The Effects of Mentha × piperita Essential Oil on C. albicans Growth, Transition, Biofilm Formation, and the Expression of Secreted Aspartyl Proteinases Genes
Abstract
:Highlights
- Mentha × piperita essential oil (EO) and its vapor were able to decrease C. albicans growth.
- The EO and its vapor reduced C. albicans transition.
- The EO and its vapor inhibited biofilm formation and disrupted mature biofilms.
- The EO significantly reduced the expression of SAPs and HWP1 genes involved in C. albicans virulence.
1. Introduction
2. Materials and Methods
2.1. Effect of Various Concentrations of EO and its Vapor on C. albicans Growth
2.2. Effect of the EO and its Vapor on C. albicans Transition from Blastospore to Hyphal Form
2.3. Effect of EO on C. albicans Ultrastructure
2.4. Effect of EO on C. albicans Biofilm Formation
2.5. Effect of EO on the Disruption of Mature C. albicans Biofilms
2.6. Effect of EO on C. albicans Gene Activation/Repression
2.7. Quantitative Real-Time RT-PCR
2.8. Statistical Analysis
3. Results
3.1. Mentha × piperita Essential Oil Reduced C. albicans Growth
3.2. Mentha × piperita EO Reduced C. albicans Transition
3.3. Mentha × piperita EO Modified C. albicans Surface Structure
3.4. Mentha × piperita EO Decreased Biofilm Formation
3.5. Mentha × piperita EO Disrupted C. albicans Biofilms
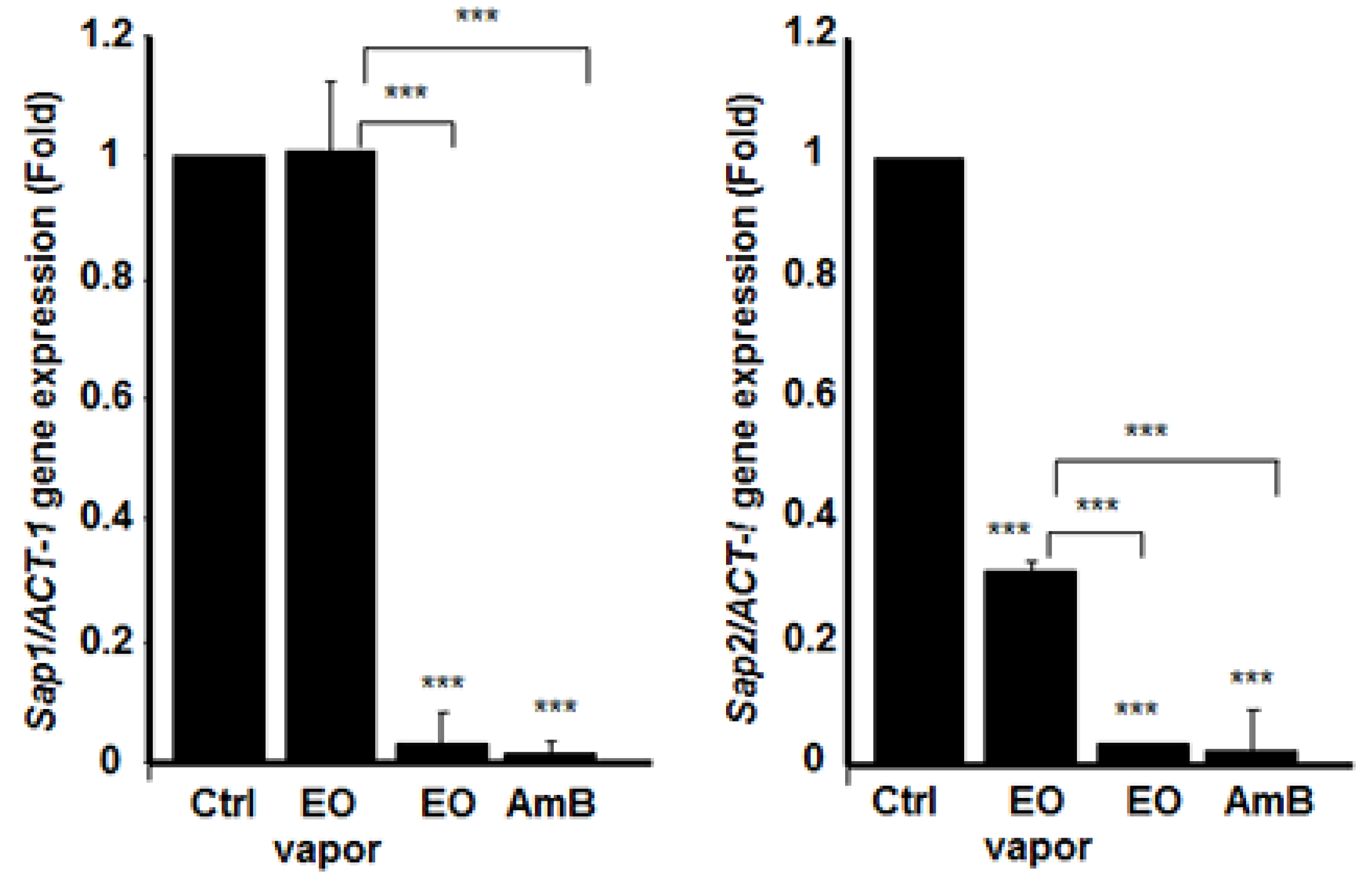
3.6. Mentha × piperita EO Decreased the Expression of Different Secreted Aspartyl Proteinases Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Poulain, D. Candida albicans, plasticity and pathogenesis. Crit. Rev. Microbiol. 2015, 41, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Cavaleiro, I.; Proença, L.; Félix, S.; Salema-Oom, M. Prevalence of yeast other than Candida albicans in denture wearers. J. Prosthodont. 2013, 22, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Grubb, S.E.; Murdoch, C.; Sudbery, P.E.; Saville, S.P.; Lopez-Ribot, J.L.; Thornhill, M.H. Candida albicans-endothelial cell interactions: A key step in the pathogenesis of systemic candidiasis. Infect. Immun. 2008, 76, 4370–4377. [Google Scholar] [CrossRef] [PubMed]
- Solis, N.V.; Park, Y.N.; Swidergall, M.; Daniels, K.J.; Filler, S.G.; Soll, D.R. Candida albicans White-Opaque Switching Influences Virulence but Not Mating during Oropharyngeal Candidiasis. Infect. Immun. 2018, 86, pii: e00774-17. [Google Scholar] [CrossRef] [PubMed]
- Höfs, S.; Mogavero, S.; Hube, B. Interaction of Candida albicans with host cells: Virulence factors, host defense, escape strategies, and the microbiota. J. Microbiol. 2016, 54, 149–169. [Google Scholar] [CrossRef]
- Lu, Y.; Su, C.; Liu, H. Candida albicans hyphal initiation and elongation. Trends Microbiol. 2014, 22, 707–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cauchie, M.; Desmet, S.; Lagrou, K. Candida and its dual lifestyle as a commensal and a pathogen. Res. Microbiol. 2017, 168, 802–810. [Google Scholar] [CrossRef]
- Tobouti, P.L.; Casaroto, A.R.; de Almeida, R.S.; de Paula Ramos, S.; Dionísio, T.J.; Porto, V.C.; Santos, C.F.; Lara, V.S. Expression of Secreted Aspartyl Proteinases in an Experimental Model of Candida albicans-Associated Denture Stomatitis. J. Prosthodont. 2016, 25, 127–134. [Google Scholar] [CrossRef]
- Silva, N.C.; Nery, J.M.; Dias, A.L. Aspartic proteinases of Candida spp.: Role in pathogenicity and antifungal resistance. Mycoses 2014, 57, 1–11. [Google Scholar] [CrossRef]
- Belmadani, A.; Semlali, A.; Rouabhia, M. Dermaseptin-S1 decreases Candida albicans growth, biofilm formation and the expression of hyphal wall protein 1 and aspartic proteasegenes. J. Appl. Microbiol. 2018, 125, 72–83. [Google Scholar] [CrossRef]
- Nobile, C.J.; Nett, J.E.; Andes, D.R.; Mitchell, A.P. Function of Candida albicans adhesin Hwp1 in biofilm formation. Eukaryot. Cell 2006, 5, 1604–1610. [Google Scholar] [CrossRef] [PubMed]
- Tumbarello, M.; Fiori, B.; Trecarichi, E.M.; Posteraro, P.; Losito, A.R.; De Luca, A.; Sanguinetti, M.; Fadda, G.; Cauda, R.; Posteraro, B. Risk factors and outcomes of candidemia caused by biofilm-forming isolates in a tertiary care hospital. PLoS ONE 2012, 7, e33705. [Google Scholar] [CrossRef] [PubMed]
- Campoy, S.; Adrio, J.L. Antifungals. Biochem. Pharmacol. 2017, 133, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Smiljkovic, M.; Kostic, M.; Stojkovic, D.; Glamoclija, J.; Sokovic, M. Could flavonoids compete with synthetic azoles in diminishing Candida albicans infections? Curr. Med. Chem. 2018. [Google Scholar] [CrossRef]
- Thompson, G.R.; Cadena, J.; Patterson, T.F. Overview of antifungal agents. Clin. Chest Med. 2009, 30, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Aoun, M. Standard antifungal therapy in neutropenic patients. Int. J. Antimicrob. Agents 2000, 16, 143–145. [Google Scholar] [CrossRef]
- Nunes, J.M.; Bizerra, F.C.; Ferreira, R.C.; Colombo, A.L. Molecular identification, antifungal susceptibility profile, and biofilm formation of clinical and environmental Rhodotorula species isolates. Antimicrob. Agents Chemother. 2013, 57, 382–389. [Google Scholar] [CrossRef]
- Clemons, K.V.; Capilla, J.; Sobel, R.A.; Martinez, M.; Tong, A.J.; Stevens, D.A. Comparative efficacies of lipid-complexed amphotericin B and liposomal amphotericin B against coccidioidal meningitis in rabbits. Antimicrob. Agents Chemother. 2009, 53, 1858–1862. [Google Scholar] [CrossRef]
- Marak, M.B.; Dhanashree, B. Antifungal Susceptibility and Biofilm Production of Candida spp. Isolated from Clinical Samples. Int. J. Microbiol. 2018, 2018, 7495218. [Google Scholar] [CrossRef]
- Shekhova, E.; Kniemeyer, O.; Brakhage, A.A. Induction of Mitochondrial Reactive Oxygen Species Production by Itraconazole, Terbinafine, and Amphotericin B as a Mode of Action against Aspergillus fumigatus. Antimicrob. Agents Chemother. 2017, 61, pii: e00978-17. [Google Scholar] [CrossRef]
- Scapaticci, M.; Bartolini, A.; Del Chierico, F.; Accardi, C.; Di Girolamo, F.; Masotti, A.; Muraca, M.; Putignani, L. Phenotypic typing and epidemiological survey of antifungal resistance of Candida species detected in clinical samples of Italian patients in a 17 months’ period. Germs 2018, 8, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Deray, G. Amphotericin B nephrotoxicity. J. Antimicrob. Chemother. 2002, 49 (Suppl. 1), 37–41. [Google Scholar] [CrossRef] [PubMed]
- Kagan, S.; Ickowicz, D.; Shmuel, M.; Altschuler, Y.; Sionov, E.; Pitusi, M.; Weiss, A.; Farber, S.; Domb, A.J.; Polacheck, I. Toxicity mechanisms of amphotericin B and its neutralization by conjugation with arabinogalactan. Antimicrob. Agents Chemother. 2012, 56, 5603–5611. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Lewinsohn, E.; Gijzen, M.; Savage, T.J.; Croteau, R. Defense mechanisms of conifers relationship of monoterpene cyclase activity to anatomical specialization and oleoresin monoterpene content. Plant Physiol. 1991, 96, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Khanna, R.; MacDonald, J.K.; Levesque, B.G. Peppermint oil for the treatment of irritable bowel syndrome: A systematic review and meta-analysis. J. Clin. Gastroenterol. 2014, 48, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, L.K.; Jawale, B.A.; Sharma, S.; Sharma, H.; Kumar, C.D.; Kulkarni, P.A. Antimicrobial activity of commercially available essential oils against Streptococcus mutans. J. Contemp. Dent. Pract. 2012, 13, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Bardaweel, S.K.; Bakchiche, B.; ALSalamat, H.A.; Rezzoug, M.; Gherib, A.; Flamini, G. Chemical composition, antioxidant, antimicrobial and Antiproliferative activities of essential oil of Mentha spicata L. (Lamiaceae) from Algerian Saharan atlas. BMC Complement. Altern. Med. 2018, 18, 201. [Google Scholar] [CrossRef]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.). Phytother. Res. 2006, 20, 619–633. [Google Scholar] [CrossRef]
- Marwa, C.; Fikri-Benbrahim, K.; Ou-Yahia, D.; Farah, A. African peppermint (Mentha piperita) from Morocco: Chemical composition and antimicrobial properties of essential oil. J. Adv. Pharm. Technol. Res. 2017, 8, 86–90. [Google Scholar]
- de Sousa Guedes, J.P.; de Souza, E.L. Investigation of damage to Escherichia coli, Listeria monocytogenes and Salmonella Enteritidis exposed to Mentha arvensis L. and M. piperita L. essential oils in pineapple and mango juice by flow cytometry. Food Microbiol. 2018, 76, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Tardugno, R.; Pellati, F.; Iseppi, R.; Bondi, M.; Bruzzesi, G.; Benvenuti, S. Phytochemical composition and in vitro screening of the antimicrobial activity of essential oils on oral pathogenic bacteria. Nat. Prod. Res. 2018, 32, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Rachitha, P.; Krupashree, K.; Jayashree, G.V.; Gopalan, N.; Khanum, F. Growth Inhibition and Morphological Alteration of Fusarium sporotrichioides by Mentha piperita Essential Oil. Pharmacogn. Res. 2017, 9, 74–79. [Google Scholar]
- Dosoky, N.S.; Satyal, P.; Pokharel, S.; Setzer, W.N. Chemical Composition, Enantiomeric Distribution, and Biological Activities of Rhododendron anthopogon Leaf Essential Oil from Nepal. Nat. Prod. Commun. 2016, 11, 1895–1898. [Google Scholar] [PubMed]
- Groll, A.H.; Giri, N.; Petraitis, V.; Petraitiene, R.; Candelario, M.; Bacher, J.S.; Piscitelli, S.C.; Walsh, T.J. Comparative efficacy and distribution of lipid formulations of amphotericin B in experimental Candida albicans infection of the central nervous system. J. Infect. Dis. 2000, 182, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Theberge, S.; Semlali, A.; Alamri, A.; Leung, K.P.; Rouabhia, M.C. albicans growth, transition, biofilm formation, and gene expression modulation by antimicrobial decapeptide KSL-W. BMC Microbiol. 2013, 13, 246. [Google Scholar] [CrossRef] [PubMed]
- Heydari, M.; Zanfardino, A.; Taleei, A.; Bushehri, A.A.S.; Hadian, J.; Maresca, V.; Sorbo, S.; Napoli, M.D.; Varcamonti, M.; Basile, A.; et al. Effect of Heat Stress on Yield, Monoterpene Content and Antibacterial Activity of Essential Oils of Mentha x piperita var. Mitcham and Mentha arvensis var. piperascens. Molecules 2018, 23, 1903. [Google Scholar] [CrossRef] [PubMed]
- Kifer, D.; Mužinić, V.; Klarić, M.Š. Antimicrobial potency of single and combined mupirocin and monoterpenes, thymol, menthol and 1,8-cineole against Staphylococcus aureus planktonic and biofilm growth. J. Antibiot. (Tokyo) 2016, 69, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Dambolena, J.S.; López, A.G.; Cánepa, M.C.; Theumer, M.G.; Zygadlo, J.A.; Rubinstein, H.R. Inhibitory effect of cyclic terpenes (limonene, menthol, menthone and thymol) on Fusarium verticillioides MRC 826 growth and fumonisin B1 biosynthesis. Toxicon 2008, 51, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Yadegarinia, D.; Gachkar, L.; Rezaei, M.B.; Taghizadeh, M.; Astaneh, S.A.; Rasooli, I. Biochemical activities of Iranian Mentha piperita L. and Myrtus communis L. essential oils. Phytochemistry 2006, 67, 1249–1255. [Google Scholar] [CrossRef]
- Singh, S.; Fatima, Z.; Hameed, S. Citronellal-induced disruption of membrane homeostasis in Candida albicans and attenuation of its virulence attributes. Rev. Soc. Bras. Med. Trop. 2016, 49, 465–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharkey, L.L.; McNemar, M.D.; Saporito-Irwin, S.M.; Sypherd, P.S.; Fonzi, W.A. HWP1 functions in the morphological development of Candida albicans downstream of EFG1, TUP1, and RBF1. J. Bacteriol. 1999, 181, 5273–5279. [Google Scholar] [PubMed]
- Ohtsu, N.; Kohari, Y.; Gotoh, M.; Yamada, R.; Nagata, Y.; Murata, M. Utilization of the Japanese Peppermint Herbal Water Byproduct of Steam Distillation as an Antimicrobial Agent. J Oleo Sci. 2018, 67, 1227–1233. [Google Scholar] [CrossRef]
- Kolecka, A.; Chorvát, D., Jr.; Bujdáková, H. The impact of growth conditions on biofilm formation and the cell surface hydrophobicity in fluconazole susceptible and tolerant Candida albicans. Folia Microbiol. (Praha) 2015, 60, 45–51. [Google Scholar] [CrossRef]
- Peixoto, L.R.; Rosalen, P.L.; Ferreira, G.L.; Freires, I.A.; de Carvalho, F.G.; Castellano, L.R.; de Castro, R.D. Antifungal activity, mode of action and anti-biofilm effects of Laurus nobilis Linnaeus essential oil against Candida spp. Arch. Oral Biol. 2017, 73, 179–185. [Google Scholar] [CrossRef]
- Beckloff, N.; Laube, D.; Castro, T.; Furgang, D.; Park, S.; Perlin, D.; Clements, D.; Tang, H.; Scott, R.W.; Tew, G.N.; et al. Activity of an antimicrobial peptide mimetic against planktonic and biofilm cultures of oral pathogens. Antimicrob. Agents Chemother. 2007, 51, 4125–4132. [Google Scholar] [CrossRef] [PubMed]
- Patrzykat, A.; Friedrich, C.L.; Zhang, L.; Mendoza, V.; Hancock, R.E. Sublethal concentrations of pleurocidin-derived antimicrobial peptides inhibit macromolecular synthesis in Escherichia coli. Antimicrob. Agents Chemother. 2002, 46, 605–614. [Google Scholar] [CrossRef]
- Puri, S.; Kumar, R.; Chadha, S.; Tati, S.; Conti, H.R.; Hube, B.; Cullen, P.J.; Edgerton, M. Secreted aspartic protease cleavage of Candida albicans Msb2 activates Cek1 MAPK signaling affecting biofilm formation and oropharyngeal candidiasis. PLoS ONE 2012, 7, e46020. [Google Scholar] [CrossRef]
- Décanis, N.; Savignac, K.; Rouabhia, M. Farnesol promotes epithelial cell defense against Candida albicans through Toll-like receptor 2 expression, interleukin-6 and human beta-defensin 2 production. Cytokine 2009, 45, 132–140. [Google Scholar] [CrossRef]







| Gene | Primer Sequence (5′ à 3′) | Amp Size (bp) |
|---|---|---|
| ACT1 | Forward: GACAATTTCTCTTTCAGCACTAGTAGTGA Reverse: GCTGGTAGAGACTTGACCAACCA | 87 |
| HWP1 | Forward: GCTCAACTTATTGCTATCGCTTATTACA Reverse: GACCGTCTACCTGTGGGACAGT | 67 |
| SAP1 | Forward: TTTCATCGCTCTTGCTATTGCTT Reverse: TGACATCAAAGTCTAAAGTGACAAAACC | 86 |
| SAP2 | Forward: TCCTGATGTTAATGTTGATTGTCAAG Reverse: TGGATCATATGTCCCCTTTTGTT | 82 |
| SAP3 | Forward: GGACCAGTAACATTTTTATGAGTTTTGAT Reverse: TGCTACTCCAACAACTTTCAACAAT | 87 |
| SAP9 | Forward: ATTTACTCCACAGTTTATCACTGAAGGT Reverse: CCACAAGAACCACCCTCAGTT | 86 |
| SAP10 | Forward: CCCGGTATCCAATAGAATCGAA Reverse: TCAGTGAATGTGACGAATTTGAAGA | 78 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benzaid, C.; Belmadani, A.; Djeribi, R.; Rouabhia, M. The Effects of Mentha × piperita Essential Oil on C. albicans Growth, Transition, Biofilm Formation, and the Expression of Secreted Aspartyl Proteinases Genes. Antibiotics 2019, 8, 10. https://doi.org/10.3390/antibiotics8010010
Benzaid C, Belmadani A, Djeribi R, Rouabhia M. The Effects of Mentha × piperita Essential Oil on C. albicans Growth, Transition, Biofilm Formation, and the Expression of Secreted Aspartyl Proteinases Genes. Antibiotics. 2019; 8(1):10. https://doi.org/10.3390/antibiotics8010010
Chicago/Turabian StyleBenzaid, Chahrazed, Amine Belmadani, Ryad Djeribi, and Mahmoud Rouabhia. 2019. "The Effects of Mentha × piperita Essential Oil on C. albicans Growth, Transition, Biofilm Formation, and the Expression of Secreted Aspartyl Proteinases Genes" Antibiotics 8, no. 1: 10. https://doi.org/10.3390/antibiotics8010010
APA StyleBenzaid, C., Belmadani, A., Djeribi, R., & Rouabhia, M. (2019). The Effects of Mentha × piperita Essential Oil on C. albicans Growth, Transition, Biofilm Formation, and the Expression of Secreted Aspartyl Proteinases Genes. Antibiotics, 8(1), 10. https://doi.org/10.3390/antibiotics8010010





