Pharmacokinetics, Withdrawal Time, and Physiological Effects of Single Oral Administration of Enrofloxacin in Dybowski’s Frog (Rana dybowskii)
Abstract
1. Introduction
2. Results
2.1. Method Verification
2.2. Pharmacokinetics and Elimination of ENR in R. dybowskii
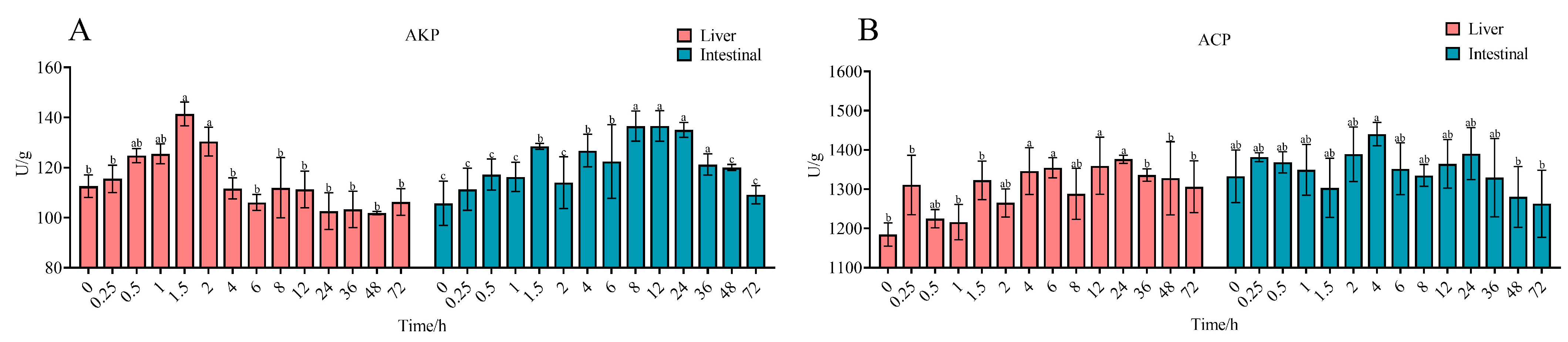
2.3. Biochemical Parameter Alterations in R. dybowskii
2.4. Expression of Keap1/Nrf2 and PI3K-Akt Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals
4.3. Experimental Design
4.4. Sample Preparation and Instrument Analysis
4.5. Pharmacokinetic and Residue Analysis
4.6. Biochemical Assays and Quantitative Real-Time PCR Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, B.-T.; Zhou, Y.; Min, M.-S.; Matsui, M.; Dong, B.-J.; Li, P.-P.; Fong, J.J. Diversity and phylogeography of Northeast Asian brown frogs allied to Rana dybowskii (Anura, Ranidae). Mol. Phylogenetics Evol. 2017, 112, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Lai, H.; Wu, W.; Cheng, R.; Lu, Y.; Peng, X. Rapid molecular identification of Rana dybowskii by species-specific primers. Anal. Biochem. 2024, 692, 115548. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, J.; Wang, D.; Wang, Y.; Hu, G.; Li, S. Isolation, Identification, and Characterisation of a Novel ST2378 Aeromonas hydrophila Strain from Naturally Diseased Frogs, Rana dybowskii. Pathogens 2024, 13, 552. [Google Scholar] [CrossRef] [PubMed]
- Densmore, C.L.; Green, D.E. Diseases of amphibians. ILAR J. 2007, 48, 235–254. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Jin, X.; Yang, D.; Wei, L.; Chen, J.; Lin, Z.; Ma, L. Identification and characterization of Serratia nematophila and Acinetobacter guillouiae from putrid-skin disease lesions in farmed Chinese spiny frog (Quasipaa spinosa). Microbiol. Spectr. 2025, 13, e0209624. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, X.; Sun, R.; Yang, Y.; Zhou, S.; Dong, J.; Yang, Q. Residue, biotransformation, risk assessment and withdrawal time of enrofloxacin in red swamp crayfish (Procambarus clarkii). Chemosphere 2022, 307, 135657. [Google Scholar] [CrossRef]
- Cook, M.A.; Wright, G.D. The past, present, and future of antibiotics. Sci. Transl. Med. 2022, 14, eabo7793. [Google Scholar] [CrossRef]
- Assis, V.R.; Robert, J.; Titon, S.C.M. Introduction to the special issue Amphibian immunity: Stress, disease and ecoimmunology. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2023, 378, 20220117. [Google Scholar] [CrossRef]
- Corum, O.; Uney, K.; Terzi, E.; Durna Corum, D.; Coskun, D.; Altan, F.; Elmas, M. Effects of temperature on the pharmacokinetics, tissue residues, and withdrawal times of Doxycycline in Rainbow Trout (Oncorhynchus mykiss) following oral administration. Vet. Sci. 2023, 10, 401. [Google Scholar] [CrossRef]
- Lulijwa, R.; Rupia, E.J.; Alfaro, A.C. Antibiotic use in aquaculture, policies and regulation, health and environmental risks: A review of the top 15 major producers. Rev. Aquac. 2020, 12, 640–663. [Google Scholar] [CrossRef]
- Badawy, S.; Yang, Y.; Liu, Y.; Marawan, M.; Ares, I.; Martínez, M.-A.; Martínez-Larrañaga, M.; Wang, X.; Anadón, A.; Martinez, M. Toxicity induced by ciprofloxacin and enrofloxacin: Oxidative stress and metabolism. Crit. Rev. Toxicol. 2021, 51, 754–787. [Google Scholar] [CrossRef]
- Grabowski, Ł.; Gaffke, L.; Pierzynowska, K.; Cyske, Z.; Choszcz, M.; Węgrzyn, G.; Węgrzyn, A. Enrofloxacin—The ruthless killer of eukaryotic cells or the last hope in the fight against bacterial infections? Int. J. Mol. Sci. 2022, 23, 3648. [Google Scholar] [CrossRef]
- Khwaza, V.; Mlala, S.; Aderibigbe, B.A. Advancements in synthetic strategies and biological effects of ciprofloxacin derivatives: A Review. Int. J. Mol. Sci. 2024, 25, 4919. [Google Scholar] [CrossRef]
- Xu, N.; Zhang, H.; Dong, J.; Yang, Y.; Liu, Y.; Zhou, S.; Zhu, X.; Ai, X. A modified withdrawal time estimation and risk assessment of enrofloxacin in grass carp (Ctenopharyngodon idella) after ad libitum medicated feed based on statistical approaches in natural cultured environments. Vet. Res. Commun. 2024, 48, 3049–3060. [Google Scholar] [CrossRef] [PubMed]
- MARAC. National Food Safety Standard- Maximum Residue Limits for Veterinary Drugs in Foods. Available online: https://www.instrument.com.cn/news/20171130/234764.shtml (accessed on 12 March 2025).
- Koc, F.; Uney, K.; Atamanalp, M.; Tumer, I.; Kaban, G. Pharmacokinetic disposition of enrofloxacin in brown trout (Salmo trutta fario) after oral and intravenous administrations. Aquaculture 2009, 295, 142–144. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, J.; Zheng, G.; Yin, Y.; Zhu, X.; Shan, Q.; Yang, Y.; Ma, L.; Li, L.; Liu, S. Pharmacokinetics, tissue distribution, and depletion of enrofloxacin and its metabolite ciprofloxacin in the northern snakehead (Channa argus) following multiple oral administration. Aquaculture 2021, 533, 736183. [Google Scholar] [CrossRef]
- Teles, J.A.; Castello Branco, L.C.; Del Bianchi, M.; Pilarski, F.; Reyes, F.G. Pharmacokinetic study of enrofloxacin in Nile tilapia (Oreochromis niloticus) after a single oral administration in medicated feed. J. Vet. Pharmacol. Ther. 2016, 39, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Long, X.-Z.; Dong, W.-J.; Xu, M.-D.; Han, X.-D.; Han, X.-Y.; Cui, L.-Y.; Tong, Q. Impact of antibiotic therapy on cutaneous and gut microbiota in Rana dybowskii amphibians: Insights and implications. Aquaculture 2024, 588, 740866. [Google Scholar] [CrossRef]
- Howard, A.M.; Papich, M.G.; Felt, S.A.; Long, C.T.; McKeon, G.P.; Bond, E.S.; Torreilles, S.L.; Luong, R.H.; Green, S.L. The pharmacokinetics of enrofloxacin in adult African clawed frogs (Xenopus laevis). J. Am. Assoc. Lab. Anim. Sci. JAALAS 2010, 49, 800–804. [Google Scholar]
- Shan, Q.; Wang, J.; Zheng, G.; Zhu, X.; Yang, Y.; Ma, L.; Zhao, C.; Li, L.; Yin, Y. Pharmacokinetics and tissue residues of enrofloxacin in the largemouth bass (Micropterus salmoides) after oral administration. J. Vet. Pharmacol. Ther. 2020, 43, 147–152. [Google Scholar] [CrossRef]
- Shan, Q.; Fan, J.; Wang, J.; Zhu, X.; Yin, Y.; Zheng, G. Pharmacokinetics of enrofloxacin after oral, intramuscular and bath administration in crucian carp (Carassius auratus gibelio). J. Vet. Pharmacol. Ther. 2018, 41, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Poapolathep, S.; Giorgi, M.; Chaiyabutr, N.; Chokejaroenrat, C.; Klangkaew, N.; Phaochoosak, N.; Wongwaipairote, T.; Poapolathep, A. Pharmacokinetics of enrofloxacin and its metabolite ciprofloxacin in freshwater crocodiles (Crocodylus siamensis) after intravenous and intramuscular administration. J. Vet. Pharmacol. Ther. 2020, 43, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Antibiotics in the aquatic environment–A review–Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Stoffregen, D.A.; Wooster, G.A.; Bustos, P.S.; Bowser, P.R.; Babish, J.G. Multiple route and dose pharmacokinetics of enrofloxacin in juvenile Atlantic salmon. J. Vet. Pharmacol. Ther. 1997, 20, 111–123. [Google Scholar] [CrossRef]
- Chan, T.; Bunce, P.E. Fluoroquinolone antimicrobial drugs. CMAJ Can. Med. Assoc. J. J. L’association Medicale Can. 2017, 189, E638. [Google Scholar] [CrossRef]
- Phu, T.M.; Douny, C.; Scippo, M.-L.; De Pauw, E.; Thinh, N.Q.; Huong, D.T.T.; Vinh, H.P.; Phuong, N.T.; Dalsgaard, A. Elimination of enrofloxacin in striped catfish (Pangasianodon hypophthalmus) following on-farm treatment. Aquaculture 2015, 438, 1–5. [Google Scholar] [CrossRef]
- Fang, W.-h.; Zhou, S.; Yu, H.-j.; Hu, L.-l.; Zhou, K.; Liang, S.-c. Pharmacokinetics and tissue distribution of enrofloxacin and its metabolite ciprofloxacin in Scylla serrata following oral gavage at two salinities. Aquaculture 2007, 272, 180–187. [Google Scholar] [CrossRef]
- Xu, W.; Zhu, X.; Wang, X.; Deng, L.; Zhang, G. Residues of enrofloxacin, furazolidone and their metabolites in Nile tilapia (Oreochromis niloticus). Aquaculture 2006, 254, 1–8. [Google Scholar] [CrossRef]
- Lucchetti, D.; Fabrizi, L.; Guandalini, E.; Podestà, E.; Marvasi, L.; Zaghini, A.; Coni, E. Long depletion time of enrofloxacin in rainbow trout (Oncorhynchus mykiss). Antimicrob. Agents Chemother. 2004, 48, 3912–3917. [Google Scholar] [CrossRef]
- Liang, J.P.; Li, J.; Li, J.T.; Liu, P.; Chang, Z.Q.; Nie, G.X. Accumulation and elimination of enrofloxacin and its metabolite ciprofloxacin in the ridgetail white prawn Exopalaemon carinicauda following medicated feed and bath administration. J. Vet. Pharmacol. Ther. 2014, 37, 508–514. [Google Scholar] [CrossRef]
- Felt, S.; Papich, M.G.; Howard, A.; Long, T.; McKeon, G.; Torreilles, S.; Green, S. Tissue distribution of enrofloxacin in African clawed frogs (Xenopus laevis) after intramuscular and subcutaneous administration. J. Am. Assoc. Lab. Anim. Sci. JAALAS 2013, 52, 186–188. [Google Scholar]
- Du, J.; Liu, Q.; Pan, Y.; Xu, S.; Li, H.; Tang, J. The research status, potential hazards and toxicological mechanisms of Fluoroquinolone antibiotics in the environment. Antibiotics 2023, 12, 1058. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Cui, Y.; Brown, P.B.; Ge, X.; Xie, J.; Xu, P. Cytotoxic effects and apoptosis induction of enrofloxacin in hepatic cell line of grass carp (Ctenopharyngodon idellus). Fish Shellfish. Immunol. 2015, 47, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Lan, M.; Li, Y.; Zhang, Z.; Guan, Y. Effects of florfenicol on the antioxidant and immune systems of Chinese soft-shelled turtle (Pelodiscus sinensis). Fish Shellfish. Immunol. 2023, 140, 108991. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Li, Z.; Li, X.; Xing, L.; Wang, S.; Sun, X.; Zhang, D. Evaluation of enrofloxacin in the Chinese soft-shelled turtle (Pelodiscus sinensis) based on the biochemical, histopathological and intestinal microbiota responses. Aquaculture 2024, 590, 741062. [Google Scholar] [CrossRef]
- Li, T.; Jin, M.; Huang, L.; Zhang, Y.; Zong, J.; Shan, H.; Kang, H.; Xu, M.; Liu, H.; Zhao, Y.; et al. Oxytetracycline-induced oxidative liver damage by disturbed mitochondrial dynamics and impaired enzyme antioxidants in largemouth bass (Micropterus salmoides). Aquatic toxicology 2023, 261, 106616. [Google Scholar] [CrossRef]
- Canning, P.; Sorrell, F.J.; Bullock, A.N. Structural basis of Keap1 interactions with Nrf2. Free. Radic. Biol. Med. 2015, 88, 101–107. [Google Scholar] [CrossRef]
- Yuan, X.; Wu, H.; Gao, J.; Geng, X.; Xie, M.; Song, R.; Zheng, J.; Wu, Y.; Ou, D. Acute deltamethrin exposure induces oxidative stress, triggers endoplasmic reticulum stress, and impairs hypoxic resistance of crucian carp. Comp. Biochem. physiology. Toxicol. Pharmacol. CBP 2023, 263, 109508. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Zhang, T.; Zhang, R.; Liu, R.; Chen, Y. Molecular mechanism on cadmium-induced activity changes of catalase and superoxide dismutase. Int. J. Biol. Macromol. 2015, 77, 59–67. [Google Scholar] [CrossRef]
- Wang, X.; Jian, S.; Zhang, S.; Wu, D.; Wang, J.; Gao, M.; Sheng, J.; Hong, Y. Enrichment of polystyrene microplastics induces histological damage, oxidative stress, Keap1-Nrf2 signaling pathway-related gene expression in loach juveniles (Paramisgurnus dabryanus). Ecotoxicol. Environ. Saf. 2022, 237, 113540. [Google Scholar] [CrossRef]
- Zhao, X.-L.; Li, P.; Zhang, S.-Q.; He, S.-W.; Xing, S.-Y.; Cao, Z.-H.; Lu, R.; Li, Z.-H. Effects of environmental norfloxacin concentrations on the intestinal health and function of juvenile common carp and potential risk to humans. Environ. Pollut. 2021, 287, 117612. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Pan, K.; Xu, H.; Song, Y.; Qi, X.; Lu, Y.; Jiang, X.; Liu, H. The effects of enrofloxacin exposure on responses to oxidative stress, intestinal structure and intestinal microbiome community of largemouth bass (Micropterus salmoides). Chemosphere 2024, 348, 140751. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-H.; Wei, X.-L.; Xu, Y.-C.; Zhang, D.-G.; Zhao, T.; Zheng, H.; Luo, Z. Waterborne enrofloxacin exposure activated oxidative stress and MAPK pathway, induced apoptosis and resulted in immune dysfunction in the gills of yellow catfish Pelteobagrus fulvidraco. Aquac. 2022, 547, 737541. [Google Scholar] [CrossRef]
- Judge, A.; Dodd, M.S. Metabolism. Essays Biochem. 2020, 64, 607–647. [Google Scholar] [CrossRef]
- Lin, Z.; Wan, G.; Wu, J.; Liu, H.; Zhang, F.; Tang, X.; Ruan, J. Toxicologic effect of short-term enrofloxacin exposure on brain of Carassius auratus var. Pengze. Sci. Total Environ. 2023, 869, 161730. [Google Scholar] [CrossRef]
- Lazado, C.C.; Strand, D.A.; Breiland, M.W.; Furtado, F.; Timmerhaus, G.; Gjessing, M.C.; Hytterød, S.; Merkin, G.V.; Pedersen, L.F.; Pittman, K.A.; et al. Mucosal immune and stress responses of Neoparamoeba perurans-infected Atlantic salmon (Salmo salar) treated with peracetic acid shed light on the host-parasite-oxidant interactions. Front. Immunol. 2022, 13, 948897. [Google Scholar] [CrossRef]
- Chang, L.; Du, S.; Wu, X.; Zhang, J.; Gan, Z. Analysis, Occurrence and exposure evaluation of antibiotic and anthelmintic residues in whole cow milk from China. Antibiotics 2023, 12, 1125. [Google Scholar] [CrossRef]
- Rahman, M.; Hoque, M.S.; Bhowmik, S.; Ferdousi, S.; Kabiraz, M.P.; van Brakel, M.L. Monitoring of pesticide residues from fish feed, fish and vegetables in Bangladesh by GC-MS using the QuEChERS method. Heliyon 2021, 7, e06390. [Google Scholar] [CrossRef]
- González-Curbelo, M.Á.; Varela-Martínez, D.A.; Riaño-Herrera, D.A. Pesticide-Residue analysis in soils by the QuEChERS Method: A Review. Molecules 2022, 27, 4323. [Google Scholar] [CrossRef]
- EMA. Guideline on Determination of Withdrawal Periods for Edible Tissues. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-determination-withdrawal-periods-edible-tissues-revision-1_en.pdf (accessed on 12 March 2025).
- Deng, F.; Wang, D.; Yu, Y.; Lu, T.; Li, S. Systemic immune response of rainbow trout exposed to Flavobacterium psychrophilum infection. Fish Shellfish. Immunol. 2024, 144, 109305. [Google Scholar] [CrossRef]

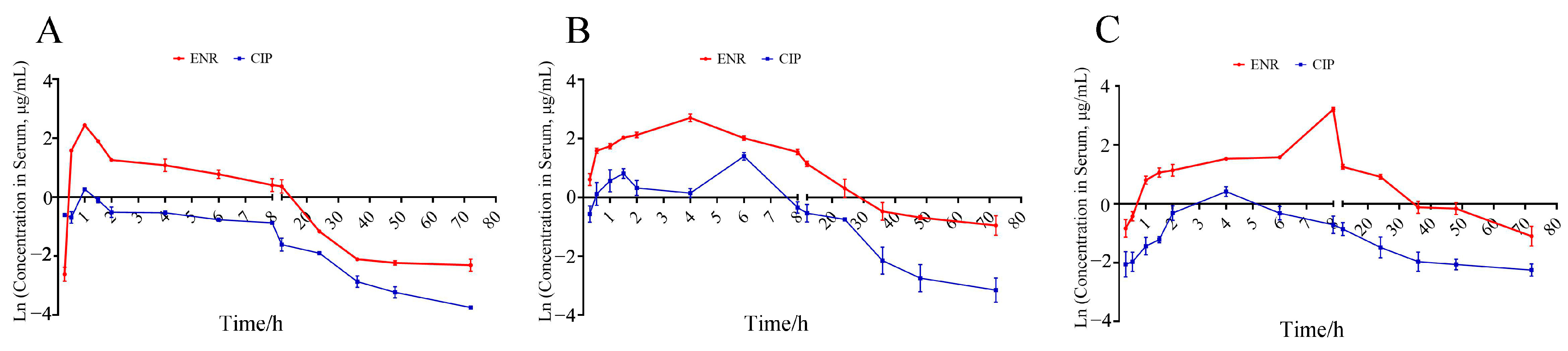




| ENR | CIP | ||||||
|---|---|---|---|---|---|---|---|
| Parameters | Unit | Serum | Liver | Kidney | Serum | Liver | Kidney |
| Cmax | μg/mL (or μg/g) | 11.51 | 11.28 | 9.11 | 1.31 | 4.07 | 1.39 |
| Tmax | h | 1 | 4 | 8 | 1 | 6 | 4 |
| AUC0−t | (μg/mL) ⋅h or μg/g ⋅h | 65.04 | 142.81 | 141.21 | 10.10 | 32.69 | 18.74 |
| MRT | h | 14.71 | 17.02 | 20.04 | 15.03 | 14.14 | 21.97 |
| T1/2λz | h | 12.76 | 16.77 | 17.90 | 28.42 | 13.18 | 81.79 |
| CL/F | mL/h/kg | 0.15 | 0.07 | 0.06 | 0.90 | 0.29 | 0.32 |
| Gene Name | Forward Primer (5′→3′) | Reserve Primer (5′→3′) | Accession Number |
|---|---|---|---|
| GST | GCTGATACGGCGCTATCAC | TCTCTCAGAGCCGGTCATGT | XM_040361832.1 |
| PI3K | TGGAATCAACTGCCGAGCC | CTGGTGACAGGGTTAAGGG | XM_040349408.1 |
| IL1β | ACGTTTGAGTGCCTGTTTGATG | CACTGGTACGGTTGTTCCCT | XM_040345988.1 |
| IRS4 | CTTCAAGGAGGTCTGCAGG | CTTGTGGACAGGCAAAGGC | XM_040323899.1 |
| JNK1 | GGTCTGATCCCAGCACATT | TAGCATTGCCATGAGCCCA | XM_040333563.1 |
| Keap1 | GCGCACTAGGAGTCATGTT | GCTGTTTGTCCGGTGGTTTC | XM_040346421.1 |
| Akt | GCTCTCCGAGCGTAACTCC | CGCGGTTTTCAGTCAGTGTG | XM_040332369.1 |
| IGF | GATGTACTGTGCTCCTGCCA | TGCACTTCCTTCTGGGCTTT | XM_040344284.1 |
| AP-1 | ACCGCCTTCACTTTCACAA | TTCGAGTTTCCTCTTCCGGC | XM_040362001.1 |
| SOD1 | AGGCATGTTGGAGACTTGG | ACTGCTGTGCGTCCAATGAT | XM_040338866.1 |
| CAT | TGACTGGCATAACACCCCC | TACCAGGTCCGAAAACAGG | XM_040328095.1 |
| GYS1 | ACGACCGGGAAGCAAATGAT | ATTCGTGAAGTGAGCCAGG | XM_040325737.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, J.; Zhao, R.; Li, S.; Hu, G.; Wang, D. Pharmacokinetics, Withdrawal Time, and Physiological Effects of Single Oral Administration of Enrofloxacin in Dybowski’s Frog (Rana dybowskii). Antibiotics 2025, 14, 417. https://doi.org/10.3390/antibiotics14040417
Wang Y, Wang J, Zhao R, Li S, Hu G, Wang D. Pharmacokinetics, Withdrawal Time, and Physiological Effects of Single Oral Administration of Enrofloxacin in Dybowski’s Frog (Rana dybowskii). Antibiotics. 2025; 14(4):417. https://doi.org/10.3390/antibiotics14040417
Chicago/Turabian StyleWang, Yanan, Jing Wang, Ran Zhao, Shaowu Li, Guo Hu, and Di Wang. 2025. "Pharmacokinetics, Withdrawal Time, and Physiological Effects of Single Oral Administration of Enrofloxacin in Dybowski’s Frog (Rana dybowskii)" Antibiotics 14, no. 4: 417. https://doi.org/10.3390/antibiotics14040417
APA StyleWang, Y., Wang, J., Zhao, R., Li, S., Hu, G., & Wang, D. (2025). Pharmacokinetics, Withdrawal Time, and Physiological Effects of Single Oral Administration of Enrofloxacin in Dybowski’s Frog (Rana dybowskii). Antibiotics, 14(4), 417. https://doi.org/10.3390/antibiotics14040417





