Abstract
Objectives: To establish the risk of being a carrier of multidrug-resistant bacteria (MDR) upon ICU admission, according to the risk factors (RFs) from the Spanish “Resistencia Zero” (RZ) project checklist, using machine learning methodology. Methods: A retrospective cohort study, conducted with a consecutive sample of patients admitted to the ICU between 2014 and 2016. The study analyzed the RZ RFs for MDR, as well as other pathological variables and comorbidities. The study group was randomly divided into a development group (70%) and a validation group (30%). Several machine learning models were used: binary logistic regression, CHAID-type decision tree, and the XGBOOST methodology (version 2.1.0) with SHAP analysis. Results: Data from 2459 patients were analyzed, of whom 210 (8.2%) were carriers of MDR. The risk grew with the accumulation of RF. Binary logistic regression identified colonization or previous infection by MDR, prior antibiotic treatment, living in a nursing home, recent hospitalization, and renal failure as the most influential factors. The CHAID tree detected MDR in 56% of patients with previous colonization or infection, a figure that increased to almost 74% if they had also received antibiotic therapy. The XGBOOST model determined that variables related to antibiotic treatment were the most important. Conclusions: The RZ RFs have limitations in predicting MDR upon ICU admission, and machine learning models offer certain advantages. Not all RFs have the same importance, but their accumulation increases the risk. There is a group of patients with no identifiable RFs, which complicates decisions on preventive isolation.
1. Introduction
Multidrug-resistant bacteria (MDR) are an escalating concern and are considered a priority in public health. They have been linked to severe infections with poor therapeutic outcomes, increased hospital stays, and higher healthcare costs [1,2,3,4,5,6]. Intensive care units (ICUs) have been identified as major contributors to the emergence of MDR within hospitals, making them key targets for controlling these pathogens [7]. However, epidemiological surveillance studies have shown that a considerable proportion of patients are already carriers of MDR upon ICU admission, either as an infection or colonization [8,9,10].
In response, the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (SEMICYUC), supported by the Ministry of Health, launched the “Resistencia Zero” (RZ) project in 2014. This initiative aimed to reduce the incidence of ICU-acquired infections caused by MDR pathogens by 20%. Secondary objectives included understanding the epidemiology of MDR infections in Spanish ICUs, distinguishing between imported and ICU-acquired MDR cases, promoting and strengthening safety policies within ICUs, and implementing evidence-based safe practices [11,12].
RZ project recommendations include active screening for MDR in all patients upon ICU admission and at least weekly thereafter. Contact precaution measures—hand hygiene, disposable gloves and gowns, and, if possible, single-patient rooms—should be applied to patients at high risk of carrying MDR according to a risk factor (RF) checklist. This checklist includes six recognized RFs: recent hospital admission and antibiotic therapy, prior MDR carriage, renal replacement therapy, and certain chronic conditions that increase colonization risk, such as chronic ulcers and cystic fibrosis.
However, as demonstrated in some studies [8,9], the sensitivity and specificity of these RFs, recognized in the literature and the RZ checklist, are insufficient to efficiently prevent MDR transmission and guide empirical antibiotic therapy.
The application of machine learning (ML) techniques, using variables present at ICU admission, may provide better risk stratification for MDR carriage. Among ML techniques, we aim to use those with clinical interpretability, an appealing feature for their implementation in clinical settings [13].
The goal of this study is to determine the risk of MDR carriage at ICU admission based on the RF checklist from the RZ project, using machine learning methodology.
2. Results
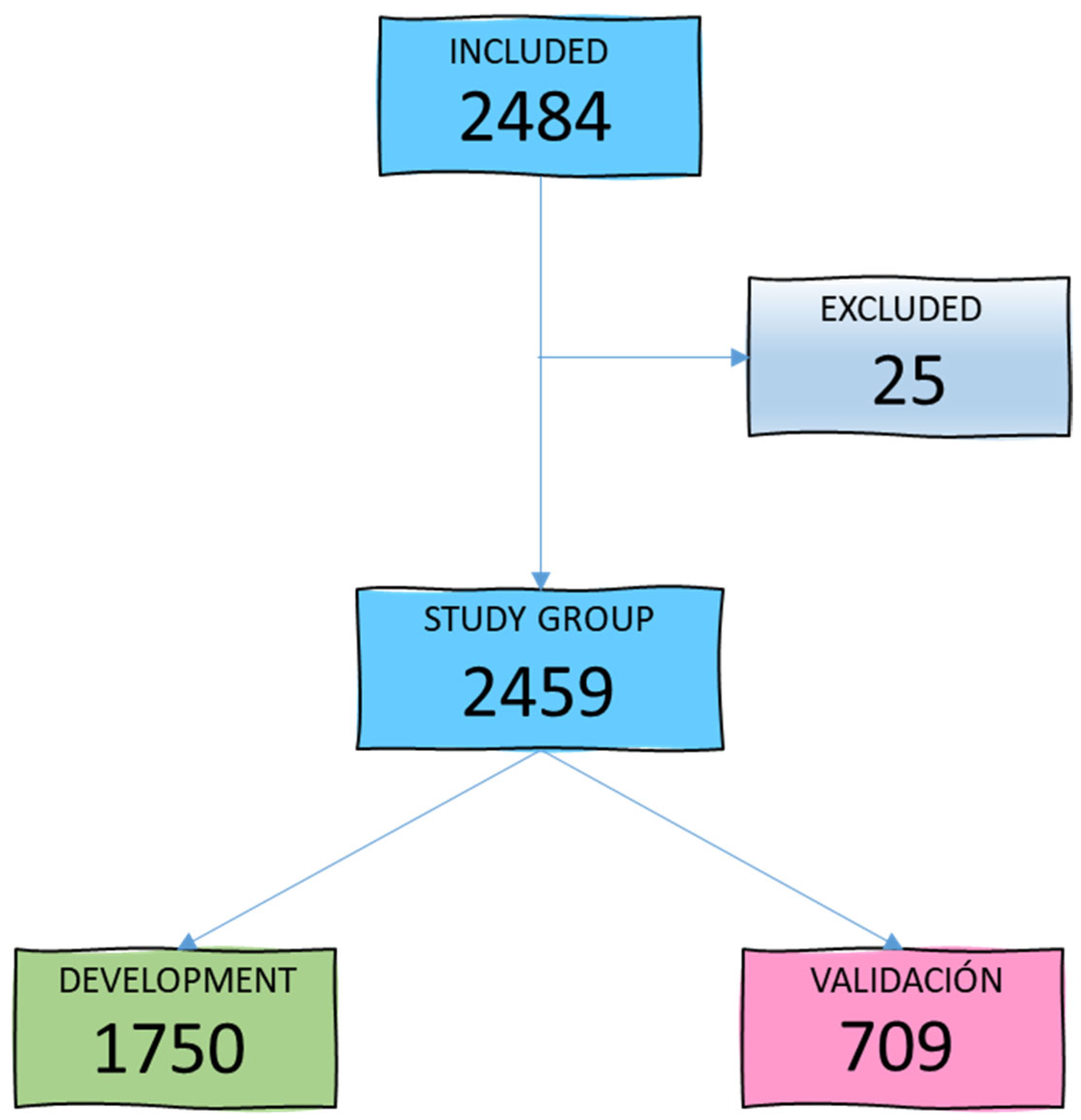
During the study period, 2484 patients aged between 15 and 98 years (mean age 59.4 years) were admitted to our ICU. A total of 25 patients were excluded due to loss of follow-up or lack of microbiological cultures within the first 48 h of admission, leaving 2459 patients for the study group. Figure 1 illustrates the patient selection diagram for the development (GD) and validation (GV) groups.
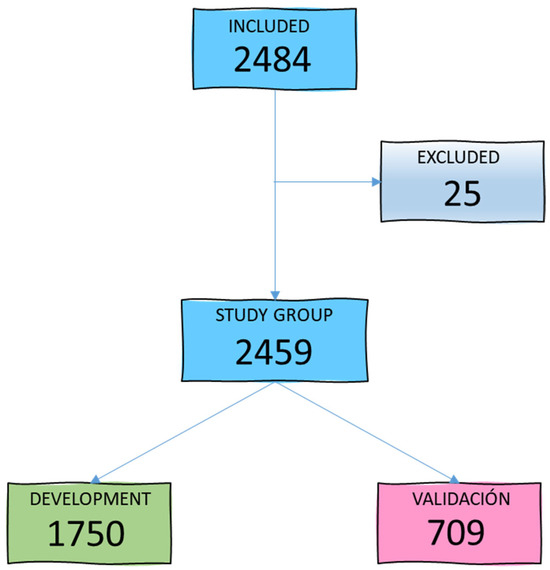
Figure 1.
Flowchart of study group selection.
Of the patients, 62.9% (1547) were male. Among all admissions, 803 (32.7%) met one or more criteria from the RZ checklist, leading to the application of contact precautions for these patients. Table 1 describes the demographic characteristics of the GD and GV groups. Approximately one-quarter of ICU admissions presented at least one risk factor for MDR colonization, with prior hospitalization being the most frequent RF, followed by prior antibiotic use.

Table 1.
Demographic characteristics of ICU patients (n = 2459), by Development and Validation groups.
A total of 210 patients (8.2%) were identified as MDR carriers, with 222 MDRs isolated (some patients carried more than one MDR). Among these, 92 patients (43.8%) carried extended-spectrum beta-lactamase-producing Enterobacterales (ESBL), 78 (37.1%) carried methicillin-resistant Staphylococcus aureus (MRSA), 24 (11.4%) carried MDR Pseudomonas aeruginosa, 13 (6.2%) carried Acinetobacter baumannii, 5 (2.4%) carried carbapenemase-producing bacteria, and 10 (4.8%) carried other MDR Gram-negative bacteria. In 57 cases (27.1%), MDR presence was associated with infection; in the remainder, it represented colonization. Table 2 details the characteristics of patients with isolated MDR in the GD.

Table 2.
Demographic characteristics of ICU patients according to the isolated MDR organism (n = 210).
Of the MDR carriers, 132 patients (62.8%) had risk criteria according to the RZ checklist. Among these, 50 patients (38%) had one RF, 53 (40%) had two, and 29 (22%) had three or more RFs, indicating an accumulation of risk. The presence of more RFs correlated with a higher probability of MDR carriage (p < 0.001). In 78 MDR carriers (37%), none of the RFs from the RZ project were identified. Table 3 also provides univariable risk values (odds ratios with 95% confidence intervals) and highlights candidate variables for inclusion in multivariable models.

Table 3.
Demographic characteristics of the development group (n = 1750), according to detection of MDR carriers.
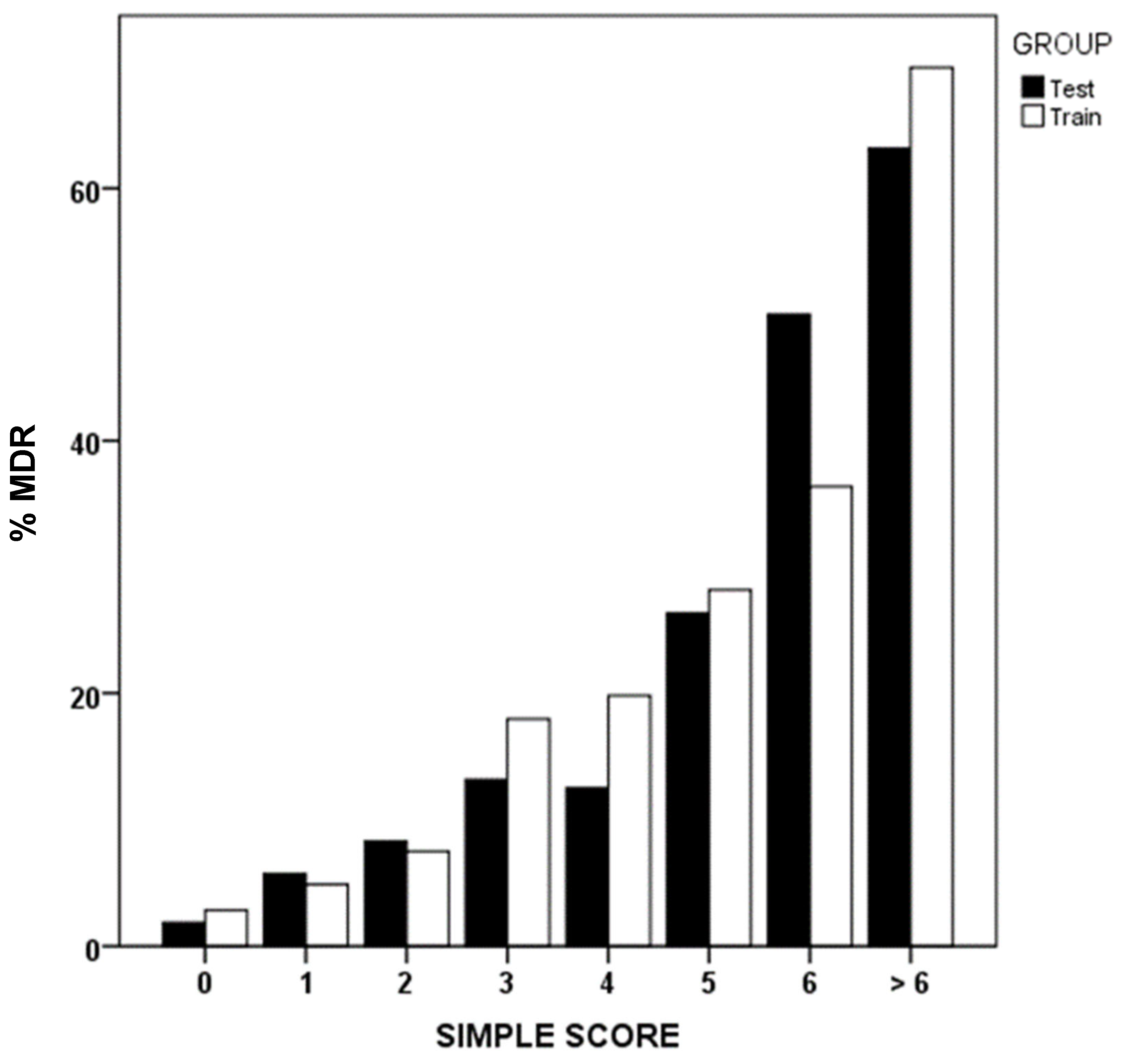
Using binary logistic regression, we identified that prior MDRB colonization or infection, prior antibiotic use, institutionalization, recent hospitalization, and renal failure were the most influential factors associated with MDR presence upon ICU admission (Table 4). Applying the simple score methodology, scores are assigned to each of these variables, reflecting the order of importance and weight of each one as a risk factor. Figure 2 shows MDR prevalence in GD and GV groups, based on the sum of the simple score.

Table 4.
Logistic regression model of factors influencing the presence of MDR bacteria.
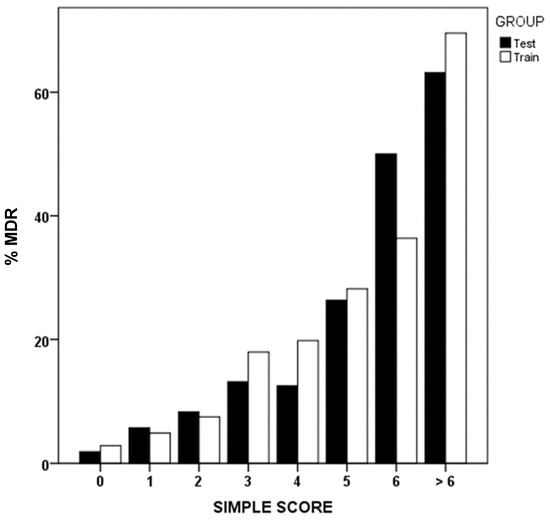
Figure 2.
Simple score values and percentage of multidrug-resistant bacteria (MDR). Development (test) and validation (train) groups.
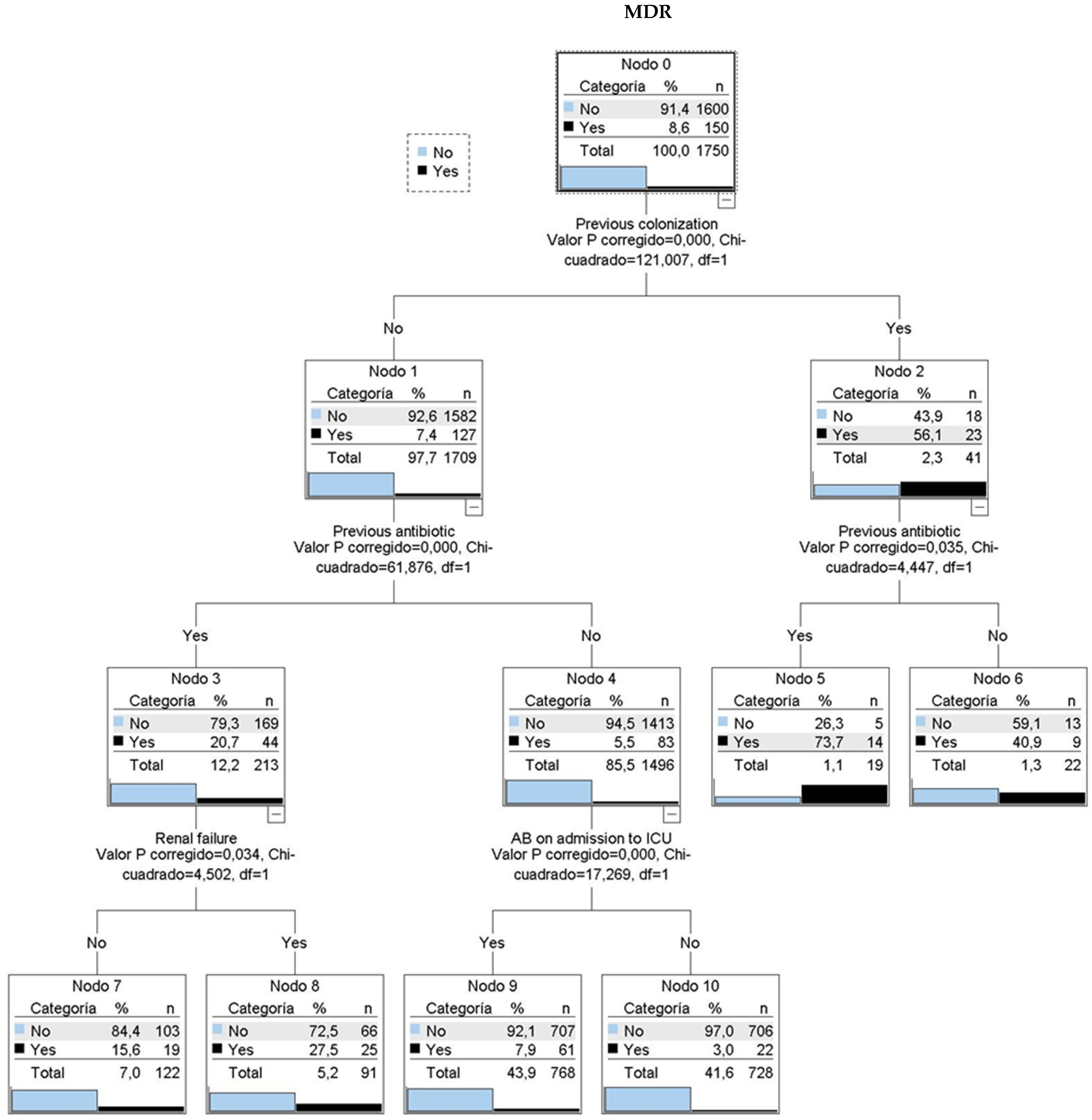
A CHAID classification tree (Figure 3) identified four key variables: prior colonization, prior antibiotic use, renal failure, and indication for antibiotic therapy at ICU admission. Among patients with prior colonization or infection, 56% had MDR upon ICU admission. This percentage increased to nearly 74% with prior antibiotic use. Patients without prior colonization showed 7.4% MDR prevalence, which rose to 20.7% with prior antibiotic use and 27.5% with additional renal failure history.
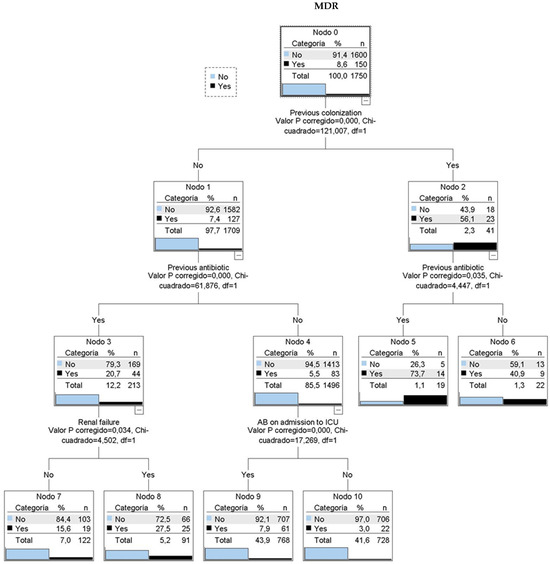
Figure 3.
CHAID classification tree. 6 classification rules. MDR: multidrug resistant bacteria. AB: antibiotic treatment. Nodo: node. Categoria: category. Valor P corregido: adjusted p-value. Chi-cuadrado: Chi-square.
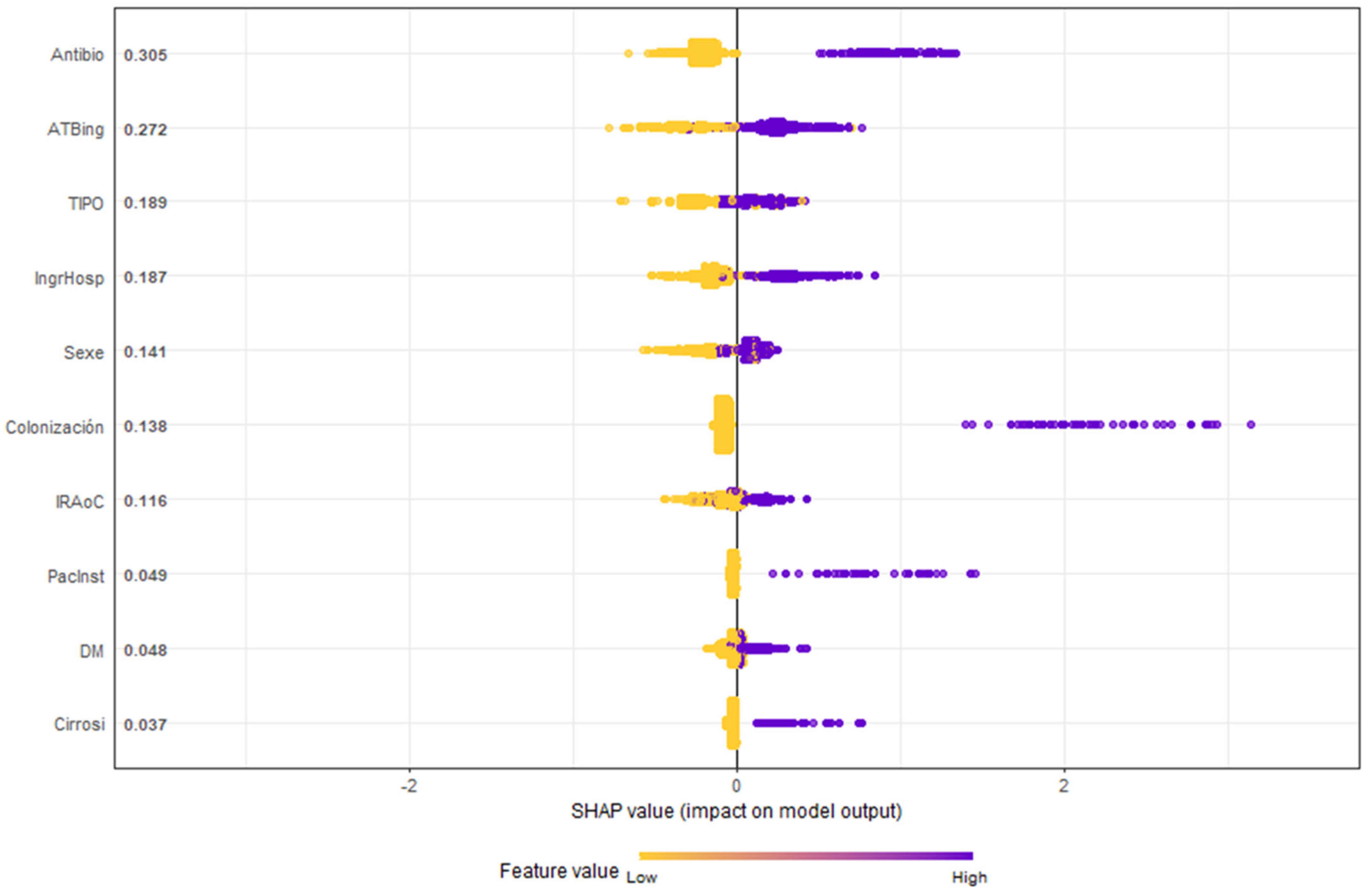
Figure 4 highlights the SHAP methodology, ranking variables by importance. Notably, antibiotic use before or at ICU admission emerged as the most significant RF.
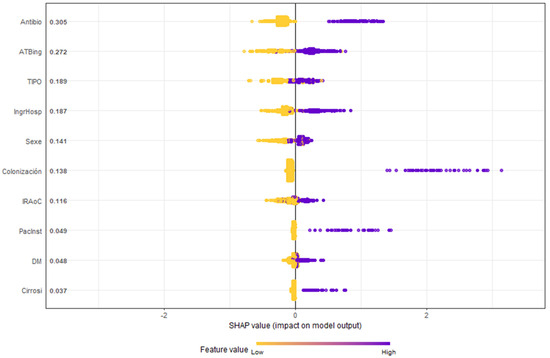
Figure 4.
Honeycomb chart shows the evaluation of the variables of the GXBOOST model with SHAP analysis. Antibio: previous antibiotic therapy (7 days in prior month); ATBing: antibiotic therapy upon ICU admission; TIPO: type, urgent medical or surgical diagnosis; IngrHosp: previous hospital admission (5 days in prior 3 months); Sexe: male; Colonización: colonization by MDR; IRAoC: acute or chronic renal failure; PacInst: residence in a nursing home; DM: diabetes mellitus; cirrosi: hepatic cirrhosis.
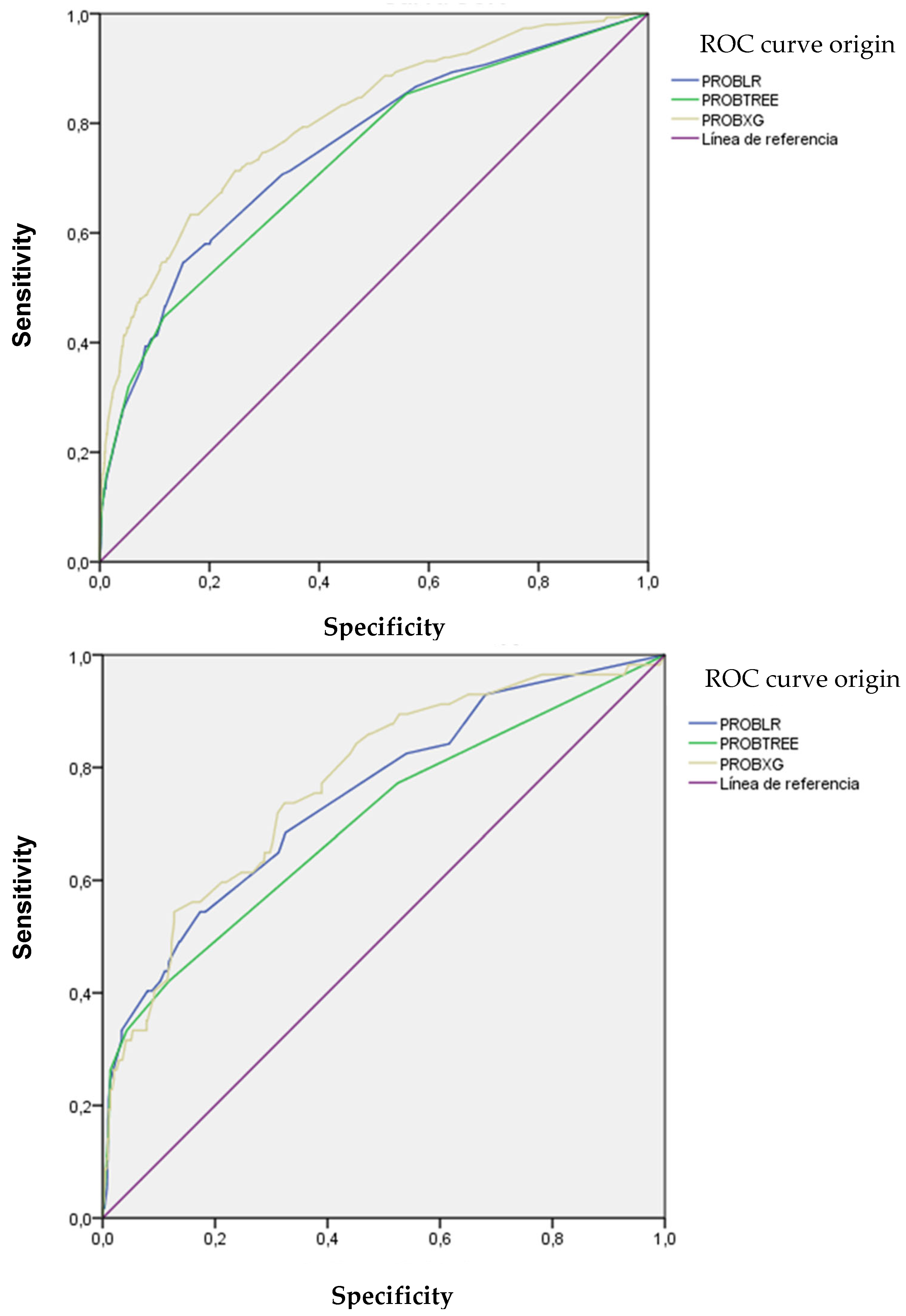
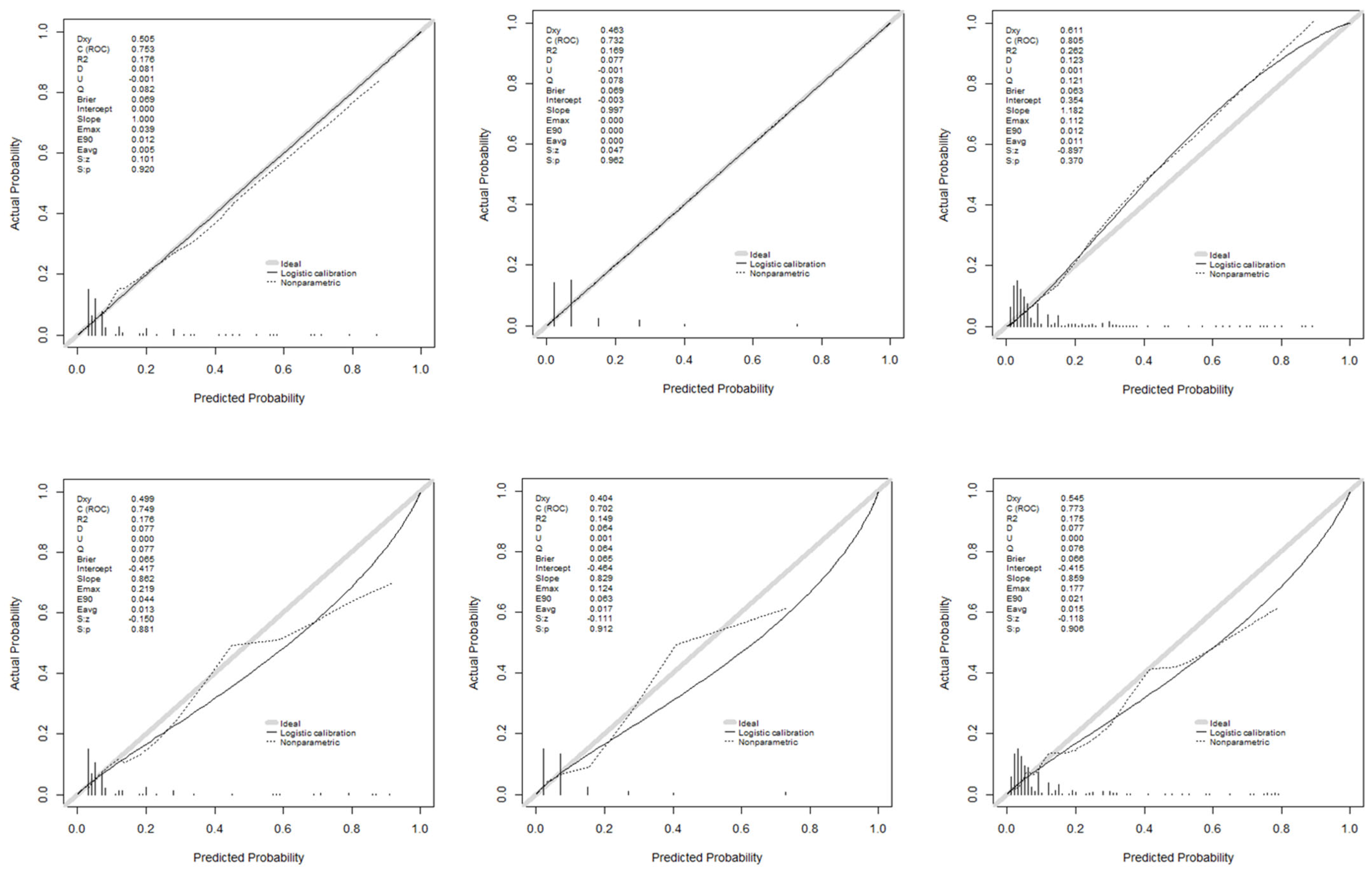
Figure 5 illustrates the discriminatory capacity of the models in GD and GV, measured by the area under the ROC curve (AUROC). The XGBoost model achieved the highest values, maintaining discriminatory power in GV. Figure 6 presents calibration curves, which show acceptable values, albeit with some loss of calibration in GV.
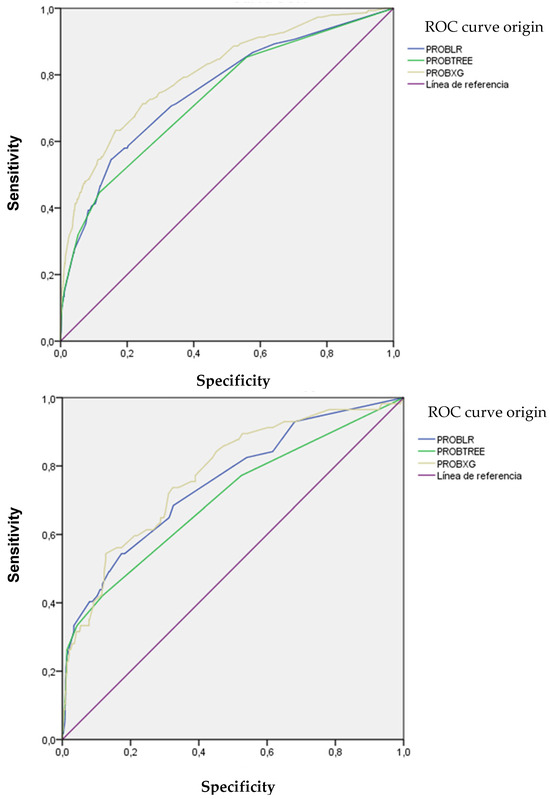
Figure 5.
ROC and AUC curves of the models used according to development and validation groups.
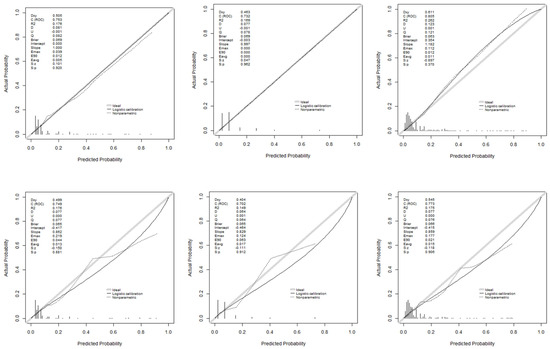
Figure 6.
Calibration curves of the models used: development and validation groups.
Applying the RZ criteria, isolating 32.7% of patients effectively captured 62.8% of MDR carriers. A simple score > 1 improved MDR identification to 70.5% while isolating 37.1% of patients. Using the CHAID tree without the antibiotic indication variable, isolating 14.4% of patients achieved 56% MDR identification. Adding this variable increased isolation to 57.3% and detection to 83.1%. This left 1051 patients undetected by these criteria, of whom 35 were MDR carriers. Annex 1 describes these 1051 patients (35 MDR vs. 1016 non-MDR). Undetected MDRs were associated with male gender, unplanned admissions, higher APACHE II scores, and no significant difference in mortality.
3. Discussion
The timely identification of MDR upon admission to ICU has clinical and epidemiological implications. Early detection allows for the rapid initiation of appropriate infection control measures, such as selective isolation and contact precautions, which are essential for reducing cross-transmission and subsequent ICU-acquired infections [14,15]. Rapid identification also facilitates timely and appropriate antimicrobial treatment, which is associated with better patient outcomes, including lower mortality and shorter ICU stays [16,17].
When a patient is admitted to the ICU, we can add to the problems caused by their illness if we isolate them because they may be a carrier of MDR. While it is true that isolation and other contact precautions reduce the transmission of MDR, protecting healthcare workers and other patients and preventing outbreaks [18], it also has undesirable effects, such as negative psychological impact, impaired care, adverse reactions, and increased costs related to human, material, and logistical resources [19,20,21,22,23,24]. Patients, especially if they are conscious, tend to become socially and emotionally isolated and may perceive their admission as more depersonalized. Therefore, isolating all patients upon admission to the ICU is not an optimal strategy, but it is necessary to identify patients at higher risk of being MDR carriers in order to optimize healthcare and improve the risk–benefit ratio of contact precautions [25,26,27,28].
The isolation criteria of the RZ project achieve acceptable but not optimal performance: one-third of patients who were MDR carriers were not isolated. A study in Spain published in 2021 [8] reports the unnecessary isolation of almost 70% of patients with RF according to the RZ project. These authors objectively state that a history of colonization or infection by an MDR was the only RF associated with the presence of an MDR upon admission to the ICU. On the other hand, in the Padilla-Serrano series [10], antibiotic therapy prior to admission to the ICU and admission after surgery were the main RFs for rectal colonization by ESBL-producing Enterobacterales. Therefore, it is necessary to look for other models to identify all (or almost all) patients who will be MDR carriers at the time of admission to the ICU. With our models, we improved the number of identifications, although they would require us to isolate a higher percentage of patients upon admission to the unit.
We have detected a significant number of patients with MDR who did not present specific RFs included in the RZ project checklist. For example, in approximately half of the patients in whom MRSA and A. baumannii were detected, we were unable to identify any risk factors. It is necessary to know the specific profile of each ICU, based on the premise that the really important information is the incidence of infections and MDR in a unit at a specific time [25]. The ENVIN-HELICS program, with a computerized database for nosocomial infection surveillance applicable to Spanish ICUs, achieves this objective [29,30,31,32].
Not all MDR species have the same RFs for colonizing or infecting a patient. We have also found that not all RFs are equally important, as described in some other studies [10,25,33,34,35]. In addition, our results show that it is easier to assign a higher probability of being an MDR carrier when there is a history of previous colonization, in patients with prolonged hospital stays, or recent use of antibiotic therapy. Our simple score model finds RFs that are more important, such as a history of being an MDR carrier, and that the accumulation of risk factors increases the probability of being an MDR carrier upon admission to the ICU.
The decision rules provided by the CHAID tree model are easy to interpret and help us to identify groups of patients in whom preventive isolation is effective or subgroups of patients who are more problematic in terms of identifying risk factors.
A more complex model, such as XGBOOST, finds better discrimination but becomes a black box when we want to interpret how it classifies risk groups. The SHAP methodology helps us to give importance to variables and provides some explanation of the model. The RF hierarchy it shows helps us to give importance to the use of antibiotics, both before and upon admission to the ICU, as an RF for identifying groups of patients with a high probability of being MDR carriers.
In summary, in our study, we propose machine learning techniques to improve existing predictive models for early detection of MDR upon admission to the ICU. In our series, if we use the RZ risk factor criteria, isolating 32.7% of patients results in effective isolation in 62.8% of MDR carriers. If we use the criterion of a value greater than 1 in the simple score, isolating 37.1% of patients results in an MDR yield of 70.5%. If we use the CHAID tree without the variable “antibiotic treatment” on admission to the ICU, isolating 14.4% of patients gives us a BMR yield of 56%. If we add that variable, we isolate 57.3% of patients to obtain a BMR yield of 83.1%.
Our work has some limitations, such as having been carried out in a single center and the limited sample size, although it was greater than 2000 patients. Other machine learning models could also have been used, but we chose those with the ability to interpret or explain the importance of the RFs found. Our results will need to be validated in other units.
4. Materials and Methods
This was a retrospective, observational study conducted at a single center, the Intensive Care Unit of Arnau de Vilanova University Hospital in Lleida (HUAV), a 22-bed multidisciplinary ICU. Data were collected from April 2014 to December 2016, during the implementation of the RZ program in Spain.
Patients included were those admitted to the ICU who underwent active MDR screening through mucosal swabs (nasal, pharyngeal, axillary, rectal) within the first 48 h, as per RZ recommendations, in addition to diagnostic cultures from clinical samples (blood, urine, sputum, tracheal or bronchoalveolar aspirates, surgical wound swabs, or others) based on medical criteria. Patients under 15 years old and those without microbiological cultures performed were excluded.
Patients and/or their families were informed about the microbiological procedures and preventive isolation policy. The HUAV ethics committee (CEIC-3025) approved the study. The development of the models followed the recommendations from the Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD) initiative [36].
The study group was randomly divided into a development group (DG) and validation group (VG) (70:30). A patient was considered an MDR carrier at admission if any of the surveillance cultures or clinical samples collected within the first 48 h tested positive for MDR. MDR bacteria included methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus spp. (VRE), extended-spectrum beta-lactamase-producing Enterobacterales (ESBL), carbapenemase-producing gram-negative bacteria, multidrug-resistant Pseudomonas aeruginosa (resistant to more than three common antibiotic families), and carbapenem-resistant Acinetobacter baumannii. Other gram-negative bacteria resistant to three or more antibiotic families or producing other resistance mechanisms, such as AMP-C or Stenotrophomonas maltophilia, were classified as “others” [11,12].
Variables collected at ICU admission included patient data from the ENVIN-HELICS registry [29] (available at http://hws.vhebron.net/envin-helics/, accessed on 31 March 2024): age, sex, diabetes mellitus (DM), acute or chronic renal failure, immunosuppression, previous malignancy, liver cirrhosis, chronic obstructive pulmonary disease (COPD), malnutrition, and organ transplant. Other data included the source of admission (community, nursing home, other institution, hospital ward, or another ICU), reason for admission (medical, elective surgery, emergency surgery, trauma, or coronary), and whether antibiotic treatment was indicated at ICU admission.
The RZ RFs included hospitalization for more than five days in the last three months, institutionalization, history of MDR carriage, antibiotic therapy for more than seven days in the month before admission, hemodialysis or peritoneal dialysis, and chronic conditions with a high incidence of colonization/infection by MDR (cystic fibrosis, bronchiectasis, chronic ulcers, etc.) [11,12].
Models were developed in the DG and validated in the VG.
Binary logistic regression was used for variable selection, including those with a univariable p-value < 0.1 in the multivariable model. A stepwise approach selected significant variables, and coefficients were rounded to the nearest integer for a scoring system (simple score).
A decision tree model using CHAID (chi-square automatic interaction detection) employed cross-validation (five partitions) and a stopping rule with a minimum terminal node size of 10 records.
An XGBoost model was also developed using gradient-boosted classification trees to improve total error. Parameters included a maximum tree depth of 4 and a learning rate of 0.05. The SHAP (SHapley Additive exPlanations) analysis was used to interpret variable importance in the XGBoost model [37].
The models’ accuracy was assessed in both DG and VG by calculating sensitivity (S), specificity (E), positive predictive value (PPV), negative predictive value (NPV), and correct classification percentage. Discrimination was assessed using ROC curves and the area under the curve (AUC), while calibration was evaluated with calibration curves.
Statistical analysis described categorical variables as percentages and continuous variables as medians (interquartile range) due to non-normal distribution (Kolmogorov–Smirnov test). The Mann–Whitney test was used for continuous variables, and the chi-square test for categorical variables, with p < 0.05 considered significant. Analyses were performed using SPSS software (version 29.0) and R statistics 4.0.3 with the lrm and SHAPforxgboost packages (version 0.1.3).
5. Conclusions
In conclusion, we can say that the risk factors on the checklist that lead to the preventive isolation of patients upon admission to the ICU according to the RZ project have limitations, and the models created with machine learning offer certain advantages. Not all risk factors are equally important, and the decision rules provided by the classification trees identify groups of patients with specific characteristics. The use of antibiotics, both before and upon admission to the ICU, is an RF to be considered. There is a group of patients, whose specific characteristics may vary in each ICU, in whom we did not find any identifiable RFs to consider preventive isolation. And finally, the decision to perform more or fewer isolations, balancing the sensitivity and specificity of the RF and the presented tools, should be made in each unit, depending on the prevalence of MRB, with stricter criteria in settings with high colonization rates.
Author Contributions
Conceptualization, M.P.M., J.T.C. and S.C.-B.; methodology, M.P.M. and J.T.C.; software, J.T.C. and S.C.-B.; validation, M.P.M., J.T.C. and X.N.C.; formal analysis, J.T.C.; investigation, S.C.-B. and J.T.C.; data curation, S.C.-B., M.M.T., G.J.J., M.V.V. and B.B.G.; writing—original draft preparation, S.C.-B.; writing—review and editing, M.P.M., J.T.C. and X.N.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Arnau de Vilanova University Hospital (protocol code CEIC 3025 approved in March 2024).
Informed Consent Statement
Informed consent was not required according to the ethics committee due to the characteristics of the study, but all patients and/or family members were informed.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to privacy and ethical reasons.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ATB | antibiotic |
| MDR | multidrug resistant bacteria |
| ICU | intensive care unit |
| RZ | Resistencia Zero Project |
| RF | risk factors |
| ESBL | extended-spectrum beta-lactamase-producing Enterobacterales |
| MRSA | methicillin-resistant Staphylococcus aureus |
| SEMICYUC | Sociedad Española de Medicina Intensiva y Unidades Coronarias |
| ML | machine learning |
| HUAV | Arnau de Vilanova University Hospital |
| DG | development group |
| VG | validation group |
| VRE | vancomycin-resistant Enterococcus spp. |
| DM | diabetes mellitus |
| COPD | chronic obstructive pulmonary disease |
| CKD | chronic kidney disease |
| ED | emergency department |
| LR | logistic regression |
| CHAID | Chi-square automatic interaction detection |
| SHAP | SHapley Additive exPlanations |
| S | sensitivity |
| E | specificity |
| PPV | positive predictive value |
| NPV | negative predictive value |
| AUC | area under the curve |
References
- Caǧlayan, Ç.; Barnes, S.L.; Pineles, L.L.; Harris, A.D.; Klein, E.Y. A Data-Driven Framework for Identifying Intensive Care Unit Admissions Colonized With Multidrug-Resistant Organisms. Front. Public Health 2022, 10, 853757. [Google Scholar] [CrossRef]
- Cosgrove, S.E.; Sakoulas, G.; Perencevich, E.N.; Schwaber, M.J.; Karchmer, A.W.; Carmeli, Y. Comparison of Mortality Associated with Methicillin-Resistant and Methicillin-Susceptible Staphylococcus aureus Bacteremia: A Meta-analysis. Clin. Infect. Dis. 2003, 36, 53–59. [Google Scholar] [CrossRef]
- DiazGranados, C.A.; Zimmer, S.M.; Klein, M.; Jernigan, J.A. Comparison of Mortality Associated with Vancomycin-Resistant and Vancomycin-Susceptible Enterococcal Bloodstream Infections: A Meta-analysis. Clin. Infect. Dis. 2005, 41, 327–333. [Google Scholar] [CrossRef]
- Cosgrove, S.E.; Qi, Y.; Kaye, K.S.; Harbarth, S.; Karchmer, A.W.; Carmeli, Y. The Impact of Methicillin Resistance in Staphylococcus aureus Bacteremia on Patient Outcomes: Mortality, Length of Stay, and Hospital Charges. Infect. Control Hosp. Epidemiol. 2005, 26, 166–174. [Google Scholar] [CrossRef]
- Song, X.; Srinivasan, A.; Plaut, D.; Perl, T.M. Effect of Nosocomial Vancomycin-Resistant Enterococcal Bacteremia on Mortality, Length of Stay, and Costs. Infect. Control Hosp. Epidemiol. 2003, 24, 251–256. [Google Scholar] [CrossRef]
- Maragakis, L.L.; Perencevich, E.N.; Cosgrove, S.E. Clinical and economic burden of antimicrobial resistance. Expert Rev. Anti-Infect. Ther. 2008, 6, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Strich, J.R.; Palmore, T.N. Preventing Transmission of Multidrug-Resistant Pathogens in the Intensive Care Unit. Infect. Dis. Clin. N. Am. 2017, 31, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, A.A.; Lumbreras, D.J.; Valbuena, B.L.; Abellán, A.N.; Pérez, I.T.; Calderón, V.E.; Delgado, D.V.; Márquez, I.C.; Manzanedo, S.G.; Calvo, L.L.d.l.O.; et al. Analysis of the predictive value of preventive isolation criteria in the intensive care unit. Med. Intensiv. (Engl. Ed.) 2021, 45, 205–210. [Google Scholar]
- Carvalho-Brugger, S.; Torner, M.M.; Jiménez, G.J.; Badallo, O.; Lerma, F.Á.; Trujillano, J.; Casals, F.X.N.; Palomar, M. Preventive isolation criteria for the detection of multidrug-resistant bacteria in patients admitted to the Intensive Care Unit: A multicenter study within the Zero Resistance program. Med. Intensiv. (Engl. Ed.) 2023, 47, 629–637. [Google Scholar] [CrossRef]
- Padilla-Serrano, A.; Serrano-Castañeda, J.; Carranza-González, R.; García-Bonillo, M. Factores de riesgo de colonización por enterobacterias multirresistentes e impacto clínico. Rev. Esp. Quimioter. 2018, 31, 257–262. [Google Scholar]
- Ministerio de Sanidad, Servicios Sociales e Igualdad. Prevención de la Emerg. de Bact. Multirresistentes en el Paciente Crítico—“Proy. Resist. ZERO” (RZ). 2014. Available online: https://seguridaddelpaciente.sanidad.gob.es/practicasSeguras/seguridadPacienteCritico/docs/PROYECTO_RZ_-_VERSION_FINAL_26MARZO_2014.pdf (accessed on 4 April 2024).
- Montero, J.G.; Lerma, F.Á.; Galleymore, P.R.; Martínez, M.P.; Rocha, L.Á.; Gaite, F.B.; Rodríguez, J.Á.; González, M.C.; Moreno, I.F.; Baño, J.R.; et al. Combatting resistance in intensive care: The multimodal approach of the Spanish ICU “Zero Resistance” program. Crit. Care 2015, 19, 114. [Google Scholar] [CrossRef][Green Version]
- Yi, F.; Yang, H.; Chen, D.; Qin, Y.; Han, H.; Cui, J.; Bai, W.; Ma, Y.; Zhang, R.; Yu, H. XGBoost-SHAP-based interpretable diagnostic framework for alzheimer’s disease. BMC Med. Inform. Decis. Mak. 2023, 23, 137. [Google Scholar] [CrossRef]
- Ledoux, G.; Six, S.; Lawson, R.; Labreuche, J.; Blazejewski, C.; Wallet, F.; Duhamel, A.; Nseir, S. Impact of a targeted isolation strategy at intensive-care-unit-admission on intensive-care-unit-acquired infection related to multidrug-resistant bacteria: A prospective uncontrolled before–after study. Clin. Microbiol. Infect. 2016, 22, 888.e11–888.e18. [Google Scholar] [CrossRef]
- Kernéis, S.; Lucet, J.C. Controlling the Diffusion of Multidrug-Resistant Organisms in Intensive Care Units. Semin. Respir. Crit. Care Med. 2019, 40, 558–568. [Google Scholar] [CrossRef]
- Kalın, G.; Alp, E.; Chouaikhi, A.; Roger, C. Antimicrobial Multidrug Resistance: Clinical Implications for Infection Management in Critically Ill Patients. Microorganisms 2023, 11, 2575. [Google Scholar] [CrossRef]
- McCrink, K.A.; DeRonde, K.J.; Jimenez, A.; Rosello, G.; Natori, Y.; Claeys, K.C.; Martinez, O.V.; De Pascale, B.; Perez-Cardona, A.; Abbo, L.M.; et al. Impact of a real-time diagnostic and antimicrobial stewardship workflow on time to appropriate therapy for infections caused by multidrug-resistant Gram-negative organisms. Int. J. Antimicrob. Agents 2023, 61, 106811. [Google Scholar] [CrossRef]
- Gbaguidi-Haore, H.; Legast, S.; Thouverez, M.; Bertrand, X.; Talon, D. Ecological Study of the Effectiveness of Isolation Precautions in the Management of Hospitalized Patients Colonized or Infected With Acinetobacter baumannii. Infect. Control Hosp. Epidemiol. 2008, 29, 1118–1123. [Google Scholar] [CrossRef]
- Abad, C.; Fearday, A.; Safdar, N. Adverse effects of isolation in hospitalised patients: A systematic review. J. Hosp. Infect. 2010, 76, 97–102. [Google Scholar] [CrossRef]
- Marra, A.R.; Edmond, M.B.; Schweizer, M.L.; Ryan, G.W.; Diekema, D.J. Discontinuing contact precautions for multidrug-resistant organisms: A systematic literature review and meta-analysis. Am. J. Infect. Control 2018, 46, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.J.; Wenzel, R.P.; Bearman, G. Contact precautions for endemic MRSA and VRE: Time to retire legal mandates. JAMA 2017, 318, 329–330. [Google Scholar] [CrossRef] [PubMed]
- Saint, S.; Higgins, L.A.; Nallamothu, B.K.; Chenoweth, C. Do physicians examine patients in contact isolation less frequently? A brief report. Am. J. Infect. Control 2003, 31, 354–356. [Google Scholar] [CrossRef] [PubMed]
- Thomas Stelfox, H.; Bates, D.W.; Redelmeier, D.A. Safety of Patients Isolated for Infection Control. JAMA 2003, 290, 1899–1905. Available online: http://jama.jamanetwork.com/ (accessed on 30 March 2024). [CrossRef] [PubMed]
- Zahar, J.R.; Garrouste-Orgeas, M.; Vesin, A.; Schwebel, C.; Bonadona, A.; Philippart, F.; Ara-Somohano, C.; Misset, B.; Timsit, J.F. Impact of contact isolation for multidrug-resistant organisms on the occurrence of medical errors and adverse events. Intensiv. Care Med. 2013, 39, 2153–2160. [Google Scholar] [CrossRef]
- Álvarez Lerma, F.; Granado Solano, J.; García Sanz, A.; López Martínez, C.; Herrera Sebastián, R.; Salvat Cobeta, C.; Rey Pérez, A.; Balaguer Blasco, R.M.; Plasencia, V.; Horcajada, J.P. Optimización de los aislamientos preventivos en una UCI polivalente mediante la aplicación de un plan de intervención. Med. Intensiv. 2015, 39, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Cooper, B.S.; Stone, S.P.; Kibbler, C.C.; Cookson, B.D.; Roberts, J.A.; Medley, G.F.; Duckworth, G.; Lai, R.; Ebrahim, S. Isolation measures in the hospital management of methicillin resistant Staphylococcus aureus (MRSA): Systematic review of the literature. BMJ 2004, 329, 533. [Google Scholar] [CrossRef][Green Version]
- Djibré, M.; Fedun, S.; Le Guen, P.; Vimont, S.; Hafiani, M.; Fulgencio, J.-P.; Parrot, A.; Denis, M.; Fartoukh, M. Universal versus targeted additional contact precautions for multidrug-resistant organism carriage for patients admitted to an intensive care unit. Am. J. Infect. Control 2017, 45, 728–734. [Google Scholar] [CrossRef]
- Domenech De Cellès, M.; Zahar, J.R.; Abadie, V.; Guillemot, D. Limits of patient isolation measures to control extended-spectrum beta-lactamase-producing Enterobacteriaceae: Model-based analysis of clinical data in a pediatric ward. BMC Infect. Dis. 2013, 13, 187. Available online: http://www.biomedcentral.com/1471-2334/13/187 (accessed on 30 March 2024). [CrossRef]
- GTEIS—Semicyuc. Sociedad Española de Medicina Intensiva, Crítica y Unidades Coronarias Grupo de Trabajo de Enfermedades Infecciosas “Estudio Nacional de Vigilancia de Infección Nosocomial en uci”. Envin-Helics 2023. Available online: https://hws.vhebron.net/envin-helics/ (accessed on 31 March 2024).
- López-Pueyo, M.J.; Barcenilla-Gaite, F.; Amaya-Villar, R.; Garnacho-Montero, J. Multirresistencia antibiotica en unidades de criticos. Med. Intensiv. 2011, 35, 41–53. [Google Scholar] [CrossRef]
- Álvarez-Lerma, F.; Palomar, M.; Olaechea, P.; Otal, J.J.; Insausti, J.; Cerdá, E.; Grupo de estudio de Vigilancia de Infección Nosocomial en UCI. Estudio nacional de vigilancia de infección nosocomial en unidades de cuidados intensivos. Informe evolutivo de los años 2003–2005. Med Intensiv. 2007, 31, 6–17. [Google Scholar] [CrossRef]
- López-Pueyo, M.J.; Olaechea-Astigarraga, P.; Palomar-Martínez, M.; Insausti-Ordeñana, J.; Álvarez-Lerma, F. Quality control of the surveillance programme of ICU-acquired infection (ENVIN-HELICS registry) in Spain. J. Hosp. Infect. 2013, 84, 126–131. [Google Scholar] [CrossRef]
- Lucet, J.C.; Chevret, S.; Durand-Zaleski, I.; Chastang, C.; Régnier, B. Prevalence and Risk Factors for Carriage of Methicillin-Resistant Staphylococcus aureus at Admission to the Intensive Care Unit Results of a Multicenter Study. Arch. Intern. Med. 2003, 163, 181–188. Available online: http://archinte.jamanetwork.com/ (accessed on 12 October 2023). [CrossRef]
- Vasudevan, A.; Mukhopadhyay, A.; Li, J.; Yuen, E.G.Y.; Tambyah, P.A. A prediction tool for nosocomial multi-drug resistant gram-negative bacilli infections in critically ill patients - prospective observational study. BMC Infect. Dis. 2014, 14, 615. [Google Scholar] [CrossRef] [PubMed]
- Zasowski, E.J.; Trinh, T.D.; Claeys, K.C.; Dryden, M.; Shlyapnikov, S.; Bassetti, M.; Carnelutti, A.; Khachatryan, N.; Kurup, A.; Cejudo, A.P.; et al. International Validation of a Methicillin-Resistant Staphylococcus aureus Risk Assessment Tool for Skin and Soft Tissue Infections. Infect. Dis. Ther. 2022, 11, 2253–2263. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G.M. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. BMJ 2015, 7, 350. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, S.M.; Erion, G.; Chen, H.; DeGrave, A.; Prutkin, J.M.; Nair, B.; Katz, R.; Himmelfarb, J.; Bansal, N.; Lee, S.-I. From local explanations to global understanding with explainable AI for trees. Nat. Mach. Intell. 2020, 2, 56–67. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).