Frequent Klebsiella pneumoniae Urinary Tract Infections in a Patient Treated with Ruxolitinib
Abstract
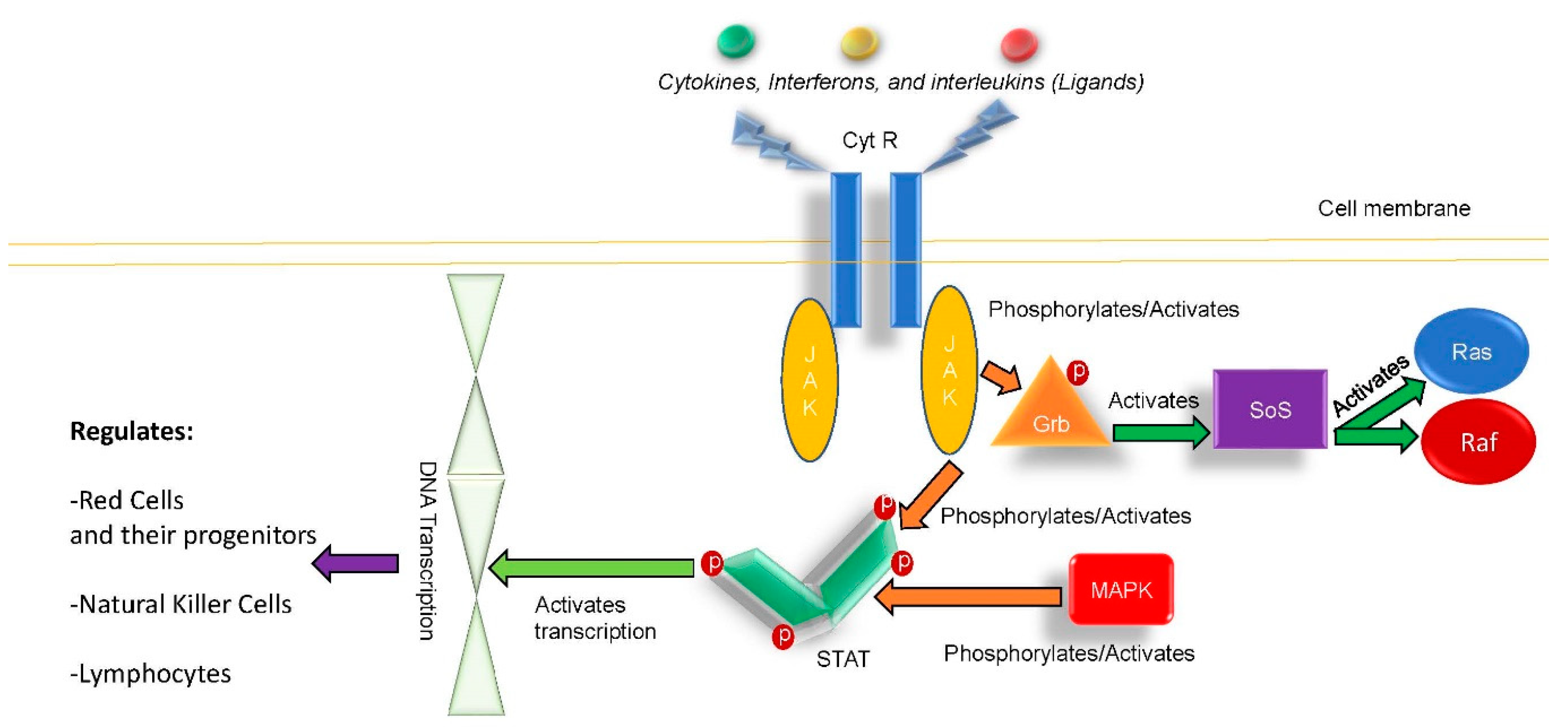
:1. Introduction
Case Report
2. Discussion
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Becker, H.; Engelhardt, M.; von Bubnoff, N.; Wäsch, R. Ruxolitinib. Recent Results Cancer Res. 2014, 201, 249–257. [Google Scholar] [PubMed]
- Raedler, L.A. Jakafi (Ruxolitinib): First FDA-Approved Medication for the Treatment of Patients with Polycythemia Vera. Am. Health Drug Benefits 2015, 8, 75–79. [Google Scholar] [PubMed]
- Assi, T.; Baz, E. Current applications of therapeutic phlebotomy. Blood Transfus. 2014, 12 (Suppl. 1), s75–s83. [Google Scholar] [PubMed]
- Vannucchi, A.M.; Kiladjian, J.J.; Griesshammer, M.; Masszi, T.; Durrant, S.; Passamonti, F.; Harrison, C.N.; Pane, F.; Zachee, P.; Mesa, R.; et al. Ruxolitinib versus Standrad Therapy for the Treatment of Polycythemia Vera. N. Engl. J. Med. 2015, 372, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Zandvakili, I.; Conboy, C.; Ayed, A. Ruxolitinib as first-line treatment in secondary hemophagocytic lymphohistiocytosis: A second experience. Am. J. Hematol. 2018, 93, E123–E125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seif, F.; Khoshmirsafa, M.; Aazami, H.; Mohsenzadegan, M.; Sedighi, G.; Bahar, M. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun. Signal. 2017, 15, 23. [Google Scholar] [CrossRef]
- Zarzour, A.; Tabarroki, A.; Taftaf, R.; Visconte, V.; Ai, J.; Hamilton, B.K.; Papadantonakis, N.; Lichtin, A.E.; Horwitz, L.J.; Advani, A.S.; et al. Rates of Infection in Myelofibrosis PatientsTreated with Ruxolitinib. Blood 2014, 124, 1835. [Google Scholar]
- Lussana, F.; Cattaneo, M.; Rambaldi, A. Ruxolitinib-associated infections: A systematic review and meta-analysis. Am. J. Hematol. 2018, 93, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Prakash, K.; Richman, D. A case report of disseminated histoplasmosis and concurrent cryptococcal meningitis in a patient treated with ruxolitinib. BMC Infect. Dis. 2019, 19, 287. [Google Scholar] [CrossRef] [PubMed]
- Heine, A.; Bossart, P.; Wolf, D. Ruxolitinib is a potent immunosuppressive compound: Is it time for anti-infective prophylaxis? Blood 2013, 122, 3843–3844. [Google Scholar] [CrossRef] [PubMed]
- Manduzio, P. Ruxolitinib in myelofibrosis: To be or not to be an immune disruptor. Ther. Clin. Risk Manag. 2017, 13, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Kusano, Y.; Terui, Y.; Ueda, K. Klebsiella pneumoniae primary liver abscess associated with ruxolitinib. Ann. Hematol. 2016, 95, 1561–1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sylvine, P.; Thomas, S.; Pirayaeh, E. Infections associated with ruxolitinib: Study in the French Pharmacovigilance database. Ann. Hematol. 2018, 97, 913–914. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, J.; Hoffman, R. A comprehensive review and analysis of the effect of ruxolitinib therapy on the survival of patients with myelofibrosis. Blood 2013, 121, 4832–4837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Reference | Age | Gender | Race | Indication | Infection Location |
|---|---|---|---|---|---|
| 12 | 78 | M | NR | PMF | Liver abscess |
| 13 | NR | NR | NR | NR | UTI |
| CC | 75 | M | Caucasian | PCV | UTI |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanna, R.M.; Khalid, M.; El-Nour, L.A.; Selamet, U. Frequent Klebsiella pneumoniae Urinary Tract Infections in a Patient Treated with Ruxolitinib. Antibiotics 2019, 8, 150. https://doi.org/10.3390/antibiotics8030150
Hanna RM, Khalid M, El-Nour LA, Selamet U. Frequent Klebsiella pneumoniae Urinary Tract Infections in a Patient Treated with Ruxolitinib. Antibiotics. 2019; 8(3):150. https://doi.org/10.3390/antibiotics8030150
Chicago/Turabian StyleHanna, Ramy M., Maham Khalid, Lama Abd El-Nour, and Umut Selamet. 2019. "Frequent Klebsiella pneumoniae Urinary Tract Infections in a Patient Treated with Ruxolitinib" Antibiotics 8, no. 3: 150. https://doi.org/10.3390/antibiotics8030150
APA StyleHanna, R. M., Khalid, M., El-Nour, L. A., & Selamet, U. (2019). Frequent Klebsiella pneumoniae Urinary Tract Infections in a Patient Treated with Ruxolitinib. Antibiotics, 8(3), 150. https://doi.org/10.3390/antibiotics8030150




