LC-MS/MS Tandem Mass Spectrometry for Analysis of Phenolic Compounds and Pentacyclic Triterpenes in Antifungal Extracts of Terminalia brownii (Fresen)
Abstract
1. Introduction
2. Results
2.1. Antifungal Effects of Extracts of Terminalia brownii Stem Bark and Wood
2.2. Results from the Phytochemical Screening of Antifungal Ethyl Acetate Extracts of T. brownii Stem Wood and Bark
2.2.1. TLC Results
2.2.2. HPLC-UV/DAD Results
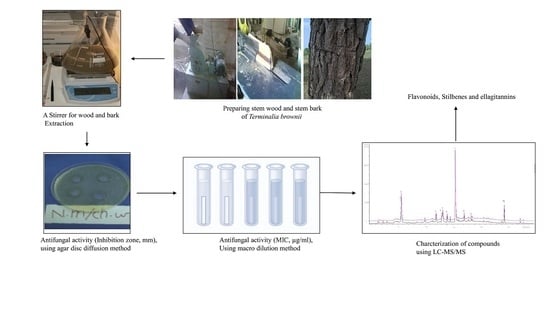
2.2.3. LC-MS/MS Results
3. Discussion
4. Materials and Methods
4.1. Collection of Plant Material
4.2. Extraction
4.3. Thin Layer Chromatography (TLC)
4.4. Solid Phase Extraction (SPE)
4.5. Reversed Phase High Performance Liquid Chromatography Coupled to Diode Array Detection (HPLC-UV/DAD)
4.6. LC-Triple Quadrupole Mass Spectrometric Analysis (LC-MS and LC-MS/MS Tandem Mass Spectrometry)
4.7. Antifungal Assays
4.7.1. Fungal Strains
4.7.2. Agar Well Diffusion Method
4.7.3. Agar Dilution Method
4.7.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Klemptner, L.R.; Sherwood, J.S.; Tugizimana, F.; Dubery, I.A.; Piater, L.A. Ergosterol, an orphan fungal microbe-associated molecular pattern (MAMP). Mol. Plant Pathol. 2014, 15, 747–761. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.K.; Cunningham, A.A.; Patel, N.G.; Morales, F.J.; Epstein, P.R.; Daszak, P. Emerging infectious diseases of plants: Pathogen pollution, climate change and agrotechnology drivers. Trends Ecol. Evol. 2004, 19, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Kieren, A.M.; Thomas, P.; David, D. Aspergillosis: Pathogenesis, clinical manifestations, and therapy. Infect. Dis. Clin. N. Am. 2002, 16, 875–894. [Google Scholar]
- Walsh, T.J.; Anaissie, E.J.; Denning, D.W.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Segal, B.H.; Steinbach, W.J.; Stevens, D.A.; et al. Treatment of Aspergillosis: Clinical Practice Guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 2008, 46, 327–360. [Google Scholar] [CrossRef] [PubMed]
- Baddley, J.W. Clinical risk factors for invasive aspergillosis. Med. Mycol. 2011, 49, S7–S12. [Google Scholar] [CrossRef] [PubMed]
- De Aguirre, L.D.; Hurst, S.F.; Choi, J.S.; Shin, J.H.; Hinrikson, H.P.; Morrison, C.J. Rapid Differentiation of Aspergillus Species from Other Medically Important Opportunistic Molds and Yeasts by PCR-Enzyme Immunoassay. J. Clin. Microbiol. 2004, 42, 3495–3504. [Google Scholar] [CrossRef] [PubMed]
- Hadrich, I.; Makni, F.; Ayadi, A.; Ranque, S. Microsatellite Typing to Trace Aspergillus flavus Infections in a Hematology Unit. J. Clin. Microbiol. 2010, 48, 2396–2401. [Google Scholar] [CrossRef] [PubMed]
- Dagenais, T.R.T.; Keller, N.P. Pathogenesis of Aspergillus fumigatus in Invasive Aspergillosis. Clin. Microbiol. Rev. 2009, 22, 447–465. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services. Guidelines for Research Involving Recombinant DNA Molecules; U.S. Department of Health and Human Services: Washington, DC, USA, 1986.
- International Agency for Research on Cancer (Iarc). IARC Monograph on the Evaluation of Carcinogenic Risk of Chemicals to Humans; Aflatoxins; International Agency for Research on Cancer: Lyon, France, 1987; Suppl 7, pp. 83–87. [Google Scholar]
- Fountain, J.C.; Scully, B.T.; Ni, X.; Kemerait, R.C.; Lee, R.D.; Chen, Z.Y.; Guo, B. Environmental influences on maize-Aspergillus flavus interactions and aflatoxin production. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Gourama, H.; Bullerman, L.B. Aspergillus flavus and Aspergillus parasiticus: Aflatoxigenic Fungi of concern in Foods and Feeds. J. Food Prot. 1995, 12, 1395–1404. [Google Scholar] [CrossRef]
- Willinger, B.; Kopetzky, G.; Harm, F.; Apfalter, P.; Makristathis, A.; Berer, A.; Bankier, A.; Winkle, S. CASE REPORTS Disseminated Infection with Nattrassia mangiferae in an Immunosuppressed Patient. J. Clin. Microbiol. 2004, 42, 478–480. [Google Scholar] [CrossRef] [PubMed]
- Sigler, L.; Summerbell, R.C.; Poole, L.; Wieden, M.; Sutton, D.A.; Rinaldi, M.G.; Aguirre, M.; Estes, G.W.; Galgiani, J.N. Invasive Nattrassia mangiferae Infections: Case Report, Literature Review, and Therapeutic and Taxonomic Appraisal. J. Clin. Microbiol. 1997, 35, 433–440. [Google Scholar] [PubMed]
- Frankel, D.H.; Rippon, J.W. Hendersonula toruloidea infection in man. Index cases in the non-endemic North American host, and a review of the literature. Mycopathologia 1989, 105, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Atanasova-Penichon, V.; Bernillon, S.; Marchegay, G.; Lornac, A.; Pinson-Gadais, L.; Ponts, N.; Zehraoui, E.; Barreau, C.; Richard-Forget, F. Bioguided Isolation, Characterization, and Biotransformation by Fusarium verticillioides of Maize Kernel Compounds that Inhibit Fumonisin Production. Mol. Plant Microbe Interact. 2014, 27, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.; Hua, H.; Selvaraj, J.N.; Yuan, Y.; Zhao, Y.; Zhou, L.; Liu, Y. Degradation of fumonisin B1 by cinnamon essential oil. Food Control 2014, 38, 37–40. [Google Scholar] [CrossRef]
- Dornbusch, H.J.; Buzina, W.; Summerbell, R.C.; Lass-Flörl, C.; Lackner, H.; Schwinger, W.; Sovinz, P.; Urban, C. Fusarium verticillioides abscess of the nasal septum in an immunosuppressed child: Case report and identification of the morphologically atypical fungal strain. J. Clin. Microbiol. 2005, 43, 1998–2001. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Bomfim, N.; Nakassugi, L.P.; Oliveira, J.F.P.; Kohiyama, C.Y.; Mossini, S.A.G.; Grespan, R.; Nerilo, S.B.; Mallmann, C.A.; Filho, B.A.A.; Machinski, M. Antifungal activity and inhibition of fumonisin production by Rosmarinus officinalis L. essential oil in Fusarium verticillioides (Sacc.) Nirenberg. Food Chem. 2015, 166, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C. Phytotoxicity: An Overview of the Physiological Responses of Plants Exposed to Fungicides. Jpn. J. Bot. 2012, 4. [Google Scholar] [CrossRef]
- Opiyo, S.A.; Manguro, L.O.A.; Owuor, P.O.; Ochieng, C.O.; Ateka, E.M.; Lemmen, P. Antimicrobial Compounds from Terminalia brownii against Sweet Potato Pathogens. J. Nat. Prod. 2011, 1, 116–120. [Google Scholar] [CrossRef]
- Chung, W.H.; Chung, W.C.; Ting, P.F.; Ru, C.C.; Huang, H.C.; Huang, J.W. Nature of Resistance to Methyl Benzimidazole Carbamate Fungicides in Fusarium oxysporum f.sp. lilii and F. oxysporum f.sp. gladioli in Taiwan. J. Phytopathol. 2009, 157, 742–747. [Google Scholar] [CrossRef]
- Villa, F.; Cappitelli, F.; Cortesi, P.; Kunova, A. Fungal Biofilms: Targets for the Development of Novel Strategies in Plant Disease Management. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Mazu, T.K.; Bricker, B.A.; Flores-Rozas, H.; Ablordeppey, S.Y. The Mechanistic Targets of Antifungal Agents: An Overview. Mini Rev. Med. Chem. 2016, 16, 555–578. [Google Scholar] [CrossRef] [PubMed]
- Troskie, A.M.; de Beer, A.; Vosloo, J.A.; Jacobs, K.; Rautenbach, M. Inhibition of agronomically relevant fungal phytopathogens by tyrocidines, cyclic antimicrobial peptides isolated from Bacillus aneurinolyticus. Microbiology 2014, 160, 2089–2101. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.A. Natural Fungicides obtained from Plants. In Fungicides for Plant and Animal Diseases; Dhanasekaran, D., Thajuddin, N., Panneerselvam, A., Eds.; InTech: Croatia, Balkans, 2012; pp. 1–28. ISBN 978-953-307-804-5. Available online: https://cdn.intechopen.com/pdfs-wm/26021.pdf (accessed on 1 May 2017).
- Singh, A.K.; Kumar, P.; Nidhi, R.; Gade, R.M. Allelopathy—A Sustainable Alternative and Eco-Friendly Tool for Plant Disease Management. Plant Dis. Sci. 2012, 7, 127–134. [Google Scholar]
- Rodrigues, A.M.; Theodoro, P.N.; Eparvier, V.; Basset, C.; Silva, M.R.; Beauchêne, J.; Espíndola, L.S.; Stien, D. Search for Antifungal Compounds from the Wood of Durable Tropical Trees. J. Nat. Prod. 2010, 73, 1706–1707. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Dikshit, A.K. Biopesticides: An eco-friendly approach for pest control. J. Biopestic. 2010, 3, 186–188. [Google Scholar]
- Shinde, S.L.; Wadje, S.S. Efficacy of terminalia bark extracts against seed-borne pathogens checked by paper disc method. Res. J. Pharm. Biol. Chem. Sci. 2011, 2, 602–607. [Google Scholar]
- Valsaraj, R.; Pushpangadan, P.; Smitt, U.W.; Adsersen, A.; Christensen, S.; Sittie, A.; Nyman, U.; Nielsen, C.; Olsen, C.E. New Anti-HIV-1, Antimalarial, and Antifungal Compounds from Terminalia bellerica. J. Nat. Prod. 1997, 60, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Wickens, G.E. Flora of Tropical East Africa: Combretaceae; East African Community: Arusha, East Africa’s Tanzania, 1973; p. 99. [Google Scholar]
- Nguyen, D.-M.-C.; Seo, D.-J.; Park, R.-D.; Lee, B.-J.; Jung, W.-J. Chitosan beads combined with Terminalia nigrovenulosa bark enhance suppressive activity to Fusarium solani. Ind. Crop Prod. 2013, 50, 462–467. [Google Scholar] [CrossRef]
- Samie, A.; Mashau, F. Antifungal activities of fifteen Southern African medicinal plants against five Fusarium species. J. Med. Plants Res. 2013, 7, 1839–1848. [Google Scholar]
- Fabry, W.; Okemo, P.; Ansorg, R. Fungistatic and fungicidal activity of East African medicinal plants. Mycoses 1996, 39, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Baba-Moussa, F.; Akpagana, K.; Bouchet, P. Antifungal activities of seven West African Combretaceae used in traditional medicine. J. Ethnopharmacol. 1999, 66, 335–338. [Google Scholar] [CrossRef]
- Nguyen, D.-M.-C.; Seo, D.-J.; Lee, H.B.; Kim, I.S.; Kim, K.Y.; Park, R.D.; Jung, W.J. Antifungal activity of gallic acid purified from Terminalia nigrovenulosa bark against Fusarium solani. Microb. Pathog. 2013, 56, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Eldeen, I.M.S.; Van Staaden, J. Antimycobacterial activity of some trees used in South African traditional medicine. S. Afr. J. Bot. 2007, 73, 248–251. [Google Scholar] [CrossRef]
- Eldeen, I.M.S.; Elgorashi, E.E.; Mulholland, D.A.; Van Staden, J. Anolignan B: A bioactive compound from the roots of Terminalia sericea. J. Ethnopharmacol. 2005, 103, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Conrad, J.; Vogler, B.; Reeb, S.; Klaiber, I.; Roos, G.; Vasquiez, E.; Setzer, M.C.; Kraus, W. Isoterchebulin and 4,6-O-isoterchebuloyl-d-glucose, novel hydrolysable tannins from Terminalia macroptera. J. Nat. Prod. 2001, 64, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Conrad, J.; Vogler, B.; Klaiber, I.; Roos, G.; Walter, U.; Kraus, W. Two triterpene esters from Terminalia macroptera bark. Phytochemistry 1998, 48, 647–650. [Google Scholar] [CrossRef]
- Silva, O.; Duarte, A.; Pimentel, M.; Viegas, S.; Barroso, H.; Machado, J.; Pires, I.; Cabrita, J.; Gomes, E. Antimicrobial activity of Terminalia macroptera root. J. Ethnopharmacol. 1997, 57, 203–207. [Google Scholar] [CrossRef]
- Kuete, V.; Tabopda, T.K.; Ngameni, B.; Nana, F.; Tshikalange, T.E.; Ngadjui, B.T. Antimycobacterial, antibacterial and antifungal activities of Terminalia superba (Combretaceae). S. Afr. J. Bot. 2010, 76, 125–131. [Google Scholar] [CrossRef]
- Joseph, C.C.; Moshi, M.J.; Innocent, E.; Nkunya, M.H.H. Isolation of a stilbene glycoside and other constituents of Terminalia sericeae. Afr. J. Tradit. Complement. Altern. Med. 2007, 4, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Pfundstein, B.; El Desouky, S.K.; Hull, W.E.; Haubner, R.; Erben, G.; Owen, R.W. Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): Characterization, quantitation and determination of antioxidant capacities. Phytochemistry 2010, 71, 1132–1148. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.H. Terminalia brownii Fresen. Seed Leafl. 2010, 148, 363–374. [Google Scholar]
- Mosango, D.M. Terminalia brownii. In Plant Resources of Tropical Africa 11(2): Medicinal plants 2; Schmelzer, G.H., Gurib-Fakim, A., Eds.; PROTA Foundation: Wageningen, The Netherlands, 2013; pp. 245–248. [Google Scholar]
- Shuping, D.S.S.; Eloff, J.N. The use of plants to protect plants and food against fungal pathogens: A review. Afr. J. Tradit. Complement. (Ajtcam) 2017, 14, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Machumi, F.; Midiwo, J.O.O.; Jacob, M.R.; Khan, S.I.; Tekwani, B.L.; Zhang, H.; Walker, L.A.; Muhaamed, L. Phytochemical, antimicrobial and antiplasmodial investigation of Terminalia brownii. J. Nat. Prod. Commun. 2013, 8, 761–764. [Google Scholar]
- Salih, E.Y.A.; Kanninen, M.; Sipi, M.; Luukkanen, O.; Hiltunen, R.; Vuorela, H.; Julkunen-Tiitto, R.; Fyhrquist, P. Tannins, flavonoids and stilbenes in extracts of African savanna woodland trees Terminalia brownii, Terminalia laxiflora and Anogeissus leiocarpus showing promising antibacterial potential. S. Afr. J. Bot. 2017, 108, 370–386. [Google Scholar] [CrossRef]
- Yamauchi, K.; Mitsunaga, T.; Muddathir, A.M. Screening for melanogenesis-controlled agents using Sudanese medicinal plants and identification of active compounds in the methanol extract of Terminalia brownii. J. Wood Sci. 2016, 62, 285–293. [Google Scholar] [CrossRef]
- Negishi, H.; Maoka, T.; Njelekela, M.; Yasui, N.; Juman, S.; Mtabaji, J.; Miki, T.; Nara, Y.; Yamori, Y.; Ikeda, K. New chromone derivative terminalianone from African plant Terminalia brownii Fresen (Combretaceae) in Tanzania. J. Asian Nat. Prod. Res. 2011, 13, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Mbwambo, Z.H.; Moshi, M.J.; Masimba, P.J.; Kapingu, M.C.; Nondo, R.S. Antimicrobial activity and brine shrimp toxicity of extracts of Terminalia brownii roots and stem. BMC Complement. Altern. Med. 2007, 7, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Masoko, P.; Picard, J.; Eloff, J.N. Antifungal activities of six South African Terminalia Species (Combretaceae). J. Ethnopharmacol. 2005, 99, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Elegami, A.A.; El-Nima, E.I.; El Tohami, M.S.; Muddathir, A.K. Antimicrobial activity of some species of the family Combretaceae. Phytother. Res. 2002, 16, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, F. Analytical Microbiology, 2nd ed.; Academic Press: New York, NY, USA, 1972; p. 11. [Google Scholar]
- Wagner, H.; Bladt, S. Plant Drug Analysis: A Thin Layer Chromatography Atlas, 2nd ed.; Springer: New York, NY, USA, 1996; 320p. [Google Scholar]
- Stecher, G.; Huck, C.; Popp, M.; Bonn, G.K. Determination of flavonoids and stilbenes in red wine and related biological products by HPLC and HPLC–ESI–MS–MS. J. Anal. Chem. 2001, 371, 73–80. [Google Scholar] [CrossRef]
- Otto, A.; Simoneit, B.R.; Wilde, V.; Kuntzmann, L.; Pűttmann, W. Terpenoids Composition of three fossil resins from Cretaceae and tertiary Conifers. Rev. Palaeobatony Palynol. 2002, 120, 203–215. [Google Scholar] [CrossRef]
- Van der Doelen, G.A.; van den Berg, K.J.; Boon, J.J.; Shibayama, N.; De La Rie, E.R.; Genuit, W.J.L. Analysis of fresh triterpenoid resin and aged triterpenoid varnishes by HPLC. APCI.MS/MS. J. Chromatogr. A 1998, 809, 21–37. [Google Scholar] [CrossRef]
- Liu, M.; Katerere, D.R.; Gray, A.I.; Seidel, V. Phytochemical and antifungal studies on Terminalia mollis and Terminalia brachystemma. Fitoterapia 2009, 80, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Bai, L.; Li, X.; Xiong, J.; Xu, P.; Xue, M. Structural analysis of metabolites of asiatic acid and its analogue madecassic acid in zebrafish using LC/IT-MSn. Molecules 2015, 20, 3001–3019. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, P.F.; Justino, G.C. Structural analysis of flavonoids and related compounds-a review of spectroscopic applications. In Phytochemicals—A Global Perspective of Their Role in Nutrition and Health; InTech: Croatia, Balkans, 2012; pp. 1–26. [Google Scholar]
- Marzouk, M.S.; El-Toumy, S.A.; Moharram, F.A. Pharmacologically active ellagitannins from Terminalia myriocarpa. Planta Med. 2002, 68, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Oeirichs, P.B.; Pearce, C.M.; Zhu, J.; Filippich, L.J. Isolation and structure determination of terminalin A toxic condense tannin from Terminalia oblongata. Nat. Toxins 1994, 2, 144–150. [Google Scholar] [CrossRef]
- Canedo, E.M.; Fill, T.P.; Pereira-Filho, E.R.; Rodrigues-Filho, E. Enzymatic Potential of Mucor inaequisporus for Naringin Biotransformation, Accessed by Fractional Factorial Design and Mass Spectrometry Analysis. J. Anal. Bioanal. Tech. 2014, S6. [Google Scholar] [CrossRef]
- Lee, M.K.; Bok, S.H.; Jeong, T.S.; Moon, S.S.; Lee, S.E.; Park, Y.B.; Choi, M.S. Supplementation of naringenin and it is synthetics derivatives altars antioxidant enzyme activities of erythrocyte and liver high cholesterol-fed rats. Bioorg. Med. Chem. 2002, 10, 2239–2244. [Google Scholar] [CrossRef]
- Fabre, N.; Rustan, I.; de Hoffmann, E.; Quetin-Leclercq, J. Determination of Flavone, Flavonol, and Flavanone Aglycones by Negative Ion Liquid Chromatography Electrospray Ion Trap Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2001, 12, 707–715. [Google Scholar] [CrossRef]
- Cuyckens, F.; Claeys, M. Mass spectrometry in the structural analysis of flavonoids. J. Mass Spectrom. 2004, 39, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Nuengchamnong, N.; Ingkaninan, K. An on-line LC-MS/DPPH approach towards the quality control of antioxidative ingredients in Sahastara. Songklanakarin J. Sci. Technol. 2017, 39, 123–129. [Google Scholar] [CrossRef]
- Xiao, H.-T.; Tsang, S.-W.; Qin, H.-Y.; Choi, F.F.; Yang, Z.-J.; Han, Q.-B.; Chen, H.-B.; Xu, H.-X.; Shen, H.; Lu, A.-P.; et al. A bioactivity-guided study on the anti-diarrheal activity of Polygonum chinense Linn. J. Ethnopharmacol. 2013, 149, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, J.P.; Osbourn, A.E. Fungal resistance to plant antibiotics as a mechanism of pathogenesis. Microbiol. Mol. Biol. Rev. 1999, 63, 708–724. [Google Scholar] [PubMed]
- Smaili, A.; Mazoir, N.; Aicha Rifai, L.; Koussa, T.; Makroum, K.; Benharref, A.; Faize, L.; Alburquerque, N.; Burgos, L.; Belfaiza, M.; et al. Antimicrobial Activity of two Semisynthetic Triterpene Derivatives from Euphorbia officinarum Latex against Fungal and Bacterial Phytopathogens. Nat. Prod. Commun. 2017, 12, 331–336. [Google Scholar]
- Sirdaarta, J.; Matthews, B.; Cock, I.E. Kakadu plum fruit extracts inhibit growth of the bacterial triggers of rheumatoid arthritis: Identification of stilbene and tannin components. J. Funct. Food 2015, 17, 610–620. [Google Scholar] [CrossRef]
- Cock, I.E.; van Vuuren, S.F. Anti-Proteus activity of some South African medicinal plants: Their potential for the prevention of rheumatoid arthritis. Inflammopharmacology 2014, 22, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Sebastià, N.; Montoro, A.; León, Z.; Soriano, J.M. Searching trans-resveratrol in fruits and vegetables: A preliminary screening. J. Food Sci. Technol. 2017, 54, 842–845. [Google Scholar] [CrossRef] [PubMed]
- Zainal, N.; Chang, C.-P.; Cheng, Y.-L.; Wu, Y.-W.; Anderson, R.; Wan, S.-W.; Chen, C.-L.; Ho, T.-S.; AbuBakar, S.; Lin, Y.S. Resveratrol treatment reveals a novel role for HMGB1 in regulation of the type 1 interferon response in dengue virus infection. Sci. Rep. UK 2017, 7, 42998. [Google Scholar] [CrossRef] [PubMed]
- Caruso, F.; Mendoza, L.; Castro, P.; Cotoras, M.; Aguirre, M.; Matsuhiro, B.; Isaacs, M.; Rossi, M.; Viglianti, A.; Antonioletti, R. Antifungal Activity of Resveratrol against Botrytiscinerea Is Improved Using 2-Furyl Derivatives. PLoS ONE 2011, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Hwang, I.A.; Sung, W.S.; Kang, H.; Kang, B.S.; Seu, Y.B.; Lee, D.G. Fungicidal effect of resveratrol on human infectious fungi. Arch. Pharm. Res. 2005, 28, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Phenols, Polyphenols and Tannins: An Overview. In Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet, 1st ed.; Crozier, A., Clifford, M.N., Ahihara, H., Eds.; Blackwell Publishing: Oxford, UK, 2006; pp. 1–25. [Google Scholar]
- Silva, L.P.; de Angelis, C.D.; Bonamin, F.; Kushima, H.; Mininel, F.J.; dos Santos, L.C.; Delella, F.K.; Felisbino, S.L.; Vilegas, W.; da Rocha, L.R.M.; et al. Terminalia catappa L.: A medicinal plant from the Caribbean pharmacopeia with anti-Helicobacter pylori and antiulcer action in experimental rodent models. J. Ethnopharmacol. 2015, 159, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Cerdá, B.; Cerón, J.J.; Tomás-Barberán, F.A.; Espín, J.C. Repeated oral administration of high doses of the pomegranate ellagitannin punicalagin to rats for 37 days is not toxic. J. Agric. Food Chem. 2003, 51, 3493–3501. [Google Scholar] [CrossRef] [PubMed]
- Glazer, I.; Masaphy, S.; Marciano, P.; Bar-Ilan, I.; Holland, D.; Kerem, Z.; Amir, R. Partial identification of antifungal compounds from Punica granatum peel extracts. J. Agric. Food Chem. 2012, 60, 4841–4848. [Google Scholar] [CrossRef] [PubMed]
- Latté, K.P.; Kolodziej, H. Antifungal effects of hydrolysable tannins and related compounds on dermatophyte, mould fungi and yeasts. Z. Naturforsch. 2000, 55c, 467–472. [Google Scholar]
- Alves, C.T.; Ferreira, I.C.; Barros, L.; Silva, S.; Azeredo, J.; Henriques, M. Antifungal activity of phenolic compounds identified in flowers from North Eastern Portugal against Candida species. Future Microbiol. 2014, 9, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Tempesti, T.C.; Alvarez, M.G.; de Arau’jo, M.F.; Ju’nior, F.E.; de Carvalho, M.G.; Durantini, E.N. Antifungal activity of a novel quercetin derivative bearing a trifluoromethyl group on Candida albicans. Med. Chem. Res. 2012, 21, 2217–2222. [Google Scholar] [CrossRef]
- Weidenbörner, M.; Hindorf, H.; Jha, H.C.; Tsotsonos, P. Antifungal activity of flavonoids against storage fungi of the genus Aspergillus. Phytochemistry 1990, 29, 1103–1105. [Google Scholar] [CrossRef]
- Céspedes, C.L.; Salazar, J.R.; Ariza-Castolo, A.; Yamaguchi, L.; Avila, J.G.; Aqueveque, P.; Kubo, I.; Alarcón, J. Biopesticides from plants: Calceolaria integrifolia sl. Environ. Res. 2014, 132, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Mou, Y.; Zhao, J.; Wang, J.; Zhou, L.; Wang, M.; Wang, D.; Han, J.; Yu, Z.; Yang, F. Flavonoids from Halostachys caspica and Their Antimicrobial and Antioxidant Activities. Molecules 2010, 15, 7933–7945. [Google Scholar] [CrossRef] [PubMed]
- Orhan, D.D.; Özçelik, B.; Özgen, S.; Ergun, F. Antibacterial, antifungal, and antiviral activities of some flavonoids. Microbiol. Res. 2010, 165, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Tokarzewski, S.; Ziółkowska, G.; Nowakiewicz, A. Susceptibility testing of Aspergillus niger strains isolated from poultry to antifungal drugs-a comparative study of the disk diffusion, broth microdilution (M 38-A) and Etest® methods. Pol. J. Vet. Sci. 2012, 15, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Guarro, J.; Soler, L.; Rinaldi, M.G. Pathogenicity and antifungal susceptibility of Chaetomium species. EUR J. Clin. Microbiol. 1995, 14, 613–618. [Google Scholar] [CrossRef]




| Fungal Strain | Stem Wood Extracts | Stem Bark Extracts | Amphotericin-B | |||
|---|---|---|---|---|---|---|
| IZ | MIC | IZ | MIC | IZ | MIC | |
| Aspergillus niger | ||||||
| Pt | NA | NA | ||||
| CHCl3 | 12 ± 0.9 | 13 ± 0.4 | ||||
| EtOAc | 17± 0.7 | 500 | 17 ± 0.8 | 500 | 35 ± 0.01 | 31.25 |
| aqueous | 17± 0.5 | 16.5 ± 0.4 | ||||
| Aspergillus flavus | ||||||
| Pt | NA | NA | ||||
| CHCl3 | 14 ± 0.5 | 14 ± 0.9 | ||||
| EtOAc | 18.5 ± 0.4 | 500 | 18.5 ± 0.8 | 500 | 28 ± 0.03 | 125 |
| aqueous | 18 ± 0.9 | 18 ± 0.5 | ||||
| Nattrassia mangiferae | ||||||
| Pt | NA | NA | ||||
| CHCl3 | 12 ± 0.5 | 12± 0.7 | ||||
| EtOAc | 19 ± 0.4 | 250 | 18.5 ± 0.4 | 250 | 30 ± 0.04 | 62.5 |
| aqueous | 18.5 ± 0.4 | 19 ± 0.4 | ||||
| Fusarium verticillioides | ||||||
| Pt | NA | NA | ||||
| CHCl3 | 13 ± 0.6 | 11 ± 0.9 | ||||
| EtOAc | 20 ± 0.4 | 250 | 19 ± 0.2 | 250 | 31 ± 0.03 | 62.5 |
| aqueous | 19 ± 0.3 | 18 ± 0.7 | ||||
| Peak No | Rt (min) | [M-H] (m/z) | CID Mn Main Fragment Ions (m/z) | Identified Compound | Molecular Formula | Exact Mass (Calc.) |
|---|---|---|---|---|---|---|
| 1 | 6.8 | 469 | 425, 407, 379, 353, 300, 271 | oleanane type triterpenoid | - | - |
| 2 | 6.8 | 491 | 447, 429, 411, 401, 385, 301 | oleanane type triterpenoid | - | - |
| 3 | 11.1 | 541 | 532, 425, 397, 301, 273, 227, 199, 169 | cis-resveratrol-3-O-β-galloyl-glucoside | C27H26O12 | 542.1416 |
| 4 | 13.2 | 541 | 532, 424, 407, 300, 275, 227, 199, 169 | trans-resveratrol-3-O-β-galloyl-glucoside | C27H26O12 | 542.1416 |
| 5 | 14.1 | 483 | 451, 433, 407, 305, 405, 377 | Methyl-(S)-flavogallonate | C22H12O13 | 484.0273 |
| 6 | 14.4 | 601 | 583, 301, 299, 271, 243, 215 | Gallagic acid dilactone | C28H10O16 | 601.9964 |
| 7 | 15.3 | 433 | 300, 314, 229, 271, 132 | Naringenin-4′-methoxy-7-pyranoside | - | - |
| 8 | 16.8 | 625 | 301, 284, 256, 229, 201,185, 129 | Quercetin-7-β-O-diglucoside | C27H30O17 | 626.1473 |
| 9 | 18.2 | 633 | 481, 463, 421, 387, 305, 275, 300, 169 | Corilagin derivative | - | - |
| 10 | 18.4 | 585 | 301, 284, 257, 229, 201, 185, 153, 132 | Quercetin-7-O-galloyl-glucoside | - | - |
| 11 | 19.1 | 725 | 665, 503, 409, 441, 379, 391 | Unknown ellagitannin | - | - |
| 12 | 25.5 | 343 | 328, 313, 298, 285, 270, 257 | 5,6-dihydroxy-3′,4′,7-trimethoxy-flavone | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salih, E.Y.A.; Fyhrquist, P.; Abdalla, A.M.A.; Abdelgadir, A.Y.; Kanninen, M.; Sipi, M.; Luukkanen, O.; Fahmi, M.K.M.; Elamin, M.H.; Ali, H.A. LC-MS/MS Tandem Mass Spectrometry for Analysis of Phenolic Compounds and Pentacyclic Triterpenes in Antifungal Extracts of Terminalia brownii (Fresen). Antibiotics 2017, 6, 37. https://doi.org/10.3390/antibiotics6040037
Salih EYA, Fyhrquist P, Abdalla AMA, Abdelgadir AY, Kanninen M, Sipi M, Luukkanen O, Fahmi MKM, Elamin MH, Ali HA. LC-MS/MS Tandem Mass Spectrometry for Analysis of Phenolic Compounds and Pentacyclic Triterpenes in Antifungal Extracts of Terminalia brownii (Fresen). Antibiotics. 2017; 6(4):37. https://doi.org/10.3390/antibiotics6040037
Chicago/Turabian StyleSalih, Enass Y. A., Pia Fyhrquist, Ashraf M. A. Abdalla, Abdelazim Y. Abdelgadir, Markku Kanninen, Marketta Sipi, Olavi Luukkanen, Mustafa K. M. Fahmi, Mai H. Elamin, and Hiba A. Ali. 2017. "LC-MS/MS Tandem Mass Spectrometry for Analysis of Phenolic Compounds and Pentacyclic Triterpenes in Antifungal Extracts of Terminalia brownii (Fresen)" Antibiotics 6, no. 4: 37. https://doi.org/10.3390/antibiotics6040037
APA StyleSalih, E. Y. A., Fyhrquist, P., Abdalla, A. M. A., Abdelgadir, A. Y., Kanninen, M., Sipi, M., Luukkanen, O., Fahmi, M. K. M., Elamin, M. H., & Ali, H. A. (2017). LC-MS/MS Tandem Mass Spectrometry for Analysis of Phenolic Compounds and Pentacyclic Triterpenes in Antifungal Extracts of Terminalia brownii (Fresen). Antibiotics, 6(4), 37. https://doi.org/10.3390/antibiotics6040037