Mapping the AMR Infection Landscape in Bihar: Implications for Strengthening Policy and Clinical Practice
Abstract
1. Introduction
1.1. Related Research in the Context of LMICs
1.2. The Bihar AMR Context
2. Results
2.1. Baseline Laboratory Capacity Assessment
2.2. Actions Taken to Improve Data Systems for AMR Testing and Reporting
2.3. Geographic Distribution of AMR Diagnostic Facilities
2.4. Demographic Distribution of Clinical Samples Across Facilities
2.5. Year-Wise Distribution of Samples Received by Different Hospital Facilities in Bihar
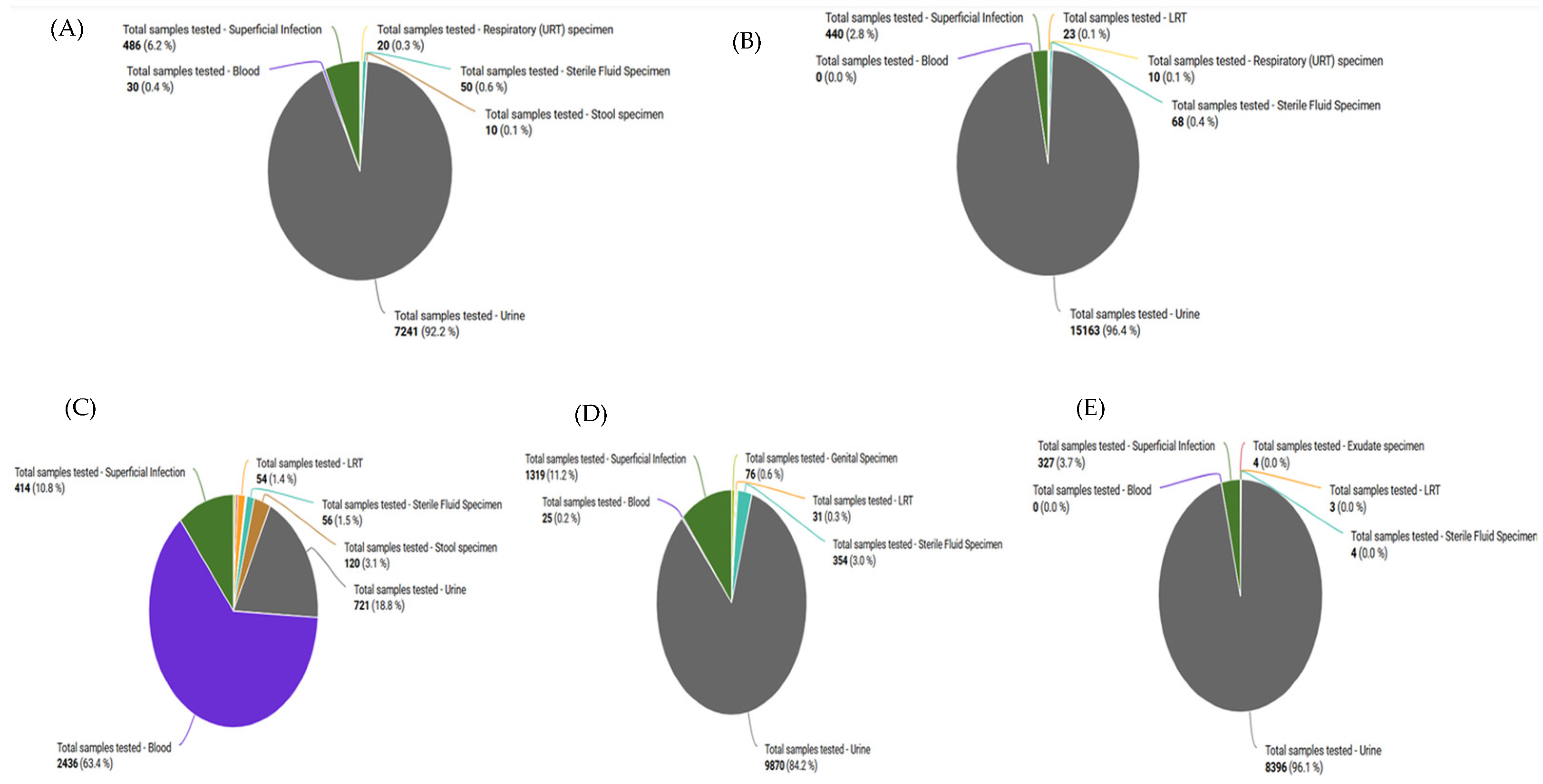
2.6. Sample Types Received from the Different Hospitals
2.7. Number of Samples Received from Different Departments
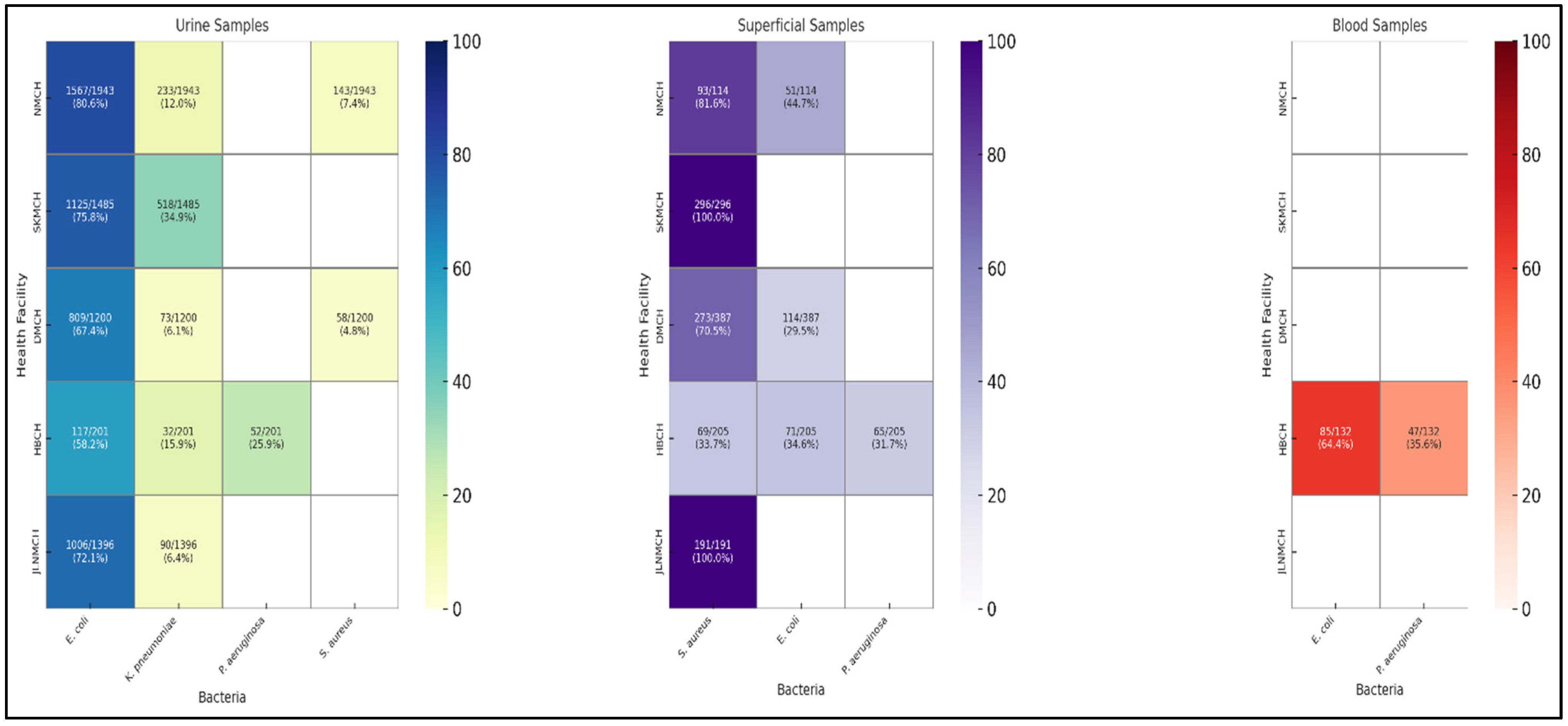
2.8. Distribution of Bacterial Isolates from Clinical Samples
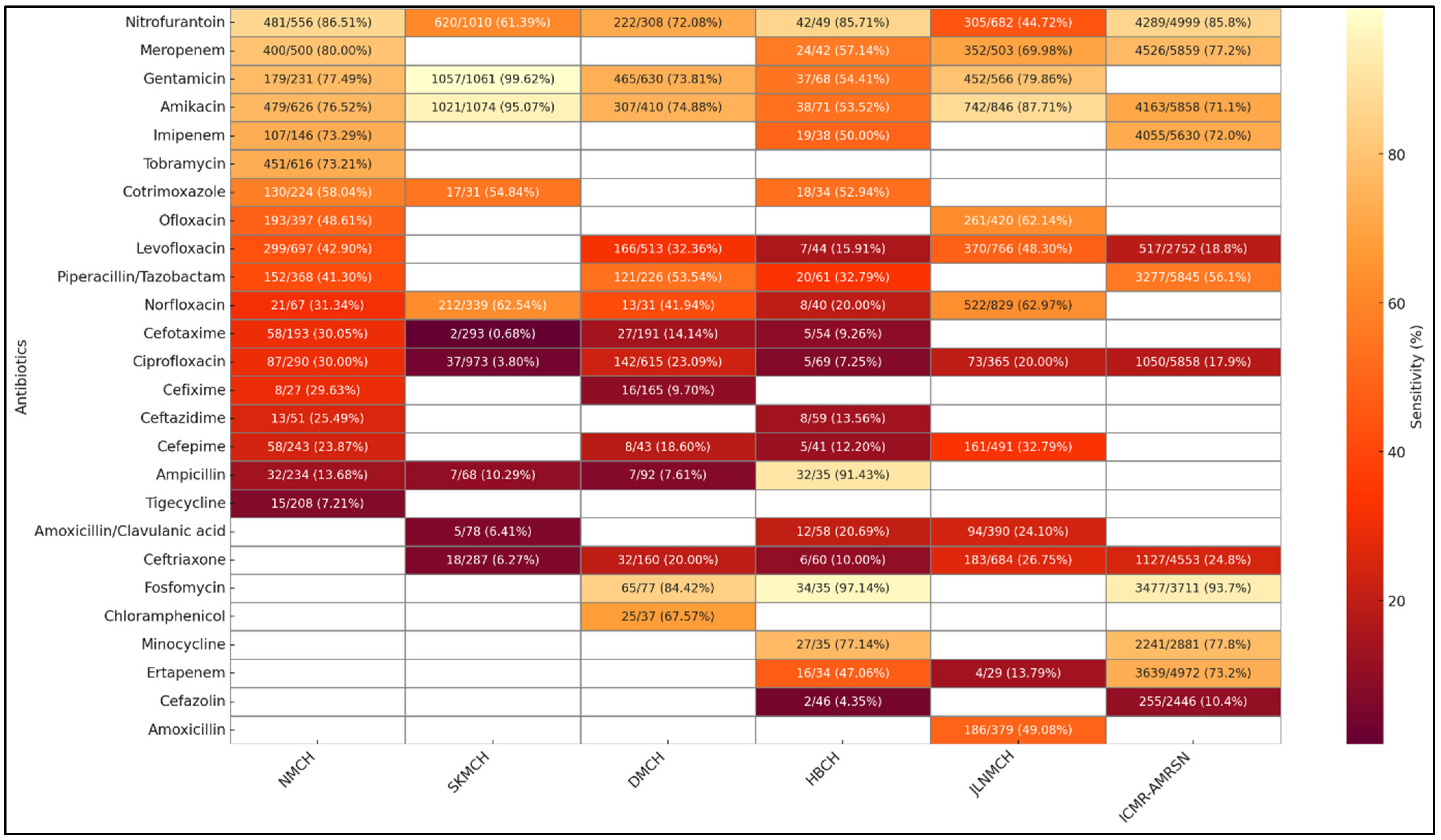
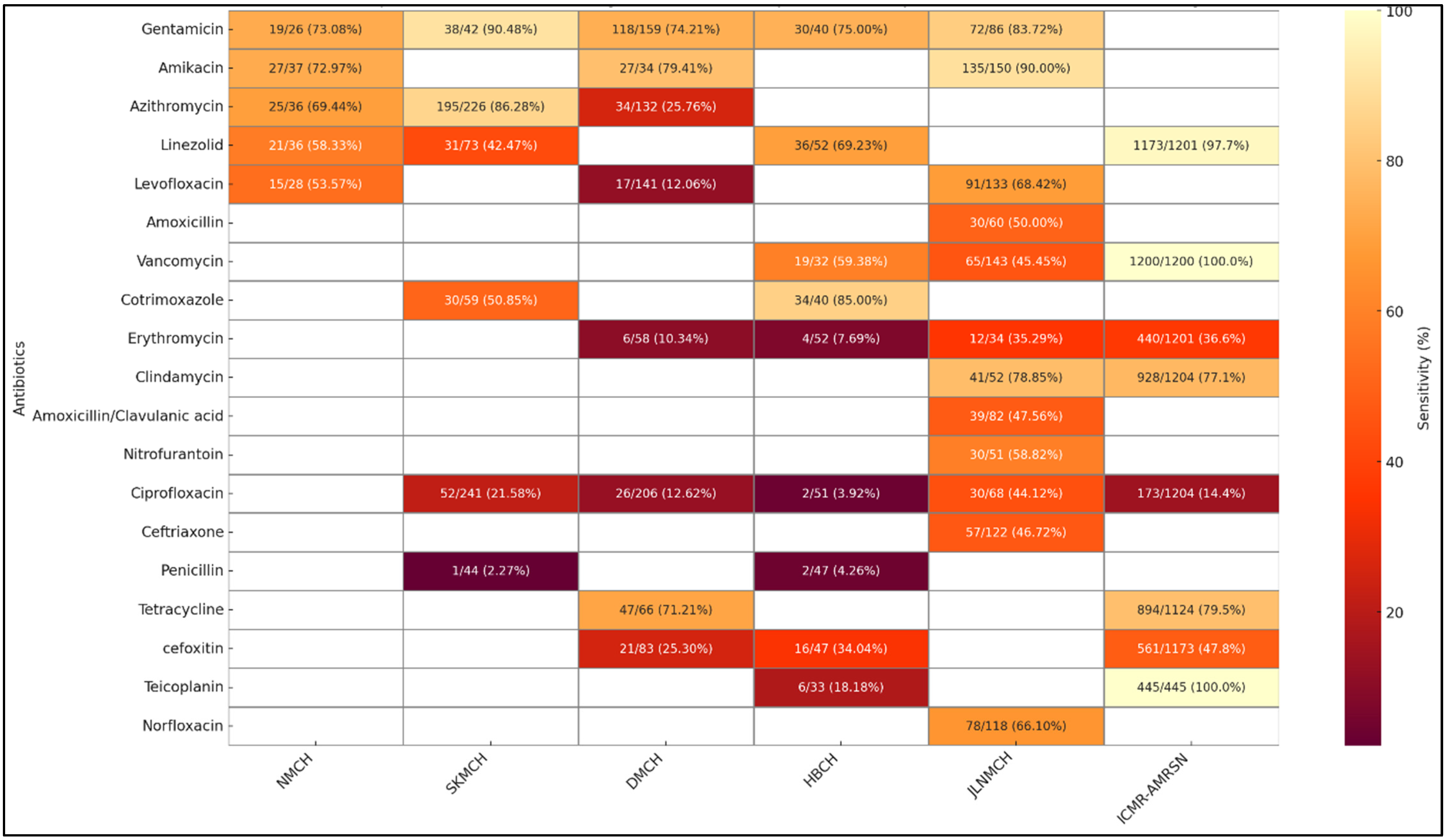
2.9. Facility-Wise Variation in Susceptibility Patterns from Clinical Isolates
3. Discussion
3.1. AMR Trends Within and Across Facilities
3.2. Comparison with National and LMICs AMR Trends
3.3. Beyond Pathogens: Social and Spatial Drivers of AMR
3.4. Implications for Policy and Clinical Practice
- 1.
- Real-time Digital Surveillance
- 2.
- Data-Informed Procurement and EML Alignment
- 3.
- Strengthening Institutional Stewardship Mechanisms
- 4.
- Improving Access to Diagnostics
4. Material and Methods
4.1. Study Sites and Catchment Area Demographics
4.2. Study Timeline
4.3. Study Team
4.4. Spatial Analysis of Diagnostic Access
4.5. Baseline Laboratory Assessment
4.6. Clinical Data
4.7. Clinical Sample Processing and Bacterial Identification
4.8. Antibiotic Susceptibility Testing (AST)
4.9. Digital Integration Process and Observational Insights
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malik, S.; Shirvankar, C.M.; Jacob, R.K.; Adhya, D.G.; Sinha, S.; Bhattacharya, S.; Walia, K.; Das Bhattacharya, S. A systematic review and meta-analysis to develop a landscape map of antibiotic resistance for six WHO priority pathogens in east and north-east India from 2011 to 2022. Indian J. Med. Microbiol. 2024, 52, 100732. [Google Scholar] [CrossRef] [PubMed]
- Hendriksen, R.S.; Munk, P.; Njage, P.; Van Bunnik, B.; McNally, L.; Lukjancenko, O.; Röder, T.; Nieuwenhuijse, D.; Pedersen, S.K.; Kjeldgaard, J.; et al. Global monitoring of antimicrobial resistance based on metagenomics analyses of urban sewage. Nat. Commun. 2019, 10, 1124. [Google Scholar] [CrossRef]
- Holt, K.E.; Megan, E.C.; Clare, C.; James, H.C.; Zoe, A.; Dyson, N.F.; Rebecca, E.G.; Mollie, V.; Gwenan, M.K. Tools and challenges in the use of routine clinical data for antimicrobial resistance surveillance. npj Antimicrob. Resist. 2025, 3, 37. [Google Scholar] [CrossRef] [PubMed]
- What, Y. Bihar: Disease Burden Profile, 1990 to 2016; ICMR, PHFI, IHME: New Delhi, India, 2017; pp. 7–10.
- NITI Aayog. N. SDG India Index & Dashboard 2020–2021: Partnerships in the Decade of Action. 2021. Available online: https://www.niti.gov.in/sites/default/files/2025-04/SDG_India_Index_2020.pdf (accessed on 30 May 2025).
- Ministry of Health and Family Welfare. India TB Report 2024. Available online: https://tbcindia.mohfw.gov.in/wp-content/uploads/2024/10/TB-Report_for-Web_08_10-2024-1.pdf (accessed on 30 May 2025).
- National Health Systems Resource Centre (NHSRC). Bihar Health Dossier 2021. Available online: https://nhsrcindia.org/sites/default/files/practice_image/HealthDossier2021/Bihar.pdf (accessed on 30 May 2025).
- Sulis, G.; Sayood, S.; Gandra, S. Antimicrobial resistance in low- and middle-income countries: Current status and future directions. Expert Rev. Anti-Infect. Ther. 2021, 20, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Malekzadeh, A.; Michels, K.; Wolfman, C.; Anand, N.; Sturke, R. Strengthening research capacity in LMICs to address the global NCD burden. Glob. Health Action 2020, 13, 1846904. [Google Scholar] [CrossRef]
- Ashley, E.A.; Shetty, N.; Patel, J.; van Doorn, R.; Limmathurotsakul, D.; Feasey, A.N.; Okeke, I.N.; Peacock, S.J. Harnessing alternative sources of antimicrobial resistance data to support surveillance in low-resource settings. J. Antimicrob. Chemother. 2019, 74, 541–546. [Google Scholar] [CrossRef]
- Iskandar, K.; Molinier, L.; Hallit, S.; Sartelli, M.; Hardcastle, T.C.; Haque, M.; Lugova, H.; Dhingra, S.; Sharma, P.; Islam, S.; et al. Surveillance of antimicrobial resistance in low- and middle-income countries: A scattered picture. Antimicrob. Resist. Infect. Control. 2021, 10, 63. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Ajulo, S.; Awosile, B.; Negatie, B.A. Global antimicrobial resistance and use surveillance system (GLASS 2022): Investigating the relationship between antimicrobial resistance and antimicrobial consumption data across the participating countries. PLoS ONE 2024, 19, e0297921. [Google Scholar] [CrossRef]
- Sihombing, B.; Bhatia, R.; Srivastava, R.; Aditama, T.Y.; Laxminarayan, R.; Rijal, S. Response to antimicrobial resistance in South-East Asia region. Lancet Reg. Health-Southeast. Asia 2023, 18, 100306. [Google Scholar] [CrossRef]
- Poudyal, N.; Holm, M.; Joh, H.S.; Gautam, S.; Sujan, M.J.; Kwon, S.Y.; Sahikh, A.; Shaw, A.; Gallagher, P.; Prifti, K.; et al. Effective Stakeholder Engagement for Collation, Analysis and Expansion of Antimicrobial Resistance (AMR) Data: A CAPTURA Experience. Clin. Infect. Dis. 2023, 77 (Suppl. S7), S519–S527. [Google Scholar] [CrossRef] [PubMed]
- Walia, K.; Madhumathi, J.; Veeraraghavan, B.; Chakrabarti, A.; Kapil, A.; Ray, P.; Singh, H.; Sistla, S.; Ohri, V. Establishing Antimicrobial Resistance Surveillance & Research Network in India. Indian J. Med. Res. 2019, 149, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.; Rupali, P.; Opintan, J.A.; Jaoko, W.; Feasey, N.A.; Peacock, S.J.; Ashley, E.A. Laboratory informatics capacity for effective antimicrobial resistance surveillance in resource-limited settings. Lancet Infect. Dis. 2021, 21, e170–e174. [Google Scholar] [CrossRef]
- Kumari, N.; Kumar, M.; Katiyar, A.; Kumar, A.; Priya, P.; Kumar, B.; Biswas, N.R.; Kaur, P. Genome-wide identification of carbapenem-resistant Gram-negative bacterial (CR-GNB) isolates retrieved from hospitalized patients in Bihar, India. Sci. Rep. 2022, 12, 8477. [Google Scholar] [CrossRef]
- Kumar, D.; Sagar, S.; Narain, P.; Soni, R.; Suman, S.; Srivastva, R.K. Demographic study and trends of antimicrobial resistance pattern of pseudomonas aeruginosa, isolated from various clinical samples, in a tertiary care hospital, at PMCH, Patna Bihar, India. Eur. J. Cardiovasc. Med. 2024, 14, 238. [Google Scholar]
- Kumar, V.; Murali, S.; Goldberg, J.; Alonso, B.; Moretó-Planas, L.; Reid, A.; Harshana, A.; Burza, S.; Mahajan, R. Antibiotic susceptibility patterns of pathogens isolated from hospitalized patients with advanced HIV disease (AHD) in Bihar, India. JAC-Antimicrob. Resist. 2023, 6, dlad151. [Google Scholar] [CrossRef]
- Kaur, J.; Kaur, J.; Dhama, A.S.; Jindal, S.; Walia, K.; Singh, H. Strengthening the Surveillance of Antimicrobial Resistance in India Using Integrative Technologies. Front. Public Health 2022, 10, 861888. [Google Scholar] [CrossRef] [PubMed]
- Torumkuney, D.; Poojary, A.; Shenoy, B.; Nijhara, P.; Dalal, K.; Manenzhe, R. Country data on AMR in India in the context of community-acquired respiratory tract infections: Links between antibiotic susceptibility, local and international antibiotic prescribing guidelines, access to medicine and clinical outcome. J. Antimicrob. Chemother. 2022, 77 (Suppl. S1), i10–i17. [Google Scholar] [CrossRef]
- Gandra, S.; Joshi, J.; Trett, A.; Lamkang, A.S.; Laxminarayan, R. Scoping Report on Antimicrobial Resistance in India; Center for Disease Dynamics, Economics & Policy: Washington, DC, USA, 2017. [Google Scholar]
- Rafique, H.; Hussain, N.; Saeed, M.U.; Iqbal, H.M.; Azim, G.; Bilal, M. Linezolid-resistance Staphylococcus aureus―Prevalence, Emerging Resistance Mechanisms, Challenges and Perspectives. J. Pure Appl. Microbiol. 2022, 16, 7753. [Google Scholar] [CrossRef]
- Shakya, S.; Edwards, J.; Gupte, H.A.; Shrestha, S.; Shakya, B.M.; Parajuli, K.; Kattel, H.P.; Shrestha, P.S.; Ghimire, R.; Thekkur, P. High multidrug resistance in urinary tract infections in a tertiary hospital, Kathmandu, Nepal. Public Health Action 2021, 11, 24–31. [Google Scholar] [CrossRef]
- Chereau, F.; Opatowski, L.; Tourdjman, M.; Vong, S. Risk assessment for antibiotic resistance in South East Asia. BMJ 2017, 358, j3393. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. GLASS Report: Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022; WHO: Geneva, Switzerland, 2022; Available online: https://iris.who.int/bitstream/handle/10665/379632/9789240103986-eng.pdf (accessed on 30 May 2025).
- Das, T.; Nath, C.; Das, P.; Ghosh, K.; Logno, T.A.; Debnath, P.; Dash, S.; Devnath, H.S.; Das, S.; Islam, M.Z. High prevalence of ciprofloxacin resistance in Escherichia coli isolated from chickens, humans and the environment: An emerging one health issue. PLoS ONE 2023, 18, e0294043. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Shrestha, R.; Koju, P.; Tamrakar, S.; Rai, A.; Shrestha, P.; Madhup, S.K.; Katuwal, N.; Shrestha, A.; Shrestha, A.; et al. The Resistance Patterns in E. coli Isolates among Apparently Healthy Adults and Local Drivers of Antimicrobial Resistance: A Mixed-Methods Study in a Suburban Area of Nepal. Trop. Med. Infect. Dis. 2022, 7, 133. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, A.; Nachimuthu, R. Antibiotic resistance, biofilm forming ability, and clonal profiling of clinical isolates of Staphylococcus aureus from southern and northeastern India. Asian Biomed. 2022, 16, 191–199. [Google Scholar] [CrossRef]
- Haque, T.A.; Urmi, U.L.; Islam, A.B.M.M.K.; Ara, B.; Nahar, S.; Mosaddek, A.S.M.; Luguva, H.; Kumar, S.; Jahan, D.; Rahman, N.A.A.; et al. Detection of qnr genes and gyrA mutation to quinolone phenotypic resistance of UTI pathogens in Bangladesh and the implications: Resistance UTI pathogens Bangladesh. J. Appl. Pharm. Sci. 2022, 12, 122–130. [Google Scholar]
- Shen, X.; Shen, J.; Pan, Y.; Cheng, J.; Chai, J.; Bowker, K.; MacGowan, A.; Oliver, I.; Lambert, H.; Wang, D. Clinical diagnosis and treatment of common respiratory tract infections in relation to microbiological profiles in rural health facilities in China: Implications for antibiotic stewardship. BMC Fam. Pract. 2021, 22, 87. [Google Scholar] [CrossRef]
- Omulo, S.; Lofgren, E.T.; Lockwood, S.; Thumbi, S.M.; Bigogo, G.; Ouma, A.; Verani, J.R.; Juma, B.; Njenga, M.K.; Kariuki, S.; et al. Carriage of antimicrobial-resistant bacteria in a high-density informal settlement in Kenya is associated with environmental risk-factors. Antimicrob. Resist. Infect. Control 2021, 10, 18. [Google Scholar] [CrossRef]
- Upadhaya, S.; Acharya, J.; Zolfo, M.; Nair, D.; Kharel, M.; Shrestha, A.; Shrestha, B.; Madhup, S.K.; Raghubanshi, B.R.; Kattel, H.P.; et al. Has Data Quality of an Antimicrobial Resistance Surveillance System in a Province of Nepal Improved between 2019 and 2022? Trop. Med. Infect. Dis. 2023, 8, 399. [Google Scholar] [CrossRef]
- Indian Council of Medical Research. Standard Operating Procedures Bacteriology: Antimicrobial Resistance Surveillance and Research Network; ICMR: New Delhi, India, 2015. Available online: https://www.icmr.gov.in/icmrobject/custom_data/pdf/resource-guidelines/Standard_Operating_Procedures_Bacteriology_1stEdition.pdf (accessed on 30 May 2025).
- VET01-A4; Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals. CLSI: Wayne, PA, USA, 2013.
- M100; Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. CLSI: Wayne, PA, USA, 2020.
- Indian Council of Medical Research. Annual Report of the Antimicrobial Resistance Surveillance Network (AMRSN)-2023; ICMR: New Delhi, India, 2023. Available online: https://www.icmr.gov.in/icmrobject/uploads/Documents/1725536060_annual_report_2023.pdf (accessed on 30 May 2025).
- Sahay, S.; Rangaswamy, V.; Mukherjee, A. Microbiology Lab Assessment-Government Hospitals in Bihar; HISP: New Delhi, India, 2022. [Google Scholar]






| Hospital Sno | Daily Patient Load | Samples Tested/Day | Testing Rate * (%) | Reporting Digital/Manual | Use of Data in the Hospital |
|---|---|---|---|---|---|
| DMCH, Darbhanga | 1800 | 20–25 | 1.25 | Manual | No |
| SKMCH, Muzaffarpur | 1800–2000 | 15–20 | 0.9 | Manual | No |
| PMCH, Patna | 1500–3000 | 60–80 | 3.1 | Manual | No |
| ANMCH, Gaya | 1000 | 17–18 | 1.75 | Manual | Yes |
| JLNMC, Bhagalpur | 1500 | 30–35 | 2.17 | Manual | No |
| JNKTMCH | 650 | No data | -- | Manual | No |
| IGIMS, Patna | 3000 | 100–150 | 4.17 | Digital | Yes |
| VIMS, Pawapuri | 2000 | 15–20 | 1.0 | Manual | No |
| GMC, Bettiah | 600 | No data | -- | Manual | No |
| Hospital | District | Samples Received | Population (2011) | Samples per 100,000 |
|---|---|---|---|---|
| DMCH | Darbhanga | 12,144 | 3,937,385 | 308.5 |
| HBCH | Muzaffarpur | 3871 | 4,801,062 | 80.7 |
| SKMCH | Muzaffarpur | 7895 | 4,801,062 | 164.4 |
| JLNMCH | Bhagalpur | 8759 | 3,032,226 | 288.9 |
| NMCH | Patna | 15,787 | 5,772,804 | 273.4 |
| Age Group (Years) | DMCH | HBCH | SKMCH | JLNMCH | NMCH |
|---|---|---|---|---|---|
| 0–9 | 844 | 807 | 584 | 888 | 1306 |
| 10–19 | 1909 | 509 | 1018 | 1222 | 3335 |
| 20–29 | 4803 | 215 | 2897 | 2125 | 6217 |
| 30–39 | 1882 | 438 | 1789 | 1541 | 2350 |
| 40–59 | 1945 | 1123 | 1256 | 1996 | 2002 |
| 60–79 | 724 | 748 | 324 | 895 | 544 |
| >80 | 37 | 31 | 27 | 92 | 33 |
| Total | 12,144 | 3871 | 7895 | 8759 | 15,787 |
| District | Population (2011 Census) | % Rural Population | No. of PHCs | No. of CHCs | Major Disease Burden (%) |
|---|---|---|---|---|---|
| Patna | 5,772,804 | 57.6% | 84 | 14 | Diarrhea (7%), TB (3.6%), LRI (10.4%) |
| Muzaffarpur | 4,801,062 | 89.6% | 102 | 17 | Diarrhea (8%), TB (3.9%), LRI (9.8%) |
| Bhagalpur | 3,032,226 | 79.5% | 65 | 11 | Diarrhea (6.8%), TB (4.1%), LRI (8.7%) |
| Darbhanga | 3,937,385 | 85.5% | 90 | 15 | Diarrhea (7.9%), TB (4.5%), LRI (9.2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modgil, V.; Sahay, S.; Taneja, N.; Qayyumi, B.; Singh, R.; Mukherjee, A.; Bhoi, B.; Arora, G. Mapping the AMR Infection Landscape in Bihar: Implications for Strengthening Policy and Clinical Practice. Antibiotics 2025, 14, 684. https://doi.org/10.3390/antibiotics14070684
Modgil V, Sahay S, Taneja N, Qayyumi B, Singh R, Mukherjee A, Bhoi B, Arora G. Mapping the AMR Infection Landscape in Bihar: Implications for Strengthening Policy and Clinical Practice. Antibiotics. 2025; 14(7):684. https://doi.org/10.3390/antibiotics14070684
Chicago/Turabian StyleModgil, Vinay, Sundeep Sahay, Neelam Taneja, Burhanuddin Qayyumi, Ravikant Singh, Arunima Mukherjee, Bibekananda Bhoi, and Gitika Arora. 2025. "Mapping the AMR Infection Landscape in Bihar: Implications for Strengthening Policy and Clinical Practice" Antibiotics 14, no. 7: 684. https://doi.org/10.3390/antibiotics14070684
APA StyleModgil, V., Sahay, S., Taneja, N., Qayyumi, B., Singh, R., Mukherjee, A., Bhoi, B., & Arora, G. (2025). Mapping the AMR Infection Landscape in Bihar: Implications for Strengthening Policy and Clinical Practice. Antibiotics, 14(7), 684. https://doi.org/10.3390/antibiotics14070684






