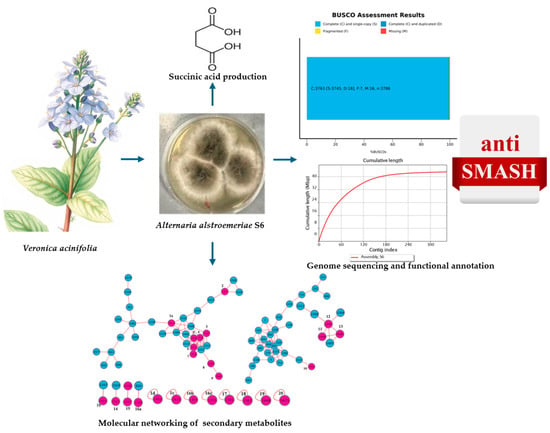
Genomic and Metabolomic Analysis of the Endophytic Fungus Alternaria alstroemeriae S6 Isolated from Veronica acinifolia: Identification of Anti-Bacterial Properties and Production of Succinic Acid
Abstract
1. Introduction
2. Results
2.1. Anti-Bacterial Activities and Molecular Identification of A. alstromeriae S6
2.2. Genome Assembly and Functional Annotation of A. alstromeriae S6
2.3. Secondary Metabolite Biosynthetic Potential of A. alstroemeriae S6
2.4. Natural Product Isolation and Metabolomic Profiling
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. High-Performance Computing Setup
4.3. Isolation of Endophytic Fungi
4.4. Extraction of Fungal Secondary Metabolites
4.5. Anti-Microbial Activity of the Extract of Fungus
4.6. Identification of Endophytic Fungi
4.7. Genome Sequencing and Assembly
4.8. Gene Prediction and Annotation
4.9. Identification of Secondary Metabolite Biosynthetic Clusters
4.10. Purification and Structure Elucidation of Succinic Acid
4.11. LC-MS/MS Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BLAST | Basic Local Alignment Search Tool |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| COG | Cluster of Orthologous Groups |
| CAZyme | Carbohydrate Activity Enzyme |
| antiSMASH | Antibiotics and Secondary Metabolite Analysis Shell |
| BGCs | Biosynthetic Gene Clusters |
| GNPS | Global Natural Products Social Molecular Networking |
References
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; International Natural Product Sciences Taskforce; Supuran, C.T. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Eshbakova, K.A.; Zakirova, R.P.; Khasanova, K.I.; Bobakulov, K.M.; Aisa, H.A.; Sagdullaev, S.S.; Nosov, A.M. Phenylpropanoids from Callus Tissue of Ajuga turkestanica. Chem. Nat. Compd. 2019, 55, 28–31. [Google Scholar] [CrossRef]
- Ashurova, L.N.; Bobakulov, K.M.; Ramazonov, N.S.; Sasmakov, S.A.; Ashirov, O.N.; Azimova, S.S.; Abdullaev, N.D. Essential Oil from the Aerial Part of Saponaria griffithiana and S. officinalis. Chem. Nat. Compd. 2021, 57, 970–972. [Google Scholar] [CrossRef]
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Ngalang, M.D.; Salleh, W.M.N.H.W.; Salihu, A.S.; Ghani, N.A.; Eshboev, F. Secondary Metabolites Isolated from the Rhizomes of Boesenbergia Albosanguinea and Its Acetylcholinesterase Inhibitory Activity. Chem. Nat. Compd. 2025, 61, 542–544. [Google Scholar] [CrossRef]
- He, T.; Li, X.; Iacovelli, R.; Hackl, T.; Haslinger, K. Genomic and Metabolomic Analysis of the Endophytic Fungus Fusarium sp. VM-40 Isolated from the Medicinal Plant Vinca minor. J. Fungi 2023, 9, 704. [Google Scholar] [CrossRef] [PubMed]
- Eshboev, F.; Karakozova, M.; Abdurakhmanov, J.; Bobakulov, K.; Dolimov, K.; Abdurashidov, A.; Baymirzaev, A.; Makhnyov, A.; Terenteva, E.; Sasmakov, S.; et al. Antimicrobial and Cytotoxic Activities of the Secondary Metabolites of Endophytic Fungi Isolated from the Medicinal Plant Hyssopus officinalis. Antibiotics 2023, 12, 1201. [Google Scholar] [CrossRef]
- Abdullaeva, Y.; Mardonova, G.; Eshboev, F.; Cardinale, M.; Egamberdieva, D. Harnessing Chickpea Bacterial Endophytes for Improved Plant Health and Fitness. AIMS Microbiol. 2024, 10, 489–506. [Google Scholar] [CrossRef]
- Rustamova, N.; Bozorov, K.; Efferth, T.; Yili, A. Novel secondary metabolites from endophytic fungi: Synthesis and biological properties. Phytochem. Rev. 2020, 19, 425–448. [Google Scholar] [CrossRef]
- Eshboev, F.; Egamberdieva, D. Medicinal plant-associated endophytic fungi: Metabolites and bioactivity. In Plant Endophytes and Secondary Metabolites; Egamberdieva, D., Parray, J.A., Davranov, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 95–104. [Google Scholar]
- Tiwari, P.; Bae, H. Endophytic Fungi: Key Insights, Emerging Prospects, and Challenges in Natural Product Drug Discovery. Microorganisms 2022, 10, 360. [Google Scholar] [CrossRef]
- Lou, J.; Fu, L.; Peng, Y.; Zhou, L. Metabolites from Alternaria Fungi and Their Bioactivities. Molecules 2013, 18, 5891–5935. [Google Scholar] [CrossRef]
- El-Hady, N.A.A.A.; Elsayed, A.I.; Wadan, K.M.; El-Saadany, S.S.; El-Sayed, A.S.A. Bioprocessing of Camptothecin from Alternaria brassicicola, an Endophyte of Catharanthus roseus, with a Strong Antiproliferative Ctivity and Inhibition to Topoisomerases. Microb. Cell Fact. 2024, 23, 214. [Google Scholar] [CrossRef]
- Nguyen, D.Q.; Nguyen, N.L.; Nguyen, V.T.; Nguyen, T.H.G.; Do, T.T.T.; Nguyen, T.H.; Dung, D.H.; Nguyen, T.K.L.; Nguyen, Q.H.; Le, T.T.; et al. Isolation and Identification of Vincristine and Vinblastine Producing Endophytic Fungi from Catharanthus roseus (L.) G. Don. Russ. J. Plant Physiol. 2023, 70, 188. [Google Scholar] [CrossRef]
- Fu, Y.; Li, X.; Yuan, X.; Zhang, Z.; Wei, W.; Xu, C.; Song, J.; Gu, C. Alternaria alternata F3, a Novel Taxol-Producing Endophytic Fungus Isolated from the Fruits of Taxus cuspidata: Isolation, Characterization, Taxol Yield Improvement, and Antitumor Activity. Appl. Biochem. Biotechnol. 2024, 196, 2246–2269. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-F.; Yue, F.; Wan, X.-R.; Li, C.; Sun, Y.; Pei, Y.-H. Antimicrobial Perylenequinones Isolated from the Endophytic Fungus Alternaria alstroemeriae. Phytochem. Lett. 2025, 65, 64–67. [Google Scholar] [CrossRef]
- Verma, V.; Srivastava, A.; Garg, S.K.; Singh, V.P.; Arora, P.K. Incorporating Omics-Based Tools into Endophytic Fungal Research. Biotechnol. Notes 2024, 5, 1–7. [Google Scholar] [CrossRef]
- Eshboev, F.; Mamadalieva, N.; Nazarov, P.A.; Hussain, H.; Katanaev, V.; Egamberdieva, D.; Azimova, S. Antimicrobial Action Mechanisms of Natural Compounds Isolated from Endophytic Microorganisms. Antibiotics 2024, 13, 271. [Google Scholar] [CrossRef]
- Cain, J.W.; Miller, K.I.; Kalaitzis, J.A.; Chau, R.; Neilan, B.A. Genome Mining of a Fungal Endophyte of Taxus yunnanensis (Chinese Yew) Leads to the Discovery of a Novel Azaphilone Polyketide, Lijiquinone. Microb. Biotechnol. 2020, 13, 1415–1427. [Google Scholar] [CrossRef]
- Ignjatović, Đ.; Živković, J.; Tovilović, G.; Šavikin, K.; Tomić, M.; Maksimović, Z.; Janković, T. Evaluation of Angiogenic and Neuroprotective Potential of Different Extracts from three Veronica species. Front. Life Sci. 2015, 8, 107–116. [Google Scholar] [CrossRef]
- Živković, J.; Barreira, J.C.M.; Stojković, D.; Ćebović, T.; Santos-Buelga, C.; Maksimović, Z.; Ferreira, I.C.F.R. Phenolic Profile, Antibacterial, Antimutagenic and Antitumour Evaluation of Veronica urticifolia Jacq. J. Funct. Foods 2014, 9, 192–201. [Google Scholar] [CrossRef]
- Saraswathi, M.; Meshram, S.H.; Siva, B.; Misra, S.; Suresh Babu, K. Isolation, Purification and Structural Elucidation of Mellein from Endophytic Fungus Lasiodiplodia Theobromae Strain (Sjf-1) and Its Broad-Spectrum Antimicrobial and Pharmacological Properties. Lett. Appl. Microbiol. 2022, 75, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Jayasuriya, H.; Silverman, K.C.; Zink, D.L.; Jenkins, R.G.; Sanchez, M.; Pelaez, F.; Vilella, D.; Lingham, R.B.; Singh, S.B. Clavaric Acid: A Triterpenoid Inhibitor of Farnesyl-Protein Transferase from Clavariadelphus truncatus. J. Nat. Prod. 1998, 61, 1568–1570. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Fang, F.; Zhang, C.G.; Zhang, X.W.; Omura, S. Heme-dependent radical generation from antimalarial fungal metaholites, radicicol and heptelidic acid. J. Antibiot. 1998, 51, 451–453. [Google Scholar] [CrossRef] [PubMed]
- Sangermano, F.; Masi, M.; Vivo, M.; Ravindra, P.; Cimmino, A.; Pollice, A.; Evidente, A.; Calabrò, V. Higginsianins A and B, Two Fungal Diterpenoid α-Pyrones with Cytotoxic Activity against Human Cancer Cells. Toxicol. Vitr. 2019, 61, 104614. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Saraswatula, V.G.; Saha, B.K. Thermal Expansion in Alkane Diacids—Another Property Showing Alternation in an Odd–Even Series. Cryst. Growth Des. 2013, 13, 3651–3656. [Google Scholar] [CrossRef]
- Liang, Y.; Xie, L.; Liu, K.; Cao, Y.; Dai, X.; Wang, X.; Lu, J.; Zhang, X.; Li, X. Bergapten: A review of its pharmacology, pharmacokinetics, and toxicity. Phytother. Res. 2021, 35, 6131–6147. [Google Scholar] [CrossRef]
- Zaher, A.M.; Moharram, A.M.; Davis, R.; Panizzi, P.; Makboul, M.A.; Calderón, A.I. Characterisation of the Metabolites of an Antibacterial Endophyte Botryodiplodia theobromae Pat. of Dracaena draco L. by LC-MS/MS. Nat. Prod. Res. 2015, 29, 2275–2281. [Google Scholar] [CrossRef]
- Robert, F.R.; Mary, P.P.; Charles, J.K.; Philip, W.Q.; Iwao, M.; Koji, N.; Janet, F.; Jon, C. Lychnopholic Acid, a Novel Trioxygenated Caryophyllene Derivative from Lychnophora Affinis Gardn. J. Am. Chem. Soc. 1978, 100, 7437–7439. [Google Scholar] [CrossRef]
- Miguel, O.G.; Lima, E.O.; Morais, V.M.F.; Gomes, S.T.A.; Delle Monache, F.; Bella Cruz, A.; Bella Cruz, R.C.; Filho, V.C. Antimicrobial Activity of Constituents Isolated from Lychnophora salicifolia (Asteraceae). Phytother. Res. 1996, 10, 694–696. [Google Scholar] [CrossRef]
- Lang, G.; Kalvelage, T.; Peters, A.; Wiese, J.; Imhoff, J.F. Linear and Cyclic Peptides from the Entomopathogenic Bacterium Xenorhabdus nematophilus. J. Nat. Prod. 2008, 71, 1074–1077. [Google Scholar] [CrossRef]
- Wu, A.; Lu, J.; Zhong, G.; Lu, L.; Qu, Y.; Zhang, C. Xanthotoxin (8-methoxypsoralen): A review of its chemistry, pharmacology, pharmacokinetics, and toxicity. Phytother. Res. 2022, 36, 3805–3832. [Google Scholar] [CrossRef] [PubMed]
- Soumoy, L.; Ghanem, G.E.; Saussez, S.; Journe, F. Bufalin for an innovative therapeutic approach against cancer. Pharmacol. Res. 2022, 184, 106442. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Chen, T.; Wang, M.; Chen, Y.; Zheng, Q.; Feng, X.; Li, S.; Wang, J. Tanshinone I Inhibits Metastasis of Cervical Cancer Cells by Inducing BNIP3/NIX-Mediated Mitophagy and Reprogramming Mitochondrial Metabolism. Phytomedicine 2022, 98, 153958. [Google Scholar] [CrossRef] [PubMed]
- Piispanen, J.; Bergmann, U.; Karhu, J.; Kauppila, T.; Witzell, J.; Kaitera, J. Diversity and Abundance of Culturable Fungal Endophytes in Leaves of Susceptible and Resistant Alternate Hosts of Cronartium pini and C. Ribicola. Eur. J. Plant Pathol. 2024, 170, 79–89. [Google Scholar] [CrossRef]
- Li, J.; Phookamsak, R.; Jiang, H.; Bhat, D.J.; Camporesi, E.; Lumyong, S.; Kumla, J.; Hongsanan, S.; Mortimer, P.E.; Xu, J.; et al. Additions to the Inventory of the Genus Alternaria Section Alternaria (Pleosporaceae, Pleosporales) in Italy. J. Fungi 2022, 8, 898. [Google Scholar] [CrossRef]
- Feng, C.; Zheng, W.; Han, L.; Wang, J.-K.; Zha, X.-P.; Xiao, Q.; He, Z.-J.; Kang, J.-C. AaLaeA Targets AaFla1 to Mediate the Production of Antitumor Compound in Alternaria alstroemeria. J. Basic Microbiol. 2024, 64, 68–80. [Google Scholar] [CrossRef]
- Xie, Q.; Jia, Y.; Tao, J.; Bu, T.; Wang, Q.; Shen, N.; Zhang, X.; Xiao, Y.; Ye, L.; Chen, Z.; et al. Chemical Constituents and Biological Activities of Endophytic Fungi from Fagopyrum dibotrys. PeerJ 2024, 12, e18529. [Google Scholar] [CrossRef]
- Ming, Q.; Huang, X.; He, Y.; Qin, L.; Tang, Y.; Liu, Y.; Huang, Y.; Zhang, H.; Li, P. Genome Mining and Screening for Secondary Metabolite Production in the Endophytic Fungus Dactylonectria alcacerensis CT-6. Microorganisms 2023, 11, 968. [Google Scholar] [CrossRef]
- Mohanta, T.K.; Bae, H. The Diversity of Fungal Genome. Biol. Proced. Online 2015, 17, 8. [Google Scholar] [CrossRef]
- Biémont, C. Genome Size Evolution: Within-Species Variation in Genome Size. Heredity 2008, 101, 297–298. [Google Scholar] [CrossRef]
- Tao, J.; Bai, X.; Zeng, M.; Li, M.; Hu, Z.; Hua, Y.; Zhang, H. Whole-Genome Sequence Analysis of an Endophytic Fungus Alternaria sp. SPS-2 and Its Biosynthetic Potential of Bioactive Secondary Metabolites. Microorganisms 2022, 10, 1789. [Google Scholar] [CrossRef]
- Garron, M.-L.; Henrissat, B. The Continuing Expansion of CAZymes and Their Families. Curr. Opin. Chem. Biol. 2019, 53, 82–87. [Google Scholar] [CrossRef]
- Aghdam, S.A.; Brown, A.M.V. Deep Learning Approaches for Natural Product Discovery from Plant Endophytic Microbiomes. Environ. Microbiome 2021, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Blin, K.; Kim, H.U.; Medema, M.H.; Weber, T. Recent development of antiSMASH and other computational approaches to mine secondary metabolite biosynthetic gene clusters. Brief. Bioinform. 2019, 20, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Deepika, V.B.; Murali, T.S.; Satyamoorthy, K. Modulation of genetic clusters for synthesis of bioactive molecules in fungal endophytes: A review. Microbiol. Res. 2016, 182, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Li, J.; Gao, H.; Zhou, D.; Xu, H.; Cong, Y.; Zhang, W.; Xin, F.; Jiang, M. Recent Progress on Bio-Succinic Acid Production from Lignocellulosic Biomass. World J. Microbiol. Biotechnol. 2021, 37, 16. [Google Scholar] [CrossRef]
- Chae, T.U.; Ahn, J.H.; Ko, Y.-S.; Kim, J.W.; Lee, J.A.; Lee, E.H.; Lee, S.Y. Metabolic engineering for the production of dicarboxylic acids and diamines. Metab. Eng. 2020, 58, 2–16. [Google Scholar] [CrossRef]
- Sun, T.; Sun, M.-L.; Lin, L.; Gao, J.; Wang, K.; Ji, X.-J. Advancing Succinic Acid Biomanufacturing Using the Nonconventional Yeast Yarrowia lipolytica. J. Agric. Food Chem. 2025, 73, 100–109. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, P.; Maity, S.K.; Agrawal, D.; Narisetty, V.; Jacob, S.; Kumar, G.; Bhatia, S.K.; Kumar, D.; Vivekanand, V. Recent Advances in Bio-Based Production of Top Platform Chemical, Succinic Acid: An Alternative to Conventional Chemistry. Biotechnol. Biofuels Bioprod. 2024, 17, 72. [Google Scholar] [CrossRef]
- Huang, S.; Chen, X.; Yan, R.; Huang, M.; Chen, D. Isolation, Identification and Antibacterial Mechanism of the Main Antibacterial Component from Pickled and Dried Mustard (Brassica juncea Coss. Var. foliosa Bailey). Molecules 2022, 27, 2418. [Google Scholar] [CrossRef] [PubMed]
- Du, S.-Y.; Huang, H.-F.; Li, X.-Q.; Zhai, L.-X.; Zhu, Q.-C.; Zheng, K.; Song, X.; Xu, C.-S.; Li, C.-Y.; Li, Y.; et al. Anti-inflammatory properties of uvaol on DSS-induced colitis and LPS-stimulated macrophages. Chin. Med. 2020, 15, 43. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-inflammatory action and mechanisms of resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yang, Q.; Wang, Z.; Ding, Q.; Li, M.; Fang, Y.; He, Q.; Zhu, Y.Z. The anti-inflammation and anti-nociception effect of ketoprofen in rats could be strengthened through co-delivery of a H2S donor, s-propargyl-cysteine. J. Inflamm. Res. 2021, 14, 5863–5875. [Google Scholar] [CrossRef]
- Ziyadullaev, M.; Karimov, R.; Abdurazakhov, A.; Parmanov, A.; Sasmakov, S.; Abdurakhmanov, J.; Eshboev, F.; Azimova, S. Synthesis of 6-substituted 3(H)-quinazolin-4-ones and their antimicrobial activity. Pharm. Chem. J. 2023, 57, 373–377. [Google Scholar] [CrossRef]
- Adizov, S.M.; Ziyavitdinov, J.F.; Tashkhodjaev, B.; Aripova, S.F.; Eshboev, F.B.; Azimova, S.S. Alkaloids of a Cultivar of Catharanthus roseus, Their Biological Activity, and Crystal Structures of Ajmalicine and Vindolinine. Chem. Nat. Compd. 2024, 60, 1203–1206. [Google Scholar] [CrossRef]
- Nurunnabi, T.R.; Sarwar, S.; Sabrin, F.; Alam, F.; Nahar, L.; Sohrab, H.; Billah, M. Molecular Identification and Antimicrobial Activity of Endophytic Fungi Isolated from Heritiera fomes (Buch.-Ham), a Mangrove Plant of the Sundarbans. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 61. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Prjibelski, A.; Antipov, D.; Meleshko, D.; Lapidus, A.; Korobeynikov, A. Using SPAdes DE Novo Assembler. Curr. Protoc. Bioinform. 2020, 70, e102. [Google Scholar] [CrossRef]
- Mikheenko, A.; Prjibelski, A.; Saveliev, V.; Antipov, D.; Gurevich, A. Versatile Genome Assembly Evaluation with QUAST-LG. Bioinformatics 2018, 34, i142–i150. [Google Scholar] [CrossRef]
- Manni, M.; Matthew, R.; Berkeley, M.; Seppey, F.A.; Evgeny, M. Evgeny M Zdobnov, BUSCO update: Novel and streamlined workflows along with broader and deeper phylogenetic coverage for scoring of eukaryotic, prokaryotic, and viral genomes. Mol. Biol. Evol. 2021, 38, 4647–4654. [Google Scholar] [CrossRef]
- Stanke, M.; Morgenstern, B. AUGUSTUS: A Web Server for Gene Prediction in Eukaryotes That Allows User-Defined Constraints. Nucleic Acids Res. 2005, 33, W465–W467. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Binns, D.; Chang, H.-Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Del Río, R.; Giner-Lamia, Á.; Cantalapiedra, J.; Botas, C.P.; Deng, J.; Hernández-Plaza, Z.; Munar-Palmer, A.; Santamaría-Hernando, M.; Rodríguez-Herva, S.; Ruscheweyh, J.J.; et al. Functional and Evolutionary Significance of Unknown Genes from Uncultivated Taxa. Nature 2024, 626, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Cepas, J.; Szklarczyk, D.; Heller, D.; Hernández-Plaza, A.; Forslund, S.K.; Cook, H.; Mende, D.R.; Letunic, I.; Rattei, T.; Jensen, L.J.; et al. eggNOG 5.0: A Hierarchical, Functionally and Phylogenetically Annotated Orthology Resource Based on 5090 Organisms and 2502 Viruses. Nucleic Acids Res. 2019, 47, D309–D314. [Google Scholar] [CrossRef]
- Moriya, Y.; Itoh, M.; Okuda, S.; Yoshizawa, A.C.; Kanehisa, M. KAAS: An Automatic Genome Annotation and Pathway Reconstruction Server. Nucleic Acids Res. 2007, 35, W182–W185. [Google Scholar] [CrossRef]
- Zheng, J.; Ge, Q.; Yan, Y.; Zhang, X.; Huang, L.; Yin, Y. dbCAN3: Automated Carbohydrate-Active Enzyme and Substrate Annotation. Nucleic Acids Res. 2023, 51, W115–W121. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Vader, L.; Szenei, J.; Reitz, Z.L.; Augustijn, H.E.; Cediel-Becerra, J.D.D.; de Crécy-Lagard, V.; Koetsier, R.A.; Williams, S.E.; et al. antiSMASH 8.0: Extended Gene Cluster Detection Capabilities and Analyses of Chemistry, Enzymology, and Regulation. Nucleic Acids Res. 2025, 53, W32–W38. [Google Scholar] [CrossRef]
- Zdouc, M.M.; Blin, K.; Louwen, N.L.L.; Navarro, J.; Loureiro, C.; Bader, C.D.; Bailey, C.B.; Barra, L.; Booth, T.J.; Bozhüyük, K.A.J.; et al. MIBiG 4.0: Advancing Biosynthetic Gene Cluster Curation through Global Collaboration. Nucleic Acids Res. 2025, 53, D678–D690. [Google Scholar] [CrossRef]
- Schmid, R.; Heuckeroth, S.; Korf, A.; Smirnov, A.; Myers, O.; Dyrlund, T.S.; Bushuiev, R.; Murray, K.J.; Hoffmann, N.; Lu, M.; et al. Integrative Analysis of Multimodal Mass Spectrometry Data in MZmine 3. Nat. Biotechnol. 2023, 41, 447–449. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with global natural products social molecular networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]







| Samples | Inhibition Zone (Mean ± SE, n = 3) a | ||||
|---|---|---|---|---|---|
| Gram-Positive Bacteria | Gram-Negative Bacteria | Fungus | |||
| B. subtilis | S. aureus | E. coli | P. aeruginosa | C. albicans | |
| EtOAc extract b | 26.5 ± 0.5 | 21.3 ± 0.75 | 16.25 ± 0.5 | 22.6 ± 0.5 | Not active c |
| Ampicillin d | 30.4 ± 0.15 | 26.45 ± 0.2 | Not tested | Not tested | Not tested |
| Gentamicin | Not tested | Not tested | 25.15 ± 0.25 | 26.5 ± 0.3 | Not tested |
| Fluconazole | Not tested | Not tested | Not tested | Not tested | 33.0 ± 0.2 |
| Genome Features | Value |
|---|---|
| Sequencing coverage a | 73× |
| Total assembly size (Mb) b | 42.93 |
| Number of contigs c | 344 |
| Largest contig d | 882,804 |
| N50 e | 301,538 |
| N90 f | 78,886 |
| L50 g | 40 |
| L90 h | 145 |
| GC content i (%) | 54.09 |
| BUSCO j (%) | 99.7 |
| Protein-coding genes k | 13,885 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eshboev, F.; Gao, A.X.; Abdurashidov, A.; Mardieva, K.; Baymirzaev, A.; Musakhanov, M.; Yusupova, E.; Lin, S.; Yang, M.; Dong, T.T.X.; et al. Genomic and Metabolomic Analysis of the Endophytic Fungus Alternaria alstroemeriae S6 Isolated from Veronica acinifolia: Identification of Anti-Bacterial Properties and Production of Succinic Acid. Antibiotics 2025, 14, 713. https://doi.org/10.3390/antibiotics14070713
Eshboev F, Gao AX, Abdurashidov A, Mardieva K, Baymirzaev A, Musakhanov M, Yusupova E, Lin S, Yang M, Dong TTX, et al. Genomic and Metabolomic Analysis of the Endophytic Fungus Alternaria alstroemeriae S6 Isolated from Veronica acinifolia: Identification of Anti-Bacterial Properties and Production of Succinic Acid. Antibiotics. 2025; 14(7):713. https://doi.org/10.3390/antibiotics14070713
Chicago/Turabian StyleEshboev, Farkhod, Alex X. Gao, Akhror Abdurashidov, Kamila Mardieva, Asadali Baymirzaev, Mirzatimur Musakhanov, Elvira Yusupova, Shengying Lin, Meixia Yang, Tina T. X. Dong, and et al. 2025. "Genomic and Metabolomic Analysis of the Endophytic Fungus Alternaria alstroemeriae S6 Isolated from Veronica acinifolia: Identification of Anti-Bacterial Properties and Production of Succinic Acid" Antibiotics 14, no. 7: 713. https://doi.org/10.3390/antibiotics14070713
APA StyleEshboev, F., Gao, A. X., Abdurashidov, A., Mardieva, K., Baymirzaev, A., Musakhanov, M., Yusupova, E., Lin, S., Yang, M., Dong, T. T. X., Sagdullaev, S., Azimova, S., & Tsim, K. W. K. (2025). Genomic and Metabolomic Analysis of the Endophytic Fungus Alternaria alstroemeriae S6 Isolated from Veronica acinifolia: Identification of Anti-Bacterial Properties and Production of Succinic Acid. Antibiotics, 14(7), 713. https://doi.org/10.3390/antibiotics14070713