Resistance Levels and Epidemiology of Non-Fermenting Gram-Negative Bacteria in Urinary Tract Infections of Inpatients and Outpatients (RENFUTI): A 10-Year Epidemiological Snapshot
Abstract
:1. Introduction
2. Results
2.1. Demographic Characteristics, Sample Types
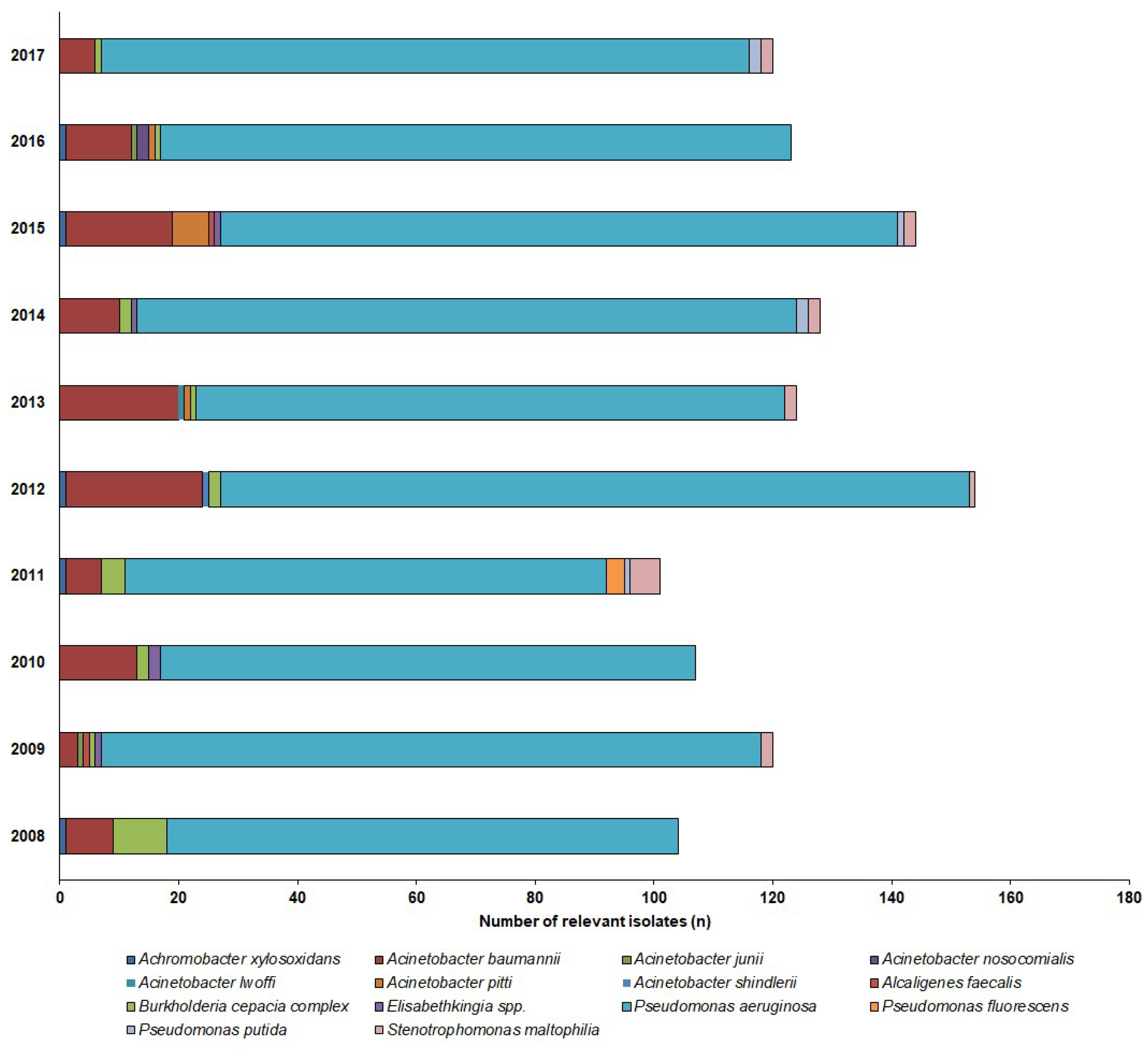
2.2. Distribution of Non-fermenting Gram-negative Bacteria in Urine Samples
2.3. Antibiotic Resistance Levels Among Urinary Non-fermenting Gram-negative Bacteria
3. Discussion
4. Materials and Methods
4.1. Study Location and Design, Data Collection
4.2. Identification of Isolates
4.3. Susceptibility Testing of Relevant Isolates
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Hooton, T.M.; Bradley, S.F.; Cardenas, D.D.; Colgan, R.; Geerlings, S.E.; Rice, J.C.; Saint, S.; Schaeffer, A.J.; Tambayh, P.A.; Tenke, P.; et al. Diagnosis, Prevention, and Treatment of Catheter-Associated Urinary Tract Infection in Adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin. Infect. Dis. 2010, 50, 625–663. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Hooton, T.M.; Naber, K.G.; Wullt, B.; Colgan, R.; Miller, L.G.; Moran, G.J.; Nicolle, L.E.; Raz, R.; Schaeffer, A.J.; et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 2011, 52, e103–e120. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, B.; Heisig, A.; Heisig, P. Uncomplicated urinary tract infections and antibiotic resistance-epidemiological and mechanistic aspects. Antibiotics 2014, 3, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B. Epidemiology of urinary tract infections: Incidence, morbidity, and economic costs. Dis. Mon. 2003, 49, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Simmering, J.E.; Tang, F.; Cavanaugh, J.E.; Polgreen, L.A.; Polgreen, P.M. The Increase in Hospitalizations for Urinary Tract Infections and the Associated Costs in the United States, 1998–2011. Open Forum. Infect. Dis. 2017, 4, ofw281. [Google Scholar] [CrossRef] [PubMed]
- Cullen, I.M.; Manecksha, R.P.; McCullagh, E.; Ahmad, S.; O’Kelly, F.; Flynn, R.; McDermott, T.E.D.; Murphy, P.; Grainger, R.; Fennell, J.P.; et al. An 11-year analysis of the prevalent uropathogens and the changing pattern of Escherichia coli antibiotic resistance in 38,530 community urinary tract infections, Dublin 1999-2009. Ir. J. Med. Sci. 2013, 182, 81–89. [Google Scholar] [CrossRef]
- Calzi, A.; Grignolo, S.; Caviglia, I.; Calevo, M.G.; Losurdo, G.; Piaggio, G.; Bandettini, R.; Castagnola, E. Resistance to oral antibiotics in 4569 Gram-negative rods isolated from urinary tract infection in children. Eur. J. Pediatr. 2016, 175, 1219–1225. [Google Scholar] [CrossRef]
- Stefaniuk, E.; Suchocka, U.; Bosacka, K.; Hryniewicz, W. Etiology and antibiotic susceptibility of bacterial pathogens responsible for community-acquired urinary tract infections in Poland. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1363–1369. [Google Scholar] [CrossRef] [Green Version]
- Adeolu, M.; Alnajar, S.; Naushad, S.; Gupta, R.S. Genome-based phylogeny and taxonomy of the “Enterobacteriales”: Proposal for Enterobacterales ord. nov. divided into the families Enterobacteriaceae, Erwiniaceae fam. nov., Pectobacteriaceae fam. nov., Yersiniaceae fam. nov., Hafniaceae fam. nov., Morganellaceae fam. nov., and Budviciaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 5575–5599. [Google Scholar]
- Pignato, S.; Giammanco, G.M.; Grimont, F.; Grimont, P.A.D.; Giammanco, G. Molecular Characterization of the Genera Proteus, Morganella, and Providencia by Ribotyping. J. Clin. Microbiol. 1999, 37, 2840–2847. [Google Scholar] [PubMed]
- Davin-Regli, A.; Pagès, J.-M. Enterobacter aerogenes and Enterobacter cloacae; versatile bacterial pathogens confronting antibiotic treatment. Front. Microbiol. 2015, 6, 392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.-H.; Kim, Y.S.; Chung, J.-W.; Kim, T.H.; Choo, E.J.; Kim, M.-N.; Kim, B.-N.; Kim, N.J.; Woo, J.H.; Ryu, J. Serratia bacteremia in a large university hospital: Trends in antibiotic resistance during 10 years and implications for antibiotic use. Infect. Control. Hosp. Epidemiol. 2002, 23, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Magyar, A.; Köves, B.; Nagy, K.; Dobák, A.; Arthanareeswaran, V.K.A.; Bálint, P.; Wagenlehner, F.; Tenke, P. Spectrum and antibiotic resistance of uropathogens between 2004 and 2015 in a tertiary care hospital in Hungary. J. Med. Microbiol. 2017, 66, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Takhar, S.S.; Moran, G.J. Diagnosis and management of urinary tract infection in the emergency department and outpatient settings. Infect. Dis. Clin. North. Am. 2014, 28, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, P.; Behzadi, E.; Ranjbar, R. Urinary tract infections and Candida albicans. Cent. European J. Urol. 2015, 68, 96–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gajdács, M.; Dóczi, I.; Ábrók, M.; Lázár, A.; Burián, K. Epidemiology of candiduria and Candida urinary tract infections in inpatients and outpatients: Results from a 10-year retrospective survey. Cent. European J. Urol. 2019, 72, 209–214. [Google Scholar] [PubMed]
- Maharjan, G.; Khadka, P.; Siddhi Shilpakar, G.; Chapagain, G.; Dhungana, G.R. Catheter-Associated Urinary Tract Infection and Obstinate Biofilm Producers. Can. J. Infect. Dis. Med. Microbiol. 2018, 2018, 7624857. [Google Scholar] [CrossRef] [PubMed]
- Clinical Microbiology Procedures Handbook, 4th ed.; Leber, A.L. (Ed.) ASM Press: Washington, DC, USA, 2016; ISBN 978-1-55581-880-7. [Google Scholar]
- Chawla, K.; Vishwanath, S.; Munim, F.C. Nonfermenting Gram-negative Bacilli other than Pseudomonas aeruginosa and Acinetobacter Spp. Causing Respiratory Tract Infections in a Tertiary Care Center. J. Glob. Infect. Dis. 2013, 5, 144–148. [Google Scholar] [PubMed]
- Gilad, J.; Schwartz, D.; Amsalem, Y. Clinical Features and Laboratory Diagnosis of Infection with the Potential Bioterrorism Agents Burkholderia Mallei and Burkholderia Pseudomallei. Int. J. Biomed. Sci. 2007, 3, 144–152. [Google Scholar] [PubMed]
- Rajkumari, N.; Mathur, P.; Gupta, A.K.; Sharma, K.; Misra, M.C. Epidemiology and outcomes of Stenotrophomonas maltophilia and Burkholderia cepacia infections among trauma patients of India: A five year experience. J. Infect. Prev. 2015, 16, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D.; Kaye, D. Urinary Tract Infections. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases (Eighth Edition), 8th ed.; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 886–913. ISBN 978-1-4557-4801-3. [Google Scholar]
- Enoch, D.A.; Birkett, C.I.; Ludlam, H.A. Non-fermentative Gram-negative bacteria. Int. J. Antimicrob. Agents 2007, 29, S33–S41. [Google Scholar] [CrossRef]
- Hotta, G.; Matsumura, Y.; Kato, K.; Nakano, S.; Yunoki, T.; Yamamoto, M.; Nagao, M.; Ito, Y.; Takakura, S.; Ichiyama, S. Risk factors and clinical charasteristics of Stenotrophomonas maltophilia bacteremia: A comparison with bacteremia due to other glucose-non fermenters. Kansenshogaku Zasshi 2013, 87, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Tohamy, S.T.; Aboshanab, K.M.; El-Mahallawy, H.A.; El-Ansary, M.R.; Afifi, S.S. Prevalence of Multidrug-Resistant Gram-Negative Pathogens Isolated from Febrile Neutropenic Cancer Patients with Bloodstream Infections in Egypt and New Synergistic Antibiotic Combinations. Available online: https://www.dovepress.com/prevalence-of-multidrug-resistant-gram-negative-pathogens-isolated-fro-peer-reviewed-fulltext-article-IDR (accessed on 20 August 2019).
- Samonis, G.; Karageorgopoulos, D.E.; Maraki, S.; Levis, P.; Dimopoulou, D.; Spernovasilis, N.A.; Kofteridis, D.P.; Falagas, M.E. Stenotrophomonas maltophilia infections in a general hospital: Patient characteristics, antimicrobial susceptibility, and treatment outcome. PLoS ONE 2012, 7, e37375. [Google Scholar] [CrossRef] [PubMed]
- Gales, A.C.; Jones, R.N.; Forward, K.R.; Liñares, J.; Sader, H.S.; Verhoef, J. Emerging importance of multidrug-resistant Acinetobacter species and Stenotrophomonas maltophilia as pathogens in seriously ill patients: Geographic patterns, epidemiological features, and trends in the SENTRY Antimicrobial Surveillance Program (1997–1999). Clin. Infect. Dis. 2001, 32, S104–S113. [Google Scholar] [CrossRef] [PubMed]
- Del Toro, M.D.; Rodríguez-Bano, J.; Herrero, M.; Rivero, A.; García-Ordoñez, M.A.; Corzo, J.; Pérez-Cano, R. Grupo Andaluz para el Estudio de las Enfermedades Infecciosas Clinical epidemiology of Stenotrophomonas maltophilia colonization and infection: A multicenter study. Medicine (Baltimore) 2002, 81, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Gajdács, M.; Urbán, E. Prevalence and Antibiotic Resistance of Stenotrophomonas maltophilia in Respiratory Tract Samples: A 10-Year Epidemiological Snapshot. Health Serv. Res. Manag. Epidemiol. 2019, 6, 2333392819870774. [Google Scholar]
- Schubert, S.; Kostrzewa, M. MALDI-TOF MS in the Microbiology Laboratory: Current Trends. Curr. Issues Mol. Biol. 2017, 23, 17–20. [Google Scholar] [CrossRef] [Green Version]
- Nagy, E. MALDI-TOF Mass Spectrometry in Microbiology; Kostrzewa, M., Schubert, S., Eds.; Caister Academic Press: Norfolk, UK, 2016; ISBN 978-1-910190-41-8. [Google Scholar]
- Fernández-Olmos, A.; García-Castillo, M.; Morosini, M.-I.; Lamas, A.; Máiz, L.; Cantón, R. MALDI-TOF MS improves routine identification of non-fermenting Gram negative isolates from cystic fibrosis patients. J. Cyst. Fibros. 2012, 11, 59–62. [Google Scholar] [CrossRef] [Green Version]
- Schaumann, R.; Knoop, N.; Genzel, G.H.; Losensky, K.; Rosenkranz, C.; Stîngu, C.S.; Schellenberger, W.; Rodloff, A.C.; Eschrich, K. Discrimination of Enterobacteriaceae and Non-fermenting Gram Negative Bacilli by MALDI-TOF Mass Spectrometry. Open Microbiol. J. 2013, 7, 118–122. [Google Scholar] [CrossRef] [Green Version]
- Gautam, V.; Sharma, M.; Singhal, L.; Kumar, S.; Kaur, P.; Tiwari, R.; Ray, P. MALDI-TOF mass spectrometry: An emerging tool for unequivocal identification of non-fermenting Gram-negative bacilli. Indian J. Med. Res. 2017, 145, 665–672. [Google Scholar] [PubMed]
- Jiménez-Guerra, G.; Heras-Cañas, V.; Gutiérrez-Soto, M.; Del Pilar Aznarte-Padial, M.; Expósito-Ruiz, M.; Navarro-Marí, J.M.; Gutiérrez-Fernández, J. Urinary tract infection by Acinetobacter baumannii and Pseudomonas aeruginosa: Evolution of antimicrobial resistance and therapeutic alternatives. J. Med. Microbiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Zarakolu, P.; Hasçelik, G.; Unal, S. Antimicrobial susceptibility pattern of nosocomial gram negative pathogens: Results from MYSTIC study in Hacettepe University Adult Hospital (2000–2004). Mikrobiyol. Bul. 2006, 40, 147–154. [Google Scholar] [PubMed]
- Labarca, J.A.; Salles, M.J.C.; Seas, C.; Guzmán-Blanco, M. Carbapenem resistance in Pseudomonas aeruginosa and Acinetobacter baumannii in the nosocomial setting in Latin America. Crit. Rev. Microbiol. 2016, 42, 276–292. [Google Scholar] [PubMed]
- Mittal, R.; Aggarwal, S.; Sharma, S.; Chhibber, S.; Harjai, K. Urinary tract infections caused by Pseudomonas aeruginosa: A minireview. J. Infect. Public Health 2009, 2, 101–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frontiers|Editorial: Pseudomonas and Acinetobacter: From Drug Resistance to Pathogenesis|Cellular and Infection Microbiology. Available online: https://www.frontiersin.org/articles/10.3389/fcimb.2018.00068/full (accessed on 20 August 2019).
- Asif, M.; Alvi, I.A.; Rehman, S.U. Insight into Acinetobacter baumannii: Pathogenesis, global resistance, mechanisms of resistance, treatment options, and alternative modalities. Infect. Drug Resist. 2018, 11, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Abbott, I.J.; Slavin, M.A.; Turnidge, J.D.; Thursky, K.A.; Worth, L.J. Stenotrophomonas maltophilia: Emerging disease patterns and challenges for treatment. Expert Rev. Anti. Infect. Ther. 2011, 9, 471–488. [Google Scholar] [CrossRef] [PubMed]
- Van Delden, C. Virulence Factors in Pseudomonas Aeruginosa. In Virulence and Gene Regulation; Ramos, J.-L., Ed.; Springer: Boston, MA, USA, 2004; pp. 3–45. ISBN 978-1-4419-9084-6. [Google Scholar]
- McGowan, J.E. Resistance in nonfermenting gram-negative bacteria: Multidrug resistance to the maximum. Am. J. Med. 2006, 119, S29–S36; discussion S62–S70. [Google Scholar] [CrossRef] [PubMed]
- Nicasio, A.M.; Kuti, J.L.; Nicolau, D.P. The current state of multidrug-resistant gram-negative bacilli in North America. Pharmacotherapy 2008, 28, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Abbo, L.M.; Hooton, T.M. Antimicrobial Stewardship and Urinary Tract Infections. Antibiotics (Basel) 2014, 3, 174–192. [Google Scholar] [CrossRef]
- Morrissey, I.; Hackel, M.; Badal, R.; Bouchillon, S.; Hawser, S.; Biedenbach, D. A Review of Ten Years of the Study for Monitoring Antimicrobial Resistance Trends (SMART) from 2002 to 2011. Pharmaceuticals (Basel) 2013, 6, 1335–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navon-Venezia, S.; Ben-Ami, R.; Carmeli, Y. Update on Pseudomonas aeruginosa and Acinetobacter baumannii infections in the healthcare setting. Curr. Opin. Infect. Dis. 2005, 18, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Kempf, M.; Rolain, J.-M. Emergence of resistance to carbapenems in Acinetobacter baumannii in Europe: Clinical impact and therapeutic options. Int. J. Antimicrob. Agents 2012, 39, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Dobbin, C.; Maley, M.; Harkness, J.; Benn, R.; Malouf, M.; Glanville, A.; Bye, P. The impact of pan-resistant bacterial pathogens on survival after lung transplantation in cystic fibrosis: Results from a single large referral centre. J. Hosp. Infect. 2004, 56, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Hadjiliadis, D.; Steele, M.P.; Chaparro, C.; Singer, L.G.; Waddell, T.K.; Hutcheon, M.A.; Davis, R.D.; Tullis, D.E.; Palmer, S.M.; Keshavjee, S. Survival of lung transplant patients with cystic fibrosis harboring panresistant bacteria other than Burkholderia cepacia, compared with patients harboring sensitive bacteria. J. Heart Lung Transplant. 2007, 26, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Gajdács, M. Extra deaths due to pandrug resistant bacteria: A survey of the literature. Egészségfejlesztés 2019, 60, 31–36. [Google Scholar]
- Sader, H.S.; Jones, R.N. Antimicrobial susceptibility of uncommonly isolated non-enteric Gram-negative bacilli. Int. J. Antimicrob. Agents 2005, 25, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Codjoe, F.S.; Donkor, E.S. Carbapenem Resistance: A Review. Med. Sci (Basel) 2017, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Mikuniya, T.; Kato, Y.; Ida, T.; Maebashi, K.; Monden, K.; Kariyama, R.; Kumon, H. Treatment of Pseudomonas aeruginosa biofilms with a combination of fluoroquinolones and fosfomycin in a rat urinary tract infection model. J. Infect. Chemother. 2007, 13, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Adegoke, A.A.; Stenström, T.A.; Okoh, A.I. Stenotrophomonas maltophilia as an Emerging Ubiquitous Pathogen: Looking Beyond Contemporary Antibiotic Therapy. Front. Microbiol. 2017, 8, 2276. [Google Scholar] [CrossRef]
- Gajdács, M.; Urbán, E. Epidemiological Trends and Resistance Associated with Stenotrophomonas maltophilia Bacteremia: A 10-Year Retrospective Cohort Study in a Tertiary-Care Hospital in Hungary. Diseases 2019, 7, 41. [Google Scholar]
- Denton, M.; Kerr, K.G. Microbiological and clinical aspects of infection associated with Stenotrophomonas maltophilia. Clin. Microbiol. Rev. 1998, 11, 57–80. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.-H.; Kang, C.-I.; Cornejo-Juárez, P.; Yeh, K.-M.; Wang, C.-H.; Cho, S.Y.; Gözel, M.G.; Kim, S.-H.; Hsueh, P.-R.; Sekiya, N.; et al. Fluoroquinolones versus trimethoprim-sulfamethoxazole for the treatment of Stenotrophomonas maltophilia infections: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2019, 25, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Gajdács, M.; Ábrók, M.; Lázár, A.; Burián, K. Comparative Epidemiology and Resistance Trends of Common Urinary Pathogens in a Tertiary-Care Hospital: A 10-Year Surveillance Study. Medicina 2019, 55, 356. [Google Scholar] [CrossRef]
- Gajdács, M.; Urbán, E. Comparative Epidemiology and Resistance Trends of Proteae in Urinary Tract Infections of Inpatients and Outpatients: A 10-Year Retrospective Study. Antibiotics 2019, 8, 91. [Google Scholar] [CrossRef]
- Gajdács, M.; Urbán, E. Resistance Trends and Epidemiology of Citrobacter-Enterobacter-Serratia in Urinary Tract Infections of Inpatients and Outpatients (RECESUTI): A 10-Year Survey. Medicina (Kaunas) 2019, 55, 285. [Google Scholar] [CrossRef]
- MacDougall, C. Beyond Susceptible and Resistant, Part I: Treatment of Infections Due to Gram-Negative Organisms with Inducible β-Lactamases. J. Pediatr. Pharmacol. Ther. 2011, 16, 23–30. [Google Scholar]
- ur Rahman, S.; Ali, T.; Ali, I.; Khan, N.A.; Han, B.; Gao, J. The Growing Genetic and Functional Diversity of Extended Spectrum Beta-Lactamases. Available online: https://www.hindawi.com/journals/bmri/2018/9519718/ (accessed on 20 August 2019).
- Bookstaver, P.B.; Bland, C.M.; Griffin, B.; Stover, K.R.; Eiland, L.S.; McLaughlin, M. A Review of Antibiotic Use in Pregnancy. Pharmacotherapy 2015, 35, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Behzad, P.; Issakhanian, L. Antimicrobial Agents and Urinary Tract Infections. Available online: http://www.eurekaselect.com/172819/article (accessed on 20 August 2019).
- Gupta, S.; Govil, D.; Kakar, P.N.; Prakash, O.; Arora, D.; Das, S.; Govil, P.; Malhotra, A. Colistin and polymyxin B: A re-emergence. Indian J. Crit. Care Med. 2009, 13, 49–53. [Google Scholar] [PubMed]
- Gajdács, M. The Concept of an Ideal Antibiotic: Implications for Drug Design. Molecules 2019, 24, 892. [Google Scholar] [CrossRef]
- Artero, A.; Alberola, J.; Eiros, J.M.; Nogueira, J.M.; Cano, A. Pyelonephritis in pregnancy. How adequate is empirical treatment? Rev. Esp. Quimioter. 2013, 26, 30–33. [Google Scholar] [PubMed]
- Catry, B.; Latour, K.; Bruyndonckx, R.; Diba, C.; Geerdens, C.; Coenen, S. Characteristics of the antibiotic regimen that affect antimicrobial resistance in urinary pathogens. Antimicrob. Resist. Infect. Control 2018, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Latour, K.; Jans, B.; Coenen, S.; Preal, R.; Catry, B. Antibiograms of consecutive urinary tract samples in elderly. Antimicrob. Resist. Infect. Control 2013, 2, 22. [Google Scholar] [CrossRef]
- Al-Hasan, M.N.; Eckel-Passow, J.E.; Baddour, L.M. Influence of referral bias on the clinical characteristics of patients with Gram-negative bloodstream infection. Epidemiol. Infect. 2011, 139, 1750–1756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hospital Bed Count and Patient Turnover Report 2017. National Health Insurance Fund of Hungary. Available online: http://www.neak.gov.hu/felso_menu/szakmai_oldalak/publikus_forgalmi_adatok/gyogyito_megelozo_forgalmi_adat/fekvobeteg_szakellatas/korhazi_agyszam.html (accessed on 20 August 2019).
- Gajdács, M.; Urbán, E. The relevance of anaerobic bacteria in brain abscesses: A ten-year retrospective analysis (2008–2017). Infect. Dis. (London) 2019, 51, 779–781. [Google Scholar] [CrossRef] [PubMed]
- Benkő, R.; Matuz, M.; Hajdú, E.; Bor, A.; Doró, P.; Viola, R.; Soós, G. Antibiotic use in the Hungarian hospitals in the last two decades (1996–2015). Orv. Hetil. 2016, 157, 1839–1846. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, R.; Cantón, R.; Brown, D.F.J.; Giske, C.G.; Heisig, P.; MacGowan, A.P.; Mouton, J.W.; Nordmann, P.; Rodloff, A.C.; Rossolini, G.M.; et al. EUCAST expert rules in antimicrobial susceptibility testing. Clin. Microbiol. Infect. 2013, 19, 141–160. [Google Scholar] [CrossRef] [Green Version]


| Tested Antibiotics | 2008–2012 | 2013–2017 | ||||
|---|---|---|---|---|---|---|
| Outpatients | Inpatients | Statistics a | Outpatients | Inpatients | Statistics a | |
| Amikacin | 5.7% (n = 4) | 57.0% (n = 39) | p < 0.001 | 10.95% (n = 8) | 51.0% (n = 34) | p < 0.001 |
| Gentamicin | 7.1% (n = 6) | 59.3% (n = 40) | p < 0.001 | 10.95% (n = 8) | 41.6% (n = 28) | p < 0.001 |
| Tobramycin | 6.3% (n = 5) | 37.4% (n = 25) | p = 0.022 | 10.95% (n = 8) | 26.9% (n = 19) | p = 0.036 |
| Ciprofloxacin | 10.0% (n = 8) | 61.1% (n = 41) | p < 0.001 | 17.8% (n = 12) | 43.7% (n = 29) | p < 0.001 |
| Levofloxacin | 7.1% (n = 6) | 53.7% (n = 36) | p < 0.001 | 16.4% (n = 11) | 38.6% (n = 26) | p < 0.001 |
| Imipenem | 5.7% (n = 4) | 16.7% (n = 11) | p = 0.041 | 8.2% (n = 6) | 24.7% (n = 16) | p = 0.019 |
| Meropenem | 5.7% (n = 4) | 22.2% (n = 15) | p = 0.046 | 6.8% (n = 5) | 20.8% (n = 14) | p = 0.028 |
| SMX/TMP b | 11.4% (n = 10) | 46.3% (n = 31) | p < 0.001 | 27.4% (n = 20) | 23.4% (n = 15) | n.s. |
| Colistin | 0% (n = 3) | 0% (n = 2) | - | 0% (n = 8) | 0% (n = 11) | - |
| Tested Antibiotics | 2008–2012 | 2013–2017 | ||||
|---|---|---|---|---|---|---|
| Outpatients | Inpatients | Statistics a | Outpatients | Inpatients | Statistics a | |
| Amikacin | 18.3% (n = 52) | 22.1% (n = 116) | n.s. | 13.1% (n = 38) | 13.1% (n = 69) | n.s. |
| Gentamicin | 31.1% (n = 89) | 47.4% (n = 247) | p = 0.043 | 13.1% (n = 38) | 25.9% (n = 135) | p = 0.043 |
| Tobramycin | 28.6% (n = 82) | 44.2% (n = 231) | p = 0.038 | 18.2% (n = 52) | 22.7% (n = 119) | n.s. |
| Ciprofloxacin | 34.5% (n = 99) | 51.2% (n = 268) | p = 0.033 | 31.6% (n = 91) | 38.2% (n = 200) | n.s. |
| Levofloxacin | 39.4% (n = 113) | 54.8% (n = 286) | p = 0.033 | 33.9% (n = 98) | 41.5% (n = 217) | n.s. |
| Imipenem | 10.9% (n = 31) | 22.8% (n = 119) | p = 0.042 | 16.2% (n = 47) | 28.3% (n = 148) | p = 0.036 |
| Meropenem | 12.7% (n = 36) | 24.7% (n = 129) | p = 0.04 | 11.9% (n = 34) | 26.3% (n = 138) | p = 0.029 |
| Ceftazidime | 9.6% (n = 29) | 23.1% (n = 121) | p = 0.036 | 13.0% (n = 37) | 15.1% (n = 79) | n.s. |
| Cefepime | 14.9% (n = 43) | 23.3% (n = 122) | p = 0.045 | 9.5% (n = 27) | 12.1% (n = 63) | n.s. |
| Piperacillin/tazobactam | 11.2% (n = 32) | 21.9% (n = 115) | p = 0.045 | 16.9% (n = 48) | 18.4% (n = 96) | n.s. |
| Colistin | 0% (n = 2) | 0% (n = 3) | - | 0% (n = 10) | 0% (n = 12) | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajdács, M.; Burián, K.; Terhes, G. Resistance Levels and Epidemiology of Non-Fermenting Gram-Negative Bacteria in Urinary Tract Infections of Inpatients and Outpatients (RENFUTI): A 10-Year Epidemiological Snapshot. Antibiotics 2019, 8, 143. https://doi.org/10.3390/antibiotics8030143
Gajdács M, Burián K, Terhes G. Resistance Levels and Epidemiology of Non-Fermenting Gram-Negative Bacteria in Urinary Tract Infections of Inpatients and Outpatients (RENFUTI): A 10-Year Epidemiological Snapshot. Antibiotics. 2019; 8(3):143. https://doi.org/10.3390/antibiotics8030143
Chicago/Turabian StyleGajdács, Márió, Katalin Burián, and Gabriella Terhes. 2019. "Resistance Levels and Epidemiology of Non-Fermenting Gram-Negative Bacteria in Urinary Tract Infections of Inpatients and Outpatients (RENFUTI): A 10-Year Epidemiological Snapshot" Antibiotics 8, no. 3: 143. https://doi.org/10.3390/antibiotics8030143
APA StyleGajdács, M., Burián, K., & Terhes, G. (2019). Resistance Levels and Epidemiology of Non-Fermenting Gram-Negative Bacteria in Urinary Tract Infections of Inpatients and Outpatients (RENFUTI): A 10-Year Epidemiological Snapshot. Antibiotics, 8(3), 143. https://doi.org/10.3390/antibiotics8030143







