Potential Inhibitory Effect of the Peptide Melittin Purified from Apis mellifera Venom on CTX-M-Type Extended-Spectrum β-Lactamases of Escherichia coli
Abstract
:1. Introduction
2. Results
2.1. Protein Analysis of Melittin
2.2. Antimicrobial Activity of Melittin
2.3. Effect of Melittin on Hydrolytic Activity
2.4. Effect of Melittin on blaCTX-M Expression
3. Discussion
4. Methods
4.1. Bacterial Strains
4.2. Purification and Characterization of Melittin
4.3. Determination of the Minimum Inhibitory Concentration (MIC) of Melittin
4.4. Growth Curve Assay
4.5. Measurement of Hydrolytic Activity
4.6. Effect of Melittin on blaCTX-M RNA Expression
4.6.1. RNA Extraction
4.6.2. Quantitative Real-Time PCR (RT-qPCR)
4.6.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castanheira, M.; Simner, P.J.; Bradford, P.A. Extended-Spectrum β-Lactamases: An Update on Their Characteristics, Epidemiology and Detection. JAC-Antimicrob. Resist. 2021, 3, dlab092. [Google Scholar] [CrossRef]
- Husna, A.; Rahman, M.M.; Badruzzaman, A.T.M.; Sikder, M.H.; Islam, M.R.; Rahman, M.T.; Alam, J.; Ashour, H.M. Extended-Spectrum -Lactamases (ESBL): Challenges and Opportunities. Biomedicines 2023, 11, 2937. [Google Scholar] [CrossRef]
- Yu, K.; Huang, Z.; Xiao, Y.; Bai, X.; Gao, H.; Wang, D. Epidemiology and Molecular Characterization of CTX-M-Type ESBLs Producing Escherichia coli Isolated from Clinical Settings. J. Glob. Antimicrob. Resist. 2024, 36, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Cantón, R.; González-Alba, J.M.; Galán, J.C. CTX-M Enzymes: Origin and Diffusion. Front. Microbiol. 2012, 3, 110. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Health Organization Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Abodakpi, H.; Wanger, A.; Tam, V.H. What the Clinical Microbiologist Should Know About Pharmacokinetics/Pharmacodynamics in the Era of Emerging Multidrug Resistance: Focusing on β-Lactam/β-Lactamase Inhibitor Combinations. Clin. Lab. Med. 2019, 39, 473–485. [Google Scholar] [CrossRef]
- Brogden, K.A. Antimicrobial Peptides: Pore Formers or Metabolic Inhibitors in Bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Marcos, J.F.; Gandía, M. Antimicrobial Peptides: To Membranes and Beyond. Expert Opin. Drug Discov. 2009, 4, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Cresti, L.; Cappello, G.; Pini, A. Antimicrobial Peptides towards Clinical Application—A Long History to Be Concluded. Int. J. Mol. Sci. 2024, 25, 4870. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, M. The Success of Natural Products in Drug Discovery. Pharmacol. Pharm. 2013, 4, 17–31. [Google Scholar] [CrossRef]
- Ratcliffe, N.; Azambuja, P.; Mello, C.B. Recent Advances in Developing Insect Natural Products as Potential Modern Day Medicines. Evid. Based Complement. Altern. Med. 2014, 2014, 904958. [Google Scholar] [CrossRef] [PubMed]
- Pascoal, A.; Estevinho, M.M.; Choupina, A.B.; Sousa-Pimenta, M.; Estevinho, L.M. An Overview of the Bioactive Compounds, Therapeutic Properties and Toxic Effects of Apitoxin. Food Chem. Toxicol. 2019, 134, 110864. [Google Scholar] [CrossRef]
- Abd El-Wahed, A.A.; Khalifa, S.A.M.; Sheikh, B.Y.; Farag, M.A.; Saeed, A.; Larik, F.A.; Koca-Caliskan, U.; AlAjmi, M.F.; Hassan, M.; Wahabi, H.A.; et al. Bee Venom Composition: From Chemistry to Biological Activity. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; Volume 60, pp. 459–484. ISBN 9780444641816. [Google Scholar]
- Memariani, H.; Memariani, M.; Shahidi-Dadras, M.; Nasiri, S.; Akhavan, M.M.; Moravvej, H. Melittin: From Honeybees to Superbugs. Appl. Microbiol. Biotechnol. 2019, 103, 3265–3276. [Google Scholar] [CrossRef]
- Jamasbi, E.; Mularski, A.; Separovic, F. Model Membrane and Cell Studies of Antimicrobial Activity of Melittin Analogues. Curr. Top. Med. Chem. 2016, 16, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, A.; Domadia, P.N.; Bhattacharjya, S. Structural and Thermodynamic Analyses of the Interaction between Melittin and Lipopolysaccharide. Biochim. Biophys. Acta—Biomembr. 2007, 1768, 3282–3291. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Li, C.; Li, M.; Zong, X.; Han, D.; Chen, Y. Antimicrobial Peptide Melittin against Xanthomonas Oryzae Pv. Oryzae, the Bacterial Leaf Blight Pathogen in Rice. Appl. Microbiol. Biotechnol. 2016, 100, 5059–5067. [Google Scholar] [CrossRef]
- Park, C.; Lee, D.G. Melittin Induces Apoptotic Features in Candida albicans. Biochem. Biophys. Res. Commun. 2010, 394, 170–172. [Google Scholar] [CrossRef]
- Abarca, G.; Herrera, M.L. Betalactamasas: Su Importancia En La Clínica y Su Detección En El Laboratorio. Rev. Médica del Hosp. Nac. Niños Dr. Carlos Sáenz Herrera 2001, 36, 77–104. [Google Scholar]
- Mora, M.; Tripaldi, P.; Muñoz, G.; Piña, A.; Torres, K. Modelo in Silico Con Actividad Contra La Betalactamasa TEM-1 Presente En Escherichia Coli. Ateneo 2024, 25, 15–37. [Google Scholar]
- Xuan, J.; Feng, W.; Wang, J.; Wang, R.; Zhang, B.; Bo, L.; Chen, Z.S.; Yang, H.; Sun, L. Antimicrobial Peptides for Combating Drug-Resistant Bacterial Infections. Drug Resist. Updat. 2023, 68, 100954. [Google Scholar] [CrossRef] [PubMed]
- Al-Ani, I.; Zimmermann, S.; Reichling, J.; Wink, M. Pharmacological Synergism of Bee Venom and Melittin with Antibiotics and Plant Secondary Metabolites against Multi-Drug Resistant Microbial Pathogens. Phytomedicine 2015, 22, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Lowenstein, H.; Mulfinger, M. Methods and Compositions for the Treatment of Mammalian Infections Employing Medicaments Comprising Hymenoptera Venom, Proteinageous or Polypeptide Components Thereof, or Analogues of Such Proteinaceous or Polypeptide Components. U.S. Patent 2001/0021697 A1, 13 September 2001. [Google Scholar]
- Giacometti, A.; Cirioni, O.; Kamysz, W.; D’Amato, G.; Silvestri, C.; Del Prete, M.S.; Łukasiak, J.; Scalise, G. Comparative Activities of Cecropin A, Melittin, and Cecropin A-Melittin Peptide CA(1-7)M(2-9)NH2 against Multidrug-Resistant Nosocomial Isolates of Acinetobacter baumannii. Peptides 2003, 24, 1315–1318. [Google Scholar] [CrossRef] [PubMed]
- Akbari, R.; Hakemi-Vala, M.; Pashaie, F.; Bevalian, P.; Hashemi, A.; Bagheri, K.P. Highly Synergistic Effects of Melittin with Conventional Antibiotics against Multidrug-Resistant Isolates of Acinetobacter baumannii and Pseudomonas aeruginosa. Microb. Drug Resist. 2019, 25, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Bardbari, A.M.; Arabestani, M.R.; Karami, M.; Keramat, F.; Aghazadeh, H.; Alikhani, M.Y.; Bagheri, K.P. Highly Synergistic Activity of Melittin with Imipenem and Colistin in Biofilm Inhibition against Multidrug-Resistant Strong Biofilm Producer Strains of Acinetobacter baumannii. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Brown-Skrobot, S.; Novick, R.P.; Projan, S.J. Inhibition of Expression of Beta-Lactamase Using Esters of Fatty Acid Alcohols. U.S. Patent 5,466,685, 14 November 1995. [Google Scholar]
- Kaderabkova, N.; Bharathwaj, M.; Furniss, R.C.D.; Gonzalez, D.; Palmer, T.; Mavridou, D.A.I. The Biogenesis of β-Lactamase Enzymes. Microbiology 2022, 168, 001217. [Google Scholar] [CrossRef]
- Rangama, S.; Lidbury, I.D.E.A.; Holden, J.M.; Borsetto, C.; Murphy, A.R.J.; Hawkey, P.M.; Wellington, E.M.H. Mechanisms Involved in the Active Secretion of CTX-M-15 β-Lactamase by Pathogenic Escherichia coli ST131. Antimicrob. Agents Chemother. 2021, 65, e0066321. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.F.M.; Sani, A.A.; Zapata, T.B.; de Sousa, D.S.M.; Rossini, B.C.; dos Santos, L.D.; Rall, V.L.M.; Riccardi, C.d.S.; Fernandes Júnior, A. Synergistic Antibacterial Efficacy of Melittin in Combination with Oxacillin against Methicillin-Resistant Staphylococcus aureus (MRSA). Microorganisms 2023, 11, 2868. [Google Scholar] [CrossRef] [PubMed]
- Nehme, H.; Ayde, H.; El Obeid, D.; Sabatier, J.M.; Fajloun, Z. Potential Inhibitory Effect of Apis Mellifera’s Venom and of Its Two Main Components—Melittin and Pla2—On Escherichia coli F1f0-Atpase. Antibiotics 2020, 9, 824. [Google Scholar] [CrossRef] [PubMed]
- Mäntsälä, P.; Lehtinen, H. Secretion of β-Lactamase by Escherichia coli in Vivo and in Vitro: Effect of Cerulenin. Antonie Van Leeuwenhoek 1982, 48, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Queenan, A.M.; Foleno, B.; Gownley, C.; Wira, E.; Bush, K. Effects of Inoculum and β-Lactamase Activity in AmpC- and Extended-Spectrum β-Lactamase (ESBL)-Producing Escherichia coli and Klebsiella pneumoniae Clinical Isolates Tested by Using NCCLS ESBL Methodology. J. Clin. Microbiol. 2004, 42, 269–275. [Google Scholar] [CrossRef]
- Händel, N.; Otte, S.; Jonker, M.; Brul, S.; Kuile, B.H.T. Factors That Affect Transfer of the IncI1 β-Lactam Resistance Plasmid PESBL-283 between E. coli Strains. PLoS ONE 2015, 10, e0123039. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, T.S.B.; Overgaard, M.; Nielsen, S.S.; Bortolaia, V.; Jelsbak, L.; Sommer, M.; Guardabassi, L.; Olsen, J.E. CTX-M-1 β-Lactamase Expression in Escherichia coli Is Dependent on Cefotaxime Concentration, Growth Phase and Gene Location. J. Antimicrob. Chemother. 2015, 70, 62–70. [Google Scholar] [CrossRef]
- Andersson, D.I.; Hughes, D. Microbiological Effects of Sublethal Levels of Antibiotics. Nat. Rev. Microbiol. 2014, 12, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Al Musaimi, O.; Jaradat, D.M.M. Advances in Therapeutic Peptides Separation and Purification. Separations 2024, 11, 233. [Google Scholar] [CrossRef]
- Beaubier, S.; Przybylski, R.; Bodin, A.; Nedjar, N.; Dhulster, P.; Kapel, R. Ultrafiltration Fractionation of Bovine Hemoglobin Hydrolysates: Prediction of Separation Performances for Optimal Enrichment in Antimicrobial Peptide. Membranes 2021, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Simal-Gandara, J. Bee Venom: An Updating Review of Its Bioactive Molecules and Its Health Applications. Nutrients 2020, 12, 3360. [Google Scholar] [CrossRef] [PubMed]
- Mercer, D.K.; Torres, M.D.T.; Duay, S.S.; Lovie, E.; Simpson, L.; von Köckritz-Blickwede, M.; de la Fuente-Nunez, C.; O’Neil, D.A.; Angeles-Boza, A.M. Antimicrobial Susceptibility Testing of Antimicrobial Peptides to Better Predict Efficacy. Front. Cell. Infect. Microbiol. 2020, 10, 326. [Google Scholar] [CrossRef] [PubMed]
- Lima, W.G.; Batista Filho, F.L.; Lima, I.P.; Simião, D.C.; Brito, J.C.M.; da Cruz Nizer, W.S.; Cardoso, V.N.; Fernandes, S.O.A. Antibacterial, Anti-Biofilm, and Anti-Adhesive Activities of Melittin, a Honeybee Venom-Derived Peptide, against Quinolone-Resistant Uropathogenic Escherichia coli (UPEC). Nat. Prod. Res. 2022, 36, 6381–6388. [Google Scholar] [CrossRef] [PubMed]
- Moerman, L.; Bosteels, S.; Noppe, W.; Willems, J.; Clynen, E.; Schoofs, L.; Thevissen, K.; Tytgat, J.; Van Eldere, J.; Van Der Walt, J.; et al. Antibacterial and Antifungal Properties of α-Helical, Cationic Peptides in the Venom of Scorpions from Southern Africa. Eur. J. Biochem. 2002, 269, 4799–4810. [Google Scholar] [CrossRef]
- Loyola, S.; Concha-Velasco, F.; Pino-Dueñas, J.; Vasquez-Luna, N.; Juarez, P.; Lllanos, C.; Salvatierra, G.; Tamariz, J.; Lescano, G.A. Antimicrobial Resistance Patterns and Dynamics of Extended-Spectrum β-Lactamase-Producing Uropathogenic Escherichia coli in Cusco, Peru. Antibiotics 2021, 10, 485. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Murakami, J.; López, G.; Paredes, M.; Cuya, G.; Talledo, M. Production and Characterization of Bee Venom in Chulucanas, Piura. Agroindustrial Sci. 2019, 9, 205–210. [Google Scholar] [CrossRef]
- Cuya, G. Análisis Molecular de La Fracción <10 KDa de La Apitoxina de Apis Mellifera y Su Efecto Sobre La Apoptosis Celular. Bachelor’s Thesis, Universidad Nacional Agraria La Molina, Lima, Peru, 2019. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 10th ed.; CLSI Document M07-A10; CLSI: Wayne, PA, USA, 2015; p. 35. ISBN 1-56238-988-2. [Google Scholar]
- Dolzani, L.; Milan, A.; Scocchi, M.; Lagatolla, C.; Bressan, R.; Benincasa, M. Sub-MIC Effects of a Proline-Rich Antibacterial Peptide on Clinical Isolates of Acinetobacter baumannii. J. Med. Microbiol. 2019, 68, 1253–1265. [Google Scholar] [CrossRef] [PubMed]
- Isgren, C.M.; Edwards, T.; Pinchbeck, G.L.; Winward, E.; Adams, E.R.; Norton, P.; Timofte, D.; Maddox, T.W.; Clegg, P.D.; Williams, N.J. Emergence of Carriage of CTX-M-15 in Faecal Escherichia coli in Horses at an Equine Hospital in the UK; Increasing Prevalence over a Decade (2008–2017). BMC Vet. Res. 2019, 15, 268. [Google Scholar] [CrossRef] [PubMed]
- Ruzauskas, M.; Siugzdiniene, R.; Klimiene, I.; Virgailis, M.; Mockeliunas, R.; Vaskeviciute, L.; Zienius, D. Prevalence of Methicillin-Resistant Staphylococcus haemolyticus in Companion Animals: A Cross-Sectional Study. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 56. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
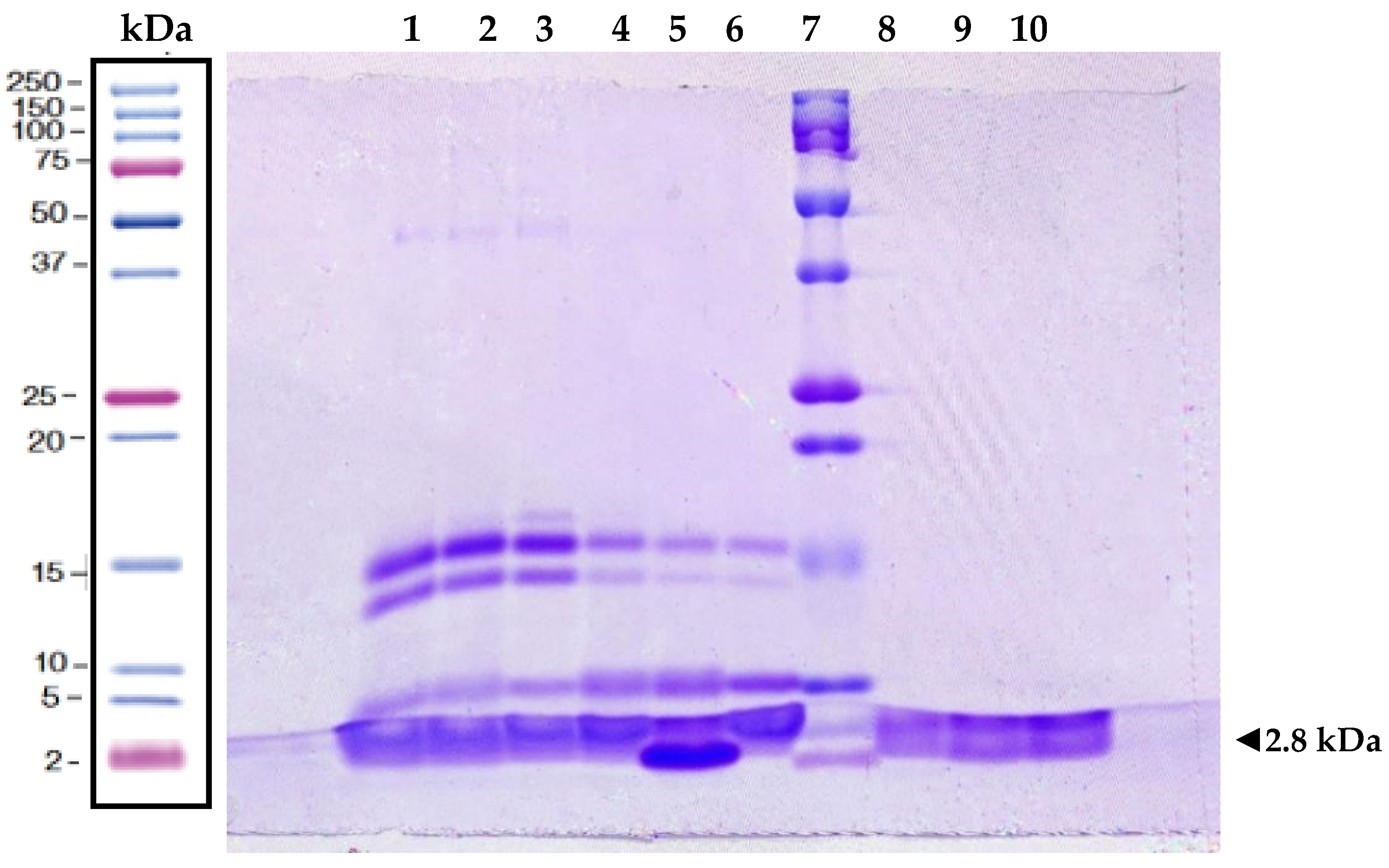

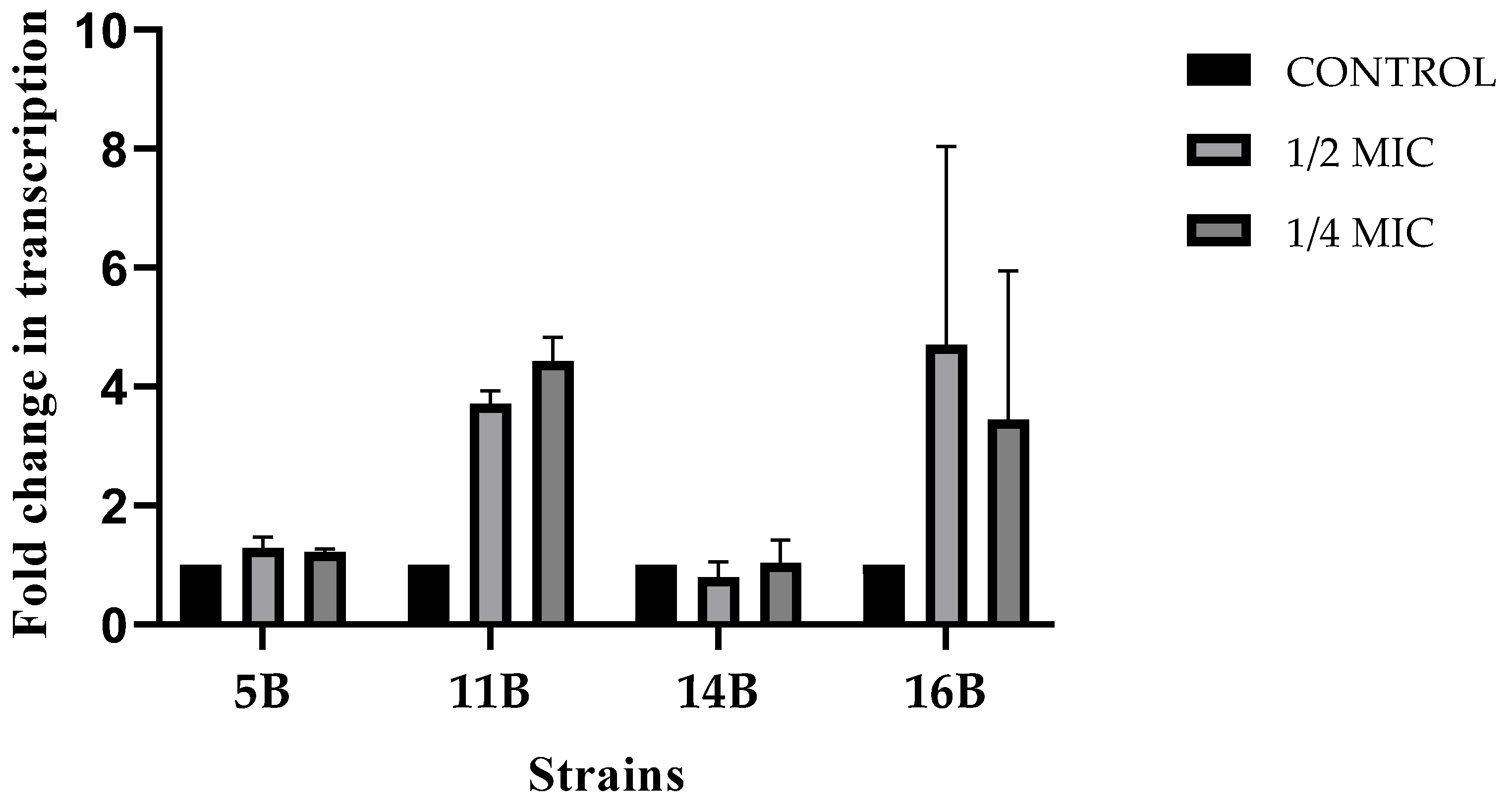
| Cepa | MIC µg/mL | 1/2 MIC µg/mL | 1/4 MIC µg/mL |
|---|---|---|---|
| 5B | 60 | 30 | 15 |
| 11B | 60 | 30 | 15 |
| 12B | 50 | 25 | 12.5 |
| 14B | 80 | 40 | 20 |
| 16B | 60 | 30 | 15 |
| Hydrolytic Activity of β-Lactamases mU/mg Protein a | |||
|---|---|---|---|
| Strain | Control | 1/4MIC | 1/2MIC b |
| 5B | 4327 | 4589 (−6.1%) | 3292 (23.9%) |
| 11B | 2561 | 2745 (−7.2%) | 1684 (34.2%) |
| 12B | 3322 | 3671 (−10.5%) | 2156 (35.1%) |
| 14B | 897 | 607 (32.3%) | 294 (67.2%) |
| 16B | 1438 | 977 (32.1%) | 872 (39.4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Alcántara, S.; Napan, M.A.C.; Campana, G.L.; Ortiz, J.T. Potential Inhibitory Effect of the Peptide Melittin Purified from Apis mellifera Venom on CTX-M-Type Extended-Spectrum β-Lactamases of Escherichia coli. Antibiotics 2025, 14, 403. https://doi.org/10.3390/antibiotics14040403
Ramos-Alcántara S, Napan MAC, Campana GL, Ortiz JT. Potential Inhibitory Effect of the Peptide Melittin Purified from Apis mellifera Venom on CTX-M-Type Extended-Spectrum β-Lactamases of Escherichia coli. Antibiotics. 2025; 14(4):403. https://doi.org/10.3390/antibiotics14040403
Chicago/Turabian StyleRamos-Alcántara, Sheril, María Alejandra Cornejo Napan, Giovanni Lopez Campana, and Jesus Tamariz Ortiz. 2025. "Potential Inhibitory Effect of the Peptide Melittin Purified from Apis mellifera Venom on CTX-M-Type Extended-Spectrum β-Lactamases of Escherichia coli" Antibiotics 14, no. 4: 403. https://doi.org/10.3390/antibiotics14040403
APA StyleRamos-Alcántara, S., Napan, M. A. C., Campana, G. L., & Ortiz, J. T. (2025). Potential Inhibitory Effect of the Peptide Melittin Purified from Apis mellifera Venom on CTX-M-Type Extended-Spectrum β-Lactamases of Escherichia coli. Antibiotics, 14(4), 403. https://doi.org/10.3390/antibiotics14040403







