/Turabian StyleSchweickert, Birgitta, Niklas Willrich, Marcel Feig, Marc Schneider, Michael Behnke, Luis Alberto Peña Diaz, Christine Geffers, Imke Wieters, Karin Gröschner, Doreen Richter, and et al. 2025. "Kinetic Patterns of Antibiotic Consumption in German Acute Care Hospitals from 2017 to 2023" Antibiotics 14, no. 3: 316. https://doi.org/10.3390/antibiotics14030316
APA StyleSchweickert, B., Willrich, N., Feig, M., Schneider, M., Behnke, M., Peña Diaz, L. A., Geffers, C., Wieters, I., Gröschner, K., Richter, D., Hoffmann, A., Eckmanns, T., & Abu Sin, M. (2025). Kinetic Patterns of Antibiotic Consumption in German Acute Care Hospitals from 2017 to 2023. Antibiotics, 14(3), 316. https://doi.org/10.3390/antibiotics14030316






