Antimicrobial Susceptibility Profiles of Commensal Enterococcus spp. Isolates from Turkeys in Hungarian Poultry Farms Between 2022 and 2023
Abstract
:1. Introduction
2. Results
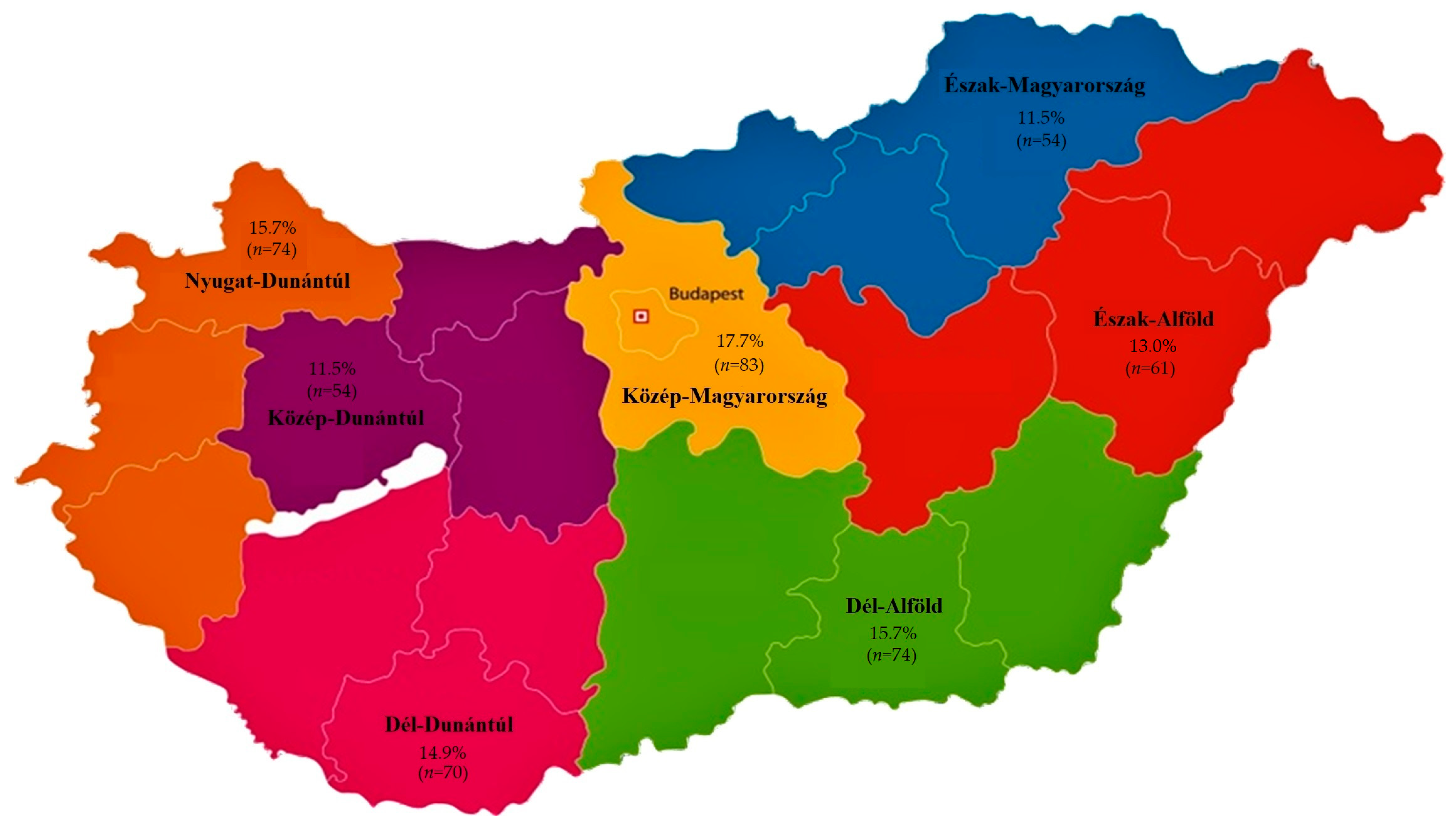
2.1. Regional Distribution and Origin of Samples
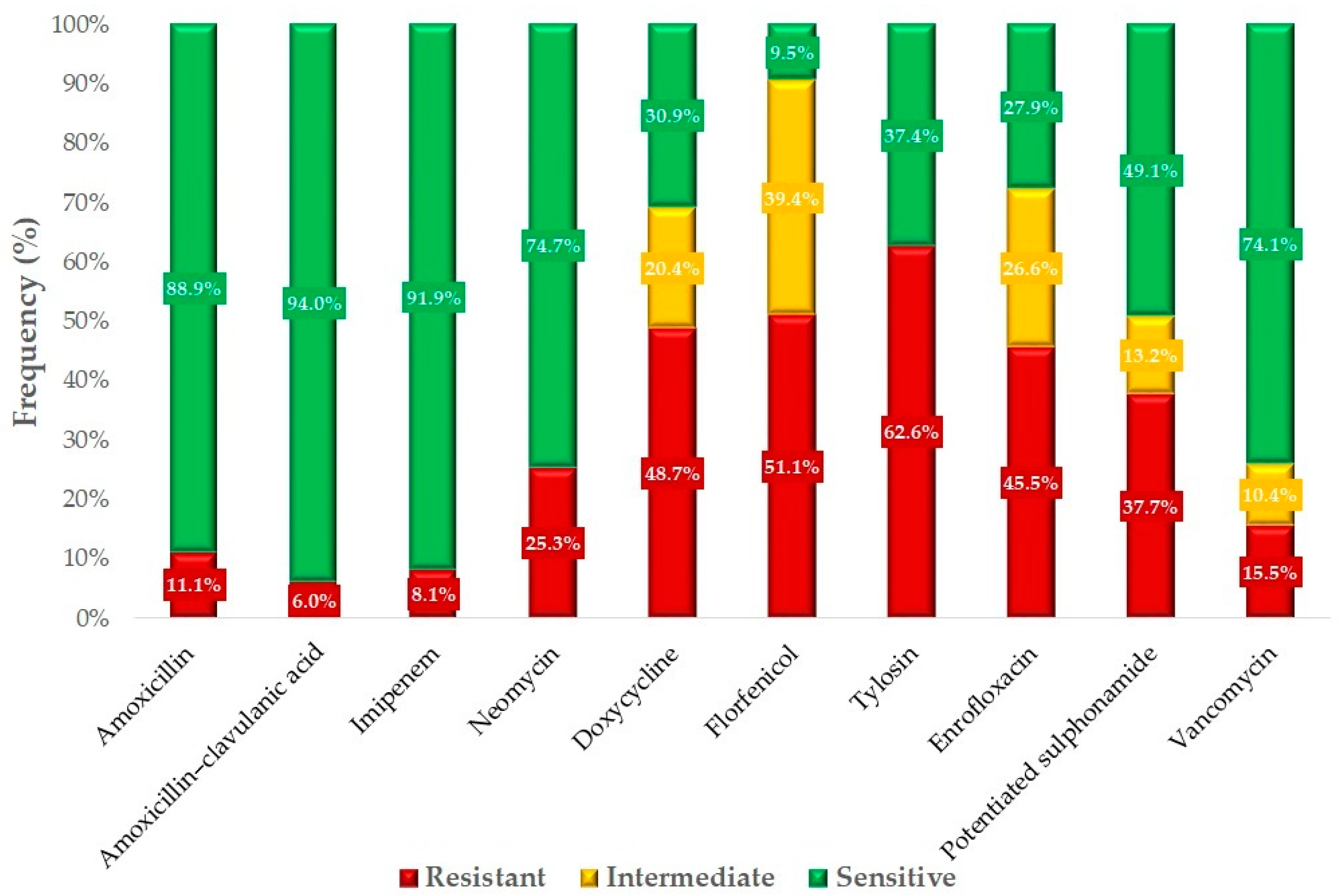
2.2. Antimicrobial Susceptibility Testing
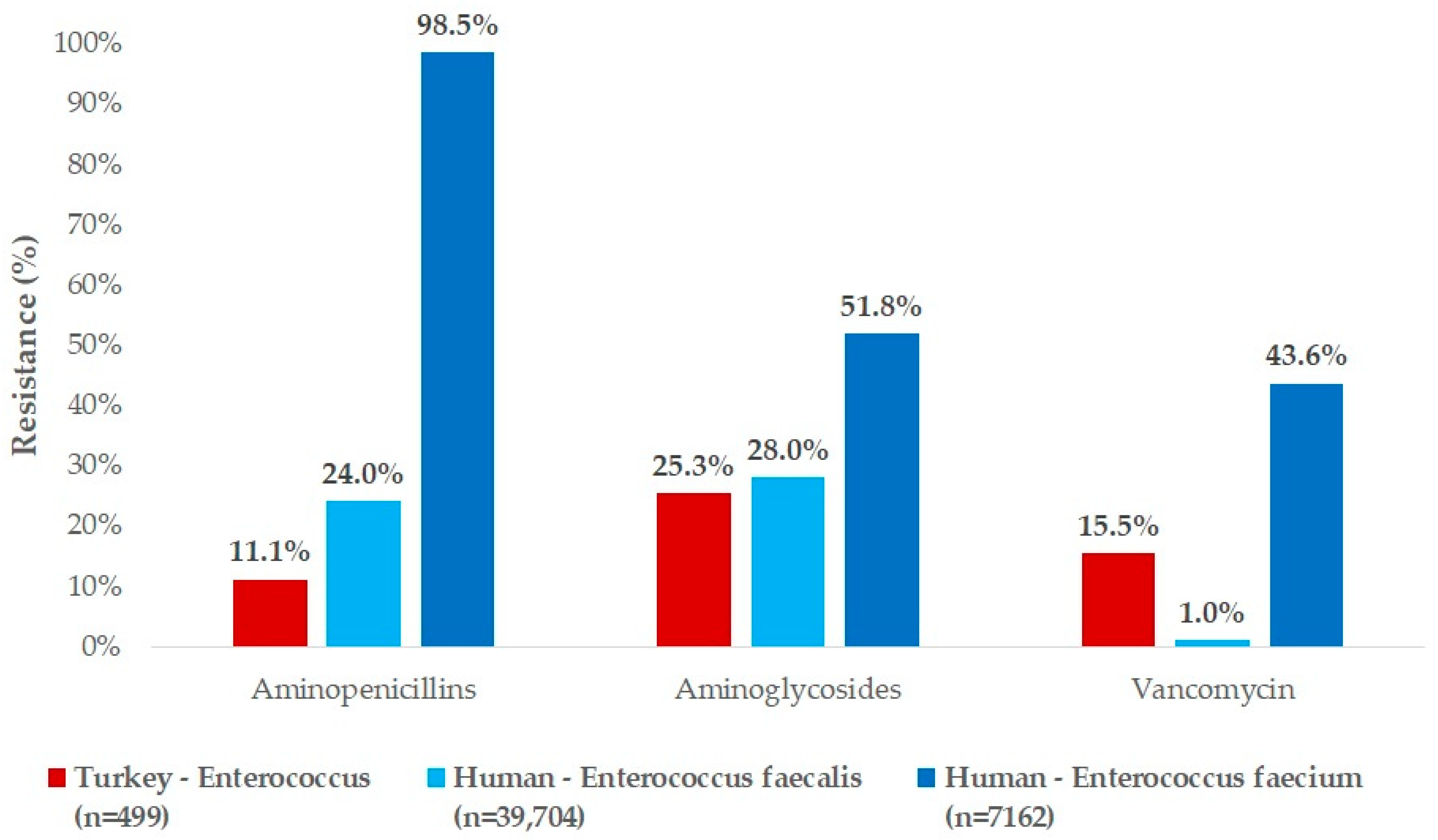
3. Discussion
4. Materials and Methods
4.1. The Origin of Samples and Human Data
4.2. Minimum Inhibitory Concentration (MIC) Determination
4.3. The Determination of Epidemiological Cut-Off Values
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2020/2021. EFSA J. 2023, 21, e07867. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Report from the Task Force on Zoonoses Data Collection Including Guidance for Harmonized Monitoring and Reporting of Antimicrobial Resistance in Commensal Escherichia coli and Enterococcus spp. from Food Animals. EFSA J. 2008, 6, 141r. [Google Scholar] [CrossRef]
- Miller, W.R.; Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance in Enterococci. Expert Rev. Anti-Infect. Ther. 2014, 12, 1221–1236. [Google Scholar] [CrossRef]
- Lebreton, F.; Willems, R.J.L.; Gilmore, M.S. Enterococcus Diversity, Origins in Nature, and Gut Colonization. In Enterococci: From Commensals to Leading Causes of Drug Resistant Infection; Massachusetts Eye and Ear Infirmary: Boston, MA, USA, 2014. [Google Scholar]
- Ramos, S.; Silva, V.; Dapkevicius, M.d.L.E.; Igrejas, G.; Poeta, P. Enterococci, from Harmless Bacteria to a Pathogen. Microorganisms 2020, 8, 1118. [Google Scholar] [CrossRef]
- Prabaker, K.; Weinstein, R.A. Trends in Antimicrobial Resistance in Intensive Care Units in the United States. Curr. Opin. Crit. Care 2011, 17, 472–479. [Google Scholar] [CrossRef]
- Werner, G.; Coque, T.M.; Hammerum, A.M.; Hope, R.; Hryniewicz, W.; Johnson, A.; Klare, I.; Kristinsson, K.G.; Leclercq, R.; Lester, C.H.; et al. Emergence and Spread of Vancomycin Resistance among Enterococci in Europe. Euro Surveill 2008, 13, 19046. [Google Scholar]
- Love, R.M. Enterococcus faecalis—A Mechanism for Its Role in Endodontic Failure. Int. Endod. J. 2001, 34, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Schleifer, K.H.; Kilpper-Bälz, R. Transfer of Streptococcus faecalis and Streptococcus faecium to the Genus Enterococcus Nom. Rev. as Enterococcus faecalis Comb. Nov. and Enterococcus faecium Comb. Nov. Int. J. Syst. Evol. Microbiol. 1984, 34, 31–34. [Google Scholar] [CrossRef]
- Paulsen, I.T.; Banerjei, L.; Myers, G.S.A.; Nelson, K.E.; Seshadri, R.; Read, T.D.; Fouts, D.E.; Eisen, J.A.; Gill, S.R.; Heidelberg, J.F.; et al. Role of Mobile DNA in the Evolution of Vancomycin-Resistant Enterococcus faecalis. Science 2003, 299, 2071–2074. [Google Scholar] [CrossRef]
- Huycke, M.M.; Sahm, D.F.; Gilmore, M.S. Multiple-Drug Resistant Enterococci: The Nature of the Problem and an Agenda for the Future. Emerg. Infect. Dis. 1998, 4, 239–249. [Google Scholar] [CrossRef]
- Gilmore, M.S.; Lebreton, F.; van Schaik, W. Genomic Transition of Enterococci from Gut Commensals to Leading Causes of Multidrug-Resistant Hospital Infection in the Antibiotic Era. Curr. Opin. Microbiol. 2013, 16, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.; Alonso, C.A.; Ruiz-Ripa, L.; León-Sampedro, R.; Del Campo, R.; Coque, T.M. Antimicrobial Resistance in Enterococcus spp. of Animal Origin. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Ghosh, A.; Zurek, L. Chapter 9—Antibiotic Resistance in Enterococci: A Food Safety Perspective. In Antimicrobial Resistance and Food Safety; Chen, C.-Y., Yan, X., Jackson, C.R., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 155–180. ISBN 978-0-12-801214-7. [Google Scholar]
- Aarestrup, F.M.; Butaye, P.; Witte, W. Nonhuman Reservoirs of Enterococci. In The Enterococci; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2002; pp. 55–99. ISBN 978-1-68367-230-2. [Google Scholar]
- Farkas, M.; Könyves, L.; Csorba, S.; Farkas, Z.; Józwiák, Á.; Süth, M.; Kovács, L. Biosecurity Situation of Large-Scale Poultry Farms in Hungary According to the Databases of National Food Chain Safety Office Centre for Disease Control and Biosecurity Audit System of Poultry Product Board of Hungary in the Period of 2021–2022. Magy. Állatorvosok Lapja 2024, 146, 723–742. [Google Scholar] [CrossRef]
- Palmer, K.L.; Kos, V.N.; Gilmore, M.S. Horizontal Gene Transfer and the Genomics of Enterococcal Antibiotic Resistance. Curr. Opin. Microbiol. 2010, 13, 632–639. [Google Scholar] [CrossRef]
- Benmazouz, I.; Kövér, L.; Kardos, G. The Rise of Antimicrobial Resistance in Wild Birds: Potential AMR Sources and Wild Birds as AMR Reservoirs and Disseminators: Literature Review. Magy. Állatorvosok Lapja 2024, 146, 91–105. [Google Scholar] [CrossRef]
- Sharma, P.; Gupta, S.K.; Barrett, J.B.; Hiott, L.M.; Woodley, T.A.; Kariyawasam, S.; Frye, J.G.; Jackson, C.R. Comparison of Antimicrobial Resistance and Pan-Genome of Clinical and Non-Clinical Enterococcus cecorum from Poultry Using Whole-Genome Sequencing. Foods 2020, 9, 686. [Google Scholar] [CrossRef]
- Jócsák, G.; Schilling-Tóth, B.; Bartha, T.; Tóth, I.; Ondrašovičová, S.; Kiss, D.S. Metal Nanoparticles—Immersion in the “tiny” World of Medicine. Magy. Állatorvosok Lapja 2025, 147, 115–127. [Google Scholar] [CrossRef]
- Sebők, C.; Márton, R.A.; Meckei, M.; Neogrády, Z.; Mátis, G. Antimicrobial Peptides as New Tools to Combat Infectious Diseases. Magy. Állatorvosok Lapja 2024, 146, 181–191. [Google Scholar] [CrossRef]
- Hetényi, N.; Bersényi, A.; Hullár, I. Physiological Effects of Medium-Chain Fatty Acids and Triglycerides, and Their Potential Use in Poultry and Swine Nutrition: A Literature Review. Magy. Állatorvosok Lapja 2024, 146, 651–659. [Google Scholar] [CrossRef]
- Kovács, L.; Nagy, D.; Könyves, L.; Jerzsele, Á.; Kerek, Á. Antimicrobial Properties of Essential Oils—Animal Health Aspects. Magy. Állatorvosok Lapja 2023, 145, 497–510. [Google Scholar] [CrossRef]
- Pomothy, J.M.; Barna, R.F.; Gere, E. The Effects of the Rosmarinic Acid in Livestock Animals: Literature Review. Magy. Állatorvosok Lapja 2020, 142, 567–576. [Google Scholar]
- Kerek, Á.; Szabó, Á.; Dobra, P.F.; Bárdos, K.; Ózsvári, L.; Fehérvári, P.; Bata, Z.; Molnár-Nagy, V.; Jerzsele, Á. Determining the In Vivo Efficacy of Plant-Based and Probiotic-Based Antibiotic Alternatives against Mixed Infection with Salmonella enterica and Escherichia coli in Domestic Chickens. Vet. Sci. 2023, 10, 706. [Google Scholar] [CrossRef]
- Jerzsele, Á.; Somogyi, Z.; Szalai, M.; Kovács, D. Effects of Fermented Wheat Germ Extract on Artificial Salmonella Typhimurium Infection in Broiler Chickens. Magy. Állatorvosok Lapja 2020, 142, 77–85. [Google Scholar]
- Olasz, Á.; Jerzsele, Á.; Balta, L.; Dobra, P.F.; Kerek, Á. In Vivo Efficacy of Different Extracts of Propolis in Broiler Salmonellosis. Magy. Állatorvosok Lapja 2023, 145, 461–475. [Google Scholar] [CrossRef]
- Kerek, Á.; Csanády, P.; Jerzsele, Á. Antibacterial Efficiency of Propolis—Part 1. Magy. Állatorvosok Lapja 2022, 144, 285–298. [Google Scholar]
- Kerek, Á.; Csanády, P.; Tuska-Szalay, B.; Kovács, L.; Jerzsele, Á. In Vitro Efficacy of Hungarian Propolis against Bacteria, Yeast, and Trichomonas gallinae Isolated from Pigeons—A Possible Antibiotic Alternative? Resources 2023, 12, 101. [Google Scholar] [CrossRef]
- Kovács, D.; Palkovicsné Pézsa, N.; Farkas, O.; Jerzsele, Á. Usage of Antibiotic Alternatives in Pig Farming: Literature Review. Magy. Állatorvosok Lapja 2021, 143, 281–282. [Google Scholar]
- Essősy, M.; Fodor, I.; Ihnáth, Z.; Karancsi, Z.; Kovács, D.; Szalai, K.V.; Szentmiklósi, D.; Jerzsele, Á. The Possibilities of Antibiotic-Free Broiler-Hen Fattening, with Special Reference to the Use of Pre- and Probiotics. Magy. Állatorvosok Lapja 2020, 142, 397–407. [Google Scholar]
- Mag, P.; Németh, K.; Somogyi, Z.; Jerzsele, Á. Antibacterial therapy based on pharmacokinetic/pharmacodynamic models in small animal medicine-1. Literature review. Magy. Állatorvosok Lapja 2023, 145, 419–438. [Google Scholar] [CrossRef]
- Jung, A.; Rautenschlein, S. Comprehensive Report of an Enterococcus cecorum infection in a Broiler Flock in Northern Germany. BMC Vet. Res. 2014, 10, 311. [Google Scholar] [CrossRef]
- Borst, L.B.; Suyemoto, M.M.; Sarsour, A.H.; Harris, M.C.; Martin, M.P.; Strickland, J.D.; Oviedo, E.O.; Barnes, H.J. Pathogenesis of Enterococcal Spondylitis Caused by Enterococcus cecorum in Broiler Chickens. Vet. Pathol. 2017, 54, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Jung, A.; Chen, L.R.; Suyemoto, M.M.; Barnes, H.J.; Borst, L.B. A Review of Enterococcus cecorum Infection in Poultry. Avian Dis. 2018, 62, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Herdt, P.; Defoort, P.; Steelant, J.; Swam, H.; Tanghe, L.; Goethem, S.V.; Vanrobaeys, M. Enterococcus cecorum Osteomyelitis and Arthritis in Broiler Chickens. Vlaams Diergeneeskd. Tijdschr. 2009, 78, 44–48. [Google Scholar]
- Wilson, G.; Horsey, B.; Stone, R. Implementing Concepts from Green Logistics in the Turkey Production Supply Chain. Front. Sustain. 2024, 5, 1416964. [Google Scholar] [CrossRef]
- Semin, A.; Sharapova, V.; Kondratenko, I.; Talu, S. Prospects for the Development of Poultry Production in the Domestic Poultry Subcomplex; Atlantis Press: Dordrecht, The Netherlands, 2021; pp. 508–511. [Google Scholar]
- Kálmán, Á.; Szőllősi, L. Global Tendencies in Turkey Meat Production, Trade and Consumption. Acta Agrar. Debr. 2023, 83–89. [Google Scholar] [CrossRef]
- AVEC. 2022 Annual Report; AVEC—Association of Poultry Processors and Poultry Trade in the EU: Brussels, Belgium. 2022. Available online: https://avec-poultry.eu/resources/annual-reports/2022-annual-report/ (accessed on 16 March 2025).
- Központi Statisztikai Hivatal Magyar Statisztikai Évkönyv; XXIV; Központi Statisztikai Hivatal (KSH): Budapest, Hungary, 2023; ISBN 9771215786409.
- Kasza, G.; Oláh, J.; Popp, J.; Lakner, Z.; Fekete, L.; Pósa, E.; Nugraha, W.S.; Szakos, D. Food Miles on the Shelves: The Share of Local Food Products in the Hungarian Retail Sector. Agric. Food Econ. 2024, 12, 3. [Google Scholar] [CrossRef]
- Mertens, E.; Colizzi, C.; Peñalvo, J.L. Ultra-Processed Food Consumption in Adults across Europe. Eur. J. Nutr. 2022, 61, 1521–1539. [Google Scholar] [CrossRef]
- Török, Á.; Yeh, C.-H.; Menozzi, D.; Balogh, P.; Czine, P. Consumers’ Preferences for Processed Meat: A Best–Worst Scaling Approach in Three European Countries. Agric. Food Econ. 2023, 11, 33. [Google Scholar] [CrossRef]
- Nielsen, S.S.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; Gortazar Schmidt, C.; Herskin, M.; Michel, V.; et al. Assessment of Animal Diseases Caused by Bacteria Resistant to Antimicrobials: Poultry. EFSA J. 2021, 19, e07114. [Google Scholar] [CrossRef]
- Rehman, M.A.; Yin, X.; Zaheer, R.; Goji, N.; Amoako, K.K.; McAllister, T.; Pritchard, J.; Topp, E.; Diarra, M.S. Genotypes and Phenotypes of Enterococci Isolated From Broiler Chickens. Front. Sustain. Food Syst. 2018, 2, 83. [Google Scholar] [CrossRef]
- Woźniak-Biel, A.; Bugla-Płoskońska, G.; Burdzy, J.; Korzekwa, K.; Ploch, S.; Wieliczko, A. Antimicrobial Resistance and Biofilm Formation in Enterococcus spp. Isolated from Humans and Turkeys in Poland. Microb. Drug Resist. 2019, 25, 277–286. [Google Scholar] [CrossRef]
- Kempf, I.; Le Roux, A.; Perrin-Guyomard, A.; Mourand, G.; Le Devendec, L.; Bougeard, S.; Richez, P.; Le Pottier, G.; Eterradossi, N. Effect of In-Feed Paromomycin Supplementation on Antimicrobial Resistance of Enteric Bacteria in Turkeys. Vet. J. 2013, 198, 398–403. [Google Scholar] [CrossRef]
- Makarov, D.A.; Ivanova, O.E.; Pomazkova, A.V.; Egoreva, M.A.; Prasolova, O.V.; Lenev, S.V.; Gergel, M.A.; Bukova, N.K.; Karabanov, S.Y. Antimicrobial Resistance of Commensal Enterococcus faecalis and Enterococcus faecium from Food-Producing Animals in Russia. Vet. World 2022, 15, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Boulianne, M.; Arsenault, J.; Daignault, D.; Archambault, M.; Letellier, A.; Dutil, L. Drug Use and Antimicrobial Resistance among Escherichia coli and Enterococcus spp. Isolates from Chicken and Turkey Flocks Slaughtered in Quebec, Canada. Can. J. Vet. Res. 2016, 80, 49–59. [Google Scholar]
- Roy, K.; Islam, M.S.; Paul, A.; Ievy, S.; Talukder, M.; Sobur, M.A.; Ballah, F.M.; Khan, M.S.R.; Rahman, M.T. Molecular Detection and Antibiotyping of Multi-Drug Resistant Enterococcus faecium from Healthy Broiler Chickens in Bangladesh. Vet. Med. Sci. 2022, 8, 200–210. [Google Scholar] [CrossRef]
- Schwaiger, K.; Schmied, E.-M.V.; Bauer, J. Comparative Analysis on Antibiotic Resistance Characteristics of Listeria spp. and Enterococcus spp. Isolated from Laying Hens and Eggs in Conventional and Organic Keeping Systems in Bavaria, Germany. Zoonoses Public Health 2010, 57, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Osman, K.M.; Badr, J.; Orabi, A.; Elbehiry, A.; Saad, A.; Ibrahim, M.D.S.; Hanafy, M.H. Poultry as a Vector for Emerging Multidrug Resistant Enterococcus spp.: First Report of Vancomycin (van) and the Chloramphenicol-Florfenicol (Cat-Fex-Cfr) Resistance Genes from Pigeon and Duck Faeces. Microb. Pathog. 2019, 128, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.E.; Fleige, C.; Layer, F.; Neumann, B.; Kresken, M.; Werner, G. Determination of a Tentative Epidemiological Cut-Off Value (ECOFF) for Dalbavancin and Enterococcus faecium. Antibiotics 2021, 10, 915. [Google Scholar] [CrossRef]
- Agersø, Y.; Torpdahl, M.; Zachariasen, C.; Seyfarth, A.; Hammerum, A.M.; Nielsen, E.M. Tentative Colistin Epidemiological Cut-off Value for Salmonella spp. Foodborne Pathog. Dis. 2012, 9, 367–369. [Google Scholar] [CrossRef]
- Laurentie, J.; Mourand, G.; Grippon, P.; Furlan, S.; Chauvin, C.; Jouy, E.; Serror, P.; Kempf, I. Determination of Epidemiological Cutoff Values for Antimicrobial Resistance of Enterococcus cecorum. J. Clin. Microbiol. 2023, 61, e0144522. [Google Scholar] [CrossRef]
- Barnácz, F.; Kerek, Á.; Csirmaz, B.; Román, I.L.; Gál, C.; Horváth, Á.; Hajduk, E.; Szabó, Á.; Jerzsele, Á.; Kovács, L. The Status of Antimicrobial Resistance in Domestic Poultry with Different Breeding Purposes in Hungary between 2022–2023. Magy. Állatorvosok Lapja 2024, 146, 339–356. [Google Scholar] [CrossRef]
- Sulayyim, H.J.A.; Ismail, R.; Hamid, A.A.; Ghafar, N.A. Antibiotic Resistance during COVID-19: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 11931. [Google Scholar] [CrossRef] [PubMed]
- Zacharopoulos, G.V.; Manios, G.A.; Papadakis, M.; Koumaki, D.; Maraki, S.; Kassotakis, D.; De Bree, E.; Manios, A. Comparative Activities of Ampicillin and Teicoplanin against Enterococcus faecalis Isolates. BMC Microbiol. 2023, 23, 5. [Google Scholar] [CrossRef]
- Billström, H.; Lund, B.; Sullivan, Å.; Nord, C.E. Virulence and Antimicrobial Resistance in Clinical Enterococcus Faecium. Int. J. Antimicrob. Agents 2008, 32, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Taji, A.; Heidari, H.; Ebrahim-Saraie, H.S.; Sarvari, J.; Motamedifar, M. High Prevalence of Vancomycin and High-Level Gentamicin Resistance in Enterococcus faecalis Isolates. Acta Microbiol. Immunol. Hung. 2019, 66, 203–217. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, F.; Shiely, F.; Byrne, S.; O’Brien, D.; Ronayne, A.; Fleming, A. Antimicrobial Use and Antimicrobial Resistance in Enterobacterales and Enterococcus faecium: A Time Series Analysis. J. Hosp. Infect. 2022, 120, 57–64. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; Volume CLSI standards M07. [Google Scholar]
- Brian, V.L. VET01SEd5; Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated From Animals. 5th ed. The Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2024. Available online: https://clsi.org/standards/products/veterinary-medicine/documents/vet01s/ (accessed on 8 May 2022).
- Jahan, M.; Krause, D.O.; Holley, R.A. Antimicrobial Resistance of Enterococcus Species from Meat and Fermented Meat Products Isolated by a PCR-Based Rapid Screening Method. Int. J. Food Microbiol. 2013, 163, 89–95. [Google Scholar] [CrossRef]
- Bywater, R.; McConville, M.; Phillips, I.; Shryock, T. The Susceptibility to Growth-Promoting Antibiotics of Enterococcus faecium Isolates from Pigs and Chickens in Europe. J. Antimicrob. Chemother. 2005, 56, 538–543. [Google Scholar] [CrossRef]
- Turnidge, J.; Kahlmeter, G.; Kronvall, G. Statistical Characterisation of Bacterial Wild-Type MIC Value Distributions and the Determination of Epidemiological Cut-off Values. Clin. Microbiol. Infect. 2006, 12, 418–425. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Fay, M.P.; Proschan, M.A. Wilcoxon-Mann-Whitney or t-test? On Assumptions for Hypothesis Tests and Multiple Interpretations of Decision Rules. Stat. Surv. 2010, 4, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Dunn, O.J. Multiple Comparisons among Means. J. Am. Stat. Assoc. 1961, 56, 52–64. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal Component Analysis: A Review and Recent Developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Sibson, R. SLINK: An Optimally Efficient Algorithm for the Single-Link Cluster Method. Comput. J. 1973, 16, 30–34. [Google Scholar] [CrossRef]



| Antibiotics | Respiratory–Cloacal | Meat–Breeding | 3 Young–4 Adult | 5 Small–6 Medium |
|---|---|---|---|---|
| p-Values | ||||
| Doxycycline | 0.0020 * | 0.2439 | 0.2439 | 0.7508 |
| Vancomycin | 0.2667 | <0.0001 * | <0.0001 * | 0.0031 * |
| Enrofloxacin | 0.4012 | 0.2107 | 0.2107 | <0.0001 * |
| 1 Amoxicillin–clavulanic acid | 0.6169 | 0.0019 * | 0.0019 * | 0.1105 |
| Florfenicol | 0.6949 | 0.0063 * | 0.0063 * | 0.5336 |
| 2 Potentiated sulfonamide | 0.1269 | 0.1423 | 0.1423 | <0.0001 * |
| Amoxicillin | 0.6966 | <0.0001 * | <0.0001 * | 0.0294 * |
| Imipenem | 0.0887 * | 0.0109 * | 0.0109 * | 0.0106 * |
| Neomycin | 0.4533 | <0.0001 * | <0.0001 * | <0.0001 * |
| Tylosin | 0.1241 | 0.0001 * | 0.0001 * | 0.2714 |
| Antibiotic | 1 BP * | 0.001 | 0.002 | 0.004 | 0.008 | 0.016 | 0.031 | 0.063 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | 1024 | MIC50 | MIC90 | 2 ECOFF | 3 T |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| µg/mL | µg/mL | |||||||||||||||||||||||||
| Enrofloxacin | 1 4 | 1 | 7 | 15 | 3 | 2 | 21 | 82 | 91 | 34 | 61 | 47 | 31 | 24 | 27 | 11 | 8 | 3 | 2 | 2 | 64 | - | 64 | |||
| 0.2% | 1.5% | 3.2% | 0.6% | 0.4% | 4.5% | 17.4% | 19.4% | 7.2% | 13.0% | 10.0% | 6.6% | 5.1% | 5.7% | 2.3% | 1.7% | 0.6% | 0.4% | |||||||||
| 4 Potentiated sulfonamide | 1 4 | 1 | 3 | 20 | 44 | 38 | 53 | 72 | 42 | 20 | 8 | 19 | 13 | 59 | 78 | 1 | 16 | - | - | |||||||
| 0.2% | 0.6% | 4.3% | 9.4% | 8.1% | 11.3% | 15.3% | 8.9% | 4.3% | 1.7% | 4.0% | 2.8% | 12.6% | 16.6% | |||||||||||||
| Tylosin | 8 | 1 | 0 | 2 | 2 | 4 | 71 | 72 | 24 | 13 | 7 | 1 | 3 | 9 | 26 | 74 | 161 | 256 | 1024 | - | - | |||||
| 0.2% | 0.0% | 0.4% | 0.4% | 0.9% | 15.1% | 15.3% | 5.1% | 2.8% | 1.5% | 0.2% | 0.6% | 1.9% | 5.5% | 15.7% | 34.3% | |||||||||||
| Florfenicol | 1 8 | 2 | 6 | 37 | 185 | 141 | 46 | 12 | 24 | 16 | 0 | 1 | 8 | 32 | 8 | 16 | ||||||||||
| 0.4% | 1.3% | 7.9% | 39.4% | 30.0% | 9.8% | 2.6% | 5.1% | 3.4% | 0.0% | 0.2% | ||||||||||||||||
| Doxycycline | 1 16 | 1 | 2 | 0 | 17 | 9 | 2 | 37 | 25 | 52 | 96 | 126 | 64 | 39 | 8 | 32 | 1 | - | ||||||||
| 0.2% | 0.4% | 0.0% | 3.6% | 1.9% | 0.4% | 7.9% | 5.3% | 11.1% | 20.4% | 26.8% | 13.6% | 8.3% | ||||||||||||||
| Amoxicillin | 1 16 | 1 | 0 | 0 | 1 | 3 | 4 | 4 | 20 | 49 | 96 | 132 | 59 | 38 | 11 | 4 | 6 | 3 | 6 | 12 | 10 | 11 | 1 | 32 | - | 8 |
| 0.2% | 0.0% | 0.0% | 0.2% | 0.6% | 0.9% | 0.9% | 4.3% | 10.4% | 20.4% | 28.1% | 12.6% | 8.1% | 2.3% | 0.9% | 1.3% | 0.6% | 1.3% | 2.6% | 2.1% | 2.3% | ||||||
| Imipenem | 1 16 | 15 | 0 | 1 | 9 | 7 | 6 | 8 | 6 | 20 | 78 | 126 | 85 | 40 | 31 | 24 | 3 | 1 | 6 | 1 | 2 | 1 | 1 | 8 | 4 | 32 |
| 3.2% | 0.0% | 0.2% | 1.9% | 1.5% | 1.3% | 1.7% | 1.3% | 4.3% | 16.6% | 26.8% | 18.1% | 8.5% | 6.6% | 5.1% | 0.6% | 0.2% | 1.3% | 0.2% | 0.4% | 0.2% | ||||||
| 5 Amoxicillin–clavulanic acid | 1 16 | 2 | 0 | 0 | 1 | 3 | 5 | 3 | 14 | 43 | 102 | 110 | 92 | 53 | 14 | 10 | 12 | 5 | 1 | 1 | 4 | - | 16 | |||
| 0.4% | 0.0% | 0.0% | 0.2% | 0.6% | 1.1% | 0.6% | 3.0% | 9.1% | 21.7% | 23.4% | 19.6% | 11.3% | 3.0% | 2.1% | 2.6% | 1.1% | 0.2% | |||||||||
| Vancomycin | 1 32 | 1 | 0 | 5 | 8 | 20 | 130 | 183 | 1 | 43 | 6 | 5 | 2 | 7 | 29 | 12 | 18 | 2 | 256 | 4 | 4 | |||||
| 0.2% | 0.0% | 1.1% | 1.7% | 4.3% | 27.7% | 38.9% | 0.2% | 9.1% | 1.3% | 1.1% | 0.4% | 1.5% | 6.2% | 2.6% | 3.8% | |||||||||||
| Neomycin | 1024 | 2 | 1 | 6 | 10 | 8 | 17 | 10 | 26 | 62 | 68 | 67 | 74 | 119 | 256 | 1024 | 256 | - | ||||||||
| 0.4% | 0.2% | 1.3% | 2.1% | 1.7% | 3.6% | 2.1% | 5.5% | 13.2% | 14.5% | 14.3% | 15.7% | 25.3% | ||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerek, Á.; Szabó, Á.; Barnácz, F.; Csirmaz, B.; Kovács, L.; Jerzsele, Á. Antimicrobial Susceptibility Profiles of Commensal Enterococcus spp. Isolates from Turkeys in Hungarian Poultry Farms Between 2022 and 2023. Antibiotics 2025, 14, 331. https://doi.org/10.3390/antibiotics14040331
Kerek Á, Szabó Á, Barnácz F, Csirmaz B, Kovács L, Jerzsele Á. Antimicrobial Susceptibility Profiles of Commensal Enterococcus spp. Isolates from Turkeys in Hungarian Poultry Farms Between 2022 and 2023. Antibiotics. 2025; 14(4):331. https://doi.org/10.3390/antibiotics14040331
Chicago/Turabian StyleKerek, Ádám, Ábel Szabó, Franciska Barnácz, Bence Csirmaz, László Kovács, and Ákos Jerzsele. 2025. "Antimicrobial Susceptibility Profiles of Commensal Enterococcus spp. Isolates from Turkeys in Hungarian Poultry Farms Between 2022 and 2023" Antibiotics 14, no. 4: 331. https://doi.org/10.3390/antibiotics14040331
APA StyleKerek, Á., Szabó, Á., Barnácz, F., Csirmaz, B., Kovács, L., & Jerzsele, Á. (2025). Antimicrobial Susceptibility Profiles of Commensal Enterococcus spp. Isolates from Turkeys in Hungarian Poultry Farms Between 2022 and 2023. Antibiotics, 14(4), 331. https://doi.org/10.3390/antibiotics14040331







