Inhibition of Bacterial and Fungal Biofilm Formation by 675 Extracts from Microalgae and Cyanobacteria
Abstract
:1. Introduction
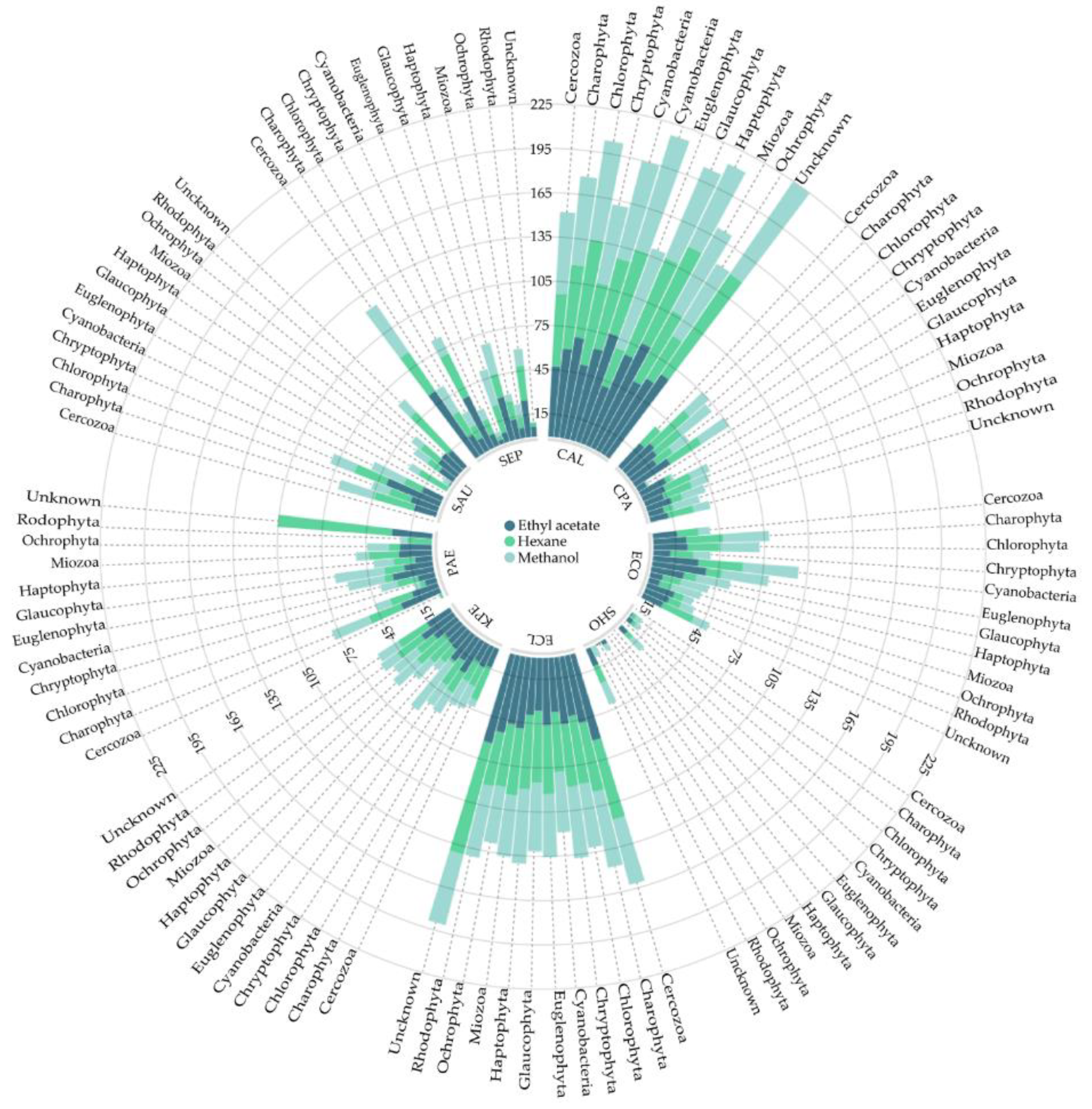
2. Results
3. Discussion
4. Materials and Methods
4.1. Microalgae and Cyanobacteria Extracts
4.2. Microorganisms and Culture Conditions
4.3. Minimal Biofilm Inhibitory Concentration (MBIC)
4.4. Statical Analysis and Data Processing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Flemming, H.-C.; Wingender, J.; Szewzyk. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Roilides, E.; Simitsopoulou, M.; Katragkou, A.; Walsh, T.J. How biofilms evade host defenses. Microbiol. Spectr. 2015, 3, 1–10. [Google Scholar] [CrossRef]
- Domenech, M.; Ramos-Sevillano, E.; García, E.; Moscoso, M.; Yuste, J. Biofilm formation avoids complement immunity and phagocytosis of Streptococcus pneumoniae. Infect. Immun. 2013, 81, 2606–2615. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S. Antimicrobial Tolerance in Biofilms. Microbiol. Spectr. 2015, 3, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed]
- Mclean, R.J.C.; Lam, J.S.; Graham, L.L. Training the biofilm generation—A tribute to J. W. Costerton. J. Bacteriol. 2012, 194, 6706–6711. [Google Scholar] [CrossRef] [PubMed]
- Garrett, T.R.; Bhakoo, M.; Zhang, Z. Bacterial adhesion and biofilms on surfaces. Prog. Nat. Sci. 2008, 18, 1049–1056. [Google Scholar] [CrossRef]
- Gbejuade, H.O.; Lovering, A.M.; Webb, J.C. The role of microbial biofilms in prosthetic joint infections—A review. Acta Orthop. 2015, 86, 147–158. [Google Scholar] [CrossRef]
- Coates, A.R.; Halls, G.; Hu, Y. Novel classes of antibiotics or more of the same? Br. J. Pharm. 2011, 163, 184–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, N.R.; Lobritz, M.A.; Collins, J.J. Microbial persistence and the road to drug resistance. Cell Host Microbe 2013, 13, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [PubMed]
- Silva-Dias, A.; Miranda, I.M.; Branco, J.; Monteiro-Soares, M.; Pina-Vaz, C.; Rodrigues, A.G. Adhesion, biofilm formation, cell surface hydrophobicity, and antifungal planktonic susceptibility: Relationship among Candida spp. Front. Microbiol. 2015, 6, 205. [Google Scholar] [CrossRef] [PubMed]
- Iñigo, M.; Del Pozo, J.L. Fungal biofilms: From bench to bedside. Rev. Esp. Quim. 2018, 31 (Suppl. 1), 35–38. [Google Scholar]
- Rodríguez-Cerdeira, C.; Gregorio, M.C.; Molares-Vila, A.; López-Barcenas, A.; Fabbrocini, G.; Bardhi, B.; Sinani, A.; Sánchez-Blanco, E.; Arenas-Guzmán, R.; Hernandez-Castro, R. Biofilms and vulvovaginal candidiasis. Colloids Surf. B Biointerfaces 2019, 174, 110–125. [Google Scholar] [CrossRef] [PubMed]
- Motuhi, S.-E.; Mehiri, M.; Payri, C.; La Barre, S.; Bach, S. Marine natural products from New Caledonia—A Review. Mar. Drugs 2016, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2017, 34, 235–294. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, A.; Naughton, L.; Montánchez, I.; Dobson, A.; Rai, D. Current status and future prospects of marine natural products (MNPs) as antimicrobials. Mar. Drugs 2017, 15, 272. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2010, 27, 165–237. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Antibacterial derivatives of marine algae: An overview of pharmacological mechanisms and applications. Mar. Drugs 2016, 14, 81. [Google Scholar] [CrossRef]
- De Morais, M.G.; Vaz, B.D.S.; De Morais, E.G.; Costa, J.A.V. Biologically active metabolites synthesized by microalgae. Biomed. Res. Int. 2015, 2015, 835761. [Google Scholar] [CrossRef]
- Singh, S.; Kate, B.N.; Banecjee, U.C. Bioactive compounds from cyanobacteria and microalgae: An overview. Crit. Rev. Biotechnol. 2005, 25, 73–95. [Google Scholar] [CrossRef] [PubMed]
- Bergsson, G.; Hilmarsson, H.; Thormar, H. Antibacterial, antiviral and antifungal activities of lipids. In Lipids and Essential Oils As Antimicrobial Agents; Thormar, H., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2010. [Google Scholar]
- Michalak, I.; Chojnacka, K. Algae as production systems of bioactive compounds. Eng. Life Sci. 2013, 2013, 1–29. [Google Scholar] [CrossRef]
- Lauritano, C.; Andersen, J.H.; Hansen, E.; Albrigtsen, M.; Escalera, L.; Esposito, F.; Helland, K.; Hanssen, K. Ø.; Romano, G.; Ianora, A. Bioactivity Screening of Microalgae for Antioxidant, Anti-Inflammatory, Anticancer, Anti-Diabetes, and Antibacterial Activities. Front. Mar. Sci. 2016, 3, 1405. [Google Scholar] [CrossRef]
- Sanchez-Baracaldo, P. Origin of marine planktonic cyanobacteria. Sci. Rep. 2015, 5, 17418. [Google Scholar] [CrossRef] [PubMed]
- Field, C.B. Primary Production of the Biosphere: Integrating Terrestrial and Oceanic Components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaubert, M.; Bouly, J.P.; Ribera d’Alcalà, M.; Falciatore, A. Light sensing and responses in marine microalgae. Curr. Opin. Plant. Biol. 2017, 37, 70–77. [Google Scholar] [CrossRef]
- Ramirez, J.; Brown, R.; Rainey, T. A review of hydrothermal liquefaction bio-crude properties and prospects for upgrading to transportation fuels. Energies 2015, 8, 6765–6794. [Google Scholar] [CrossRef]
- Stenz, L.; Franã§ois, P.; Fischer, A.; Huyghe, A.; Tangomo, M.; Hernandez, D.; Cassat, J.; Linder, P.; Schrenzel, J.; François, P. Impact of oleic acid (cis-9-octadecenoic acid) on bacterial viability and biofilm production inStaphylococcus aureus. FEMS Microbiol. Lett. 2008, 287, 149–155. [Google Scholar] [CrossRef]
- Davies, D.G.; Marques, C.N.H. A fatty acid messenger is responsible for inducing dispersion in microbial biofilms. J. Bacteriol. 2009, 191, 1393–1403. [Google Scholar] [CrossRef]
- Marques, C.N.H.; Davies, D.G.; Sauer, K. Control of biofilms with the fatty acid signaling molecule cis-2-Decenoic acid. Pharmaceuticals 2015, 8, 816–835. [Google Scholar] [CrossRef]
- Cuellar-Bermudez, S.P.; Aguilar-Hernandez, I.; Cardenas-Chavez, D.L.; Ornelas-Soto, N.; Romero-Ogawa, M.A.; Parra-Saldivar, R. Extraction and purification of high-value metabolites from microalgae: Essential lipids, astaxanthin and phycobiliproteins. Microb. Biotechnol. 2015, 8, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.J.; Nechev, I.; Tsvetkova, H.; Najdenski, K.; Popov, S.S. Compounds with antibacterial activity from the freshwater alga Spirogyra crassa. Genet. Plant. Physiol. 2011, 1, 31–37. [Google Scholar] [CrossRef]
- Niedermeyer, T.H. Anti-infective natural products from Cyanobacteria. Planta Med. 2015, 81, 1309–1325. [Google Scholar] [CrossRef] [PubMed]
- Ciofu, O.; Rojo-Molinero, E.; Macià, M.D.; Oliver, A. Antibiotic treatment of biofilm infections. APMIS 2017, 125, 304–319. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, M.; Zhou, Z.; Dong, J.; Zhang, J.; Xia, Y.; Shu, R. Antibacterial and antibiofilm activities of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) against periodontopathic bacteria. Microb. Pathog. 2016, 99, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Ramos, V.; Morais, J.; Castelo-Branco, R.; Pinheiro, Â.; Martins, J.; Regueiras, A.; Pereira, A.L.; Lopes, V.R.; Frazão, B.; Gomes, D.; et al. Cyanobacterial diversity held in mBRCs as a biotechnological asset: The case study of the newly established LEGE Culture Collection. J. Appl. Phycol. 2018, 30, 1437–1451. [Google Scholar] [CrossRef] [PubMed]
- Kotai, J. Instructions for the Preparation of Modiwed Nutrient Solution Z8 for Algae, Vol 11/69; Norwegian Institute for Water Research: Oslo, Norway, 1972; Volume 11, p. 5. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; 24th Information Supplement, M100-S24, vol. 34, no. 1; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2014. [Google Scholar]
- Ali, L.; Khambaty, F.; Diachenko, G. Investigating the suitability of the Calgary biofilm device for assessing the antimicrobial efficacy of new agents. Bioresour. Technol. 2006, 97, 1887–1893. [Google Scholar] [CrossRef]

| Phylum | Escherichia coli | Klebsiella pneumoniae | Enterobacter cloacae | Pseudomonas aeruginosa | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | MBIC50 | MBIC90 | GM | Range | MBIC50 | MBIC90 | GM | Range | MBIC50 | MBIC90 | GM | Range | MBIC50 | MBIC90 | GM | |
| Cercozoa | 32–128 | 32 | 128 | 50.8 | 32–128 | 32 | 128 | 50.8 | 32–128 | 32 | 128 | 50.8 | 16–64 | 16 | 64 | 25.4 |
| Charophyta | 32–2048 | 256 | 512 | 82.5 | 32–2048 | 256 | 512 | 69.4 | 16–2048 | 256 | 512 | 145.3 | 64–2048 | 256 | 1024 | 326.3 |
| Chlorophyta | 8–4096 | 512 | 1024 | 301.7 | 8–4096 | 512 | 1024 | 315.2 | 2–4096 | 256 | 1024 | 288.1 | 8–4096 | 512 | 1024 | 315.2 |
| Cryptophyta | 16–1024 | 64 | 1024 | 101.6 | 8–1024 | 64 | 1024 | 90.5 | 4–256 | 16 | 256 | 22.6 | 8–512 | 64 | 512 | 64.0 |
| Cyanobacteria | 8–2048 | 128 | 1024 | 153.7 | 16–2048 | 256 | 1024 | 197.3 | 8–2048 | 256 | 512 | 186.6 | 8–2048 | 128 | 512 | 168.7 |
| Euglenophyta | 64–256 | 128 | 256 | 128.0 | 64–256 | 64 | 256 | 101.6 | 64–256 | 128 | 256 | 128.0 | 64–256 | 128 | 256 | 128.0 |
| Glaucophyta | 64–1024 | 256 | 1024 | 256.0 | 64–1024 | 256 | 1024 | 256.0 | 64–512 | 256 | 512 | 228.1 | 64–1024 | 256 | 1024 | 256.0 |
| Haptophyta | 16–512 | 128 | 512 | 136.3 | 16–512 | 128 | 512 | 128.0 | 4–512 | 64 | 512 | 72.6 | 8–512 | 64 | 256 | 82.3 |
| Miozoa | 32–1024 | 64 | 1024 | 122.2 | 32–1024 | 64 | 1024 | 116.7 | 16–1024 | 64 | 1024 | 101.6 | 32–1024 | 64 | 1024 | 111.4 |
| Ochrophyta | 32–1024 | 128 | 512 | 149.3 | 64–1024 | 128 | 512 | 157.2 | 8–512 | 64 | 512 | 91.7 | 32–1024 | 128 | 512 | 149.3 |
| Rhodophyta | 32–512 | 64 | 512 | 90.5 | 32–512 | 64 | 512 | 90.5 | 4–128 | 16 | 128 | 22.6 | 32–256 | 64 | 256 | 80.6 |
| Unknown | 16–256 | 64 | 256 | 71.8 | 16–128 | 64 | 128 | 64.0 | 16–128 | 64 | 128 | 64.0 | 16–256 | 64 | 256 | 71.8 |
| Phylum | Staphylococcus aureus | Staphylococcus epidermidis | Staphylococcus hominis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | MBIC50 | MBIC90 | GM | Range | MBIC50 | MBIC90 | GM | Range | MBIC50 | MBIC90 | GM | |
| Cercozoa | 32–128 | 32 | 128 | 50.8 | 64–256 | 64 | 256 | 101.6 | 64–256 | 64 | 256 | 101.6 |
| Charophyta | 64–4096 | 512 | 1024 | 542.4 | 64–4096 | 512 | 1024 | 588.1 | 64–4096 | 512 | 1024 | 530.1 |
| Chlorophyta | 8–8192 | 512 | 2048 | 469.2 | 8–8192 | 512 | 2048 | 472.8 | 8–8192 | 256 | 1024 | 291.1 |
| Cryptophyta | 32–2048 | 128 | 2048 | 203.2 | 32–2048 | 128 | 2048 | 203.2 | 32–2048 | 128 | 2048 | 203.2 |
| Cyanobacteria | 16–4096 | 256 | 2048 | 297 | 16–4096 | 256 | 10,248 | 339.2 | 16–4096 | 256 | 2048 | 255.3 |
| Euglenophyta | 128–512 | 256 | 512 | 256 | 128–512 | 256 | 512 | 256 | 128–512 | 256 | 512 | 256 |
| Glaucophyta | 128–2048 | 512 | 2048 | 512 | 128–2048 | 512 | 2048 | 512 | 128–2048 | 512 | 2048 | 512 |
| Haptophyta | 32–1024 | 128 | 512 | 120.8 | 32–1024 | 256 | 1024 | 170.9 | 32–1024 | 256 | 1024 | 170.9 |
| Miozoa | 32–2048 | 128 | 1024 | 194 | 64–2048 | 128 | 1024 | 222.9 | 16–2048 | 128 | 1024 | 154 |
| Ochrophyta | 128–1024 | 256 | 1024 | 298.6 | 128–1024 | 256 | 1024 | 314.4 | 128–1024 | 256 | 512 | 249.5 |
| Rhodophyta | 64–128 | 128 | 1024 | 181 | 64–128 | 128 | 1024 | 181 | 64–128 | 128 | 1024 | 181 |
| Unknown | 16–256 | 128 | 256 | 101.6 | 32–256 | 128 | 256 | 114 | 2–128 | 64 | 128 | 45.3 |
| Phylum | Candida albicans | Candida parapsilosis | ||||||
|---|---|---|---|---|---|---|---|---|
| Range | MBIC50 | MBIC90 | GM | Range | MBIC50 | MBIC90 | GM | |
| Cercozoa | 32–64 | 32 | 64 | 40.3 | 32–128 | 32 | 128 | 80.8 |
| Charophyta | 8–128 | 16 | 64 | 23.7 | 32–4096 | 256 | 512 | 284 |
| Chlorophyta | 2–2048 | 32 | 256 | 44.7 | 8–4096 | 256 | 1024 | 224.6 |
| Cryptophyta | 4–128 | 8 | 128 | 12.7 | 16–1024 | 64 | 1024 | 101.6 |
| Cyanobacteria | 2–2048 | 32 | 256 | 39.6 | 16–4096 | 256 | 1024 | 186.6 |
| Euglenophyta | 8–16 | 8 | 16 | 12.7 | 64–256 | 128 | 256 | 128 |
| Glaucophyta | 4–256 | 8 | 256 | 16 | 64–1024 | 256 | 1024 | 256 |
| Haptophyta | 0.5–256 | 32 | 128 | 20.2 | 16–512 | 128 | 512 | 90.5 |
| Miozoa | 8–512 | 32 | 256 | 44.2 | 32–1024 | 64 | 1024 | 122.2 |
| Ochrophyta | 4–256 | 32 | 256 | 27.4 | 64–1024 | 128 | 512 | 165.5 |
| Rhodophyta | 8–64 | 16 | 64 | 20.2 | 32–512 | 64 | 512 | 90.5 |
| Unknown | 16–64 | 32 | 64 | 28.5 | 16–256 | 64 | 256 | 64 |
| Microorganism Strains | OD580 | Type |
|---|---|---|
| Escherichia coli | 1.552 | Bacteria |
| Klebsiella pneumoniae | 0.934 | Bacteria |
| Enterobacter cloacae | 0.879 | Bacteria |
| Pseudomonas aeruginosa | 0.701 | Bacteria |
| Staphylococcus aureus | 0.918 | Bacteria |
| Staphylococcus epidermidis | 1.129 | Bacteria |
| Staphylococcus hominis | 0.940 | Bacteria |
| Candida albicans | 1.014 | Yeast |
| Candida parapsilosis | 1.301 | Yeast |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cepas, V.; López, Y.; Gabasa, Y.; Martins, C.B.; Ferreira, J.D.; Correia, M.J.; Santos, L.M.A.; Oliveira, F.; Ramos, V.; Reis, M.; et al. Inhibition of Bacterial and Fungal Biofilm Formation by 675 Extracts from Microalgae and Cyanobacteria. Antibiotics 2019, 8, 77. https://doi.org/10.3390/antibiotics8020077
Cepas V, López Y, Gabasa Y, Martins CB, Ferreira JD, Correia MJ, Santos LMA, Oliveira F, Ramos V, Reis M, et al. Inhibition of Bacterial and Fungal Biofilm Formation by 675 Extracts from Microalgae and Cyanobacteria. Antibiotics. 2019; 8(2):77. https://doi.org/10.3390/antibiotics8020077
Chicago/Turabian StyleCepas, Virginio, Yuly López, Yaiza Gabasa, Clara B. Martins, Joana D. Ferreira, Maria J. Correia, Lília M.A. Santos, Flávio Oliveira, Vitor Ramos, Mariana Reis, and et al. 2019. "Inhibition of Bacterial and Fungal Biofilm Formation by 675 Extracts from Microalgae and Cyanobacteria" Antibiotics 8, no. 2: 77. https://doi.org/10.3390/antibiotics8020077
APA StyleCepas, V., López, Y., Gabasa, Y., Martins, C. B., Ferreira, J. D., Correia, M. J., Santos, L. M. A., Oliveira, F., Ramos, V., Reis, M., Castelo-Branco, R., Morais, J., Vasconcelos, V., Probert, I., Guilloud, E., Mehiri, M., & Soto, S. M. (2019). Inhibition of Bacterial and Fungal Biofilm Formation by 675 Extracts from Microalgae and Cyanobacteria. Antibiotics, 8(2), 77. https://doi.org/10.3390/antibiotics8020077








