The Preoperative Inflammatory Status Affects the Clinical Outcome in Cardiac Surgery
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Design
2.2. Operation Techniques
2.3. Definitions
2.4. Biochemistry
2.5. Follow-up
2.6. Statistical Analyses
3. Results
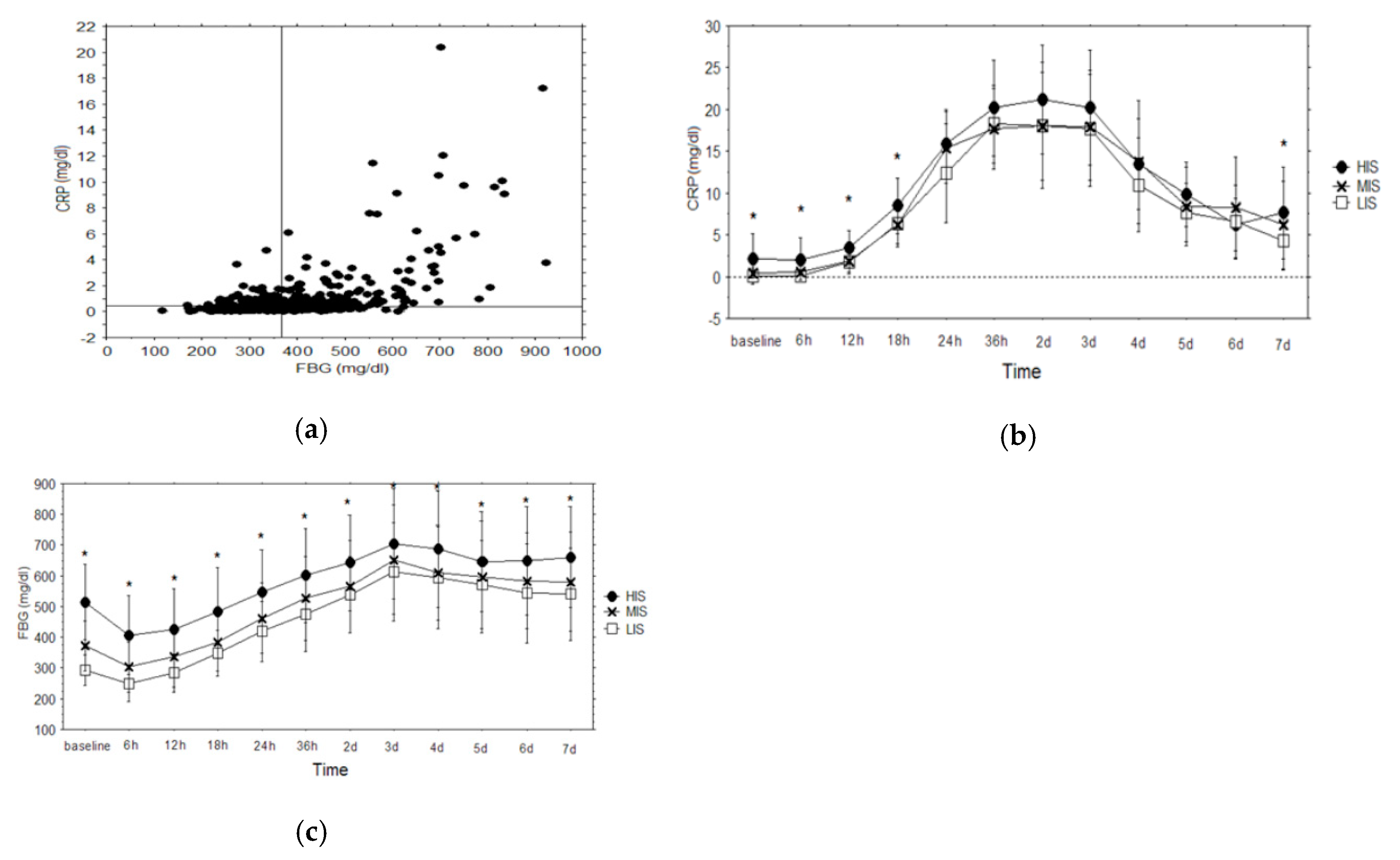
3.1. Laboratory Results
3.2. Clinical Results
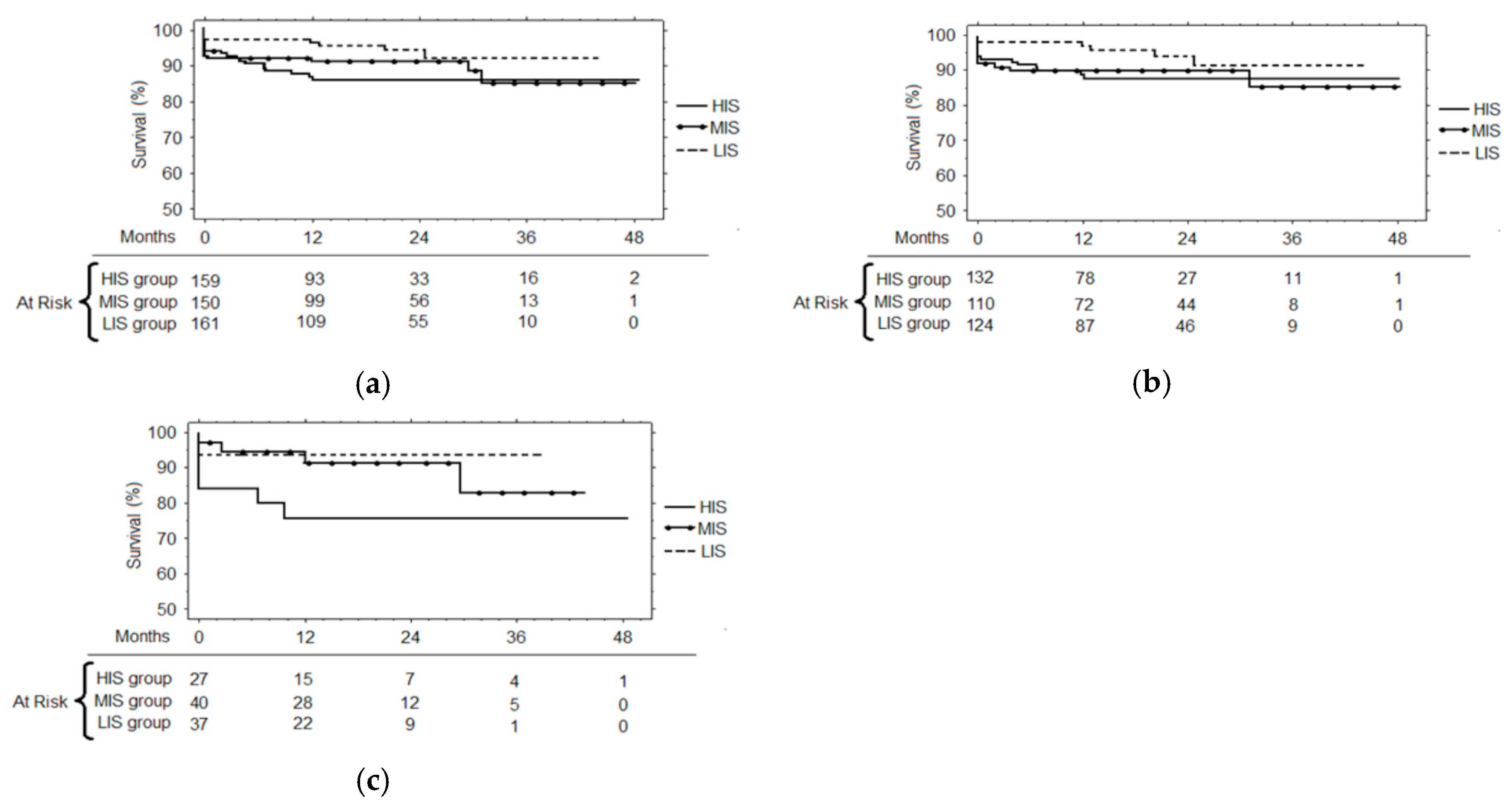
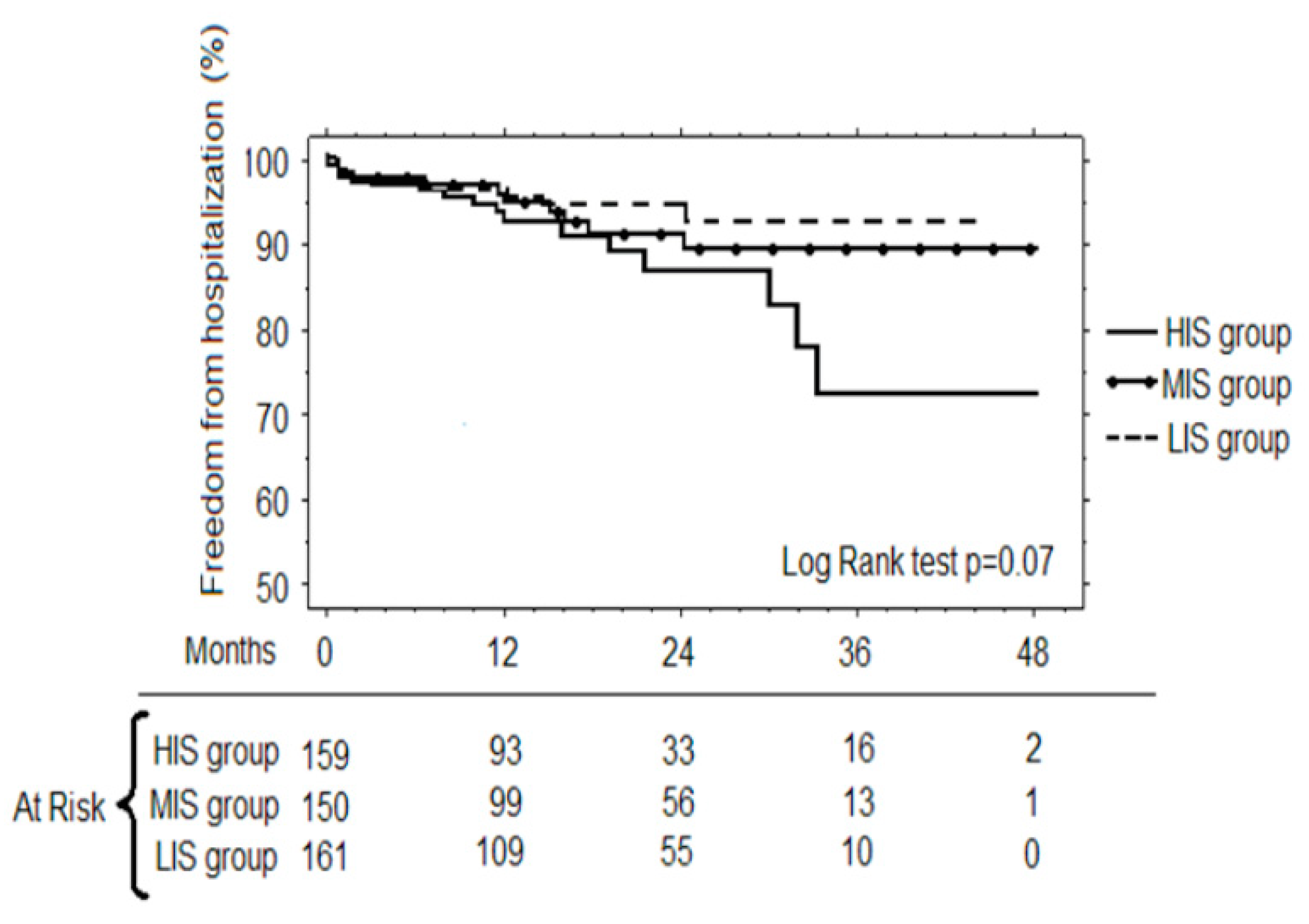
3.3. Follow-up
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pearson, T.A.; Mensah, G.A.; Alexander, R.W.; Anderson, J.L.; 3rd Cannon, R.O.; Criqui, M.; Fadl, Y.Y.; Fortmann, S.P.; Hong, Y.; Myers, G.L.; et al. Markers of Inflammation and Cardiovascular Disease. Application to Clinical and Public Health Practice. A Statement for Healthcare Professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003, 28, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Zebrack, J.S.; Anderson, J.L.; Maycock, C.A.; Horne, B.D.; Bair, T.L.; Muhlestein, J.B. Intermountain Heart Collaborative (IHC) Study Group. Usefulness of high sensitivity C-reactive protein in predicting long-term risk of death or acute myocardial infarction in patients with unstable or stable angina pectoris or acute myocardial infarction. Am. J. Cardiol. 2002, 89, 145–149. [Google Scholar] [CrossRef]
- Bickel, C.; Rupprecht, H.J.; Blankenberg, S.; Espiniola-Klein, C.; Schlitt, A.; Rippin, G.; Hafner, G.; Treude, R.; Othman, H.; Hofmann, K.P.; et al. Relation of markers of inflammation (C-reactive protein, fibrinogen, von Willebrand factor, and leukocyte count) and statin therapy to long-term mortality in patients with angiographically proven coronary artery disease. Am. J. Cardiol. 2002, 89, 901–908. [Google Scholar] [CrossRef]
- Yip, H.K.; Wu, C.J.; Chang, H.W.; Yang, C.H.; Yeh, K.H.; Chua, S.; Fu, M. Levels and values of serum high-sensitivity C-reactive protein within 6 hours after the onset of acute myocardial infarction. Chest 2004, 126, 1417–1422. [Google Scholar] [CrossRef] [PubMed]
- Chew, D.P.; Bhatt, D.L.; Robbins, M.A.; Penn, M.S.; Schneider, J.P.; Lauer, M.S.; Topol, E.J.; Ellis, S.G. Incremental prognostic value of elevated baseline C-reactive protein among established markers of risk in percutaneous coronary intervention. Circulation 2001, 104, 992–997. [Google Scholar] [CrossRef] [PubMed]
- Montalescot, G.; Ankri, A.; Vicaut, E.; Drobinski, G.; Grosgogeat, Y.; Thomas, D. Fibrinogen after coronary angioplasty. Circulation 1995, 92, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Ferlan, G.; Berthet-Bondet, M.; De Pasquale, C.; D’Agostino, D.; Deveze, J.L.; Bex, J.P. Percutaneous angioplasty in the treatment of early vein graft stenosis in patients with coronary artery bypass grafts: Observations in 5 cases treated successfully. Cuore 1990, 7, 335–340. [Google Scholar]
- Walter, D.H.; Fichtlscherer, S.; Britten, M.B.; Rosin, P.; Auch-Schwelk, W.; Schächinger, V.; Zeiher, A.M. Statin therapy, inflammation and recurrent coronary events in patients following coronary stent implantation. J. Am. Coll. Cardiol.. 2001, 38, 2006–2012. [Google Scholar] [CrossRef] [Green Version]
- Otto, C.M.; Kuusisto, J.; Reichenback, D.D.; Gown, A.M.; O’Brien, K.D. Characterization of the early lesion of “degenerative” valvular aortic stenosis. Histological and immunohistochemical studies. Circulation 1994, 90, 844–853. [Google Scholar] [CrossRef]
- Gerber, I.L.; Stewart, R.A.; Hammett, C.J.; Legget, M.E.; Oxenham, H.; West, T.M.; French, J.K.; White, H.D. Effect of aortic valve replacement on C reactive Protein in Nonrheumatic Aortic Stenosis. Am. J. Cardiol. 2003, 92, 1129–1132. [Google Scholar] [CrossRef]
- Galante, A.; Pietroiusti, A.; Vellini, M.; Piccolo, P.; Possati, G.; De Bonis, M.; Grillo, R.L.; Fontana, C.; Favalli, C. C-reactive protein is increased in patients with degenerative aortic valvular stenosis. J. Am. Coll. Cardiol. 2001, 38, 1078–1082. [Google Scholar] [CrossRef] [Green Version]
- Samsonov, M.; Lopatin, J.; Tiltz, G.P. The activated immune system and the renin-angiotensin-aldosterone system in congestive heart failure. J. Intern. Med. 1998, 243, 93–98. [Google Scholar] [CrossRef] [PubMed]
- MacGowan, G.A.; Mann, D.L.; Kormos, R.L.; Feldman, A.M.; Murali, S. Circulating interleukin-6 in severe heart failure. Eur. Heart J. 1998, 19, 990–1003. [Google Scholar] [CrossRef]
- Krasuski, R.A.; Bush, A.; Kay, J.E.; Jr Mayes, C.E.; Wang, A.; Fleming, J.; Pierce, C.; Kisslo, K.B.; Harrison, J.K.; Bashore, T.M. C-reactive protein elevation independently influences the procedural success of percutaneous balloon mitral valve commissurotomy. Am. Heart J. 2003, 146, 1099–1104. [Google Scholar] [CrossRef]
- Werdan, K. The activated immune system in congestive heart failure. From dropsy to the cytokine paradigm. J. Intern. Med. 1998, 243, 87–92. [Google Scholar]
- Alonso-Martinez, J.L.; Llorente-Diez, B.; Echegaray-Angara, M.; Olaz-Preciado, F.; Urbieta-Echezarreta, M.; Gonzalez-Arencibia, C. C-reactive protein as predictor of improvement and readmission in heart failure. Eur. J. Heart Fail. 2002, 4, 331–336. [Google Scholar] [CrossRef]
- Biancari, F.; Lahtinen, J.; Lepojarvi, S.; Rainio, P.; Salmela, E.; Pokela, R.; Lepojärvi, M.; Satta, J.; Juvonen, T.S. Preoperative C-reactive protein and outcome after coronary artery bypass. Ann. Thorac. Surg. 2003, 76, 2007–2012. [Google Scholar] [CrossRef]
- Boeken, U.; Feindt, P.; Zimmermann, N.; Kalweiy, G.; Petzold, T.; Gams, E. Increased preoperative C-reactive protein (CRP)-values without signs of an infection and complicated course after cardiopulmonary bypass (CPB) – operations. Eur. J. Cardio-thorac. Surg. 1998, 13, 541–545. [Google Scholar] [CrossRef]
- Fransen, E.J.; Maessen, J.G.; Elenbaas, T.W.; van Aarnhem, E.E.; Dieijen-Visser, M.P. Increased preoperative C-Reactive protein plasma levels as risk factor for post-operative infections. Ann. Thorac. Surg. 1999, 67, 134–138. [Google Scholar] [CrossRef]
- Mervyn, S.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Gordon, R.B.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar]
- Osmand, A.P.; Friedenson, B.; Gewurz, H.; Painter, R.H.; Hofmann, T.; Shelton, E. Characterization of C-reactive protein and the complement subcomplement C1t as homologous proteins displaying cyclic pentameric symmetry (pentraxins). Proc. Natl. Acad. Sci. USA 1977, 74, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, M.; Nasso, G.; Andreotti, F.; Minniti, G.; Iacoviello, L.; Donati, M.; Schiavello, R.; Possati, G. Preoperative C-reactive protein level and outcome following cardiac surgery. Eur. J. Cardio-thorac. Surg. 2002, 22, 521–526. [Google Scholar] [CrossRef]
- Paparella, D.; Yau, T.M.; Young, E. Cardiopulmonary bypass induced inflammation: pathophysiology and treatment. An update. Eur. J. Cardiothorac. Surg. 2002, 21, 232–244. [Google Scholar] [CrossRef] [Green Version]
- Royster, R.L.; Grosshans, D.W.; Kon, N.D. A multimodal approach to address the inflammation of cardiopulmonary bypass? J. Cardiothorac Vasc. Anesth. 2011, 25, e35. [Google Scholar] [CrossRef] [PubMed]
- Gnoni, A.; Ballini, A.; Trentadue, R.; Taurino, F.; Santacroce, L.; Ferrara, P.; Massaro, F.; Brienza, N.; Massari, A.M.; Sardaro, N.; et al. Induction of mitochondrial dysfunction in patients under cardiopulmonary by-pass: preliminary results. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8115–8123. [Google Scholar]
- O’Neil, M.P.; Fleming, J.C.; Badhwar, A.; Guo, L.R. Pulsatile versus nonpulsatile flow during cardiopulmonary bypass: microcirculatory and systemic effects. Ann. Thorac. Surg. 2012, 94, 2046–2053. [Google Scholar] [CrossRef]
- Dieleman, J.M. Corticosteroids for the inflammatory response to cardiopulmonary bypass: an update. Curr. Pharm. Des. 2013, 19, 3979–3991. [Google Scholar] [CrossRef]
- Landis, R.C.; Brown, J.R.; Fitzgerald, D.; Likosky, D.S.; Shore-Lesserson, L.; Baker, R.A.; Hammon, J.W. Attenuating the systemic inflammatory response to adult cardiopulmonary bypass: A critical review of the Evidence Base. J. Extra Corpor. Technol. 2014, 46, 197–211. [Google Scholar]
- Takeuchi, K.; Cao-Danh, H.; Friehs, I.; Glynn, P.; D’Agostino, D.; Simplaceanu, E.; McGowan, F.X.; del Nido, P.J. Administration of fructose 1,6-diphosphate during early reperfusion significantly improves recovery of contractile function in the post ischemic heart. J. Thorac. Cardiovasc Surg. 1998, 116, 335–343. [Google Scholar] [CrossRef]
- Petrosillo, G.; Di Venosa, N.; Ruggiero, F.M.; Pistolese, M.; D’Agostino, D.; Tiravanti, E.; Fiore, T.; Paradies, G. Mitochondrial dysfunction associated with cardiac ischemia/reperfusion can be attenuated by oxygen tension control. Role of oxygen-free radicals and cardiolipin. Biochim. Biophys. Acta-Bioenergetics 2005, 1710, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Giordano, P.; Scrascia, G.; D’Agostino, D.; Mastro, F.; Rotunno, C.; Conte, M.; Rociola, R.; Paparella, D. Myocardial damage following cardiac surgery: Comparison between single-dose Celsior cardioplegic solution and cold blood multi-dose cardioplegia. Perfusion 2013, 28, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Colantuono, G.; Tiravanti, E.A.; Di Venosa, N.; Cazzato, A.; Rastaldo, R.; Cagiano, R.; D’Agostino, D.; Federici, A.; Fiore, T. Hyperoxia confers myocardial protection in mechanically ventilated rats through the generation of free radicals and opening of mitochondrial ATP-sensitive potassium channels. Clin. Exp. Pharmacol. Physiol. 2008, 35, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Di Venosa, N.; Masciopinto, F.; Romito, F.M.; Altea Tiravanti, E.; Schena, S.; Fasanella, E.; D’Agostino, D.; Pappagallo, L.; Federici, A.; Fiore, T. Hypoxic reperfusion limits functional impairment following cardioplegic arrest in isolated rat heart. Minerva Anestesiologica 2001, 67, 509–517. [Google Scholar] [PubMed]
- Bortone, A.S.; D’Agostino, D.; Schena, S.; Rubini, G.; Viecca, M.; Sardaro, V.; Tucci, A.; De Luca Tupputi Schinosa, L. Instrumental validation of percutaneous transmyocardial revascularization: Follow-up at one year. Ann. Thoracic. Surg. 2000, 70, 1115–1118. [Google Scholar] [CrossRef]
- Bortone, A.S.; D’Agostino, D.; Schena, S.; Rubini, G.; Brindicci, P.; Sardaro, V.; D’Addabbo, A.; De Luca Tupputi Schinosa, L. Inflammatory response and angiogenesis after percutaneous transmyocardial laser revascularization. Ann. Thoracic. Surg. 2000, 70, 1134–1138. [Google Scholar] [CrossRef]
- Serviddio, G.; Di Venosa, N.; Federici, A.; D’Agostino, D.; Rollo, T.; Prigigallo, F.; Altomare, E.; Fiore, T.; Vendemiale, G. Brief hypoxia before normoxic reperfusion (postconditioning) protects the heart against ischemia-reperfusion injury by preventing mitochondria peroxyde production and glutathione depletion. FASEB J. 2005, 19, 354–361. [Google Scholar] [CrossRef]
- Denis, M.; Campbell, D.; Gregg, E.O. Interleukin-2 and granulocyte-macrophage colony-stimulating factor stimulate growth of a virulent strain of Escherichia Coli. Infect. Immunol. 1991, 59, 1853–1856. [Google Scholar]
- Raffaella, T.; Fiore, F.; Fabrizia, M.; Francesco, P.; Arcangela, I.; Salvatore, S.; Luigi, S.; Nicola, B. Induction of mitochondrial dysfunction and oxidative stress in human fibroblast cultures exposed to serum from septic patients. Life Sci. 2012, 91, 237–243. [Google Scholar] [CrossRef]
- Meduri, G.U.; Kanagat, S.; Stefan, J.; Tolley, E.; Schaberg, S. Cytokines IL-1beta, IL-6 and TNF-alpha enhance in vitro growth of bacteria. Am. J. Respir. Crit. Care Med. 1999, 160, 961–967. [Google Scholar] [CrossRef]
- Meduri, G.U. Clinical review: A paradigm shift: The bidirectional effect of inflammation on bacterial growth. Clinical implications for patients with acute respiratory distress syndrome. Critical Care 2002, 6, 24–29. [Google Scholar] [CrossRef]
- Furtado de Mendonca-Filho, H.T.; Gomes, R.V.; Campos, L.A.; Tura, B.; Nunes, E.M.; Gomes, R.; Bozza, F.; Bozza, P.T.; Castro-Faria-Neto, H.C. Circulating levels of macrophage migration inhibitory factor are associated with mild pulmonary dysfunction after cardiopulmonary bypass. Shock 2004, 22, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Rifai, N.; Rose, L.; Buring, J.E.; Cook, N.R. Comparison of C-reactive protein and low density lipoprotein cholesterol levels in the prediction of first cardiovascular event. N. Engl. J. Med. 2002, 347, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Cook, N.R. Clinical Usefulness of Very High and Very Low levels of C-reactive protein Across the Full Range of Framingham Risk Scores. Circulation 2004, 109, 1955–1959. [Google Scholar] [CrossRef] [PubMed]
- Dibra, A.; Mehilli, J.; Braun, S.; Hadamitzky, M.; Baum, H.; Dirschinger, J.; Schühlen, H.; Schömig, A.; Kastrati, A. Association between C-Reactive Protein Levels and Subsequent Cardiac Events among Patients with Stable Angina Treated with Coronary Artery Stenting. Am. J. Med. 2003, 114, 715–722. [Google Scholar] [CrossRef]
- Walter, D.H.; Fichtlscherer, S.; Sellwig Auch-Schwelk, W.; Schächinger, V.; Zeiher, A.M. Preprocedural C-Reactive Protein Levels and Cardiovascular Events After Coronary Stent Implantation. J. Am. Coll. Cardiol. 2001, 37, 839–846. [Google Scholar] [CrossRef]
- Rahel, B.M.; Visseren, F.L.; Suttorp, M.J.; Plokker, T.H.; Kelder, J.C.; de Jongh, B.M.; Bouter, K.P.; Diepersloot, R.J. Preprocedural serum levels of acute-phase reactants and prognosis after percutaneous coronary intervention. Cardiovasc Res. 2003, 60, 136–140. [Google Scholar] [CrossRef]
- Otsuka, M.; Hayashi, Y.; Ueda, H.; Imazu, M.; Kohno, N. Predictive value of preprocedural fibrinogen concerning coronary stenting. Atherosclerosis 2002, 164, 371–378. [Google Scholar] [CrossRef]
- D’Agostino, D.; Bottalico, L.; Santacroce, L. Infective endocarditis: What is changed in epidemiology and prophylaxis. Acta Medica Medit. 2012, 28, 311–319. [Google Scholar]
- D’Agostino, D. Infective endocarditis today. F1000 Res. 2017, 6, 2188. [Google Scholar] [CrossRef] [Green Version]
- D’Agostino, D.; Man, A.; Santacroce, L. Current trends in cardiac surgery: Clinical experience in the treatment of mediastinitis with sternal wound infection through negative pressure therapy. Acta Medica Medit. 2016, 32, 1905–1910. [Google Scholar]
- D’agostino, D.; Lacatena, C.; Santacroce, L. Postoperative mediastinitis in cardiac surgery-pathophysiology, risk factors and prevention. Acta Medica Medit. 2015, 31, 1311–1318. [Google Scholar]
- D’Agostino, D.; Losacco, T.; Santacroce, L. Clinical management of the infective endocarditis today. Acta Medica Medit. 2012, 28, 321–329. [Google Scholar]
- Prejbeanu, R.; Vermesan, H.; Dragulescu, S.I.; Vermesan, D.; Motoc, A.; Sabatini, R.; Santacroce, L.; Cagiano, R. Thromboembolic risk after knee endoprosthesis. Eur. Rev. Med. Pharmacol. Sci. 2007, 11, 297–300. [Google Scholar] [PubMed]
- Ballini, A.; Cantore, S.; Farronato, D.; Cirulli, N.; Inchingolo, F.; Papa, F.; Malcangi, G.; Inchingolo, A.D.; Dipalma, G.; Sardaro, N.; et al. Periodontal disease and bone pathogenesis: the crosstalk between cytokines and porphyromonas gingivalis. J. Biol. Regul. Homeost Agents. 2015, 29, 273–281. [Google Scholar] [PubMed]
- Vermesan, D.; Vermesan, H.; Dragulescu, S.I.; Bera, I.; Di Giovanni, A.; Sabatini, R.; Santacroce, L.; Bottalico, L.; Flace, P.; Cagiano, R. Secondary pathologic fractures in osteosarcoma: prognosis and evolution. Eur. Rev. Med. Pharmacol. Sci. 2009, 13, 71–76. [Google Scholar] [PubMed]
- Santacroce, L.; Cagiano, R.; Del Prete, R.; Bottalico, L.; Sabatini, R.; Carlaio, R.G.; Prejbeanu, R.; Vermesan, H.; Dragulescu, S.I.; Vermesan, D.; et al. Helicobacter pylori infection and gastric MALTomas: an up-to-date and therapy highlight. Clin. Ter. 2008, 159, 457–462. [Google Scholar]
- Santacroce, L.; Carlaio, R.G.; Bottalico, L. Does it make sense that diabetes is reciprocally associated with periodontal disease? Endocr. Metab. Immune Disord. Drug Targets 2010, 10, 57–70. [Google Scholar] [CrossRef]
- Giudice, G.; Cutrignelli, D.A.; Sportelli, P.; Limongelli, L.; Tempesta, A.; Gioia, G.D.; Santacroce, L.; Maiorano, E.; Favia, G. Rhinocerebral Mucormycosis with Orosinusal Involvement: Diagnostic and Surgical Treatment Guidelines. Endocr. Metab. Immune Disord. Drug Targets 2016, 16, 264–269. [Google Scholar] [CrossRef]
- Ballini, A.; Cantore, S.; Dedola, A.; Santacroce, L.; Laino, L.; Cicciù, M.; Mastrangelo, F. IL-1 haplotype analysis in periodontal disease. J. Biol. Regul. Homeost Agents 2018, 32, 433–437. [Google Scholar]
- Santacroce, L.; Cagiano, R.; Carlaio, R.G.; Del Prete, R.; Bottalico, L. Dentistry oral hygiene and endocarditis. Pathophysiology and prophylactic therapy. Recenti Progr. Med. 2008, 99, 516–521. [Google Scholar]
- Santacroce, L.; Bottalico, L.; Mangini, F. Dental hygiene procedure in a patient with Giardia lamblia infection. Int. J. Dent. Hyg. 2007, 5, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Bottalico, L.; Valenzano, A.; Leone, D.; Mangini, F.; Santacroce, L. The incidence of dental caries during childhood. A clinical and epidemiologic study in Matera (Southern Italy). Clin. Ter. 2007, 158, 409–419. [Google Scholar] [PubMed]
- Campanella, V.; Syed, J.; Santacroce, L.; Saini, R.; Ballini, A.; Inchingolo, F. Oral probiotics influence oral and respiratory tract infections in pediatric population: a randomized double-blinded placebo-controlled pilot study. Eur. Rev. Med. Pharmacol Sci. 2018, 22, 8034–8041. [Google Scholar] [PubMed]
- Santacroce, L.; Charitos, I.A.; Bottalico, L. A successful history: probiotics and their potential as antimicrobials. Expert Rev. Anti Infect. Ther. 2019, 17, 635–645. [Google Scholar] [CrossRef]
- Ballini, A.; Santacroce, L.; Cantore, S.; Bottalico, L.; Dipalma, G.; Topi, S.; Saini, R.; De Vito, D.; Inchingolo, F. Probiotics Efficacy on Oxidative Stress Values in Inflammatory Bowel Disease: A Randomized Double-Blinded Placebo-Controlled Pilot Study. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 373–381. [Google Scholar] [CrossRef]
- Ballini, A.; Santacroce, L.; Cantore, S.; Bottalico, L.; Dipalma, G.; Vito, D.; Saini, R.; Inchingolo, F. Probiotics Improve Urogenital Health in Women. Open Access Maced. J. Med. Sci. 2018, 6, 1845–1850. [Google Scholar] [CrossRef] [Green Version]
- Man, A.; Santacroce, L.; Iacob, R.; Mare, A.; Man, L. Antimicrobial Activity of Six Essential Oils Against a Group of Human Pathogens: A Comparative Study. Pathogens 2019, 8, 15. [Google Scholar] [CrossRef]
- Inchingolo, F.; Dipalma, G.; Cirulli, N.; Cantore, S.; Saini, R.S.; Altini, V.; Santacroce, L.; Ballini, A.; Saini, R. Microbiological results of improvement in periodontal condition by administration of oral probiotics. J. Biol. Regul Homeost Agents. 2018, 32, 1323–1328. [Google Scholar]
- Santacroce, L.; D’Agostino, D.; Charitos, I.A.; Bottalico, L.; Ballini, A. A short review about electrophysiology and bioimpedance: History and perspectives. Indian J. Public Health Res. Develop. 2018, 9, 577–591. [Google Scholar] [CrossRef]
- Santacroce, L.; Charitos, I.A.; Topi, S.; Bottalico, L. The Alcmaeon’s School of Croton: Philosophy and Science. Open Access Maced. J. Med. Sci. 2019, 28, 500–503. [Google Scholar] [CrossRef]
- Santacroce, L.; Bottalico, L.; Charitos, I.A. Greek Medicine Practice at Ancient Rome: The Physician Molecularist Asclepiades. Medicines (Basel) 2017, 4, 92. [Google Scholar] [CrossRef] [PubMed]
- Cantore, S.; Crincoli, V.; Boccaccio, A.; Uva, A.E.; Fiorentino, M.; Monno, G.; Bollero, P.; Derla, C.; Fabiano, F.; Ballini, A.; et al. Recent Advances in Endocrine, Metabolic and Immune Disorders: Mesenchymal Stem Cells (MSCs) and Engineered Scaffolds. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Di Serio, F.; Lovero, R.; D’Agostino, D.; Nisi, L.; Miragliotta, G.; Contino, R.; Man, A.; Ciccone, M.M.; Santacroce, L. Evaluation of procalcitonin, Vitamin D and C-reactive protein levels in septic patients with positive emocoltures. Our preliminary experience. Acta Medica Medit. 2016, 32, 1911–1914. [Google Scholar]
- Charitos, I.A.; Topi, S.; Castellaneta, F.; D’Agostino, D. Current issues and perspectives in patients with possible sepsis at Emergency Departments. Antibiotics (Basel) 2019, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, R.; Ronga, L.; Addati, G.; Magrone, R.; Abbasciano, A.; Carlo, D.D.; Santacroce, L. A Retrospective Study about the Impact of Switching from Nested PCR to Multiplex Real-Time PCR on the Distribution of the Human Papillomavirus (HPV) Genotypes. Medicina (Kaunas) 2019, 55, 418. [Google Scholar] [CrossRef] [PubMed]
| LIS | MIS | HIS | p | |
|---|---|---|---|---|
| Patients | 161 | 150 | 159 | |
| Age (years) | 63.1 ± 10.2 | 65.5 ± 9.6 | 66.2 ± 8.8 | 0.01 |
| Male | 126 (78.3%) | 104 (69.3%) | 116 (73%) | 0.19 |
| BSA (m2) | 1.79 ± 0.18 | 1.76 ± 0.16 | 1.78 ± 0.16 | 0.46 |
| Unstable Angina | 19 (11.8%) | 23 (15.3%) | 25 (15.7%) | 0.54 |
| MI < 21 days | 31 (19.3%) | 29 (19.3%) | 41 (25.8%) | 0.26 |
| Calcific Aortic Stenosis | 12 (7.4%) | 15 (10%) | 9 (5.6%) | 0.35 |
| Rheumatic valvular disease | 8 (4.9%) | 7 (4.7%) | 4 (2.5%) | 0.48 |
| LVEF (%) | 49.2 ± 8.8 | 49.9 ± 9.9 | 46.8 ± 10.6 | 0.02 |
| Hypertension | 88 (54.7%) | 94 (62.7%) | 115 (72.3%) | 0.005 |
| Diabetes | 49 (30.4%) | 41 (27.3%) | 50 (31.4%) | 0.71 |
| Hypercholesterolemia | 75 (46.6%) | 83 (55.3%) | 89 (56%) | 0.17 |
| Statins therapy | 55 (34.2%) | 61 (40.6) | 67 (42.1) | 0.29 |
| Smoking history | 37 (23%) | 34 (22.7%) | 52 (32.7%) | 0.07 |
| COPD | 39 (24.2%) | 37 (24.7%) | 46 (28.9%) | 0.57 |
| C.A. Stenosis > 60% | 7 (4.3%) | 9 (6%) | 13 (8.2%) | 0.36 |
| CVA < 21 days | 1 (0.6%) | 1 (0.7%) | 4 (2.5%) | 0.23 |
| Preop. cTnI (ng/mL) | 0.11 ± 0.42 | 0.21 ± 1.2 | 0.40 ± 1.43 | 0.15 |
| LIS | MIS | HIS | p | |
|---|---|---|---|---|
| Patients | 161 | 150 | 159 | |
| CABG | 79 (49.1%) | 72 (48%) | 81 (50.9%) | 0.87 |
| OPCAB | 38 (23.6%) | 30 (20%) | 44 (27.7%) | 0.28 |
| Aortic Root surgery | 3 (1.9%) | 3 (2%) | 6 (3.8%) | 0.48 |
| AVR | 12 (7.5%) | 17 (11.3%) | 9 (5.7%) | 0.17 |
| Mitral replacement/repair | 7 (4.3%) | 14 (9.3%) | 10 (6.3%) | 0.2 |
| Combined procedures | 16 (9.9%) | 13 (8.7%) | 8 (5%) | 0.24 |
| Other procedures | 6 (3.7%) | 1 (0.7%) | 1 (0.6%) | 0.06 |
| CPB duration (min) | 105 ± 28 | 114 ± 44 | 120 ± 34 | 0.006 |
| X-Clamp duration (min) | 58 ± 22 | 63 ± 23 | 62 ± 23 | 0.3 |
| LIS | MIS | HIS | p | |
|---|---|---|---|---|
| M 1 | ||||
| Patients | 161 | 150 | 159 | |
| Operative Mortality | 4 (2.5%) | 9 (6%) | 11 (6.9%) | 0.16 |
| Causes of death | ||||
| Cardiac death | 4 (2.5%) | 5 (3.3%) | 3 (1.8%) | 0.72 |
| Sepsis | 0 | 2 (1.3%) | 6 (3.7%) | 0.03 |
| Stroke | 0 | 1 (0.7%) | 1 (0.6%) | 0.59 |
| Bleeding | 0 | 1 (0.7%) | 1 (0.6%) | 0.59 |
| M 2 | ||||
| ICU stay (h) | 46.9 ± 28.5 | 62.1 ± 78.9 | 65.6 ± 101.2 | 0.07 |
| Overall stay (days) | 8.1 ± 4.9 | 9.9 ± 8.2 | 9.7 ± 71.8 | 0.06 |
| Mechanical ventilation > 24 h | 13 (8.1%) | 26 (17.3%) | 32 (20.1%) | 0.007 |
| Reintubation | 3 (1.9%) | 8 (5.3%) | 8 (5%) | 0.22 |
| LOS | 19 (11.8%) | 26 (17.3%) | 26 (16.4%) | 0.34 |
| IABP | 4 (2.5%) | 9 (6%) | 7 (4.4%) | 0.31 |
| cTnI peak (ng/mL) | 15.9 ± 35.2 | 15.5 ± 19.6 | 19.5 ± 49.5 | 0.56 |
| Dialysis/Ultrafiltration | 0 | 4 (2.7%) | 7 (4.4%) | 0.03 |
| AF onset | 43 (26.7%) | 41 (27.3%) | 41 (25.8%) | 0.95 |
| CVA | 0 | 4 (2.7%) | 4 (2.5%) | 0.11 |
| Sepsis | 0 | 5 (3.3%) | 6 (3.8%) | 0.05 |
| Sternal wound infection | 5 (3.1%) | 7 (4.7%) | 20 (12.6%) | 0.002 |
| Overall infections | 9 (5.6%) | 11 (7.3%) | 30 (18.9%) | 0.0002 |
| Blood loss (ml) | 840.4 ± 432.4 | 745.4 ± 406.7 | 776.2 ± 500.1 | 0.17 |
| Reopening for bleeding | 2 (1.2%) | 1 (0.7%) | 3 (1.9%) | 0.63 |
| Blood Units transfusion (mean value) | 1.5 ± 2.2 | 1.9 ± 2.7 | 2.1 ± 4.4 | 0.18 |
| FFP Units transfusion (mean value) | 0.3 ± 1.4 | 0.3 ± 1.1 | 0.5 ± 2.4 | 0.53 |
| Platelet Units transfusion (mean value) | 0.2 ± 0.9 | 0.2 ± 1.1 | 0.2 ± 1 | 0.97 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Agostino, D.; Cappabianca, G.; Rotunno, C.; Castellaneta, F.; Quagliara, T.; Carrozzo, A.; Mastro, F.; Charitos, I.A.; Beghi, C.; Paparella, D. The Preoperative Inflammatory Status Affects the Clinical Outcome in Cardiac Surgery. Antibiotics 2019, 8, 176. https://doi.org/10.3390/antibiotics8040176
D’Agostino D, Cappabianca G, Rotunno C, Castellaneta F, Quagliara T, Carrozzo A, Mastro F, Charitos IA, Beghi C, Paparella D. The Preoperative Inflammatory Status Affects the Clinical Outcome in Cardiac Surgery. Antibiotics. 2019; 8(4):176. https://doi.org/10.3390/antibiotics8040176
Chicago/Turabian StyleD’Agostino, Donato, Giangiuseppe Cappabianca, Crescenzia Rotunno, Francesca Castellaneta, Teresa Quagliara, Alessandro Carrozzo, Florinda Mastro, Ioannis Alexandros Charitos, Cesare Beghi, and Domenico Paparella. 2019. "The Preoperative Inflammatory Status Affects the Clinical Outcome in Cardiac Surgery" Antibiotics 8, no. 4: 176. https://doi.org/10.3390/antibiotics8040176
APA StyleD’Agostino, D., Cappabianca, G., Rotunno, C., Castellaneta, F., Quagliara, T., Carrozzo, A., Mastro, F., Charitos, I. A., Beghi, C., & Paparella, D. (2019). The Preoperative Inflammatory Status Affects the Clinical Outcome in Cardiac Surgery. Antibiotics, 8(4), 176. https://doi.org/10.3390/antibiotics8040176






